Abstract
Importance
Reducing early (<30 days) hospital readmissions is a policy priority aimed at improving healthcare quality. The Cumulative Complexity Model conceptualizes patient context. It predicts that highly supportive discharge interventions will enhance patient capacity to enact burdensome self-care and avoid readmissions.
Objectives
To synthesize the evidence of the efficacy of interventions to reduce early hospital readmissions and identify intervention features—including their impact on treatment burden and on patients’ capacity to enact post-discharge self-care—that might explain their varying effects.
Data Sources
We searched electronic databases (1990 until April 1st, 2013), contacted experts, and reviewed bibliographies.
Study Selection
Randomized trials that assessed the effect of interventions on all-cause or unplanned readmissions within 30 days of discharge in adult patients admitted to the hospital for a medical or surgical cause for > 24 hours and discharged to home.
Data extraction and Synthesis
Reviewer pairs extracted trial characteristics and used an activity-based coding strategy to characterize the interventions; fidelity was confirmed with authors. Blinded to trial outcomes, reviewers noted the extent to which interventions placed additional work on patients after discharge or supported their capacity for self-care in accordance with the Cumulative Complexity Model.
Main Outcome
Relative risk of all-cause or unplanned readmission with or without out of hospital deaths at 30 days post-discharge.
Results
In 42 trials, the tested interventions prevented early readmissions [pooled random effects relative risk (RR) 0.82, 95% CI 0.73 to 0.91; p=.03; I2= 32%], a finding that was consistent across patient subgroups. Trials published before 2002 reported interventions that were 1.6 times more effective than those tested later (pinteraction = .01). In exploratory subgroup analyses, interventions with many components (pinteraction <.01), involving more individuals in care delivery (pinteraction = .05), and supporting patient capacity for self-care (pinteraction = .04) were 1.4, 1.3, and 1.3 times more effective than other interventions. A post-hoc regression model showed incremental value in providing comprehensive, post-discharge support to patients and caregivers.
Conclusions
Tested interventions are effective at reducing readmissions, but more effective interventions are complex and support patient capacity for self-care. Interventions tested more recently are less effective.
Registration Number
PROSPERO, CRD42013004773
INTRODUCTION
Early hospital readmissions have been recognized as a common and costly occurrence, particularly among elderly and high risk patients. One in five Medicare beneficiaries is readmitted within 30 days, for example, at a cost of over $26 billion per year.1 To encourage improvement in the quality of care and a reduction in unnecessary health expense; policymakers, reimbursement strategists, and the United States government have made reducing 30-day hospital readmissions a national priority.2–4 Achieving this goal, however, requires more complete understanding of the underlying causes of readmission.
The Cumulative Complexity Model (CuCoM)5 is a framework developed by our research group that conceptualizes patient context as a balance between workload and capacity (Figure 1). Workload consists of all the work of being a patient and includes efforts to understand and plan for care, to enroll the support of others, and to access and use healthcare services.6,7 Capacity is determined by the quality and availability of resources that patients can mobilize to carry out this work (physical and mental health, social capital, financial resources, and environmental assets). The CuCoM is novel in its consideration of the effects of treatment burden on patient context and it illustrates how infeasible, unsupported, and context-irreverent care can lead to poor health outcomes and reduced healthcare effectiveness. Because patients recently discharged from the hospital are in a state of extreme physiologic and psychological vulnerability,8 their capacity for enacting self-care is low. The CuCoM predicts that, unless sufficient support is given to enhance patient and caregiver capacity to carry out the work of patienthood, placing highly burdensome discharge demands on these patients will lead to poor outcomes and hospital readmission.
Figure 1.
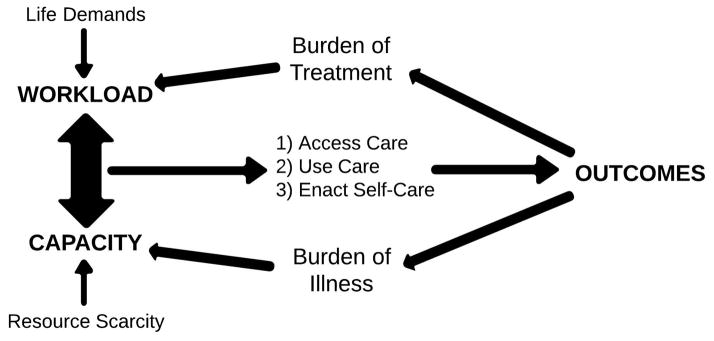
The Cumulative Complexity Model. Patient context is represented as a balance between workload and capacity. This balance must be optimized to ensure care effectiveness and improve outcomes. In turn, the outcomes achieved feedback to affect the workload-capacity balance.
To evaluate the validity of the CuCoM and provide hypothesis-generating work in the understanding of patient context, we chose to synthesize the evidence on the efficacy of interventions to reduce early hospital readmissions. In particular, we sought to determine the degree to which a number of intervention characteristics—including their impact on patient capacity and workload—might account for differences in their effectiveness.
METHODS
A registered protocol (PROSPERO CRD42013004773) guided the conduct of this review,9 which we report in adherence to the Preferred Reporting Items for Systematic Reviews and Meta-analyses (PRISMA) statement.10
Eligibility Criteria
Eligible studies were randomized trials reported in English or Spanish, since 1990, that assessed the effectiveness of peri-discharge interventions versus any comparator on the risk of early (i.e., within 30 days of discharge) all-cause or unplanned readmissions with or without out-of-hospital deaths. The intervention had to focus its efforts on the hospital to home transition, permit patients across arms to have otherwise similar inpatient experiences, and be generalizable to contexts beyond a single patient diagnosis. Adult patients had to be admitted from the community to an inpatient ward for at least 24 hours with a medical or surgical cause. Studies including obstetric or psychiatric admissions or only including discharges to skilled nursing or rehabilitation facilities were excluded.
Information Sources
In collaboration with an experienced research librarian (P.E.), we searched in April of 2013 the following databases: PubMed, Ovid MEDLINE, Ovid EMBASE, EBSCO CINAHL, and Scopus. Supplement A includes the complete search strategy. Two reviewers (T.S., A.L.) hand searched the bibliographies of included studies and recent reviews. Experts in the field were asked to identify additional references.
Study Selection
Four reviewers (A.L., M.G., J.B., T.S.) worked independently and considered the eligibility of candidate papers by examining their titles and abstracts, and then the full version of papers identified as potentially eligible by at least one reviewer. Conflicts about the eligibility of full papers were resolved by discussion and consensus. Eligibility was delayed for studies reporting outcomes incompletely, pending author contact.
Data Collection
After creating and piloting a standardized form, reviewers (A.L., M.G., J.B.) working independently and in duplicate and using a web-based program (DistillerSR; Ottawa, Canada), abstracted details about the patient population, the interventions compared, and the outcomes reported.
We abstracted details of the interventions tested verbatim from either the trial report or a cited protocol, limiting our focus to the period of hospitalization until 30 days post discharge, and identifying the “net intervention” by selecting out activities that occurred in the intervention arm but not in the control arm. These activities were coded using a taxonomy adapted from Hansen et al11 (Table 1). We also noted the number of meaningfully involved individuals participating in the intervention’s delivery and the number of meaningful interactions these individuals had with patients. “Meaningfully involved individuals” played a structured and requisite function in the delivery of central aspects of the intervention (e.g., a physician that might be contacted only as needed would not be considered meaningfully involved). Similarly, meaningful patient interactions were defined as those that were the proposed sources of the intervention’s effectiveness (e.g., a nurse visiting a patient only to deliver educational materials, but not to actually engage in educational activity, would not be considered a meaningful interaction). Two team members (A.L., M.G.) created summary descriptions of the interventions in a standardized format; these were shared with each author to confirm their fidelity to what happened in the trial.
Table 1.
Activity-based Coding Framework for Discharge Interventions
| Label | Activity Observed |
|---|---|
| Discharge Planning | Simply thinking about and formalizing an approach to prepare for discharge when this did not occur in any way in the control arm |
| Case Management | Logistical coordination of care and/or resources not specifically focused on self-management and either not occurring in control arm or occurring to lesser degree |
| Telephone Follow-up | Use of a telephone or videophone for provider-initiated communication after discharge that does not occur in the control arm |
| Telemonitoring | Use of remote technology designed for the patient to transmit objective measures of health status with or without connected subjective assessment |
| Patient Education | Patient-directed education related to diagnosis or treatment rationale but not focused on encouraging self-management and not occurring in control arm |
| Self-Management | Patient-directed education or coaching directly focused on improving patient’s ability to self-manage care needs that does not happen in control arm |
| Medication Intervention | Medication reconciliation or special education aimed at improving medication understanding or adherence; often conducted by a pharmacist but need not be |
| Home Visits | Physical visitation by intervention provider to patient’s place of residence when this does not happen in control |
| Follow-Up Scheduled | Scheduling of a follow-up visit prior to discharge when this is not done in the control arm or is done less reliably |
| Patient Centered Discharge Instructions | Some difference in the format or usability of discharge materials to make them more accessible or relevant compared to control |
| Provider Continuity | Increased provider presence on both sides of the hospital to home transition compared to control; may include involvement of PCP in inpatient care or strategic follow up with inpatient provider after discharge or “bridging” provider |
| Timely Follow-up | Post-discharge follow-up visit or communication with patient when this either does not occur or occurs at a later date in the control arm |
| Timely PCP Communication | Engagement with PCP in communication about patient status when this either does not occur or occurs at a later date in the control arm |
| Patient Hotline | Presence of an open line for patient-initiated communication when this either does not exist in the control arm or is more restricted in availability or usefulness |
| Rehab Intervention | Patient-directed rehabilitation efforts that are not entirely diagnosis-specific but aimed at improving functional status and do not exist in control |
| Streamlining | A general streamlining of services provided, often with dedicated assignment of responsibility when this does not occur in control |
| Making Requisite | Increasing the use or quality of services currently available but underutilized compared to the situation in control |
| Other | Special situations unique to the intervention (caregiver education, peer mentoring, etc) |
After calibrating judgments on a pilot sample, two raters familiar with the CuCoM (F.M., K.G.), not involved in data collection and blinded to trial results, evaluated each standardized intervention description on a scale of 1 (substantially decrease) to 4 (no effect) to 7 (substantially increase) to reflect the degree to which the intervention was likely to impact patient workload and patient capacity for self-care. The impact on patient capacity was rated with perfect agreement 50% of the time and within 1 point of difference in 42% of cases (8% differed by 2 points). Because no interventions were rated to decrease patient capacity and all average ratings fell within the range of 4 to 5.5, we elected to dichotomize the variable (threshold of ≥5 for increasing capacity) for analysis. Workload was more difficult to assess reliably: perfect agreement and minor disagreement (+/− 1 point) were seen in 29% and 44% of cases, respectively, with 27% of cases differing by 2 or more points. This variable was divided into 3 categories (increase, decrease, or no change).
For each included trial, we extracted or computed the risk of early readmission for each arm, analyzing patients as randomized (intention to treat analysis). We used the number randomized as the denominator except when the number of patients discharged was reported and differed from the number randomized. We selected the outcome to extract based on an ad-hoc hierarchy of outcomes of interest, with priority given to unplanned readmissions, then to all-cause readmissions, and finally to the composite endpoints of unplanned and all-cause readmissions plus out-of-hospital deaths, respectively. Outcomes were extracted and analyzed at the longest period of follow-up, up to 30 days from discharge. Examination of trials reporting the effect of interventions on more than one of these outcomes revealed that treatment effects were consistent across them (data not shown).
Risk of Bias
Two raters (A.L., M.K.) worked independently and in duplicate to determine the extent to which each trial was at risk of bias using a standardized form based on the Cochrane Collaboration’s tool.12 The assessment considered the quality of the randomization sequence generation, allocation concealment, blinding of outcome assessors, the potential for missing outcomes (i.e., likelihood of missing readmissions to other hospitals), and the proportion of patients lost to follow-up. For missing outcomes, “high risk of bias” was assigned when the readmissions data came from internal health system records only. To assess for publication bias, we examined a funnel plot for asymmetry and conducted Egger’s asymmetry regression13 and determined the associated p-value.
Data Synthesis
We used random effects meta-analyses to estimate pooled risk ratios and 95% confidence intervals for early readmission.14,15 We tested for heterogeneity of effect on this outcome using the Cochran Q chi-square test16 and estimated between-trial inconsistency not due to chance using the I2 statistic.17
To explore the effects of patient, intervention, and outcome characteristics on the impact of measured intervention effectiveness, we conducted planned subgroup analyses, testing variables one at a time.
Patient characteristics tested were age (average ≥65 or not), diagnosis (heart failure or other), and hospital ward (general medical or other). Intervention characteristics tested included the number of unique activities involved in the intervention, the number of unique individuals/roles meaningfully involved in its delivery, the minimum number of meaningful patient interactions occurring within 30 days, the location of the intervention activity (i.e. whether it occurred entirely during the inpatient stay, after discharge, or as a combination that “bridged” the transition), whether the intervention was rated to increase or decrease patient workload, and whether the intervention was rated to increase patient capacity (no intervention was found that decreased patient capacity for self-care). Ad-hoc variables tested were year of publication and type of outcome reported (i.e. unplanned readmissions vs. other).
Informed by the findings of the exploratory subgroup analyses and our initial hypotheses, we constructed a post-hoc meta-regression model to test a variable that reflected the degree to which discharge interventions provided comprehensive patient and caregiver support. This “comprehensive support” variable could return values within a range of 0 to 4 “points” based on whether the intervention 1.) was rated to increase patient capacity, 2.) had ≥5 (75th % of distribution) unique intervention activities, 3.) had ≥5 (75th % of distribution) meaningful patient contacts, and 4.) had ≥2 (75th % of distribution) individuals involved in its delivery. We created three categories for this variable: interventions with zero points (Category 1), interventions with one or two points (Category 2), and interventions with three or four points (Category 3). To control for changes in standard care delivery over time, we adjusted based on the year of publication variable.
RESULTS
Study Selection
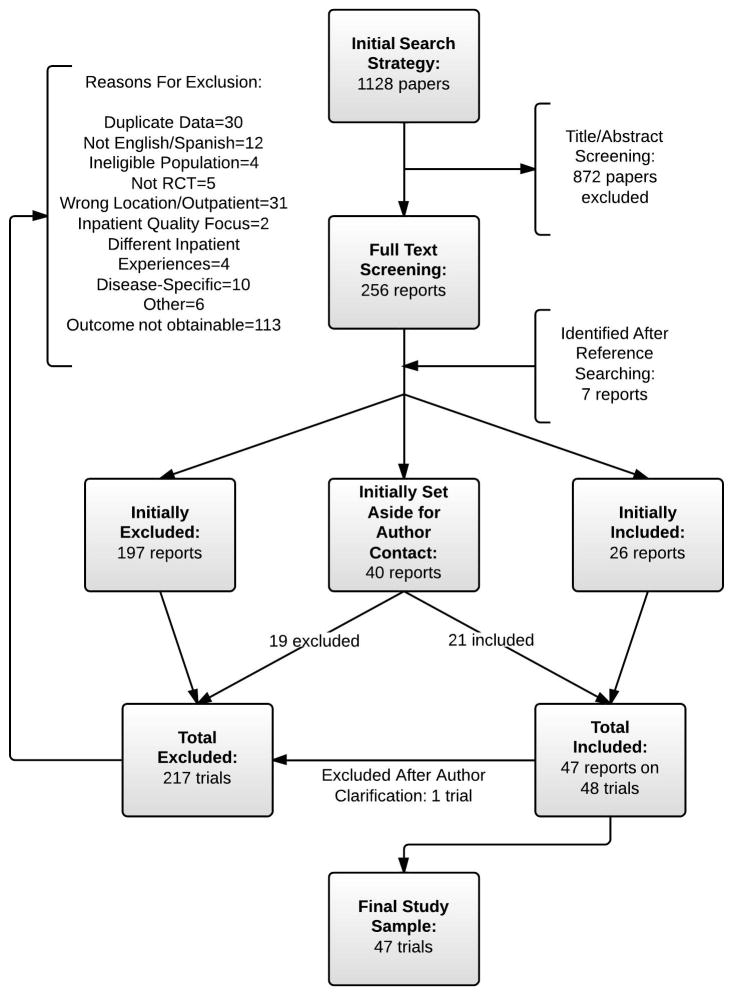
Our initial database search generated 1128 reports (Figure 2). Through abstract and title screening, 256 reports were identified for full-text review. During full-text screening (agreement 89%), 24 were selected for inclusion, and 39 were set aside for author contact prior to making a decision. Of 7 potentially eligible studies identified from bibliographies and expert consultation, 2 were included, and 1 was set aside for author contact. Of the 40 trials requiring author contact for a final eligibility decision, 21 were deemed eligible. Of the 48 apparently eligible trials, one was found ineligible after the author confirmed that readmission data was only collected for readmissions related to the index diagnosis.18 The final sample therefore comprised 47 trials from 46 reports.19–64
Figure 2.
Summary of evidence search and selection. Note that typical papers initially set aside for author inclusion were those that did not report the outcome of interest within 30 days but reported other outcomes within this time, reported the outcome within a survival analysis graph but without information about the number of patients at risk, or reported the outcome as a component of a composite outcome.
Of the 47 eligible trials, 42 contributed data for the primary meta-analysis, and 5 (those that reported numbers of readmissions rather than the number of patients readmitted) were analyzed separately.31,45,50,55,61 A complete list of excluded full-text studies and rationale for exclusion is available in Supplement B.
Study Characteristics
Table 2 describes the included trials. Many were single-center trials taking place in academic medical centers, enrolling few patients (e.g., 21 trials enrolled <200 patients), and reporting 30-day readmissions. Most interventions tested took place in both the inpatient and outpatient settings. Supplement C reports the results of the coded activity analysis. In general, interventions included anywhere from 1 to 7 unique activities. Case management, patient education, home visits, and self-management support were commonly present in net activity descriptions (Supplement C). Trial authors responded to confirmation requests for 34 of the 47 net intervention descriptions. Three authors requested minor modifications and one author made major modifications to these descriptions.
Table 2.
Study Characteristics
| [Ref] Author, Year (*cluster randomized) |
Setting | Population | Intervention | Outcome, Time reported |
N | # Activities | # People | # Interaction | Capacity | Workload | Location |
|---|---|---|---|---|---|---|---|---|---|---|---|
| 19 Melton, 2012 | 48 different states, USA | Commercially insured for 3 acute DRG’s | Risk-prioritized telephone follow-up | UR, 1 month | 3988 | 1 | 1 | 1 | 4 | 3.5 | Out |
| 20 Marusic, 2013 | Academic hospital in Croatia | Elderly pts on two or more meds for chronic disease | Specialized pharmacotherapeutic counseling | UR, 1 month | 160 | 2 | 1 | 1 | 4 | 3.5 | In |
| 21 Altfeld, 2012 | USA | Elderly pts with 7+ meds and psychosocial need | Targeted telephone follow-up program | ACR, 1 month | 906 | 2 | 1 | 1 | 5 | 2 | Out |
| 22 Davis, 2012 | Academic center, USA | Heart failure pts with mild cognitive impairment | Self-management focused education program | ACR, 1 month | 125 | 5 | 1 | 2 | 5 | 3.5 | Both |
| 23 Bowles, 2011 | Urban community, USA | Mostly black HF patients under specific home care agency | Telehomecare substitution of traditional home care | ACR, 1 month | 218 | 1 | 2 | 5 | 4 | 5.5 | Out |
| 24 *Finn, 2011 | Academic center, USA | General medical patients of academic center; about 25% discharged to SNF | Embedded nurse practitioner into academic team to improve discharge process | ACR, 1 month | 646 | 2 | 1 | 1 | 4 | 2.5 | In |
| 25 Wong, 2011 | Large general hospital, Hong Kong | Elderly general medical population in Hong Kong | Use nurse case managers and trained volunteers to improve transition through health-social partnership | ACR, 1 month | 686 | 4 | 3 | 5 | 4.5 | 3.5 | Both |
| 26 Leventhal, 2011 | University hospital in Switzerland | Elderly heart failure patients | Outpatient, interdisciplinary education and support program | ACR, 1 month | 34 | 5 | 1 | 4 | 4.5 | 4 | Out |
| 27 Rytter, 2010 | Single center in Denmark | Elderly patients from medical or geriatric ward | Use mandatory home visits to improve follow up from PCP and district nurses | ACR, 1 month | 331 | 4 | 2 | 2 | 4 | 2.5 | Out |
| 28 Koehler, 2009 | Academic center, USA | Elderly gen med pts with expected return to home or assisted living | Supplemental care bundle that shifted responsibilities from nurses to care coordinators and added follow-up | UR, 1 month | 41 | 6 | 2 | 5 | 4.5 | 4 | Both |
| 29 Braun, 2009 | Medical center, Israel | General medical patients | Use tight telephone follow-up, especially to improve compliance | ACR, 1 month | 400 | 1 | 1 | 2 | 4.5 | 3 | Out |
| 30 Courtney, 2009 | Tertiary center, Australia | Elderly general medical patients at high risk | Individualized, exercise-based care plan for elderly | UR, 1 month | 128 | 6 | 2 | 5 | 5 | 5 | Both |
| 31 Jack, 2009 | Academic, urban, safety net center, USA | General medical patients; 51% black | Standardized discharge package to minimize failures using discharge planners and pharmacists | ACRE, 1 month | 738 | 6 | 2 | 3 | 5 | 2.5 | Both |
| 32 Wakefield, 2008 | VA medical center in Iowa, USA | Males with heart failure; average age 69 | Telehealth-facilitated postdischarge support program | ACR, 1 month | 148 | 2 | 1 | 5 | 5 | 5 | Out |
| 33 Balaban, 2008 | Small community teaching hospital, USA | Culturally and linguisitically diverse general medical or surgical patients | Program to promptly reconnect patients to medical home through discharge form | ACR, 1 month | 96 | 3 | 2 | 2 | 5 | 2.5 | Both |
| 34 Wong, 2008 | Three regional hospitals in Hong Kong | Elderly patients readmitted to department of medicine | Preventive, post-discharge home visits for high risk patients | UR, 1 month | 354 | 2 | 1 | 2 | 4 | 4 | Both |
| 35 Coleman, 2006 | Single center, USA | Elderly medical patients that were in capitated delivery system; about 20% discharged to SNF | Use of Transition Coaches™ and personal health record to equip patients and caregivers to be more active in care | UR, 1 month | 750 | 5 | 1 | 5 | 4.5 | 5 | Both |
| 36 Linne, 2006 | Multiple community hospitals in Sweden | Heart failure patients; discharge disposition not reported | Use computer-based education session in discharge process | ACR, 1 month | 230 | 2 | 0 | 0 | 4 | 4.5 | Both |
| 37 Casas, 2006 | Two tertiary centers; one in Spain and one in Belgium | Elderly COPD patients | Integrated care plan to generate synergy and avoid redundancy between inpatient and outpatient care teams for COPD patients | ACR, 1 month | 155 | 7 | 3 | 5 | 5 | 4.5 | Both |
| 38 Riegel, 2006 | Two community hospitals in Southern California, USA | Mexican Americans with HF that were old and ill and poorly accultured | Telephone case management program to improve discharge transition in Mexican Americans | ACR, 1 month | 135 | 5 | 1 | 1 | 5.5 | 3 | Out |
| 39 Koelling, 2005 | University hospital, USA | Select HF patients; average age 65 | Single pre-discharge education session | ACR, 1 month | 223 | 2 | 1 | 1 | 4.5 | 4.5 | In |
| 40 Mejhert, 2004 | University hospital, Sweden | Elderly heart failure patients | Nurse-driven, protocol-based outpatient management program | ACR+D, 1 month | 196 | 3 | 2 | 1 | 5 | 4.5 | Out |
| 41 Kwok, 2004 | Two acute hospitals in Hong Kong | Elderly chronic lung disease patients at high-risk | Community nurse-supported program based on weekly home visits | UR, 1 month | 157 | 4 | 1 | 5 | 5.5 | 4.5 | Both |
| 42 *Doughty, 2002 | Single center in New Zealand | HF patients; dispositions not reported | Outpatient, integrated management program for heart failure | ACR, 1 month | 197 | 6 | 3 | 1 | 4.5 | 5 | Out |
| 43 Jaarsma, 1999 | University Hospital, Netherlands | Elderly heart failure patients; average age 73 | Nurse-led education and support program with follow-up home visit | ACR, 1 month | 179 | 3 | 1 | 3 | 4 | 3.5 | Both |
| 44 Naylor, 1999 | Two urban academic hospitals | Elderly medical and surgical patients; 45% black | Advanced practice nurse-directed program that stressed continuity, home and telephone follow-up | ACR, 1 month | 363 | 5 | 1 | 4 | 5.5 | 2.5 | Both |
| 45 Stewart, 1998 | Tertiary referral center in Australia | General medical and surgical patients; 83% considered high risk | Risk-targeted, home-based intervention by nurse and pharmacist | UR+DE, 1 month | 762 | 5 | 2 | 3 | 5 | 2.5 | Both |
| 46 Dunn, 1995 | Geriatric hospital in England | Geriatric ward patients with average age of 83 | Single home visit from public health nurse | ACR, 1 month | 204 | 2 | 1 | 1 | 4.5 | 3 | Out |
| 47 Rich, 1995 | Single academic center, USA | High risk, elderly heart failure patients | Nurse-directed, multidisciplinary intervention with home visit follow-up | ACR, 1 month | 274 | 6 | 4 | 5 | 5.5 | 2 | Both |
| 48 Naylor (med), 1994 | Single University hospital, USA | Elderly patients with or without caregiver for medical cardiac diagnosis | Individualized, comprehensive program directed by clinical nurse specialists, including home follow-up | ACR, 2 weeks | 142 | 5 | 1 | 4 | 4.5 | 2 | Both |
| 48 Naylor (surg), 1994 | Single University hospital, USA | Elderly patients with or without caregiver for surgical cardiac diagnosis | Individualized, comprehensive program directed by clinical nurse specialists, including home follow-up | ACR, 2 weeks | 134 | 5 | 1 | 4 | 4.5 | 2 | Both |
| 49 Naylor, 1990 | Urban medical center, USA | Elderly general medical or surgical patients | Comprehensive, individualized discharge planning protocol with home follow-up directed by nurse specialists | ACR, 2 weeks | 40 | 4 | 1 | 4 | 4 | 3 | Both |
| 50 Kulshreshtha, 2010 | Urban teaching hospital, USA | Heart failure patients; could enter study up to 2 weeks post discharge | Remote monitoring follow-up program for ambulatory patients | ACRE, 1 month | 150 | 2 | 1 | 5 | 5.5 | 5.5 | Out |
| 51 *Graumlich, 2009 | Tertiary teaching hospital, USA | General medical patients at high-risk of readmission | Discharge software to improve communication and address deficiencies | ACR, 1 month | 631 | 3 | 1 | 0 | 4 | 3 | In |
| 52 Atienza, 2004 | Three tertiary University hospitals, Spain | Heart failure patients; average age 68 | Hospital discharge and outpatient disease management program | ACR, 1 month | 338 | 5 | 2 | 2 | 4.5 | 4 | Both |
| 53 Riegel, 2004 | Two hospitals in suburban Southwest, USA | Heart failure patients in integrated health system; average age 73 | Use of peer mentors to improve self-care in recently-discharged patients | ACR, 1 month | 88 | 1 | 1 | 2 | 4.5 | 3 | Out |
| 54 Stowasser, 2002 | Two large hospitals in Australia | General medical and surgical patients | Medication liaison service to improve communication of medication-related issues through discharge process | UR, 1 month | 240 | 3 | 1 | 0 | 4 | 3 | In |
| 55 Li, 2012 | Academic center in New York, USA | Elderly patients and their family caregivers | Training of family caregivers to prepare for anticipated post-discharge role | ACRE, 2 weeks | 407 | 1 | 0 | 0 | 4.5 | 3.5 | In |
| 56 Shyu, 2005 | Large, single center in Taiwan | Elderly hip fracture patients | Interdisciplinary program of geriatric consultation, rehab, and discharge planning service | ACR, 1 month | 137 | 4 | 3 | 5 | 5.5 | 3.5 | Both |
| 57 Angermann, 2012 | Nine centers in Germany | Heart failure patients; average age 69 | Nurse-coordinated disease management program that emphasized a “call and care center” | ACR, 1 month | 715 | 4 | 1 | 5 | 4.5 | 4.5 | Both |
| 58 Naylor, 2004 | Six academic and community hospitals, USA | Elderly HF patients; 36% black | Advanced practice nurse-directed care program with emphasis on comorbid and chronic condition management; included home follow-up | ACR+D, 1 month | 239 | 6 | 1 | 5 | 5 | 3 | Both |
| 59 Stromberg, 2003 | One University and two county hospitals in Sweden | Elderly heart failure patients | Requisite follow-up in specialized, protocol-driven, nurse-led heart failure clinic | ACR+D, 1 month | 106 | 5 | 1 | 1 | 5 | 3.5 | Out |
| 60 Hansen, 1995 | University Hospital in Denmark | Highly selected patients from subacute geriatric ward needing home rehab, medical, and social support | Home visit follow-up program for highly-targeted elderly population | ACR, 1 month | 193 | 4 | 2 | 2 | 5 | 3.5 | Out |
| 61 *Maslove, 2009 | Single academic center, Canada | General medical patients; about 80% discharged home | Development of more useful and standardized discharge summary | ACRE, 1 month | 209 | 2 | 1 | 0 | 4 | 3.5 | In |
| 62 Forster, 2005 | Two campuses of a teaching hospital in Canada | General medical patients; average age 66 | Integration of dedicated clinical nurse specialist into care team to facilitate discharge planning process | ACR+D, 1 month | 361 | 3 | 1 | 1 | 4 | 3 | Both |
| 63 Dudas, 2001 | Single academic center, USA | General medical service patients | Pharmacy service follow-up call | ACR, 1 month | 221 | 2 | 1 | 1 | 4 | 3 | Out |
| 64 Parry, 2009 | Two community hospitals, USA | Fee for service Medicare patients in single health system; inclusion criteria desired to catch patients discharging to SNF—did not report dispositions | Use of Transition Coaches™ and personal health record to equip patients and caregivers to assert more active role in care transition | UR, 1 month | 98 | 5 | 1 | 5 | 5 | 2.5 | Both |
#Activities=number of activities in the intervention as evaluated by coding strategy from Table 1
#People=number of individuals meaningfully involved in delivery of the intervention
#Interactions=minimum number of meaningful human interactions in intervention delivery
Capacity=rated likelihood of intervention to affect patient capacity for self-care on scale of 1 to 7, where 4 is no change
Workload=rated likelihood of intervention to impose work or burden on patient on scale of 1 to 7, where 4 is no change
Location=setting (inpatient, outpatient, or both) where intervention activity occurred
ACR=all cause readmission rate; UR=unplanned readmission rate; ACR+D=all cause readmission and out of hospital deaths rate; ACRE=all cause readmission event count; UR+DE=unplanned readmission and out of hospital deaths event count; N=total number of analyzed patients from both arms (number discharged); In=all activity occurred in inpatient environment; Out=all activity occurred in outpatient environment; Both=activity occurred in both inpatient and outpatient environment
Most studies were at low risk of bias (Supplement D). The most common methodological limitation of these trials was the lack of a reliable method for dealing with missing data.
Meta-analysis
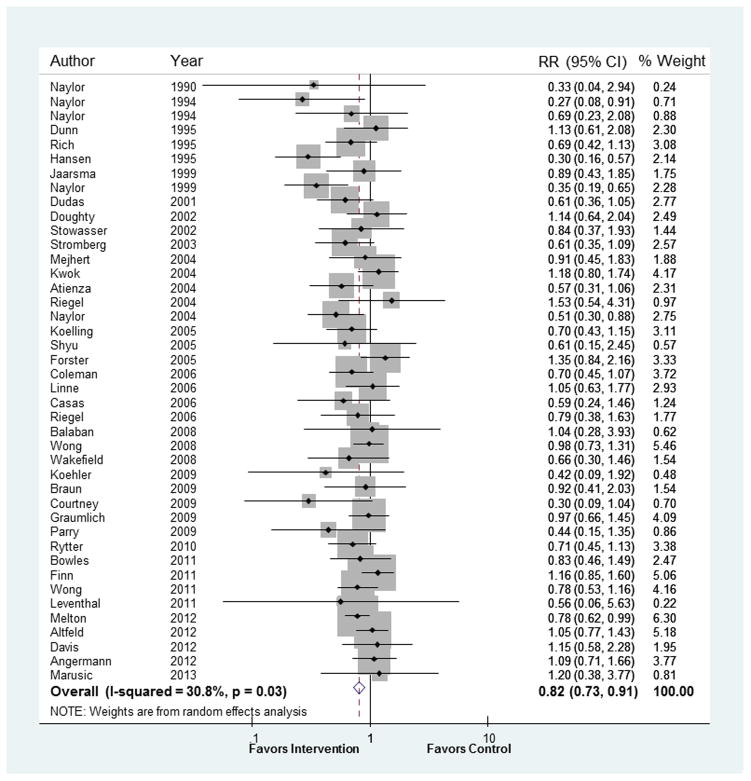
In the 42 trials reporting readmission rates, the overall pooled relative risk (RR) of readmission within 30 days was 0.82, 95% CI 0.73 to 0.91; p<.001 (Figure 3). Inconsistency across trials was low (I2=31%). Funnel plot examination showed asymmetry suggestive of publication bias in the context of smaller studies (see Supplement E) and Egger’s test was significant (p=0.02). The five trials reporting number of readmissions (rather than number of patients with readmissions) had a pooled relative risk of readmission of 0.93, 95% CI 0.72 to 1.20, I2=23%, p=0.59. While this result was consistent with the risk found in trials reporting readmission rates (pinteraction= 0.38), we opted not to include these trials in subgroup analyses.
Figure 3.
Results of primary meta-analysis. RR=relative risk.
Subgroup analyses failed to find an interaction between trial results and patient characteristics or outcome measured (Table 3). A number of intervention characteristics, however, did interact with measured effectiveness. These include whether the intervention was rated to augment patient capacity for self-care [RR 0.68, 95% CI 0.53 to 0.86 when it was and RR 0.88, 95% CI 0.80 to 0.97 when it was not; pinteraction=0.04], whether the intervention had at least five unique, component activities [RR 0.63, 95% CI 0.53 to 0.76 when it did and RR 0.91, 95% CI 0.81 to 1.01 when it did not; pinteraction<0.01], and whether the intervention had at least two individuals involved in delivery [RR 0.69, 95% CI 0.57 to 0.84 when it did and RR 0.87, 95% CI 0.77 to 0.98 when it did not; pinteraction=0.05]. Studies testing interventions more recently were associated with reduced effectiveness [RR 0.89, 95% CI 0.81 to 0.97 when published in 2002 or later and RR 0.56, 95% CI 0.40 to 0.79 when published prior to 2002; pinteraction=0.01] Other characteristics of the interventions, such as their rated effect on patient workload, and the site of delivery had no significant interaction with the intervention effect.
Table 3.
Subgroup Analyses
| Category | Study Subgroup Characteristic | Number of Studies in Subgroup* | Ratio of Relative Risks of Readmission+ (95% CI) | P Value for Interaction |
|---|---|---|---|---|
| Patient Characteristics | Patients Enrolled had CHF | 16 | 1.02 (0.82 to 1.27) | 0.83 |
| Patients Enrolled were Aged >65 | 36 | 0.87 (0.69 to 1.10) | 0.24 | |
| Patients Enrolled were from General Medical Ward | 18 | 0.97 (0.78 to 1.21) | 0.79 | |
| Intervention Characteristics | Intervention was Rated to Increase Capacity | 16 | 0.76 (0.59 to 0.99) | 0.04 |
| Intervention was Rated to Increase Workload | 5# | 0.93 (0.67 to 1.29) | 0.68 | |
| Intervention was Rated to Decrease Workload | 19# | 0.99 (0.78 to 1.25) | 0.90 | |
| b Study was Published in 2001 or Later | 24 | 1.59 (1.05 to 2.40) | 0.03 | |
| a Two or More Individuals Delivered the Intervention | 13 | 0.79 (0.63 to 1.00) | 0.05 | |
| a Five or More Meaningful Patient Interactions in the Intervention | 13 | 0.91 (0.73 to 1.14) | 0.43 | |
| a Five or More Unique Activities in the Intervention | 16 | 0.70 (0.56 to 0.86) | <0.01 | |
| Intervention had Both an Inpatient and Outpatient Component | 22 | 0.92 (0.74 to 1.15) | 0.46 | |
| Outcome Characteristics | Outcome Measured was Unplanned Readmissions | 9^ | 0.95 (0.76 to 1.21) | 0.70 |
This represents the ratio of pooled relative risks of early hospital readmission among studies that had a certain characteristic as compared to those that did not have this characteristic.
Compared to the remainder of analyzed studies (e.g. 42-N) unless otherwise noted.
Compared to “No Change,” (N=18).
Compared to “All-Cause,” (N=30).
These cut-offs were chosen as they represented the 75th percentile of distribution of each variable.
This cut-off was chosen as it represented the mid-point of study eligibility for this review.
Post-hoc Meta-Regression Analysis
Despite potential colinearity of the contributing variables, meta-regression showed a significant and incremental effect of “comprehensive support” on reducing readmissions (Table 4). Seven interventions comprised Category 328,30,37,47,56,58,64. Compared to Category 1 interventions, these were associated with a relative risk of readmission of 0.63, 95% CI 0.43 to 0.93; p=0.02. Category 3 interventions used a consistent and complex strategy that emphasized the assessment and addressing of factors related to patient context and capacity for self-care (including the impact of comorbidities, functional status, caregiver capabilities, socioeconomic factors, potential for self-management, and patient and caregiver goals for care). These interventions coordinated care across the inpatient to outpatient transition and involved multiple patient interactions; all but one28 involved patient home visits.
Table 4.
Effects of “Comprehensive Support” in Meta-Regression Analysis
| Study Characteristic | Number of Studies | Relative Risk of Readmission Compared to Reference^ | 95% Confidence Interval | P value |
|---|---|---|---|---|
| Comprehensive Support Category 1 (0 points) | 15 | 1 (reference) | ||
| Comprehensive Support Category 2 (1 or 2 points) | 20 | 0.82 | 0.66 to 1.02 | 0.07 |
| Comprehensive Support Category 3 (3 or 4 points) | 7 | 0.63 | 0.43 to 0.93 | 0.02 |
| Publication in 2002 or After | 33 | 1.47 | 1.10 to 1.96 | 0.01 |
The “Comprehensive Support” variable returned one point each for interventions that 1.) were rated to increase patient capacity, 2.) had five or more unique intervention activities, 3.) had five or more meaningful patient interactions, and 4.) had two or more individuals involved in its delivery.
This represents the adjusted effect of each characteristic on early readmission
DISCUSSION
Our Findings
The body of randomized trial evidence shows a consistent and beneficial effect of tested interventions on the risk of 30-day readmissions. Exploratory subgroup analyses suggest that effective interventions are more complex and seek to enhance patient capacity to reliably access and enact post-discharge care. Additionally, interventions tested more recently are, in general, less efficacious when compared to controls.
Our findings are consistent with the CuCoM in their suggestion that providing comprehensive and context-sensitive support to patients reduces the risk of early hospital readmission; however, we could not identify an effect of rated intervention workload on this risk.
Limitations and strengths of this review
Many studies in this review were conducted in single, academic centers; this raises questions about applicability. Also, the scales we used to evaluate intervention effects on patient workload and capacity relied on global judgments (rather than criterion-based judgments) and are original to this work. To our knowledge, no validated scale exists to assess the potential of an intervention to impose patient workload or treatment burden and/or affect a patient’s capacity for self-care. Although our raters were consistent in their assessments of interventions’ effect on patient capacity, their judgment of impact on patient workload was less reliable. Particularly, raters felt that some burdensome interventions could be beneficial if the patient had the capacity and resources to access and enact the care. Because the experience of treatment burden is not constant between patients, an ideal analysis of its effects would be based on patient-reported assessments of intervention workload. Indeed, many eligible patients declined enrollment into some studies23,28,44,50,53, often because they were not agreeable to the perceived burden of the intervention; evaluating the effect of intervention-imposed workload in such samples is of limited applicability. In general, these assessments should be regarded as hypothesis-generating and the inferences made based on subgroup analyses must be viewed as tentative (given the borderline significance of the analyses, the potential for chance findings from testing multiple hypotheses, and the possibility that some variables are correlated). Finally, despite robust efforts to obtain unpublished data, there was evidence of publication bias. The overall effect of this on our findings is not known.
This review also has many strengths. First, it provides the largest and most comprehensive assessment of discharge interventions and their effect on 30-day readmissions, including 47 randomized trials at low risk of bias. This is a stronger and less heterogeneous body of evidence than previously assembled11,65 and it includes unpublished data from 18 trials. Our study used an activity-based coding method designed to ensure appropriate characterization of each intervention and their net activities when comparing the intervention and control arms. This method contributes to the field and can be applied to future assessments of complex interventions. To our knowledge, this is also the first use of the CuCoM5 to analyze the impact of healthcare delivery interventions on patients as an explanation for their relative efficacy.
Comparison with other studies
We identified 31 more RCT’s than were accumulated in the most recent review of discharge intervention effects on 30 day readmission rates11 and we provide the first meta-analysis on this topic. Although previous studies and reviews have suggested that “bundled” interventions are of greater value,11,65 this meta-analysis provides objective support for this claim. Additionally, our study adds to and enhances the body of evidence related to the importance of patient contextual factors in affecting health outcomes.66
Implications for policy and practice
In this analysis, interventions that used a complex and supportive strategy to assess and address contextual issues and limitations in patient capacity were most effective at reducing early hospital readmissions. Many of these contacted the patient frequently, used home visits, and reported cost savings. This information can be used to guide the design and testing of future interventions. The CuCoM may also have value in helping to conceptualize the effects of healthcare interventions across diverse patient contexts, but we were unable to characterize a consistent effect of rated intervention workload on outcomes. Finally, we found that more recently tested interventions were less effective. We hypothesize that this may represent 1.) a general improvement over time in the standard of care that was not fully appreciated in control descriptions, 2.) an increased effort over time to test simpler and less comprehensive interventions, 3.) a higher likelihood over time of more diverse interventions to measure and report 30 day readmission rates (e.g. including those less focused on reducing early readmissions) and/or, 4.) a general shift away from interventions stressing human interaction toward those more “high tech” in nature. Further study is needed to determine the implications of this finding.
Conclusions
Our results suggest that most interventions tested are effective in reducing the risk of early readmissions. Some features, however, may enhance the effect of these programs. In particular, we found value in interventions that supported patients’ capacity for self-care in their transition from hospital to home. Future work intended to improve the effectiveness of healthcare delivery may benefit from consideration of the demands that healthcare interventions place on recently-discharged patients and their caregivers, and the extent to which these demands are offset by comprehensive support for implementation.
Supplementary Material
Acknowledgments
FUNDING
This publication was made possible by CTSA Grant Number UL1 TR000135 from the National Center for Advancing Translational Sciences (NCATS), a component of the National Institutes of Health (NIH). Its contents are solely the responsibility of the authors and do not necessarily represent the official view of NIH. The funding source did not participate in any part of this study.
The authors thank the following for providing unpublished data, conducting secondary analyses, assisting with study identification, and/or providing guidance and support: Agneta Björck Linné and Hans Liedholm (Malmö University Hospital, Sweden); Marcia E Leventhal, Sabina De Geest, and Kris Denhaerynck (Institute of Nursing Science, University of Basel, Switzerland); Lars Rytter, MD (Glostrup University Hospital, Denmark); Gillian A Whalley (University of Auckland, New Zealand); David Maslove, MD, FRCPC (University of Toronto, Canada); Dr. Judith Garcia-Aymerich (Universitat Pomeu Fabra-Barcelona, Spain); Bonnie J Wakefield, PhD, RN, FAAN (Iowa City Veterans Affairs Healthcare System); Kathleen Finn, MD (Massachusetts General Hospital Department of Medicine, Boston); Jon C Tilburt, MD, MPH (Mayo Clinic); Prof. Dr. med. Christiane E Angermann (Universitätsklinikum Würzburg, Denmark); Felipe Atienza, MD, PhD (Hospital General Universitario Gregorio Maranon-Madrid, Spain); Dan Gronseth (Mayo Clinic); Michael W Rich, MD (Washington University, St. Louis); Andrew Masica, MD, MSCI (Baylor Health Care System); Dr. Karen B Hirschman and Dr. Mary D Naylor (University of Pennsylvania School of Nursing); James F Graumlich, MD, FACP (University of Illinois College of Medicine at Peoria); Anna Strömberg (Linköping University Hospital, Sweden)
Footnotes
Potential conflicts of interest: The study authors report no conflicts of interest.
Author contributions: Conceptualization and study design; AL, VM. Search strategy development and conduction; PE, AL, VM, TS, MM. Report screening; AL, MG, JB, TS. Data extraction; AL, MG, JB. Intervention coding; AL, MG. Capacity and workload rating; FM, KG. Author correspondence: AL, MK. Meta-analysis oversight: MM, VM. Data analysis; ZW, AL. Quality and risk of bias assessments; MK, AL. Manuscript preparation; AL, VM, JB, FM, KG, HT, MG, MM, KB, TS, NS. Substantive collaboration and manuscript approval; all authors.
Data access, responsibility, and analysis: Dr. Leppin and Dr. Montori had full access to all the data in the study and take responsibility for the integrity of the data and the accuracy of the data analysis.
Online supplemental materials: A. the complete search strategy, B. a listing and rationale for full text exclusions, C. a summary of intervention coding, D. the risk of bias assessments, and E. a funnel plot for testing of publication bias
Contributor Information
Aaron L. Leppin, Email: Leppin.Aaron@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Michael R. Gionfriddo, Email: Gionfriddo.Michael@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Maya Kessler, Email: Kessler.Maya@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Juan Pablo Brito, Email: Brito.Juan@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Frances S. Mair, Email: Frances.Mair@glasgow.ac.uk, 1 Horselethill Road; Glasgow G12 9LX; Scotland, UK.
Katie Gallacher, Email: Katie.Gallacher@glasgow.ac.uk, 1 Horselethill Road; Glasgow G12 9LX; Scotland, UK.
Zhen Wang, Email: Wang.Zhen@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Patricia J. Erwin, Email: Erwin.Patricia@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Tanya Sylvester, Email: tsylvest@slu.edu, 1402 S. Grand Blvd; St. Louis, Missouri 63104.
Kasey Boehmer, Email: Boehmer.Kasey@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Henry H. Ting, Email: Ting.Henry@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
M. Hassan Murad, Email: Murad.Mohammad@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
Nathan D. Shippee, Email: nshippee@umn.edu, D375 Mayo MMC 729, 420 Delaware St SE; Minneapolis, MN 55455.
Victor M. Montori, Email: Montori.Victor@mayo.edu, 200 First Street SW; Rochester, Minnesota 55905.
References
- 1.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009 Apr 2;360(14):1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 2.Institute of Medicine. Rewarding Provider Performance: Aligning Incentives in Medicare. Washington, DC: 2006. [Google Scholar]
- 3.Medicare Payment Advisory Commission. Report to the Congress: promoting greater efficiency in Medicare. 2007. [Google Scholar]
- 4.Joynt KE, Jha AK. A path forward on Medicare readmissions. N Engl J Med. 2013 Mar 28;368(13):1175–1177. doi: 10.1056/NEJMp1300122. [DOI] [PubMed] [Google Scholar]
- 5.Shippee ND, Shah ND, May CR, Mair FS, Montori VM. Cumulative complexity: a functional, patient-centered model of patient complexity can improve research and practice. J Clin Epidemiol. 2012 Oct;65(10):1041–1051. doi: 10.1016/j.jclinepi.2012.05.005. [DOI] [PubMed] [Google Scholar]
- 6.Gallacher K, May CR, Montori VM, Mair FS. Understanding patients’ experiences of treatment burden in chronic heart failure using normalization process theory. Ann Fam Med. 2011 May-Jun;9(3):235–243. doi: 10.1370/afm.1249. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sav A, Kendall E, McMillan SS, et al. ‘You say treatment, I say hard work’: treatment burden among people with chronic illness and their carers in Australia. Health Soc Care Community. 2013 May 23; doi: 10.1111/hsc.12052. [DOI] [PubMed] [Google Scholar]
- 8.Krumholz HM. Post-hospital syndrome--an acquired, transient condition of generalized risk. N Engl J Med. 2013 Jan 10;368(2):100–102. doi: 10.1056/NEJMp1212324. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Leppin AL, Ting HH, Gionfriddo MR, et al. Describing the usefulness and efficacy of discharge interventions: predicting 30 day readmissions through application of the cumulative complexity model (protocol) 2013 http://minimallydisruptivemedicine.org/bibliography/
- 10.Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. PLoS Med. 2009 Jul 21;6(7):e1000097. doi: 10.1371/journal.pmed.1000097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hansen LO, Young RS, Hinami K, Leung A, Williams MV. Interventions to reduce 30-day rehospitalization: a systematic review. Ann Intern Med. 2011 Oct 18;155(8):520–528. doi: 10.7326/0003-4819-155-8-201110180-00008. [DOI] [PubMed] [Google Scholar]
- 12.Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. Bmj. 2011;343:d5928. doi: 10.1136/bmj.d5928. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sterne JA, Egger M. Funnel plots for detecting bias in meta-analysis: guidelines on choice of axis. J Clin Epidemiol. 2001 Oct;54(10):1046–1055. doi: 10.1016/s0895-4356(01)00377-8. [DOI] [PubMed] [Google Scholar]
- 14.DerSimonian R, Kacker R. Random-effects model for meta-analysis of clinical trials: An update. Contemporary Clinical Trials. 2007;28(2):105–114. doi: 10.1016/j.cct.2006.04.004. [DOI] [PubMed] [Google Scholar]
- 15.Mantel N, Haenszel W. Statistical aspects of the analysis of data from retrospective studies of disease. J Natl Cancer Inst. 1959 Apr;22(4):719–748. [PubMed] [Google Scholar]
- 16.Cohran WG. Some methods for strengthening the common x2 tests. Biometrics. 1954:10417–10451. [Google Scholar]
- 17.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. Bmj. 2003 Sep 6;327(7414):557–560. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Herrera-Espineira C, Rodriguez del Aguila MDM, Navarro Espigares JL, et al. Effect of a telephone care program after hospital discharge from a trauma surgery unit. [Spanish] Efecto de un programa de atencion telefonica tras el alta hospitalaria de una unidad de cirugia traumatologica. Gaceta Sanitaria. 2011 Mar-Apr;25(2):133–138. doi: 10.1016/j.gaceta.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 19.Melton LD, Foreman C, Scott E, McGinnis M, Cousins M. Prioritized post-discharge telephonic outreach reduces hospital readmissions for select high-risk patients. The American journal of managed care. 2012 Dec;18(12):838–844. [PubMed] [Google Scholar]
- 20.Marusic S, Gojo-Tomic N, Erdeljic V, et al. The effect of pharmacotherapeutic counseling on readmissions and emergency department visits. International journal of clinical pharmacy. 2013 Feb;35(1):37–44. doi: 10.1007/s11096-012-9700-9. [DOI] [PubMed] [Google Scholar]
- 21.Altfeld SJ, Shier GE, Rooney M, et al. Effects of an Enhanced Discharge Planning Intervention for Hospitalized Older Adults: A Randomized Trial. The Gerontologist. 2012 Sep 7; doi: 10.1093/geront/gns109. [DOI] [PubMed] [Google Scholar]
- 22.Davis KK, Mintzer M, Dennison Himmelfarb CR, Hayat MJ, Rotman S, Allen J. Targeted intervention improves knowledge but not self-care or readmissions in heart failure patients with mild cognitive impairment. European journal of heart failure. 2012 Sep;14(9):1041–1049. doi: 10.1093/eurjhf/hfs096. [DOI] [PubMed] [Google Scholar]
- 23.Bowles KH, Hanlon AL, Glick HA, et al. Clinical effectiveness, access to, and satisfaction with care using a telehomecare substitution intervention: a randomized controlled trial. International journal of telemedicine and applications. 2011;2011:540138. doi: 10.1155/2011/540138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Finn KM, Heffner R, Chang Y, et al. Improving the discharge process by embedding a discharge facilitator in a resident team. Journal of hospital medicine: an official publication of the Society of Hospital Medicine. 2011 Nov;6(9):494–500. doi: 10.1002/jhm.924. [DOI] [PubMed] [Google Scholar]
- 25.Wong FK, Ho MM, Yeung S, Tam SK, Chow SK. Effects of a health-social partnership transitional program on hospital readmission: a randomized controlled trial. Soc Sci Med. 2011 Oct;73(7):960–969. doi: 10.1016/j.socscimed.2011.06.036. [DOI] [PubMed] [Google Scholar]
- 26.Leventhal ME, Denhaerynck K, Brunner-La Rocca HP, et al. Swiss Interdisciplinary Management Programme for Heart Failure (SWIM-HF): a randomised controlled trial study of an outpatient inter-professional management programme for heart failure patients in Switzerland. Swiss Med Wkly. 2011;141:w13171. doi: 10.4414/smw.2011.13171. [DOI] [PubMed] [Google Scholar]
- 27.Rytter L, Jakobsen HN, Ronholt F, et al. Comprehensive discharge follow-up in patients’ homes by GPs and district nurses of elderly patients. A randomized controlled trial. Scand J Prim Health Care. 2010 Sep;28(3):146–153. doi: 10.3109/02813431003764466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Koehler BE, Richter KM, Youngblood L, et al. Reduction of 30-day postdischarge hospital readmission or emergency department (ED) visit rates in high-risk elderly medical patients through delivery of a targeted care bundle. Journal of Hospital Medicine (Online) 2009 Apr;4(4):211–218. doi: 10.1002/jhm.427. [DOI] [PubMed] [Google Scholar]
- 29.Braun E, Baidusi A, Alroy G, Azzam ZS. Telephone follow-up improves patients satisfaction following hospital discharge. Eur. 2009 Mar;20(2):221–225. doi: 10.1016/j.ejim.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 30.Courtney M, Edwards H, Chang A, Parker A, Finlayson K, Hamilton K. Fewer emergency readmissions and better quality of life for older adults at risk of hospital readmission: a randomized controlled trial to determine the effectiveness of a 24-week exercise and telephone follow-up program. Journal of the American Geriatrics Society. 2009 Mar;57(3):395–402. doi: 10.1111/j.1532-5415.2009.02138.x. [DOI] [PubMed] [Google Scholar]
- 31.Jack BW, Chetty VK, Anthony D, et al. A reengineered hospital discharge program to decrease rehospitalization: a randomized trial. Ann Intern Med. 2009 Feb 3;150(3):178–187. doi: 10.7326/0003-4819-150-3-200902030-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Wakefield BJ, Ward MM, Holman JE, et al. Evaluation of home telehealth following hospitalization for heart failure: a randomized trial. Telemed J E Health. 2008 Oct;14(8):753–761. doi: 10.1089/tmj.2007.0131. [DOI] [PubMed] [Google Scholar]
- 33.Balaban RB, Weissman JS, Samuel PA, Woolhandler S. Redefining and redesigning hospital discharge to enhance patient care: a randomized controlled study. J Gen Intern Med. 2008 Aug;23(8):1228–1233. doi: 10.1007/s11606-008-0618-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Wong FK, Chow S, Chung L, et al. Can home visits help reduce hospital readmissions? Randomized controlled trial. J Adv Nurs. 2008 Jun;62(5):585–595. doi: 10.1111/j.1365-2648.2008.04631.x. [DOI] [PubMed] [Google Scholar]
- 35.Coleman EA, Parry C, Chalmers S, Min SJ. The care transitions intervention: results of a randomized controlled trial. Arch Intern Med. 2006 Sep 25;166(17):1822–1828. doi: 10.1001/archinte.166.17.1822. [DOI] [PubMed] [Google Scholar]
- 36.Linne AB, Liedholm H. Effects of an interactive CD-program on 6 months readmission rate in patients with heart failure - a randomised, controlled trial [ NCT00311194] BMC Cardiovasc Disord. 2006;6:30. doi: 10.1186/1471-2261-6-30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Casas A, Troosters T, Garcia-Aymerich J, et al. Integrated care prevents hospitalisations for exacerbations in COPD patients. Eur Respir J. 2006 Jul;28(1):123–130. doi: 10.1183/09031936.06.00063205. [DOI] [PubMed] [Google Scholar]
- 38.Riegel B, Carlson B, Glaser D, Romero T. Randomized controlled trial of telephone case management in Hispanics of Mexican origin with heart failure. J Card Fail. 2006 Apr;12(3):211–219. doi: 10.1016/j.cardfail.2006.01.005. [DOI] [PubMed] [Google Scholar]
- 39.Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005 Jan 18;111(2):179–185. doi: 10.1161/01.CIR.0000151811.53450.B8. [DOI] [PubMed] [Google Scholar]
- 40.Mejhert M, Kahan T, Persson H, Edner M. Limited long term effects of a management programme for heart failure. Heart. 2004 Sep;90(9):1010–1015. doi: 10.1136/hrt.2003.014407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Kwok T, Lum CM, Chan HS, Ma HM, Lee D, Woo J. A randomized, controlled trial of an intensive community nurse-supported discharge program in preventing hospital readmissions of older patients with chronic lung disease. J Am Geriatr Soc. 2004 Aug;52(8):1240–1246. doi: 10.1111/j.1532-5415.2004.52351.x. [DOI] [PubMed] [Google Scholar]
- 42.Doughty RN, Wright SP, Pearl A, et al. Randomized, controlled trial of integrated heart failure management: The Auckland Heart Failure Management Study. Eur Heart J. 2002 Jan;23(2):139–146. doi: 10.1053/euhj.2001.2712. [DOI] [PubMed] [Google Scholar]
- 43.Jaarsma T, Halfens R, Huijer Abu-Saad H, et al. Effects of education and support on self-care and resource utilization in patients with heart failure. Eur Heart J. 1999 May;20(9):673–682. doi: 10.1053/euhj.1998.1341. [DOI] [PubMed] [Google Scholar]
- 44.Naylor MD, Brooten D, Campbell R, et al. Comprehensive discharge planning and home follow-up of hospitalized elders: a randomized clinical trial. Jama. 1999 Feb 17;281(7):613–620. doi: 10.1001/jama.281.7.613. [DOI] [PubMed] [Google Scholar]
- 45.Stewart S, Pearson S, Luke CG, Horowitz JD. Effects of home-based intervention on unplanned readmissions and out-of-hospital deaths. Journal of the American Geriatrics Society. 1998 Feb;46(2):174–180. doi: 10.1111/j.1532-5415.1998.tb02535.x. [DOI] [PubMed] [Google Scholar]
- 46.Dunn RB, Guy PM, Hardman CS, Lewis PA, Vetter NJ. Can a house call by a public health nurse improve the quality of the discharge process for geriatric patients? Clin Perform Qual Health Care. 1995 Jul-Sep;3(3):151–155. [PubMed] [Google Scholar]
- 47.Rich MW, Beckham V, Wittenberg C, Leven CL, Freedland KE, Carney RM. A multidisciplinary intervention to prevent the readmission of elderly patients with congestive heart failure. New England Journal of Medicine. 1995 Nov 2;333(18):1190–1195. doi: 10.1056/NEJM199511023331806. [DOI] [PubMed] [Google Scholar]
- 48.Naylor M, Brooten D, Jones R, Lavizzo-Mourey R, Mezey M, Pauly M. Comprehensive discharge planning for the hospitalized elderly. A randomized clinical trial. Ann Intern Med. 1994 Jun 15;120(12):999–1006. doi: 10.7326/0003-4819-120-12-199406150-00005. [DOI] [PubMed] [Google Scholar]
- 49.Naylor MD. Comprehensive discharge planning for hospitalized elderly: a pilot study. Nurs Res. 1990 May-Jun;39(3):156–161. [PubMed] [Google Scholar]
- 50.Kulshreshtha A, Kvedar JC, Goyal A, Halpern EF, Watson AJ. Use of remote monitoring to improve outcomes in patients with heart failure: A pilot trial. International journal of telemedicine and applications. 2010:870959. doi: 10.1155/2010/870959. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Graumlich JF, Novotny NL, Nace GS, et al. Patient readmissions, emergency visits, and adverse events after software-assisted discharge from hospital: Cluster randomized trial. Journal of Hospital Medicine. 2009 Sep;4(7):E11–E19. doi: 10.1002/jhm.469. [DOI] [PubMed] [Google Scholar]
- 52.Atienza F, Anguita M, Martinez-Alzamora N, et al. Multicenter randomized trial of a comprehensive hospital discharge and outpatient heart failure management program. European journal of heart failure. 2004 Aug;6(5):643–652. doi: 10.1016/j.ejheart.2003.11.023. [DOI] [PubMed] [Google Scholar]
- 53.Riegel B, Carlson B. Is individual peer support a promising intervention for persons with heart failure? J Cardiovasc Nurs. 2004;19(3):174–183. doi: 10.1097/00005082-200405000-00006. [DOI] [PubMed] [Google Scholar]
- 54.Stowasser DA, Collins DM, Stowasser M. A randomised controlled trial of medication liaison services - Patient outcomes. Journal of Pharmacy Practice and Research. 2002;32(2):133–140. [Google Scholar]
- 55.Li H, Powers BA, Melnyk BM, et al. Randomized controlled trial of CARE: an intervention to improve outcomes of hospitalized elders and family caregivers. Res Nurs Health. 2012 Oct;35(5):533–549. doi: 10.1002/nur.21491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Shyu YI, Liang J, Wu CC, et al. A pilot investigation of the short-term effects of an interdisciplinary intervention program on elderly patients with hip fracture in Taiwan. J Am Geriatr Soc. 2005 May;53(5):811–818. doi: 10.1111/j.1532-5415.2005.53253.x. [DOI] [PubMed] [Google Scholar]
- 57.Angermann CE, Stork S, Gelbrich G, et al. Mode of action and effects of standardized collaborative disease management on mortality and morbidity in patients with systolic heart failure: the Interdisciplinary Network for Heart Failure (INH) study. Circ. 2012 Jan;5(1):25–35. doi: 10.1161/CIRCHEARTFAILURE.111.962969. [DOI] [PubMed] [Google Scholar]
- 58.Naylor MD, Brooten DA, Campbell RL, Maislin G, McCauley KM, Schwartz JS. Transitional care of older adults hospitalized with heart failure: a randomized, controlled trial.[Erratum appears in J Am Geriatr Soc. 2004 Jul;52(7):1228] Journal of the American Geriatrics Society. 2004 May;52(5):675–684. doi: 10.1111/j.1532-5415.2004.52202.x. [DOI] [PubMed] [Google Scholar]
- 59.Stromberg A, Martensson J, Fridlund B, Levin LA, Karlsson JE, Dahlstrom U. Nurse-led heart failure clinics improve survival and self-care behaviour in patients with heart failure: results from a prospective, randomised trial. Eur Heart J. 2003 Jun;24(11):1014–1023. doi: 10.1016/s0195-668x(03)00112-x. [DOI] [PubMed] [Google Scholar]
- 60.Hansen FR, Poulsen H, Sorensen KH. A model of regular geriatric follow-up by home visits to selected patients discharged from a geriatric ward: a randomized controlled trial. Aging Clin. 1995 Jun;7(3):202–206. doi: 10.1007/BF03324316. [DOI] [PubMed] [Google Scholar]
- 61.Maslove DM, Leiter RE, Griesman J, et al. Electronic versus dictated hospital discharge summaries: a randomized controlled trial. J Gen Intern Med. 2009 Sep;24(9):995–1001. doi: 10.1007/s11606-009-1053-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Forster AJ, Clark HD, Menard A, et al. Effect of a nurse team coordinator on outcomes for hospitalized medicine patients. Am J Med. 2005 Oct;118(10):1148–1153. doi: 10.1016/j.amjmed.2005.04.019. [DOI] [PubMed] [Google Scholar]
- 63.Dudas V, Bookwalter T, Kerr KM, Pantilat SZ. The impact of follow-up telephone calls to patients after hospitalization. Am J Med. 2001 Dec 21;111(9B):26S–30S. doi: 10.1016/s0002-9343(01)00966-4. [DOI] [PubMed] [Google Scholar]
- 64.Parry C, Min SJ, Chugh A, Chalmers S, Coleman EA. Further application of the care transitions intervention: results of a randomized controlled trial conducted in a fee-for-service setting. Home Health Care Serv Q. 2009;28(2–3):84–99. doi: 10.1080/01621420903155924. [DOI] [PubMed] [Google Scholar]
- 65.Hesselink G, Schoonhoven L, Barach P, et al. Improving patient handovers from hospital to primary care: a systematic review. Ann Intern Med. 2012 Sep 18;157(6):417–428. doi: 10.7326/0003-4819-157-6-201209180-00006. [DOI] [PubMed] [Google Scholar]
- 66.Payne RA, Abel GA, Guthrie B, Mercer SW. The effect of physical multimorbidity, mental health conditions and socioeconomic deprivation on unplanned admissions to hospital: a retrospective cohort study. Cmaj. 2013 Mar 19;185(5):E221–228. doi: 10.1503/cmaj.121349. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.