Abstract
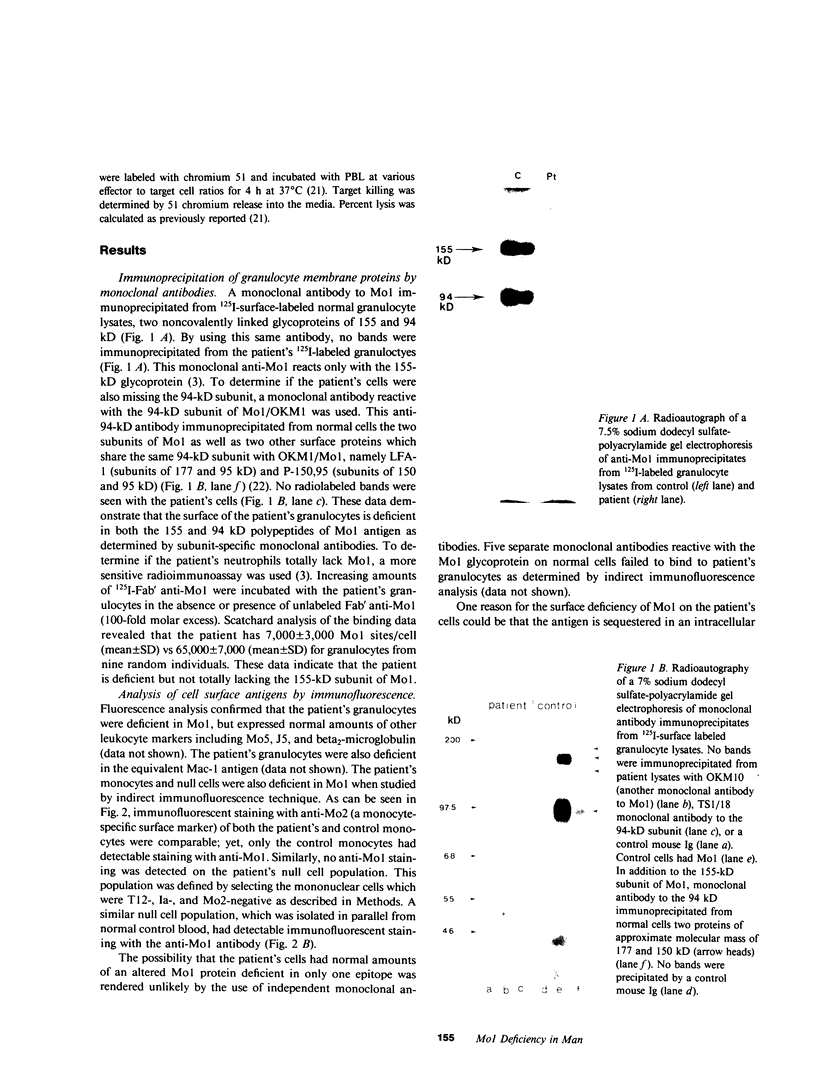
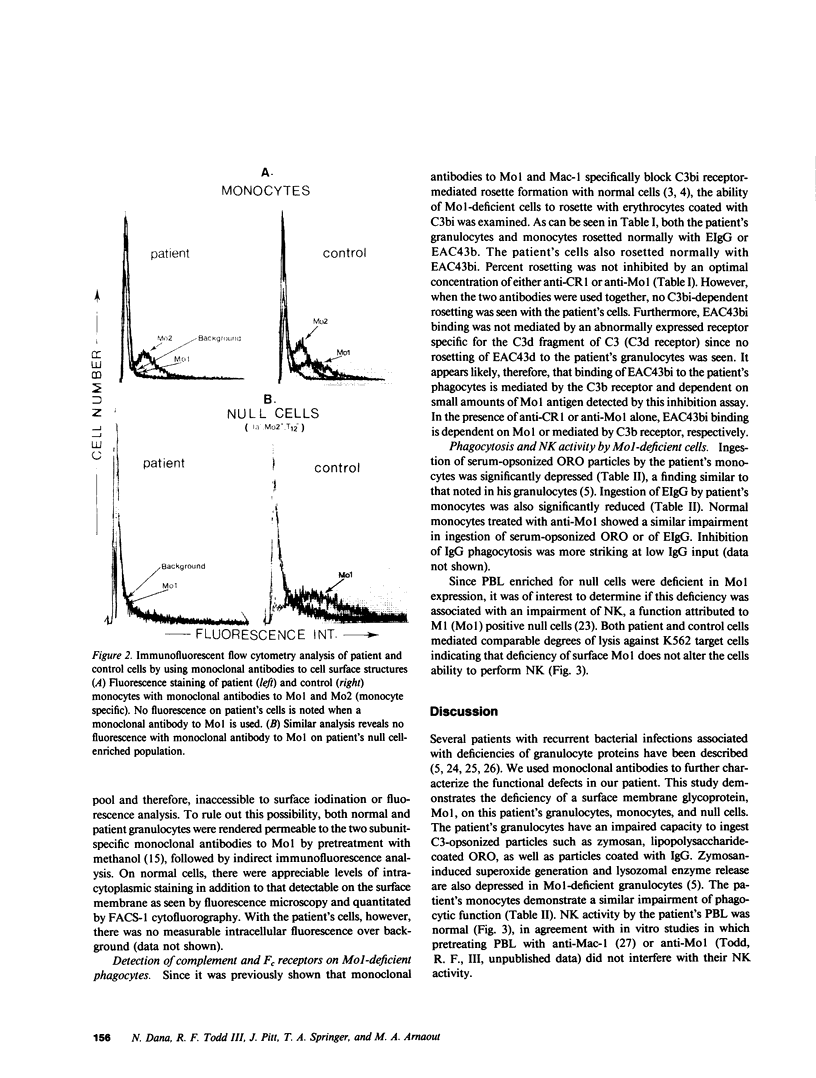
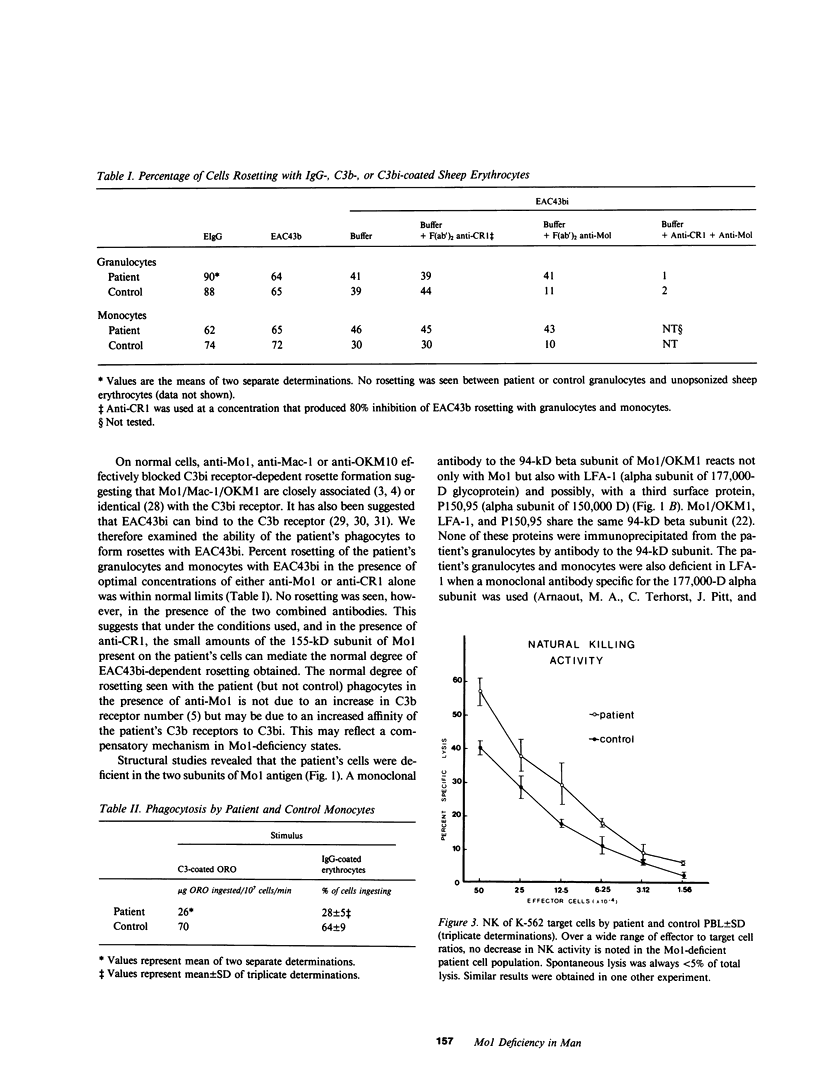
Deficiency of a granulocyte surface glycoprotein of 150,000-D had been associated with defective C3- and IgG-dependent phagocytosis in a patient with recurrent bacterial infections. By using monoclonal antibodies, we found that this patient's granulocytes, monocytes, and null cells were deficient in Mo1 (equivalent to OKM1 and Mac-1), a cell surface molecule consisting of two noncovalently linked glycoproteins of 155,000 and 94,000 D. The 155,000-D subunit is closely associated with the human complement receptor that recognizes C3bi and/or a further degradation product termed C3dg (C3bi receptor); the 94,000-D subunit has been shown to be shared, on normal cells, by two other surface membrane glycoproteins: lymphocyte function-associated antigen-1 (LFA-1) and P-150, 95. Both subunits of Mo1 were deficient on the patient's granulocytes as determined by immunoprecipitation with subunit-specific monoclonal antibodies as well as fluorescence analysis. Mol-deficient monocytes, like granulocytes, had defective C3-and IgG-dependent phagocytosis. Natural killing activity by the patient's peripheral blood leukocytes was normal. Mo1-deficient granulocytes and monocytes rosetted normally with sheep erythrocytes coated with C3bi. This rosetting was totally inhibited by a mixture of anti-Mo1 and anti-C3b (the major fragment of C3) receptor antibodies but not by either antibody alone. Since monoclonal antibodies to the 155,000-D subunit of Mo1 can inhibit C3bi receptor binding, immune phagocytosis, opsonized zymosan-induced degranulation, and superoxide generation by normal phagocytes (functions which are defective in Mo1-deficient cells), it appears likely that Mo1 deficiency may in part underlie the functional aberrations leading to recurrent bacterial infections in man.
Full text
PDF



Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Arnaout M. A., Pitt J., Cohen H. J., Melamed J., Rosen F. S., Colten H. R. Deficiency of a granulocyte-membrane glycoprotein (gp150) in a boy with recurrent bacterial infections. N Engl J Med. 1982 Mar 25;306(12):693–699. doi: 10.1056/NEJM198203253061201. [DOI] [PubMed] [Google Scholar]
- Arnaout M. A., Todd R. F., 3rd, Dana N., Melamed J., Schlossman S. F., Colten H. R. Inhibition of phagocytosis of complement C3- or immunoglobulin G-coated particles and of C3bi binding by monoclonal antibodies to a monocyte-granulocyte membrane glycoprotein (Mol). J Clin Invest. 1983 Jul;72(1):171–179. doi: 10.1172/JCI110955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ault K. A., Springer T. A. Cross-reaction of a rat-anti-mouse phagocyte-specific monoclonal antibody (anti-Mac-1) with human monocytes and natural killer cells. J Immunol. 1981 Jan;126(1):359–364. [PubMed] [Google Scholar]
- Beller D. I., Springer T. A., Schreiber R. D. Anti-Mac-1 selectively inhibits the mouse and human type three complement receptor. J Exp Med. 1982 Oct 1;156(4):1000–1009. doi: 10.1084/jem.156.4.1000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowen T. J., Ochs H. D., Altman L. C., Price T. H., Van Epps D. E., Brautigan D. L., Rosin R. E., Perkins W. D., Babior B. M., Klebanoff S. J. Severe recurrent bacterial infections associated with defective adherence and chemotaxis in two patients with neutrophils deficient in a cell-associated glycoprotein. J Pediatr. 1982 Dec;101(6):932–940. doi: 10.1016/s0022-3476(82)80013-9. [DOI] [PubMed] [Google Scholar]
- Breard J., Reinherz E. L., O'Brien C., Schlossman S. F. Delineation of an effector population responsible for natural killing and antibody-dependent cellular cytotoxicity in man. Clin Immunol Immunopathol. 1981 Jan;18(1):145–150. doi: 10.1016/0090-1229(81)90018-0. [DOI] [PubMed] [Google Scholar]
- Böyum A. Isolation of mononuclear cells and granulocytes from human blood. Isolation of monuclear cells by one centrifugation, and of granulocytes by combining centrifugation and sedimentation at 1 g. Scand J Clin Lab Invest Suppl. 1968;97:77–89. [PubMed] [Google Scholar]
- Crossley L. G. C3b inactivator and beta 1H. Methods Enzymol. 1981;80(Pt 100):112–124. [PubMed] [Google Scholar]
- Crowley C. A., Curnutte J. T., Rosin R. E., André-Schwartz J., Gallin J. I., Klempner M., Snyderman R., Southwick F. S., Stossel T. P., Babior B. M. An inherited abnormality of neutrophil adhesion. Its genetic transmission and its association with a missing protein. N Engl J Med. 1980 May 22;302(21):1163–1168. doi: 10.1056/NEJM198005223022102. [DOI] [PubMed] [Google Scholar]
- Fearon D. T. Identification of the membrane glycoprotein that is the C3b receptor of the human erythrocyte, polymorphonuclear leukocyte, B lymphocyte, and monocyte. J Exp Med. 1980 Jul 1;152(1):20–30. doi: 10.1084/jem.152.1.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gahmberg C. G., Andersson L. C., Ruutu P., Timonen T. T., Hänninen A., Vuopio P., de la Chapelle A. Decrease of the major high molecular weight surface glycoprotein of human granulocytes in monosomy-7 associated with defective chemotaxis. Blood. 1979 Aug;54(2):401–406. [PubMed] [Google Scholar]
- Gerdes J., Stein H. Physicochemical characterization of C3b receptors isolated from human erythrocytes by immunoprecipitation. Immunology. 1980 Dec;41(4):929–936. [PMC free article] [PubMed] [Google Scholar]
- Laemmli U. K. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature. 1970 Aug 15;227(5259):680–685. doi: 10.1038/227680a0. [DOI] [PubMed] [Google Scholar]
- Medicus R. G., Melamed J., Arnaout M. A. Role of human factor I and C3b receptor in the cleavage of surface-bound C3bi molecules. Eur J Immunol. 1983 Jun;13(6):465–470. doi: 10.1002/eji.1830130607. [DOI] [PubMed] [Google Scholar]
- Medof M. E., Iida K., Mold C., Nussenzweig V. Unique role of the complement receptor CR1 in the degradation of C3b associated with immune complexes. J Exp Med. 1982 Dec 1;156(6):1739–1754. doi: 10.1084/jem.156.6.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Musson R. A., Becker E. L. The inhibitory effect of chemotactic factors on erythrophagocytosis by human neutrophils. J Immunol. 1976 Aug;117(2):433–439. [PubMed] [Google Scholar]
- Nadler L. M., Stashenko P., Hardy R., Pesando J. M., Yunis E. J., Schlossman S. F. Monoclonal antibodies defining serologically distinct HLA-D/DR related Ia-like antigens in man. Hum Immunol. 1981 Feb;2(1):77–90. doi: 10.1016/0198-8859(81)90009-4. [DOI] [PubMed] [Google Scholar]
- Reinherz E. L., Meuer S., Fitzgerald K. A., Hussey R. E., Levine H., Schlossman S. F. Antigen recognition by human T lymphocytes is linked to surface expression of the T3 molecular complex. Cell. 1982 Oct;30(3):735–743. doi: 10.1016/0092-8674(82)90278-1. [DOI] [PubMed] [Google Scholar]
- Ross G. D., Newman S. L., Lambris J. D., Devery-Pocius J. E., Cain J. A., Lachmann P. J. Generation of three different fragments of bound C3 with purified factor I or serum. II. Location of binding sites in the C3 fragments for factors B and H, complement receptors, and bovine conglutinin. J Exp Med. 1983 Aug 1;158(2):334–352. doi: 10.1084/jem.158.2.334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Krensky A. M., Ware C. F., Robbins E., Strominger J. L., Burakoff S. J., Springer T. A. Three distinct antigens associated with human T-lymphocyte-mediated cytolysis: LFA-1, LFA-2, and LFA-3. Proc Natl Acad Sci U S A. 1982 Dec;79(23):7489–7493. doi: 10.1073/pnas.79.23.7489. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchez-Madrid F., Nagy J. A., Robbins E., Simon P., Springer T. A. A human leukocyte differentiation antigen family with distinct alpha-subunits and a common beta-subunit: the lymphocyte function-associated antigen (LFA-1), the C3bi complement receptor (OKM1/Mac-1), and the p150,95 molecule. J Exp Med. 1983 Dec 1;158(6):1785–1803. doi: 10.1084/jem.158.6.1785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer T., Galfré G., Secher D. S., Milstein C. Mac-1: a macrophage differentiation antigen identified by monoclonal antibody. Eur J Immunol. 1979 Apr;9(4):301–306. doi: 10.1002/eji.1830090410. [DOI] [PubMed] [Google Scholar]
- Stossel T. P., Alper C. A., Rosen F. S. Serum-dependent phagocytosis of paraffin oil emulsified with bacterial lipopolysaccharide. J Exp Med. 1973 Mar 1;137(3):690–705. doi: 10.1084/jem.137.3.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Summerhayes I. C., Cheng Y. S., Sun T. T., Chen L. B. Expression of keratin and vimentin intermediate filaments in rabbit bladder epithelial cells at different stages of benzo[a]pyrene-induced neoplastic progression. J Cell Biol. 1981 Jul;90(1):63–69. doi: 10.1083/jcb.90.1.63. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Leeman E. L., Daley J. F., Schlossman S. F. Mo2: functional properties of antigen-bearing cells. Clin Immunol Immunopathol. 1983 Jan;26(1):118–125. doi: 10.1016/0090-1229(83)90180-0. [DOI] [PubMed] [Google Scholar]
- Todd R. F., 3rd, Nadler L. M., Schlossman S. F. Antigens on human monocytes identified by monoclonal antibodies. J Immunol. 1981 Apr;126(4):1435–1442. [PubMed] [Google Scholar]
- Todd R. F., 3rd, Van Agthoven A., Schlossman S. F., Terhorst C. Structural analysis of differentiation antigens Mo1 and Mo2 on human monocytes. Hybridoma. 1982;1(3):329–337. doi: 10.1089/hyb.1.1982.1.329. [DOI] [PubMed] [Google Scholar]
- Wright S. D., Rao P. E., Van Voorhis W. C., Craigmyle L. S., Iida K., Talle M. A., Westberg E. F., Goldstein G., Silverstein S. C. Identification of the C3bi receptor of human monocytes and macrophages by using monoclonal antibodies. Proc Natl Acad Sci U S A. 1983 Sep;80(18):5699–5703. doi: 10.1073/pnas.80.18.5699. [DOI] [PMC free article] [PubMed] [Google Scholar]