Abstract
Multipotent mesenchymal stromal cells (MSC) are receiving increased attention for their non-progenitor immunomodulatory potential. Cryopreservation is commonly used for long-term storage of MSC. Post-thaw MSC proliferation is associated with a lag-phase in vitro. How this lag-phase affect MSC immunomodulatory properties is unknown. We hypothesized that in vitro there is no difference in lymphocyte suppression potential between quick-thawed cryopreserved equine cord blood (CB) MSC immediately included in mixed lymphocyte reaction (MLR) and same MSC allowed post-thaw culture time prior to inclusion in MLR. Cryopreserved CB-MSC from five unrelated foals were compared using two-way MLR. For each of the five unrelated MSC cultures, paired MLR assays of MSC allowed five days of post-thaw culture and MSC included in MLR assay immediately post-thawing were evaluated. We report no difference in the suppression of lymphocyte proliferation by CB-MSC that had undergone post-thaw culture and MSC not cultured post-thaw (p<0.0001). Also, there was no inter-donor variability between the lymphocyte suppressive properties of MSC harvested from the five different donors (p = 0.13). These findings suggest that cryopreserved CB-MSC may have clinical utility immediately upon thawing. One implication hereof is the possibility of using cryopreserved CB-MSC at third party locations without the need for cell culture equipment or competencies.
Introduction
Multipotent mesenchymal stromal cells (MSC) are receiving significant attention as a treatment option for various conditions. Currently MSC are thought to have either progenitor or non-progenitor cellular functions. Although studied extensively, progenitor cell integration into recipient tissues has only been observed in very small numbers challenging the importance of the MSC progenitor paradigm [1]–[3]. Di Nicola et al [4] observed MSC-mediated lymphocyte suppression occurred in a MSC-dose dependent, time independent, and reversible manner that did not require cell-to-cell contact in vitro. These findings resulted in a paradigm shift—from progenitor to non-progenitor functions—as a main mechanism by which undifferentiated MSC exerts therapeutic effect. Non-progenitor MSC actions that have been investigated include cell-to-cell fusion [5]–[7], organelle transfer [8],[9], reactive oxygen scavenge [10]–[13], and suppression of lymphocyte proliferation [4],[14],[15].
MSC-mediated lymphocyte suppression has been observed in equine in vitro studies. Expected equine lymphocyte proliferation following stimulation with either allogeneic lymphocytes or plant-based mitogens was suppressed by equine MSC derived from bone marrow, adipose tissue and umbilical cord blood (CB) in vitro [16]. Work in our lab confirmed the lymphocyte suppressive properties of equine CB-MSC [17]. One potential advantage of CB-MSC compared to other MSC sources is that they can be isolated and characterized prior to the donor sustaining and injury in case of autologous use. Allogeneic use of equine CB-MSC have also recently been reported in clinical cases without observed adverse reactions [18]. These reports suggest that allogeneic CB-MSC may have clinical utility as immune-modulatory agents.
Equine MSC lymphocyte suppression studies to-date have explored MSC that were maintained in culture prior to inclusion in so-called two-way mixed lymphocyte reactions (MLR) in vitro. Cryopreserved and passaged umbilical cord derived endothelial cells exhibit a proliferative lag-phase upon thawing and sub-culturing [19]. The lag phase however, was observed to be 36h longer for cryopreserved endothelial cells than non-cryopreserved cells. No difference was noted between fresh and cryopreserved/thawed human umbilical vein endothelial cells with regard to anti-inflammatory and anti-coagulant activity in vitro. The thawed cells, however, were allowed to overcome their proliferative lag phase following cryopreservation prior to inclusion in these in vitro assays [19]. Whether cryopreserved/thawed MSC exhibit an equivalent ‘functional lag-phase’ with regard to lymphocyte suppression is undetermined.
We hypothesized that in vitro there is no difference in lymphocyte suppression potential between post-thaw non-cultured (PTNC) equine CB-MSC immediately included in mixed lymphocyte reaction (MLR) and same MSC allowed post-thaw culture (PTC) prior to inclusion in MLR.
Materials and Methods
Ethics statement
This study was specifically approved by the University of Guelph Animal Care Committee with regard to the procedures of collection of equine peripheral blood lymphocytes and equine umbilical cord blood (animal use protocols 1756 and 1570). Additional research conducted using specimens of this kind does not require review by the Animal Care Committee (falls under CCAC Category of Invasiveness A) and therefore the mixed lymphocyte reactions can be considered to have been conducted in accordance with the institutional ethics guidelines. Collection of peripheral blood and cord blood was add-on procedures to the routine care of the horses. No animals were sacrificed during the study. Equine umbilical cord blood was collected on two privately owned commercial farms in Southern Ontario. Four of five samples were collected on one farm from Thoroughbred foals. One sample was collected on another farm from a Warmblood foal. Informed consent was obtained in writing from the horse owners/agents prior to sampling. The broodmares on the foaling farms are housed in large foaling boxes. Both farms are staffed 24/7 and mares are under constant video surveillance and carrying foaling alarms to allow for observed foaling and assisted delivery if needed. Umbilical cord blood was collected by the farm staff after receiving instruction by Dr. Koch. Instruction included video-review of cord blood collection. Cord blood was collected from an isolated segment of the umbilical cord after the umbilical cord had been clamped and detached from the foal. Peripheral venous blood was obtained from the Equine Research Herd owned by the Ontario Ministry of Agriculture and Food (OMAF). Once investigators have an approved animal care protocol from the University of Guelph Animal Care Committee access to these research horses are granted. In this study peripheral blood was collected from 5 adult mixed-bred horses. The adult horses on the research farm are housed in smaller groups with run-in sheds throughout the year. The horses are on pasture during the summer and during the winter they have access to large paddocks with gravel surface. Peripheral blood was collected by Drs. Williams or Koch. Collection of peripheral blood was collected under mild sedation (Xylazine HCl, 0.35–0.40 mg/kg bwt IV; Bayer, Toronto, ON) from the jugular vein following which manual pressure was applied for several minutes to aid hemostasis.
Lymphocyte collection
Equine peripheral blood lymphocytes (PBL) were isolated from equine whole blood obtained from five unrelated adult horses. 450 mL of whole blood was collected into a commercially available blood collection bag containing anticoagulant (Na citrate) and processed within 2 hours of collection by Ficoll (GE Healthcare, Mississauga, ON) density gradient separation. 35 mL of whole blood was layered over 15 mL of Ficoll-Hypaque- plus (density 1.077 g/L) within a 50 mL conical tube. Samples were centrifuged at 500 xg for 30 minutes at room temperature (RT) with no brake. The interphase was collected and washed twice with phosphate buffered saline (PBS) following centrifugation at 500 xg for 10 minutes at RT. Washed PBL were suspended in 10 mL of MLR culture medium (MLR-CM) which consisted of Roswell Park Memorial Institute (RPMI 1640, Invitrogen, Burlington, ON) culture medium, 10% heat inactivated horse serum (Invitrogen, Burlington, ON), 1% penicillin/streptomycin (Invitrogen, Burlington, ON), and 1% L-glutamine (Lonza/Cambrex, Walkersville MD). Cells were counted using an automated fluorescent based cell counter (Nucleocounter NC-100, Mandel Scientific Company, Guelph, ON), re-suspended freshly prepared cryomedium (10% DMSO in MLR-CM) and frozen in 1.8 mL cryovials at a concentration of 6×106 cells/mL. Cells were slowly frozen at a rate of −1°C/min to −80°C (Mr. Frosty, Nalgene, Mississauga, ON) before transfer for long-term storage in liquid nitrogen. At the time of use, the PBL were thawed in a 37°C water-bath, followed by centrifugation at 500 xg for 5 minutes at RT. The PBL were suspended in 5 ml MLR-CM, counted and adjusted to a concentration of 2×106 PBL/mL.
MSC collection, culture, and cryopreservation
Cryopreserved CB-MSC cultures from five unrelated foals (N = 5) were included. The CB-MSC cultures were established as previously described [20],[21]. These primary cell cultures were cryopreserved at passages ranging from P1–P3 and preserved at a concentration of 1×106/mL. Same-batch MSC vials from each of the five foals were designated to one of two treatment groups—PTC and PTNC.
MSC-PTC
Five days prior to MLR set-up, one CB-MSC cryovial from each foal was thawed in a 37°C water bath, slowly diluted into 5 mL of MSC culture medium (MSC-CM) consisting of Dulbecco's modified eagle medium containing 30% fetal bovine serum, 1% penicillin/streptomycin, and 1% L-glutamine. MSC suspensions were centrifuged at 300 xg for 5 minutes, supernatant removed and suspended in MSC-CM. An automated cell count was performed using an automated fluorescent based cell counter (Nucleocounter NC-100, Mandel Scientific Company, Guelph ON). Thawed MSC were seeded in polystyrene culture flasks at a density of 5,000 MSC/cm2 and incubated at 38°C, 5% CO2, in a humidified atmosphere for 5 days. MSC-CM was changed on day two and four of the incubation period. On the fifth day MSC were detached from the culture flasks using trypsin-EDTA and suspended in MSC-CM, and counted. Following centrifugation the MSC were suspended in MLR-CM at a concentration of 2×105/mL.
MSC-PTNC
On day five, the day of MLR set-up, the second same-batch cryovial from each foal was thawed. The MSC were suspended in MSC-CM as described above. MSC were counted and suspended in MLR-CM at a concentration of 2×105/mL.
MLR
Cryopreserved PBL were thawed in a 37°C water bath, slowly diluted into 5 mL of MLR culture media (MLR-CM). PBL suspensions were centrifuged at 300 xg for 5 minutes, the supernatant was removed, and cell pellet was suspended in MSC-CM. An automated cell count was performed using an automated fluorescent-based cell counter (Nucleocounter NC-100, Mandel Scientific Company, Guelph ON). PBL were subdivided into tubes for use as stimulator PBL and responder PBL, respectively. Stimulator PBL and CB-MSC were mitotically inactivated with 20Gy γ-radiation (Theratron 780C Cobalt 60, MDS Nordion, Ottawa ON). Irradiated stimulator PBL (PBLx) from all five horses were pooled in equal proportions at a concentration of 2×105 PBLx/mL.
Non-irradiated responder PBL (PBL), PBLx, and irradiated MSC (MSCx) were combined in a 10∶1∶1 ratio, respectively, in 96 well round bottom plates and cultured five days at 38°C, 5% CO2, in a humidified atmosphere. Lymphocyte proliferation was determined by bromodeoxyuridine (Brd-U) assay, see below for details. A series of controls were prepared on each plate including: negative control (PBL + autologous PBLx), positive control (PBL + allogeneic pool of PBLx), Brd-U staining controls (PBL + FITC or 7-AAD in the absence of Brd-U), and unstained (PBL only). On the fifth day of MLR culture Brd-U was added to each well and cultured for an additional 24 hours before fixation and staining for flow cytometry.
MLR reactions were performed in triplicate reactions using responder PBL from three different horses; resulting in nine replicates of each reaction. Identical experiments were setup using MSC that had received 5 days of PTC or PTNC MSC. These paired MLR were cultured, processed, and evaluated simultaneously.
Outcome assessment
On day 6 of MLR culture, cells were fixed and stained for Brd-U flow cytometry using a commercially available kit (BD Biosciences, Mississauga ON,) according to the manufacturers directions. In brief, MLR-CM was removed, cells were washed, fixed, and stained using FITC anti-BrdU antibody (to detect proliferative cells) and 7-AAD antibody (viability stain). After 24 hours, cells were analyzed by flow cytometry. The gate to identify resting and stimulated lymphocytes was maintained consistent throughout the experiments.
Statistical Analysis
Raw data was imported into a statistical analysis software package (SAS, SAS institute, Cary, NC). A general linear model was used to analyze the effect of treatment (MSC cell line, positive/negative controls), lymphocyte donor, and post thaw culture period using the PROC MIXED function. All two and three way interactions were initially evaluated and non-significant effects and interactions were removed from the model. Residual analysis was performed in order to determine if ANOVA assumptions were met, to detect potential outliers, and evaluate the need for data transformation. The residuals were formally tested for normality using the four tests offered by SAS (Shapiro-Wilk, Kolmogorov-Smirnov, Cramer-von Mises, Anderson-Darling) and plotted against the predicted values and variables used in the model. For the purpose of determining statistical significance α was set at 0.05.
Results
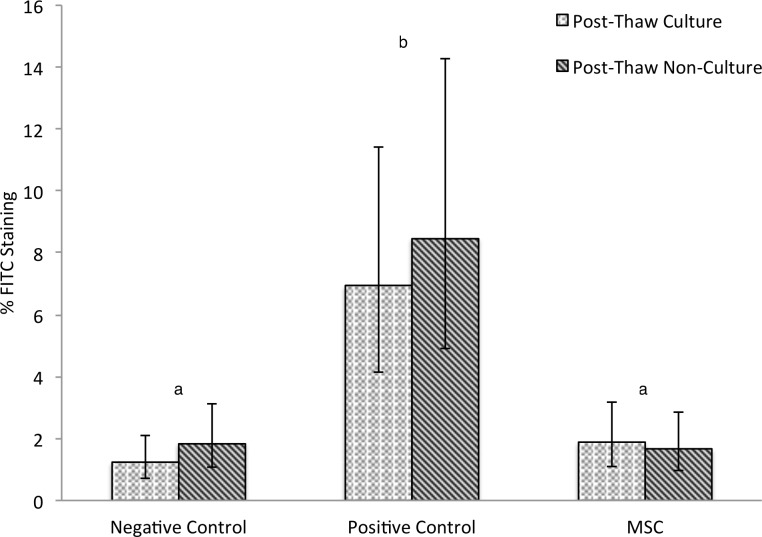
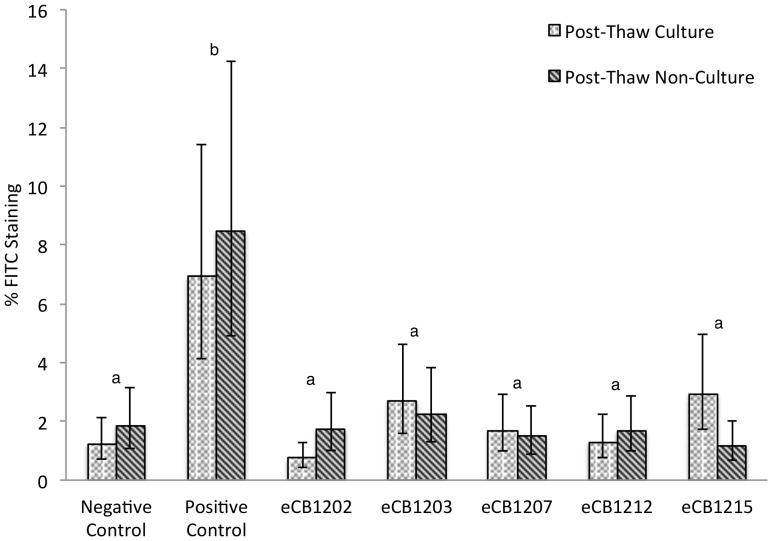
CB-MSC suppressed lymphocyte proliferation compared to the positive control, Figure 1 (p<0.0001). No difference was observed between CB-MSC cultures or between CB-MSC cultures and the negative control (p = 0.13). All MLR that included CB-MSC, no matter the post thaw status, proliferated significantly less than their associated positive control, Figures 2 and 3. Positive controls were always significantly different than negative controls (p<0.0001). The odds ratio of the positive control staining for Brd-U was 4.99 (95% CI 3.21-7.76) times the odds of any other treatment staining Brd-U positive.
Figure 1. Mean FITC anti-BrdU staining following two-way MLR.
Lymphocyte proliferation of un-stimulated (negative control) and allogeneic stimulated (positive control) compared to stimulated lymphocytes treated with MSC. Three biological replicates and three technical replicates were used in the negative control, positive control, and for evaluation of each of the five MSC cultures. Five thousand lymphocytes in each sample were observed and designated either FITC positive (proliferative in the previous 24 h) or FITC negative (not proliferative) Umbilical cord blood MSC from five cultures were either added after a 5-day post-thaw-culture period or identical MSC vials were thawed immediately prior to the experiment. No difference was observed between post-thaw-culture and post-thaw-non-culture groups. Different letters indicate statistically significant differences between means (α = 0.05). Error bars represent the 95% confidence interval.
Figure 2. FITC anti-BrdU staining of five different CB-MSC cultures.
Cryopreserved CB-MSC were either cultured five days or thawed immediately prior to MLR set-up. Each bar represents the mean percentage of FITC positive cells in nine replicates (triplicate wells for each of the three unrelated responder lymphocyte donors, 5000 lymphocytes evaluated in each sample). In no instance were significant differences observed between post-thaw-culture and post-thaw-non-culture groups. Different letters indicate statistically significant differences between means (α = 0.05). Error bars represent the 95% confidence interval.
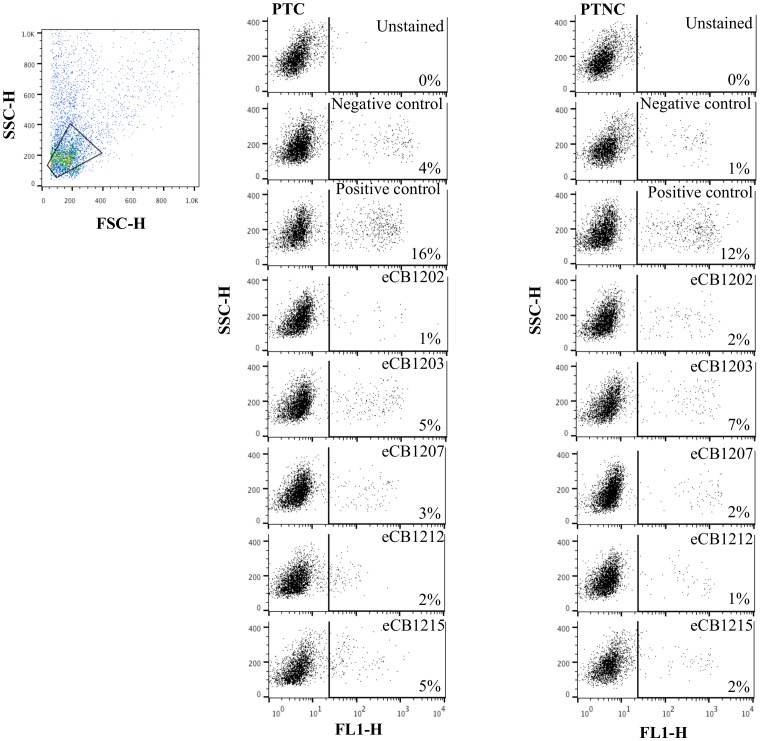
Figure 3. Cell proliferation during 2-way MLR was detected with a FITC-labelled antibody to BrdU.
Dot plots represent fluorescence of lymphocytes identified by forward and side scatter after co-culture with post-thaw-cultured (PTC) and post-thaw-non-cultured (PTNC) CB-MSC. The percentage fluorescence (right quadrant) indicates the proportion of lymphocytes proliferating in autologous (negative control), allogeneic (positive control) and allogeneic plus CB-MSC reactions. Differences in lymphocyte proliferation induced by PTC and PTNC CB-MSC were not significant.
Discussion
Cryopreserved equine CB-MSC appear to constitutively suppress lymphocyte proliferation in vitro independent of post-thaw culture period. The noted homogeneity in the lymphocyte suppressive effects among CB-MSC suggests that although minor inter-donor variation did exist, screening of individual CB-MSC cultures prior to in vivo therapy may not be warranted.
Clinical use of cryopreserved CB-MSC immediately upon thawing would allow the attending veterinarian to determine treatment time independent of MSC procurement. Following injury, time is a critical factor as inflammatory mediators and cells quickly localize to the area of injury facilitating an inflammatory response [22],[23]. Despite the importance of inflammatory events in tissue healing, acute excessive inflammatory events or chronic inflammation is often associated with impeded tissue healing or further tissue damage [22],[24],[25]. This occurs as a result of activation of the host immune responses including elevated cytokine levels, reactive oxygen species, the release of host matrix metalloproteinases, and other collagenolytic enzymes [24].
Development of an “off the shelf” cryopreserved CB-MSC therapy appears feasible using an allogeneic strategy. Although lameness was not associated with IA MSC injection into healthy joints, mild inflammatory responses to IA injection of autologous and allogeneic MSC has been reported in the horse [3],[26]. Equine allogeneic MSC from CB and adipose tissue were not associated with adverse reactions when used as treatment for tendonitis in two small case series [18],[27]. The possible immune-privileged nature or immune evasive properties of MSC indicates potential for immediate MSC treatment using a cryopreserved allogeneic MSC product.
One caveat of the study is that PTNC CB-MSC were added into the reaction wells five days before evaluation of lymphocyte proliferation. We can therefore only conclude that any recovery period required by MSC prior to being lymphocyte suppressive is less than five days. In the initial report of lymphocyte suppression in MLR [4], the effect of human bone marrow-derived MSC was reported to occur independent of the time that MSC were added. Work with cryopreserved human MSC suggests recovery of normal cellular function occurs relatively quickly post-thaw [28],[29]. These studies investigated the effect of cold and osmotic shock associated with cryopreservation on human BM-MSC and concluded that the metabolic disturbances observed in thawed MSC ceased to exist after 24 h [28] and could be minimized using proper technique [29]. We speculate that the recovery period is short, and clinically irrelevant, as no difference in lymphocyte proliferation was observed. This notion is supported by the mean response we observed within CB-MSC cultures. In three of the five CB-MSC cultures evaluated, lymphocyte proliferation was less in the wells treated with CB-MSC that were from the PTNC group. In fact, of the two CB-MSC cultures where trends towards significant differences were observed within a sample pair, the sample with the lower mean had been treated with CB-MSC from the PTNC group.
In conclusion, our findings suggest that cryopreserved equine CB-MSC may have clinical utility immediately upon thawing. One implication hereof is the possibility of using cryopreserved CB-MSC at third party locations without the need for cell culture equipment or competencies.
Acknowledgments
We would like to thank Mr. William Sears for his expertise in performing the statistical analysis and Ms. Kim Stewart for her help with irradiation of cells for the MLR assays.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.
Funding Statement
These studies were supported by The Danish Council for Independent Research - Council for Technology and Production Sciences (TGK grant #902539; http://ufm.dk/en/research-and-innovation/funding-programmes-for-research-and-innovation/find-danish-funding-programmes/dff-the-danish-council-for-independent-research) and the Equine Guelph Research Foundation (TGK grant #EG 2012 12; http://www.equineguelph.ca/research/index.php). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Sutton EJ, Boddington SE, Nedopil AJ, Henning TD, Demos SG, et al. (2009) An optical imaging method to monitor stem cell migration in a model of immune-mediated arthritis. Opt Express 17:24403–24413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Toupet K, Maumus M, Peyrafitte JA, Bourin P, van Lent PL, et al. (2013) Long-term detection of human adipose-derived mesenchymal stem cells after intraarticular injection in SCID mice. Arthritis Rheum 65:1786–1794. [DOI] [PubMed] [Google Scholar]
- 3. Pigott JH, Ishihara A, Wellman ML, Russell DS, Bertone AL (2013) Investigation of the immune response to autologous, allogeneic, and xenogeneic mesenchymal stem cells after intra-articular injection in horses. Vet Immunol Immunopathol 156:99–106. [DOI] [PubMed] [Google Scholar]
- 4. Di Nicola M, Carlo-Stella C, Magni M, Milanesi M, Longoni PD, et al. (2002) Human bone marrow stromal cells suppress T-lymphocyte proliferation induced by cellular or nonspecific mitogenic stimuli. Blood 99:3838–3843. [DOI] [PubMed] [Google Scholar]
- 5. Leri A, Kajstura J, Anversa P (2005) Cardiac stem cells and mechanisms of myocardial regeneration. Physiol Rev 85:1373–1416. [DOI] [PubMed] [Google Scholar]
- 6. Terada N, Hamazaki T, Oka M, Hoki M, Mastalerz DM, et al. (2002) Bone marrow cells adopt the phenotype of other cells by spontaneous cell fusion. Nature 416:542–545. [DOI] [PubMed] [Google Scholar]
- 7. Ying QL, Nichols J, Evans EP, Smith AG (2002) Changing potency by spontaneous fusion. Nature 416:545–548. [DOI] [PubMed] [Google Scholar]
- 8. Gallagher KL, Benfey PN (2005) Not just another hole in the wall: Understanding intercellular protein trafficking. Genes Dev 19:189–195. [DOI] [PubMed] [Google Scholar]
- 9. Rustom A, Saffrich R, Markovic I, Walther P, Gerdes HH (2004) Nanotubular highways for intercellular organelle transport. Science 303:1007–1010. [DOI] [PubMed] [Google Scholar]
- 10. Park JE, Seo YK, Yoon HH, Kim CW, Park JK, et al. (2013) Electromagnetic fields induce neural differentiation of human bone marrow derived mesenchymal stem cells via ROS mediated EGFR activation. Neurochem Int 62:418–424. [DOI] [PubMed] [Google Scholar]
- 11.Torrente D, Avila M, Cabezas R, Morales L, Gonzalez J, et al. (2013) Paracrine factors of human mesenchymal stem cells increase wound closure and reduce reactive oxygen species production in a traumatic brain injury in vitro model. Hum Exp Toxicol doi:0960327113509659 [DOI] [PubMed]
- 12. Nyamandi VZ, Johnsen VL, Hughey CC, Hittel DS, Kahn A, et al. (2014) Enhanced stem cell engraftment and modulation of hepatic reactive oxygen species production in diet-induced obesity. Obesity (Silver Spring) 22:721–9. [DOI] [PubMed] [Google Scholar]
- 13.Quintanilha LF, Takami T, Hirose Y, Fujisawa K, Murata Y, et al. (2013) Canine mesenchymal stem cells show antioxidant properties against thioacetamide-induced liver injury in vitro and in vivo. Hepatol Res 2013 doi:10.1111/hepr.12204 [DOI] [PubMed]
- 14. Keating A (2008) How do mesenchymal stromal cells suppress T cells? Cell Stem Cell 2:106–108. [DOI] [PubMed] [Google Scholar]
- 15. Zhou Y, Day A, Haykal S, Keating A, Waddell TK (2013) Mesenchymal stromal cells augment CD4+ and CD8+ T-cell proliferation through a CCL2 pathway. Cytotherapy 15:1195–1207. [DOI] [PubMed] [Google Scholar]
- 16. Carrade DD, Lame MW, Kent MS, Clark KC, Walker NJ, et al. (2012) Comparative analysis of the immunomodulatory properties of equine adult-derived mesenchymal stem cells. Cell Med 4:1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Tessier L (2013) Isolation, immunophenotyping and lymphocyte suppressive properties of equine cord blood-derived mesenchymal stromal cells. MSc thesis. University of Guelph. Available at https://atrium.lib.uoguelph.ca/xmlui/handle/10214/7507 accessed February 2014. [Google Scholar]
- 18. Kang JG, Park SB, Seo MS, KIM HS, Chae JS, et al. (2013) Characterization and clinical application of mesenchymal stem cells from equine umbilical cord blood. J Vet Sci 14:367–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Lehle K, Hoenicka M, Jacobs VR, Schmid FX, Birnbaum DE (2005) Cryopreservation of human endothelial cells for vascular tissue engineering. Cryobiology 50:154–161. [DOI] [PubMed] [Google Scholar]
- 20. Koch TGT, Heerkens TT, Thomsen PDP, Betts DH (2007) Isolation of mesenchymal stem cells from equine umbilical cord blood. BMC biotechnol 7:26. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Koch TG, Thomsen PD, Betts DH (2009) Improved isolation protocol for equine cord blood-derived mesenchymal stromal cells. Cytotherapy 11:443–447. [DOI] [PubMed] [Google Scholar]
- 22.Frisbie D (2012) Synovial joint biology and pathobiology. In: Auer JS, Stick JSeditors. Equine surgery. 4th ed. St. Louis: Saunders Elsevier; pp.1096–1114. [Google Scholar]
- 23.Osteoarthritis CJ (2003) In: Ross M, Dyson Seditors. In: Diagnosis and management of lameness in the horse. St. Louis: Saunders; pp.572–591. [Google Scholar]
- 24. Shirtliff ME, Mader JT (2002) Acute septic arthritis. Clin Microbiol Rev 15:527–544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Roy S, Bhawan J (1975) Ultrastructure of articular cartilage in pyogenic arthritis. Arch Pathol 99:44–47. [PubMed] [Google Scholar]
- 26. Carrade DD, Owens SD, Galuppo LD, Vidal MA, Ferraro GL, et al. (2011) Clinicopathologic findings following intra-articular injection of autologous and allogeneic placentally derived equine mesenchymal stem cells in horses. Cytotherapy 13:419–430. [DOI] [PubMed] [Google Scholar]
- 27. Ricco S, Renzi S, Del Bue M, Conti V, Merli E, et al. (2013) Allogeneic adipose tissue-derived mesenchymal stem cells in combination with platelet rich plasma are safe and effective in the therapy of superficial digital flexor tendonitis in the horse. Int J Immunopathol Pharmacol 26S:61–68. [DOI] [PubMed] [Google Scholar]
- 28. Francois M, Copland IB, Yuan S, Romieu-Mourez R, Waller EK, et al. (2012) Cryopreserved mesenchymal stromal cells display impaired immunosuppressive properties as a result of heat-shock response and impaired interferon-gamma licensing. Cytotherapy 14:147–152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Xu X, Liu Y, Cui Z, Wei Y, Zhang L (2012) Effects of osmotic and cold shock on adherent human mesenchymal stem cells during cryopreservation. J Biotechnol 162:224–231. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper.