Abstract
Background and Objectives:
Variety of pathological factors including viral hepatitis, alcohol and drug abuse, metabolic diseases, autoimmune diseases and congenital abnormalities can cause hepatic injury. Liver transplantation is the treatment of choice for end-stage liver diseases, however, it faces several difficulties. So the aim of the work is to evaluate the effect of bone marrow derived mesenchymal stem cells (BM-MSCs) on the liver structure in carbon tetra chloride CCL4 induced liver fibrosis in rats.
Materials and Results:
BM-MSCs were isolated and characterized from long bones of twenty male albino rats. Sixty female rats were divided into the following two groups: Group I; thirty rats which were the control group. Group II; thirty rats were injected intra-peritoneal (IP) by CCL4 twice weekly for four weeks and was further subdivided into the following three subgroups: Subgroup IIA (CCL4 alone); included ten rats which were sacrificed after this four weeks. Subgroup IIB (CCL4/MSCs); included ten rats which were IP injected by a single dose of BM-MSCs and were sacrificed after four weeks. Subgroup IIC (CCL4/recovery); included ten rats which were left for another four weeks without any intervention. Histological examination of liver specimens showed that CCl4 caused variable pathological changes with elevated liver enzymes. Injection of BM-MSCs revealed an improvement in the histological picture of the liver and its enzymatic profile. On the other hand, most of the pathological lesion were still detected in rats of recovery group.
Conclusions:
BM-MSC could restore the liver structure and function in experimental model of liver fibrosis.
Keywords: Bone Marrow, Liver, Fibrosis, CCL4, Histopathology, MSCs
Introduction
The liver is a vital organ that plays a key role in the detoxification of exogenous and endogenous substances. It also performs a wide range of metabolic activities required for homeostasis, nutrition and immune defense (1). A variety of pathological factors including viral hepatitis (especially hepatitis B and C), alcohol and drug abuse, metabolic diseases, autoimmune diseases and congenital abnormalities can cause hepatic injury. Lifestyle changes, mainly exercise withdrawal and weight gain, have probably raised the prevalence of non- alcoholic fatty liver diseases, which is one of the causes of chronic liver diseases (2). Liver fibrosis is the final stage of all chronic hepatic disease. It is a well-known fact that fibrosis has lots of important complications such as portal hypertension, hepatocellular carcinoma, hepatic encephalopathy, spontaneous bacterial peritonitis and hepato-renal syndrome (3). Liver transplantation is the treatment of choice for end-stage liver diseases, however, it faces several difficulties, including donor shortage, surgery-related complications, immunological rejection and high medical cost. Cell transplantation therapy would be the minimally invasive and alternative methods with fewer complications (4).
Mesenchymal stem cells were described as multi-potent because of their ability for differentiation into a variety of different cells and tissue lineages. MSCs could be differentiated into epithelial cells of the liver, kidney, lung, skin, gastrointestinal tract, myocytes of heart, skeletal muscles, chondrocytes, osteoblasts, adipocytes, fibroblasts and other tissues of mesenchymal origin (5). They could also differentiate into functional hepatocyte-like cells (6). Previous studies showed that BM-MSCs could engraft injured liver tissue and recover its function (7). More over MSCs exhibited a greater homing capability for the injured liver than did hematopoietic stem cells (8). So the aim of this work was to evaluate the effect of BM-MSCs on the liver structure of experimental model of liver fibrosis in rats.
Materials and Methods
Animals
Twenty young male albino rats (5∼6 weeks) weighing 120∼140 gm and sixty adult female albino rats (6∼8 weeks) with average weight 200 gm were used in this study. The animals were purchased and raised in the Medical Research Center Ain Shams University. They were housed in plastic cages with mesh wire covers and were given food and water ad libitum. The rats were killed according to the Ethics Committee recommendations of Ain Shams University.
Chemicals
Dulbecco’s modified Eagle’s medium (DMEM), fetal bovine serum (FBS), penicillin-streptomycin solution, and 0.25% trypsin − 0.02% EDTA solution were purchased from Lonza Company, Swiss. The monoclonal mouse human antibody for CD44, CD 29, CD 34, Alpha smooth muscle actine (α- SMA), Proliferating cell nuclear antigen (PCNA) and biotinylated goat anti-mouse secondary antibody were purchased from LABVISION USA. Carbon tetrachloride (CCL4) (concentration 100%) and olive oil were obtained from Algomhoria Company.
Culture and characterization of BM-MSCs
The BM were harvested from the twenty male albino rats. The BM was flushed out from the diaphysis of femoral and tibia bones with 2 mL of complete medium, consisting of 80 ml DMEM, 15 ml FBS and 5 ml of Penicillin/streptomycin mixture. The marrow plugs were expelled from the opposite ends of diaphysis into the sterile petri dish. The culture dishes, containing the BM cells, were incubated in a humidified incubator at 37°C in 5% CO2 and 95% air (by volume). Every two days, The exchange of media was done. On day nine from the isolation and culture, when the cultured cells became confluent, the MSC in culture were characterized by their adhesiveness and fibro-blast like shape (9). We also use streptavidin-biotin immune-peroxidase technique for detection of CD44, CD 29 and CD 34 gene expression. MSCs are CD44, CD 29 positive and CD 34 negative while hematopoietic cells are CD 34 positive (10).
Preparation of BM-MSC for intra-peritoneal administration
On day nine of isolation and culture, after being sure that the cultured cells were MSCs, The cells were detached by adding 0.3 ml Trypsin EDTA solution for 10 minutes. The cell suspension was centrifuged and the cell pellets were obtained. 0.1 ml of cell pellet was mixed with 0.1 ml of trypan blue suspension for 5∼15 minutes. The live cells do not take up the dye, whereas dead cells do (11). A sample of Trypan blue-cell suspension was dispensed on top of a hemocytometer slide so that the fluid entirely covered the surface of the squares of the slide. The number of viable and non-viable cells in each square was then counted, using the ordinary light microscope. The cell concentration was calculated as follows: Cells/ml= average cell count per square×dilution factor×104 (12). Total cells in the original solution=cells/ml×the original volume of fluid from which the cell sample was removed. % Cell viability=total viable cells (unstained)/total cells× 100. In the current study, Average cell count in one square was 15 Living cells and 5 dead cells, Dilution factor= 2, the original volume of fluid from which the cell sample was removed=10 ml. So, Cells/ml=20×2×104=4×105. Total cells in the original solution=4×105×10=4×106. % Cell viability=15/20×100=75%. Total viable cells were injected in each rat=4×106×75%=3×106.
CCl4-induced liver fibrosis model
Thirty female albino rats were injected by CCL4 (0.5 mg/kg of body weight) twice weekly for four weeks intraperitoneal (I.P.). The solution for injection were formed of 100 mg of CCL4 dissolved in 100 ml of olive oil. Each rat was received 0.1 ml of CCL4 dissolved in olive oil (13).
Experimental groups
Group I (control group): Thirty female albino rats were equally subdivided into three subgroups:
- Subgroup IA: Rats were used to collect liver specimen as a negative control group.
- Subgroup IB: Each rat was given 0.1 mL of olive oil by IP injection twice weekly for four weeks then the rats were sacrificed.
- Subgroup IC: Each rat was given 0.1 mL of olive oil by IP injection twice weekly for four weeks. Then the rats were injected by a single IP dose of 0.5 mL phosphate buffer saline (PBS). Then the rats were sacrificed after another four weeks.
Group II: Thirty female albino rats were injected CCL4 to induce liver fibrosis as scheduled above. Then rats were equally subdivided into the following three subgroups; subgroup IIA (CCL4 alone group) was sacrificed immediately at the end of this four weeks. subgroup IIB (CCL4/MSCs group) was injected I.P. by a single dose 3×106 of BM-MSCs suspended in 0.5 ml PBS and was left for another four weeks. Subgroup IIC (CCL4/recovery group) was left for another four weeks without any intervention.
At the end of the experiment, the rats were sacrificed using ether anesthesia. Liver specimens were collected and processed for histological study. Blood samples were also collected for biochemical analysis.
Histological study
For light microscopic examination: Liver samples were collected and fixed in 10% buffered formalin for 24 hours. Serial 5-μm paraffin sections were prepared and stained with hematoxylin and eosin (H&E), Masson Trichrome (MT) and immunohistochemical for Alpha smooth muscle actin (α-SMA) (14) and Proliferating cell nuclear antigen (PCNA)(15).
For Transmission electron microscope (TEM) examination, Small liver specimens (1 mm3) were fixed in 2.5% gluteraldehyde solution. They were then post-fixed in 1% osmium tetroxide, dehydrated and embedded in Epon. Ultrathin sections were cut, stained with uranyl acetate and lead citrate and then examined using TEM1010- EXII (Joel, Tokyo, Japan) at the electron microscopic unit Unit, Faculty of Science, Ain Shams University., Cairo, Egypt.
PCR detection of male-derived MSC
Genomic DNA was prepared from liver tissue homogenate of the rats in each group using QIAamp® DNA Mini and Blood Mini KIT, Germany. The presence or absence of the sex determination region on the Y chromosome male (sry) gene in recipient female rats was assessed by PCR. Primer sequences for sry gene (forward 5′-CATCGAAGGGTTAAAGTGCCA-3′, reverse 5′-ATAGTGTGTAG-GTTGTTGTCC-3′) were obtained from published sequences (16) and amplified a product of 104 bp was purchased from (Sigma-USA). The PCR conditions were as follows: incubation at 94°C for 4 min; 35 cycles of incubation at 94°C for 50 s, 60°C for 30 s, and 72°C for 1 min; with a final incubation at 72°C for 10 min. PCR products were separated using 2% agarose gel electrophoresis and stained with ethidium bromide. Positive (male white albino rat genomic DNA) and negative (female white albino rat genomic DNA) controls were included in each assay. Y chromosomes marker detected as Transilluminated line.
Biochemical analysis
Blood samples were collected to measure the level of Aspartate aminotransferase (AST), Alanine aminotransferase (ALT) and Serum albumin. They were determined spectrophometrically with an automatic analyzer using commercially available kits in Dr. Ali Khalifa Chemical Lab. Ain Shams University.
Morphometric analysis
Using image analyzer at Faculty of dentistry, Ain shams University, Area percentage of collagen content using Masson’s trichrome stain, Area percentage of α-SMA and the mean number of PCNA positive cells were measured. All parameters were measured in randomly chosen five fields/section in five sections in ten rats in each group at magnification 400.
Statistical analysis
The morphometric and biochemical measurements are expressed as mean±SD. Significant differences were determined by using ANOVA and post hoc tests for multiple comparisons using SPSS 9.0 computer Software. Results were considered significant at p value≤0.05.
Results
Light microscopic examination of the liver
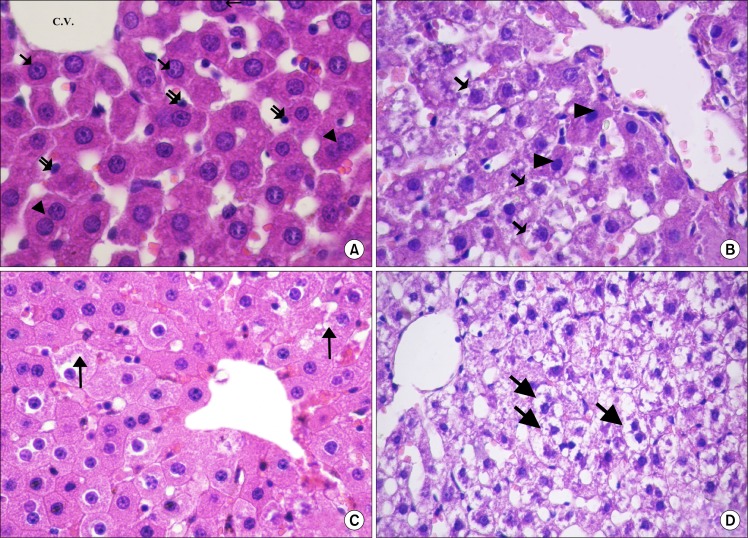
Examination of H and E stained sections of all subgroups of control rats showed nearly the same histological picture. The hepatocytes were arranged in the form of branching and anastomosing cords that radiate from the central veins and were separated by blood sinusoids, which were lined by flat endothelial cells. The hepatocytes showed acidophilic cytoplasm with single central rounded vesicular nuclei and some of the cells were binucleated (Fig. 1A). In subgroupIIA (CCL4 alone group), most of the hepatocytes contained multiple, large cytoplasmic vacuoles and some hepatocytes had deeply acidophilic cytoplasm and deeply stained nuclei (Fig. 1B). After MSCs administration, remarkable improvement in the hepatocytes was noticed as they appeared nearly similar to that of the control rats. Only few hepatocytes appeared with slight vacuolated cytoplasm (Fig. 1C). On the other hand, in the CCL4/recovery group the liver was still markedly affected. Hepatocytes appeared with highly vacuolated cytoplasm and deeply stained nuclei (Fig. 1D).
Fig. 1.
(A) Showing radiating cords of hepatocytes from the central vein (C.V.). The hepatocytes have central, rounded, vesicular nuclei (↑) and acidophilic cytoplasm. Some of the cells appear bi-nucleated (▲). Notice the lining cells (
 ) of the blood sinusoids (control H and E. ×720). (B) Most of the hepatocytes are vacuolated (
) of the blood sinusoids (control H and E. ×720). (B) Most of the hepatocytes are vacuolated (
 ). Few hepatocytes appear with acidophilic cytoplasm and deeply stained nuclei (▲) (CCL4 alone group. H and E. ×560). (C) Most of the hepatocytes have granular acidophilic cytoplasm and vesicular nuclei. Few cells show cytoplasmic vacuolation (↑) (CCL4/MSCs group. H and E. ×560). (D) Showing highly vacuolated hepatocytes with deeply stained nuclei (↑) (CCL4/Recovery group, H and E. ×400).
). Few hepatocytes appear with acidophilic cytoplasm and deeply stained nuclei (▲) (CCL4 alone group. H and E. ×560). (C) Most of the hepatocytes have granular acidophilic cytoplasm and vesicular nuclei. Few cells show cytoplasmic vacuolation (↑) (CCL4/MSCs group. H and E. ×560). (D) Showing highly vacuolated hepatocytes with deeply stained nuclei (↑) (CCL4/Recovery group, H and E. ×400).
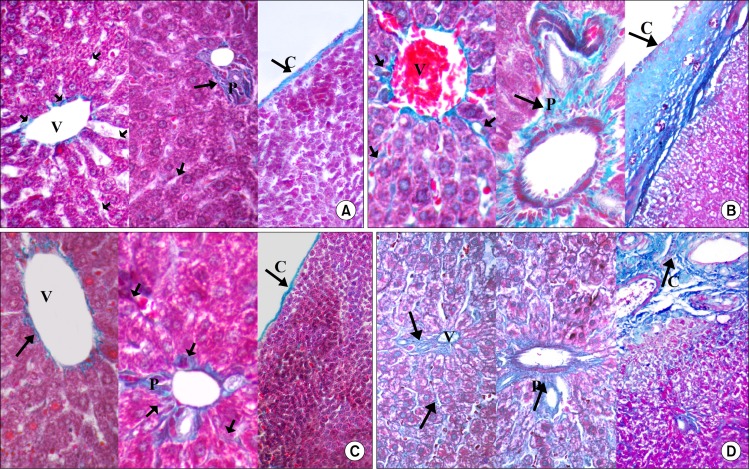
In Masson's trichrome stained sections the parenchyma of the liver in control groups appeared to be supported with a stroma of very delicate meshwork of collagenous fibers. Few collagenous fibers surrounding the central veins, in portal area and in capsules were seen (Fig. 2A). In subgroupIIA (CCL4 alone group), the stroma was well defined. There was thick connective tissue capsule and an apparent increase in the collagen fibers around the central veins, in between hepatocyte cords and in portal areas (Fig. 2B). In CCL4/MSCs group, few collagen fibers were detected (Fig. 2C) while in the CCL4/recovery group the collagen fibers were very numerous (Fig. 2D).
Fig. 2.
(A) Showing few collagen fibers (
 ) surrounding the central vein (V), in portal area (P) and in the capsule (C) in Control group. (B) Showing numerous collagen fibers (
) surrounding the central vein (V), in portal area (P) and in the capsule (C) in Control group. (B) Showing numerous collagen fibers (
 ) surrounding the central veins (V), in portal area (P) and in the capsule (C) in CCL4 alone group. (C) Showing the collagen fibers of CCL4/MSCs group which are comparable to that of the control group. (D) Showing numerous collagen fibers (↑) surrounding the central veins (V), in portal area (P) and in the capsule (C) in CCL4/Recovery group (Masson’s trichrome V×400, P×400, C×140).
) surrounding the central veins (V), in portal area (P) and in the capsule (C) in CCL4 alone group. (C) Showing the collagen fibers of CCL4/MSCs group which are comparable to that of the control group. (D) Showing numerous collagen fibers (↑) surrounding the central veins (V), in portal area (P) and in the capsule (C) in CCL4/Recovery group (Masson’s trichrome V×400, P×400, C×140).
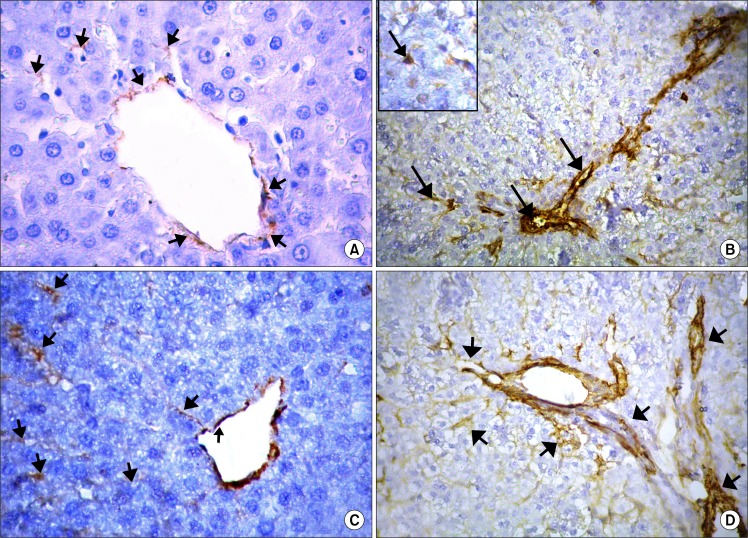
Immune-stained sections for α-SMA showed few mildly immune-reacted α-SMA positive cells, in between the hepatocytes and around the central veins in the control groups (Fig. 3A). On the other hand, in CCL4 alone group numerous strong immune-reacted α-SMA positive cells were detected in-between the hepatocytes, around the central veins as well as in the connective tissue septa between the hepatic lobules (Fig. 3B). These cells showed intense positive brownish cytoplasmic reaction. They appeared as small spindle-shaped cell bodies with multiple cytoplasmic processes. These α-SMA positive cells were apparently few in CCL4/MSCs group (Fig. 3C) and markedly increased in the CCL4/recovery group (Fig. 3D).
Fig. 3.
(A) Showing few α-SMA positive cells (
 ) around the central vein and in-between the hepatocytes (control group×560). (B) Showing an apparent increase in α-SMA positive cells (
) around the central vein and in-between the hepatocytes (control group×560). (B) Showing an apparent increase in α-SMA positive cells (
 ) around central vein, in septa between the hepatic lobules and in-between the hepatocytes. Inset: α-SMA positive cells appear spindle in shape with cytoplasmic processes (CCL4 alone group×400–inset×560). (C) Showing few α-SMA positive cells (
) around central vein, in septa between the hepatic lobules and in-between the hepatocytes. Inset: α-SMA positive cells appear spindle in shape with cytoplasmic processes (CCL4 alone group×400–inset×560). (C) Showing few α-SMA positive cells (
 ) around the central vein and in-between the hepatocytes (CCL4/MSCs group×560). (D) Showing strong positive immune reaction for α-SMA (
) around the central vein and in-between the hepatocytes (CCL4/MSCs group×560). (D) Showing strong positive immune reaction for α-SMA (
 ) around the central vein and in-between the hepatocytes (CCL4/Recovery group×400). Immunostaining for α-SMA.
) around the central vein and in-between the hepatocytes (CCL4/Recovery group×400). Immunostaining for α-SMA.
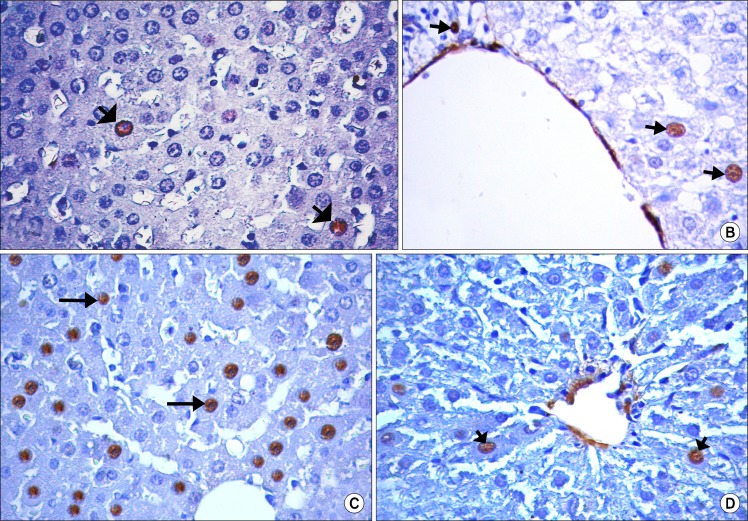
Immune-stained sections for PCNA showed that few hepatocytes had a positive nuclear reaction in the control group (Fig. 4A), CCL4 alone group (Fig. 4B) and CCL4/recovery group (Fig. 4D). While the positive PCNA hepatocytes were many in CCL4/MSCs group (Fig. 4C).
Fig. 4.
(A) Showing few hepatocytes with positive immune reaction for PCNA (
 ) in control group. (B) Showing few hepatocytes with positive immune reaction for PCNA (
) in control group. (B) Showing few hepatocytes with positive immune reaction for PCNA (
 ) in CCL4 alone group. (C) Showing many of PCNA positive hepatocytes (
) in CCL4 alone group. (C) Showing many of PCNA positive hepatocytes (
 ) in CCL4/MSCs group. (D) Showing few hepatocytes with positive immune reaction for PCNA (
) in CCL4/MSCs group. (D) Showing few hepatocytes with positive immune reaction for PCNA (
 ) in CCL4/Recovery group (Immunostaining for PCNA, ×560).
) in CCL4/Recovery group (Immunostaining for PCNA, ×560).
The electron microscope examination of the liver
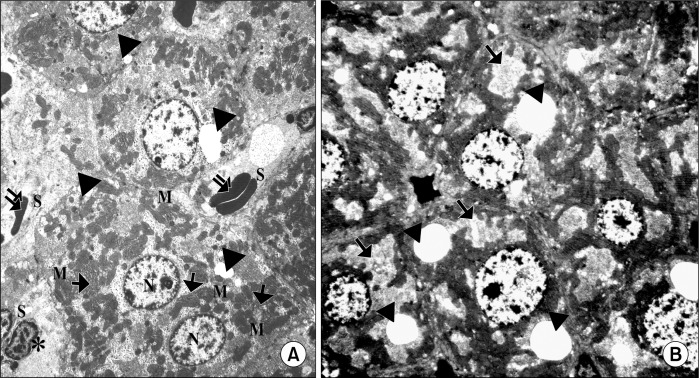
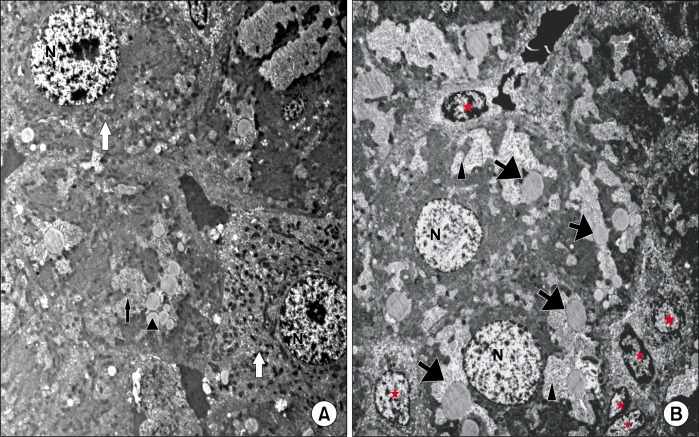
Ultrathin sections of the control group showed adjacent hepatocytes with sinusoidal spaces in-between. Hepatic stellate cells (HSCs) were seen in the perisinusoidal space. The hepatocytes appear with large rounded, central vesicular nuclei (N). The nuclei showed usual characteristic chromatin distribution. The cytoplasm contained numerous mitochondria and prominent rough endoplasmic reticulum (rER) as well as fat droplets (Fig. 5A). In CCL4 alone group, The hepatocytes showed large vacuoles and many fat droplets in their cytoplasm and an apparent reduction of mitochondria and rER. The nuclei showed abnormal distribution of the chromatin (Fig. 5B). The liver of CCL4/MSCs rats showed that the structure of most of the hepatocytes was nearly comparable to that of the control group. Hepatocytes showed numerous mitochondria, rER. and glycogen granules. Some hepatocytes contained small vacuoles and few fat droplets (Fig. 6A). The hepatocytes of rats in CCL4/recovery group appeared highly vacuolated with many fat droplets and an apparent reduction in the mitochondria and rER. HSCs were frequently seen in-between hepatocytes with large euchromatic nucleus (Fig. 6B).
Fig. 5.
(A) Showing adjacent hepatocytes with sinusoidal spaces (S) in-between. HSCs (*) are seen in the perisinusoidal space The hepatocytes appear with large rounded, central vesicular nuclei (N). The nuclei show usual characteristic chromatin distribution. The cytoplasm contains numerous mitochondria (M), rER (
 ) and fat droplets (▲) (Control group TEM×4050). (B) Showing adjacent hepatocytes. The nuclei of the hepatocytes show abnormal chromatin distribution (N). The cytoplasm contains multiple vacuoles (
) and fat droplets (▲) (Control group TEM×4050). (B) Showing adjacent hepatocytes. The nuclei of the hepatocytes show abnormal chromatin distribution (N). The cytoplasm contains multiple vacuoles (
 ) and fat droplets (▲) (CCL4 alone group TEM ×4050).
) and fat droplets (▲) (CCL4 alone group TEM ×4050).
Fig. 6.
(A) Showing hepatocytes (white
 ) that are comparable to that of the control group. They contain many mitochondria and show usual distribution of chromatin in their nuclei (N). Small vacuoles (
) that are comparable to that of the control group. They contain many mitochondria and show usual distribution of chromatin in their nuclei (N). Small vacuoles (
 ) and few fat droplets (▲) are seen in some hepatocytes (CCL4/MSCs group TEM×4050). (B) Showing irregular large vacuoles (▲) and fat droplets (
) and few fat droplets (▲) are seen in some hepatocytes (CCL4/MSCs group TEM×4050). (B) Showing irregular large vacuoles (▲) and fat droplets (
 ) inside the cytoplasm of hepatocytes. The nuclei appear with abnormal chromatin distribution (N). Notice the presence of HSCs (*) with euchromatic nuclei in between hepatocytes (CCL4/Recovery group TEM×4050).
) inside the cytoplasm of hepatocytes. The nuclei appear with abnormal chromatin distribution (N). Notice the presence of HSCs (*) with euchromatic nuclei in between hepatocytes (CCL4/Recovery group TEM×4050).
PCR detection of male-derived BMMSCs
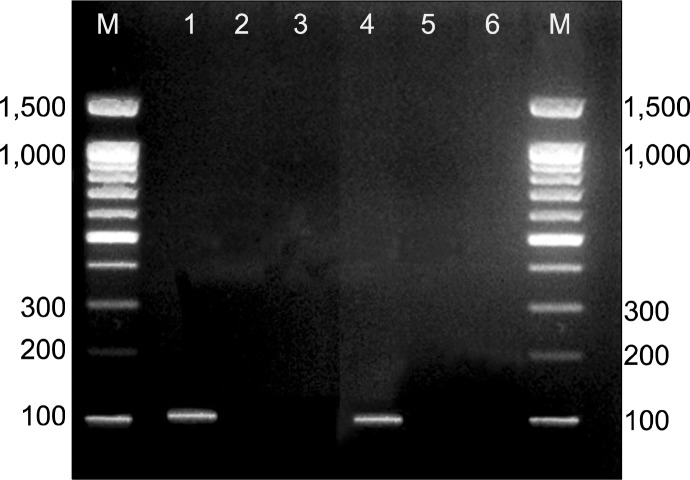
The SRY gene that was used as the Y chromosome marker was expressed in male rats from which BM-MSCs were isolated. It was also detected in female rats that were injected by male derived BM-MSCs in CCL4/MSCs group. Y chromosome marker was not detecting in the control group, CCL4 alone group and CCL4/recovery group (Fig. 7).
Fig. 7.
Showing presence of Y chromosomes in male rats from which BM-MSCs were isolated (lane 1) and female rats treated with BM-MSCs in CCL4/MSCs group (lane 4). Y chromosome marker is not detecting in female rats of the control group (lane 2), CCL4 alone group (lane 3) and CCL4/recovery group (lane 5). M: PCR marker (U.V. trans-illuminated agarose gel of PCR products of SRY gene).
Morphometric results
The area percentage of both collagen and α-SMA showed a significant increase (p<0.001) in both CCL4 alone and CCL4/recovery groups as compared to the other groups. A significant increase (p<0.001) in the area percentage of both collagen and α-SMA of CCL4/recovery group as compared to that of CCL4 alone group was detected. Non-significantly difference was noticed between the control and CCL4/MSCs groups (Table 1).
Table 1.
Showing changes in area percentage of collagen, α smooth muscle actin, mean number of PCNA positive cells/high power field (HPFs) and biochemical measurements (ALT, AST and albumin) in different groups
| Control group | CCL4 alone group | CCL4/MSCs group | CCL4/Recovery group | |
|---|---|---|---|---|
| Area percentage of collagen fibers (%) | 14.4±2.8 (■○) | 74.4±4.47 (*▲○) | 21.5±4.7 (■○) | 85.5±3.6 (*■▲) |
| Area percentage of α smooth muscle actin (%) | 14.67±3.33 (■○) | 41.43±2.1 (*▲○) | 18.4±3.8 (■○) | 58.26±4.09 (*■▲) |
| Number of PCNA/HPFs | 2.04±0.96 (▲) | 1.02±1.03 (▲) | 10.09±1.47 (*■○) | 1.21±0.03 (▲) |
| ALT (μ/L) | 57.4±6.8 (■○) | 112.6±16.1 (*▲) | 64.1±9.2 (■○) | 109.1±15.75 (*▲) |
| AST (μ/L) | 37.2±3.4 (■○) | 431.7±14.2 (*▲) | 46.5±4.7 (■○) | 434.7±16.15 (*▲) |
| ALBUMIN (g/dL) | 3.65±0.5 (■○) | 1.98±0.66 (*▲) | 3.73±0.34 (■○) | 1.72±0.53 (*▲) |
Significant difference from Control group,
Significant difference from CCL4 alone group,
Significant difference from CCL4/MSCs group,
Significant difference from CCL4/Recovery group.
The mean number of PCNA positive cells/HPFs was significantly high (p<0.001) in the CCL4/MSCs group as compared to other groups. There was non-significantly difference in its level in control, CCL4 alone group and CCL4/recovery group (Table 1).
Biochemical results
Serum ALT and AST showed a significant high level (p<0.001) in both CCL4 alone group and CCL4/recovery groups as compared to the other groups. The levels in the control and CCL4/MSCs were non-significantly different (Table 1).
There was a significant reduction (p<0.001) of serum albumin of rats of CCL4 alone and CCL4/recovery groups as compared to the other groups. While the levels in the control and CCL4/MSCs were non-significantly different and significantly high as compared to CCL4 alone and CCL4/recovery groups (Table 1).
Discussion
BM-MSCs are capable of homing to specific tissues, differentiate and stimulate a local repair response (17). So this study was performed to investigate if the BM-MSCs could restore the liver structure in the experimental model of liver fibrosis. CCL4 was used in this study to induce liver fibrosis because among the different experimental animal models of liver fibrosis, the CCL4 model was the most closely resembling that of human liver cirrhosis (18). Four weeks after CCL4 administration, light microscopic examination of the liver revealed loss of the usual hepatic architecture, many vacuoles with dark stained nuclei in most of the liver cells. Moreover, few mitochondria, few rER, many fat droplets and large irregular vacuoles were also detected by electron microscopic examination. This structural damage was due to edema of the organelles (19) and explained the significant increase in serum AST and ALT and significant decrease in serum albumin, which were observed in the present work, as compared to the control. ALT and AST are useful serum markers for inflammation and necrosis of the liver (20).
Liver fibrosis was clearly evidenced, in the present work, by a significant increase in area percentage of the collagen fibers in Masson trichrome stained section. The expression of α-SMA were determined as a marker for the activity of HSCs, which were identified as the primary cell type that mediate fibrogenesis (20). So, immune-staining of α-SMA was done to detect the activity of HSCs. There was a significant increase in the area percentage of α-SMA positive cells in CCL4 alone group as compared to that of control group. This increase in the area percentage of α-SMA reflected an increase in the activity of HSCs. The α-SMA positive cells appeared as small spindle-shaped cell bodies with multiple cytoplasmic processes. HSCs are non-parenchymal quiescent cells for vitamin A storage in control liver. In pathological conditions as in liver fibrosis, HSCs lose retinoid, changes from the star-shaped stellate cells to myofibroblasts like cell and synthesize a large amount of extra cellular matrix components including collagen, proteoglycan and adhesive glycoproteins (21). α-SMA is a good marker for the detection of this myofibroblasts like cells (22). It was explained that hepatic fibrosis is usually initiated by hepatocytes damage leading to activation of Kupffer cells and subsequent release of cytokines and growth factors. These factors activate HSCs which proliferate and transform into myofibroblasts-like cells that deposit large amounts of connective tissue components (23). Moreover, CCL4 causes oxidative stress that activates HSCs (24).
Some of female rats with CCL4 induced liver fibrosis were treated with BM-MSCs (CCL4/MSCs group) while others were left without treatment (CCL4/recovery group). Our findings revealed that the liver structure of CCL4/recovery group was still affected. The light and electron microscopic picture of the hepatocytes and also the liver function were nearly similar to that of the CCL4 alone group. Moreover there was a progressive increase in the liver fibrosis. The area percentage of collagen fibers and α-SMA positive cells were significantly increased as compared to that of CCL4 alone group.
Intra-peritoneal route was chosen for BMMSCs injection in experimental fibrotic rats. The peritoneal cavity had long been used as a favorable site for therapeutic cell transplantation, with the advantage that large volume of cells can be implanted, technically easy to perform, less traumatic and less invasive (25). The homing of the male donor cells were confirmed in the injured liver of female recipients by using PCR for the SRY gene. The Y-chromosome marker was expressed in female rats in CCL4/MSCs group. The presence of liver injury recruits homing of BM-MSCs through up regulating of cytokines such as stem cell factor-1, hepatocytes growth factor (HGF) and MMP and many of these cytokines and chemokines are chemo attractants (26).
Light and electron microscopic examination of CCL4/MSCs group revealed an improvement of the liver structure. Most of hepatocytes appeared nearly as those of the control group and regained their function. Non-significant change was noticed in the level of ALT, AST and albumin in CCL4/MSCs group as compared to that were seen in the control groups. Similarly previous finding showed that transplanted BMMSCs could restore the serum albumin level and significantly suppressed transaminase activity and liver fibrosis in the injured liver of rats. BM-MSCs differentiated into hepatic oval cells and then to hepatocyte-like cells. The damaged hepatocytes were repaired and fibrosis was resolved, resulting in an overall improvement in liver function (27).
The liver fibrosis was also resolved after BM-MSCs administration. The area percentage of the collagen fibers and area percentage of α-SMA positive cells were significantly decreased as compared to CCL4 alone group. BM-MSCs ameliorated liver fibrosis by down regulating the profibrotic genes and up-regulating anti-fibrotic hepatic genes (28). Moreover, MSCs might play an inhibition role in process of HSCs transition from the quiescent state to activated state and induce HSCs apoptosis through release of interleukin-10 (29). Recently, it was found that BM-MSCs reduced expression of collagen type I and α-SMA (30).
Upon liver injury, the body attempts to repair the damage through increasing the expression of HGF, TGF-β and other cytokines to enhance hepatocytes proliferation and initiate tissue repairing process (31). It was found that BM-MSCs secrete a variety of these cytokines and growth factors which suppress the local immune system, inhibit fibrosis and apoptosis, enhance angiogenesis and stimulate mitosis and differentiation of tissue-intrinsic stem cells (32). PCNA immune-staining technique was used in the present study to detect the percentage of proliferating liver cells. The number of PCNA positive hepatocytes were significantly higher in the CCL4/MSCs group as compared to control, CCL4 and recovery group. This result could be explained by the previous findings which reported that the normal hepatocytes were generally quiescent and replicate in a limited and regulated manner. The replicative activity of hepatocytes diminishes in advanced cirrhosis in humans and in chronic liver injury in mouse, reaching a state of replicative senescence (20). Infusion of BM-MSCs facilitated the proliferation of hepatocytes after massive hepatectomy in rats, reflected by the elevated PCNA-positive cells (33).
Conclusion
From the previously discussed results, it was concluded that BM-MSCs markedly decreased the induced liver fibrosis by CCL4 in albino rats. So, stem cell therapy offers a hope to patient waiting for liver transplantation. Further studies on the differentiation of MSCs into hepatocytes in vitro and their use in treatment of liver fibrosis were recommended.
Footnotes
Potential conflict of interest
The authors have no conflicting financial interest.
References
- 1.Lee HS, 1, Li L, Kim HK, Bilehal D, Li W, Lee DS, Kim YH. The protective effects of Curcuma longa Linn. extract on carbon tetrachloride-induced hepatotoxicity in rats via up-regulation of Nrf2. J Microbiol Biotechnol. 2010;20:1331–1338. doi: 10.4014/jmb.1002.03010. [DOI] [PubMed] [Google Scholar]
- 2.Yang FR, Fang BW, Lou JS. Effects of Haobie Yangyin Ruanjian decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2010;16:1458–1464. doi: 10.3748/wjg.v16.i12.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sporea I, Raţiu I, Bota S, Şirli R, Jurchiş A. Are different cut-off values of liver stiffness assessed by transient elastography according to the etiology of liver cirrhosis for predicting significant esophageal varices? Med Ultrason. 2013;15:111–115. doi: 10.11152/mu.2013.2066.152.is1ir2. [DOI] [PubMed] [Google Scholar]
- 4.Woo SY, Park M, Lee HJ, Kim JY, Ryu KH. Tonsil-derived stromal cells reduces CCl4-induced liver fibrosis in mice via autophagy activation (P2180) The Journal of Immunology. 2013;190:69.28. [Google Scholar]
- 5.Zhang S, Chen L, Liu T, Zhang B, Xiang D, Wang Z, Wang Y. Human umbilical cord matrix stem cells efficiently rescue acute liver failure through paracrine effects rather than hepatic differentiation. Tissue Eng Part A. 2012;18:1352–1364. doi: 10.1089/ten.tea.2011.0516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Dong X, Pan R, Zhang H, Yang C, Shao J, Xiang L. Modification of histone acetylation facilitates hepatic differentiation of human bone marrow mesenchymal stem cells. PLoS One. 2013;8:e63405. doi: 10.1371/journal.pone.0063405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nasir GA, Mohsin S, Khan M, Shams S, Ali G, Khan SN, Riazuddin S. Mesenchymal stem cells and Interleukin-6 attenuate liver fibrosis in mice. J Transl Med. 2013;11:78. doi: 10.1186/1479-5876-11-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li Q, Zhou X, Shi Y, Li J, Zheng L, Cui L, Zhang J, Wang L, Han Z, Han Y, Fan D. In vivo tracking and comparison of the therapeutic effects of MSCs and HSCs for liver injury. PLoS One. 2013;8:e62363. doi: 10.1371/journal.pone.0062363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Rochefort GY, Vaudin P, Bonnet N, Pages JC, Domenech J, Charbord P, Eder V. Influence of hypoxia on the domiciliation of mesenchymal stem cells after infusion into rats: possibilities of targeting pulmonary artery remodeling via cells therapies? Respir Res. 2005;6:125. doi: 10.1186/1465-9921-6-125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bobis S, Jarocha D, Majka M. Mesenchymal stem cells: characteristics and clinical applications. Folia Histochem Cytobiol. 2006;44:215–230. [PubMed] [Google Scholar]
- 11.Zickri MB, Ahmad NA, Maadawi ZM, Mohamady YK, Metwally HG. Effect of stem cell therapy on induced diabetic keratopathy in albino rat. Int J Stem Cells. 2012;5:57–64. doi: 10.15283/ijsc.2012.5.1.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kruse PF, Patterson MK. Tissue culture: methods and applications. New York: Academic Press; 1973. [Google Scholar]
- 13.Iredale JP, Benyon RC, Pickering J, McCullen M, Northrop M, Pawley S, Hovell C, Arthur MJ. Mechanisms of spontaneous resolution of rat liver fibrosis. Hepatic stellate cell apoptosis and reduced hepatic expression of metalloproteinase inhibitors. J Clin Invest. 1998;102:538–549. doi: 10.1172/JCI1018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Ahmed AF, Mahmoud MF, Ouf MA, El-Fathaah EA. Aminoguanidine potentiates the hepatoprotective effect of silymarin in CCL4 treated rats. Ann Hepatol. 2011;10:207–215. [PubMed] [Google Scholar]
- 15.Sun H, Yu L, Wei H, Liu G. A novel antihepatitis drug, bicyclol, prevents liver carcinogenesis in diethylnitrosamine-initiated and phenobarbital-promoted mice tumor model. J Biomed Biotechnol. 2012;2012:584728. doi: 10.1155/2012/584728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.An J, Beauchemin N, Albanese J, Abney TO, Sullivan AK. Use of a rat cDNA probe specific for the Y chromosome to detect male-derived cells. J Androl. 1997;18:289–293. [PubMed] [Google Scholar]
- 17.Fu X, Li H. Mesenchymal stem cells and skin wound repair and regeneration: possibilities and questions. Cell Tissue Res. 2009;335:317–321. doi: 10.1007/s00441-008-0724-3. [DOI] [PubMed] [Google Scholar]
- 18.Geerts AM, Vanheule E, Praet M, Van Vlierberghe H, De Vos M, Colle I. Comparison of three research models of portal hypertension in mice: macroscopic, histological and portal pressure evaluation. Int J Exp Pathol. 2008;89:251–263. doi: 10.1111/j.1365-2613.2008.00597.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tasci I, Mas N, Mas MR, Tuncer M, Comert B. Ultrastructural changes in hepatocytes after taurine treatment in CCl4 induced liver injury. World J Gastroenterol. 2008;14:4897–4902. doi: 10.3748/wjg.14.4897. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cheung PY, Zhang Q, Zhang YO, Bai GR, Lin MC, Chan B, Fong CC, Shi L, Shi YF, Chun J, Kung HF, Yang M. Effect of WeiJia on carbon tetrachloride induced chronic liver injury. World J Gastroenterol. 2006;12:1912–1917. doi: 10.3748/wjg.v12.i12.1912. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Senoo H, Yoshikawa K, Morii M, Miura M, Imai K, Mezaki Y. Hepatic stellate cell (vitamin A-storing cell) and its relative--past, present and future. Cell Biol Int. 2010;34:1247–1272. doi: 10.1042/CBI20100321. [DOI] [PubMed] [Google Scholar]
- 22.Jung YJ, Ryu KH, Cho SJ, Woo SY, Seoh JY, Chun CH, Yoo K, Moon IH, Han HS. Syngenic bone marrow cells restore hepatic function in carbon tetrachloride-induced mouse liver injury. Stem Cells Dev. 2006;15:687–695. doi: 10.1089/scd.2006.15.687. [DOI] [PubMed] [Google Scholar]
- 23.Bauer M, Schuppan D. TGFbeta1 in liver fibrosis: time to change paradigms? FEBS Lett. 2001;502:1–3. doi: 10.1016/s0014-5793(01)02655-2. [DOI] [PubMed] [Google Scholar]
- 24.Yang FR, Fang BW, Lou JS. Effects of Haobie Yangyin Ruanjian decoction on hepatic fibrosis induced by carbon tetrachloride in rats. World J Gastroenterol. 2010;16:1458–1464. doi: 10.3748/wjg.v16.i12.1458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Xu YQ, Liu ZC. Therapeutic potential of adult bone marrow stem cells in liver disease and delivery approaches. Stem Cell Rev. 2008;4:101–112. doi: 10.1007/s12015-008-9019-z. [DOI] [PubMed] [Google Scholar]
- 26.Zhang W, Walboomers XF, van Osch GJ, van den Dolder J, Jansen JA. Hard tissue formation in a porous HA/TCP ceramic scaffold loaded with stromal cells derived from dental pulp and bone marrow. Tissue Eng Part A. 2008;14:285–294. doi: 10.1089/tea.2007.0146. [DOI] [PubMed] [Google Scholar]
- 27.Oyagi S, Hirose M, Kojima M, Okuyama M, Kawase M, Nakamura T, Ohgushi H, Yagi K. Therapeutic effect of transplanting HGF-treated bone marrow mesenchymal cells into CCl4-injured rats. J Hepatol. 2006;44:742–748. doi: 10.1016/j.jhep.2005.10.026. [DOI] [PubMed] [Google Scholar]
- 28.Ali G, Masoud MS. Bone marrow cells ameliorate liver fibrosis and express albumin after transplantation in CCl4-induced fibrotic liver. Saudi J Gastroenterol. 2012;18:263–267. doi: 10.4103/1319-3767.98433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dai LJ, Li HY, Guan LX, Ritchie G, Zhou JX. The therapeutic potential of bone marrow-derived mesenchymal stem cells on hepatic cirrhosis. Stem Cell Res. 2009;2:16–25. doi: 10.1016/j.scr.2008.07.005. [DOI] [PubMed] [Google Scholar]
- 30.Shao CH, Chen SL, Dong TF, Chai H, Yu Y, Deng L, Wang Y, Cheng F. Transplantation of bone marrow-derived mesenchymal stem cells after regional hepatic irradiation ameliorates thioacetamide-induced liver fibrosis in rats. J Surg Res. 2014;186:408–416. doi: 10.1016/j.jss.2013.08.016. [DOI] [PubMed] [Google Scholar]
- 31.Paradis V, Youssef N, Dargère D, Bâ N, Bonvoust F, Deschatrette J, Bedossa P. Replicative senescence in normal liver, chronic hepatitis C, and hepatocellular carcinomas. Hum Pathol. 2001;32:327–332. doi: 10.1053/hupa.2001.22747. [DOI] [PubMed] [Google Scholar]
- 32.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 33.Yu J, Yin S, Zhang W, Gao F, Liu Y, Chen Z, Zhang M, He J, Zheng S. Hypoxia preconditioned bone marrow mesenchymal stem cells promote liver regeneration in a rat massive hepatectomy model. Stem Cell Res Ther. 2013;4:83. doi: 10.1186/scrt234. [DOI] [PMC free article] [PubMed] [Google Scholar]