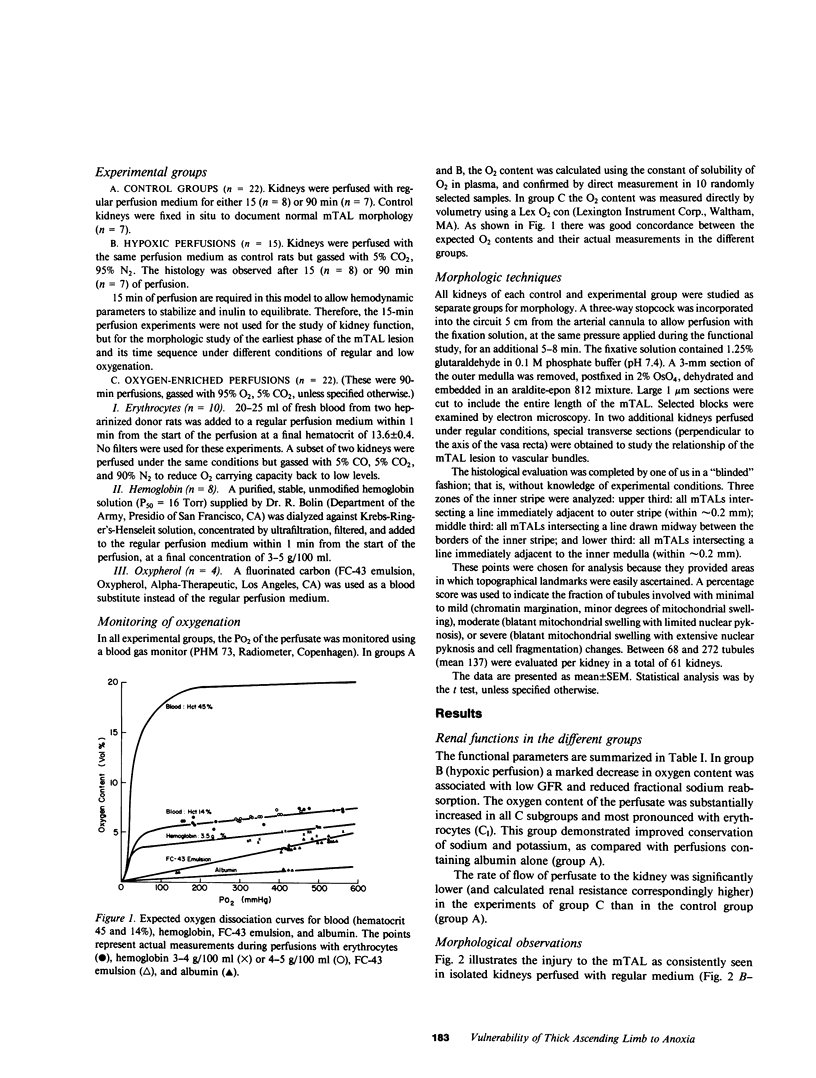
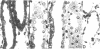Abstract
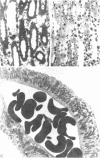
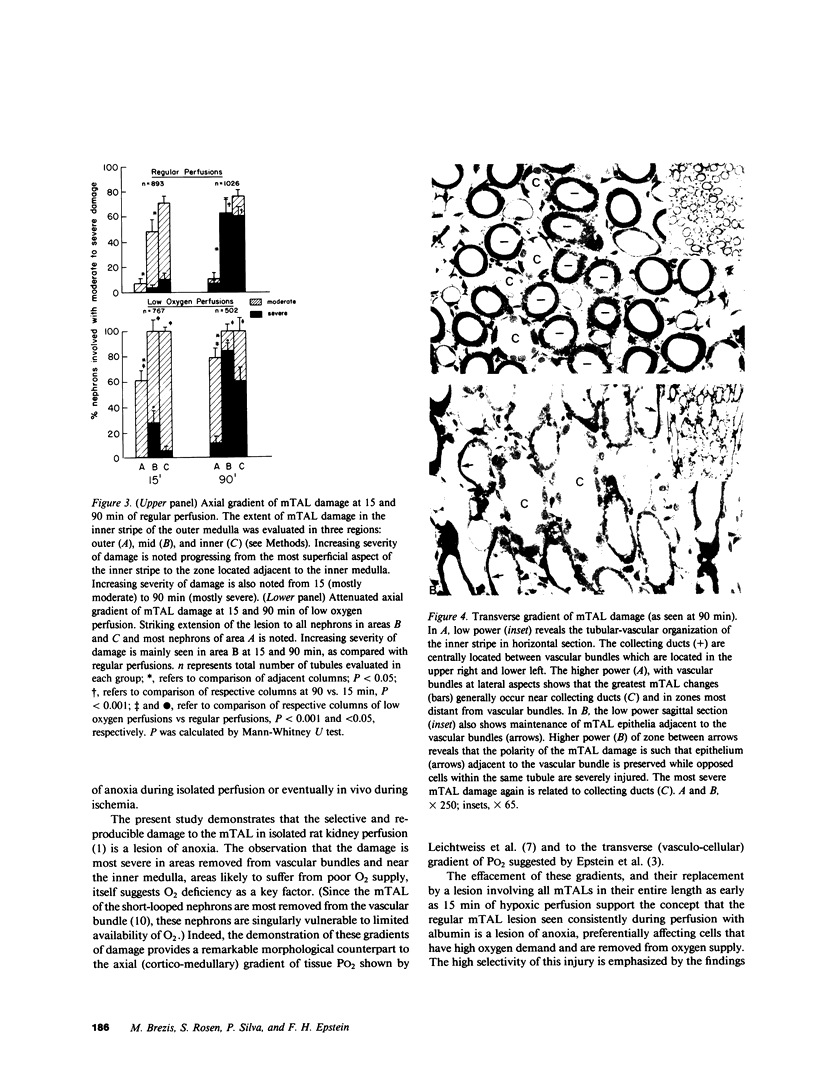
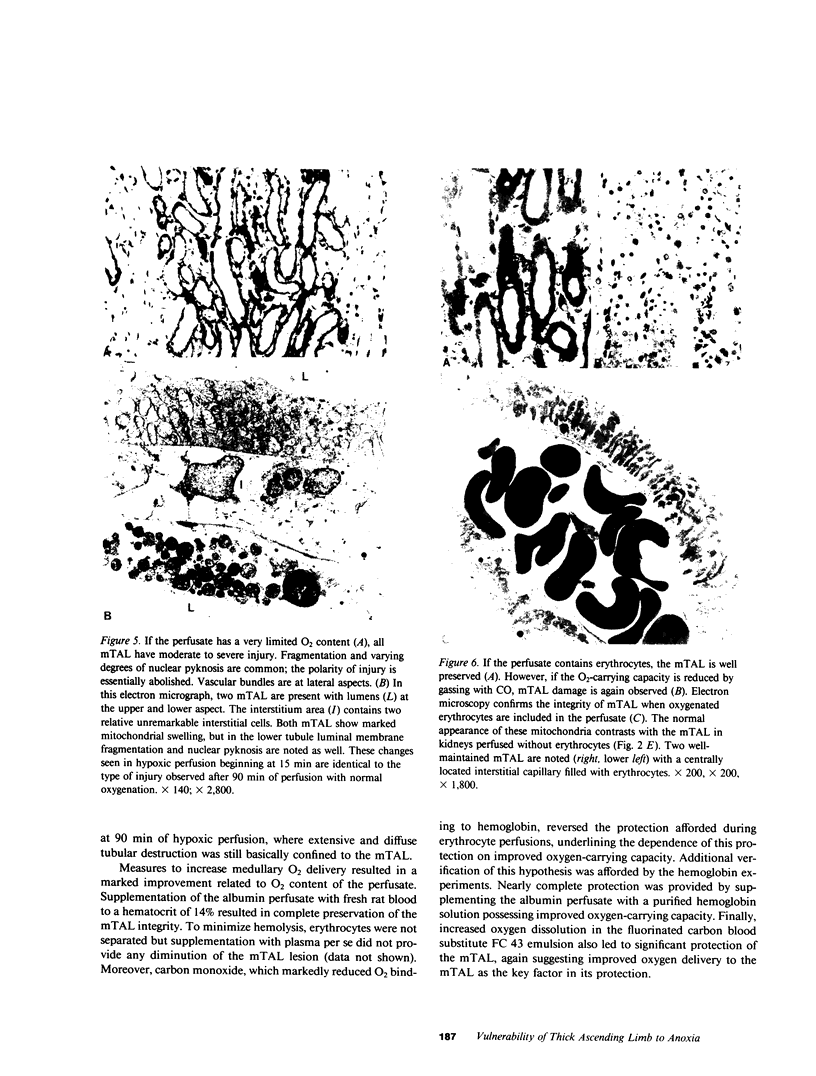
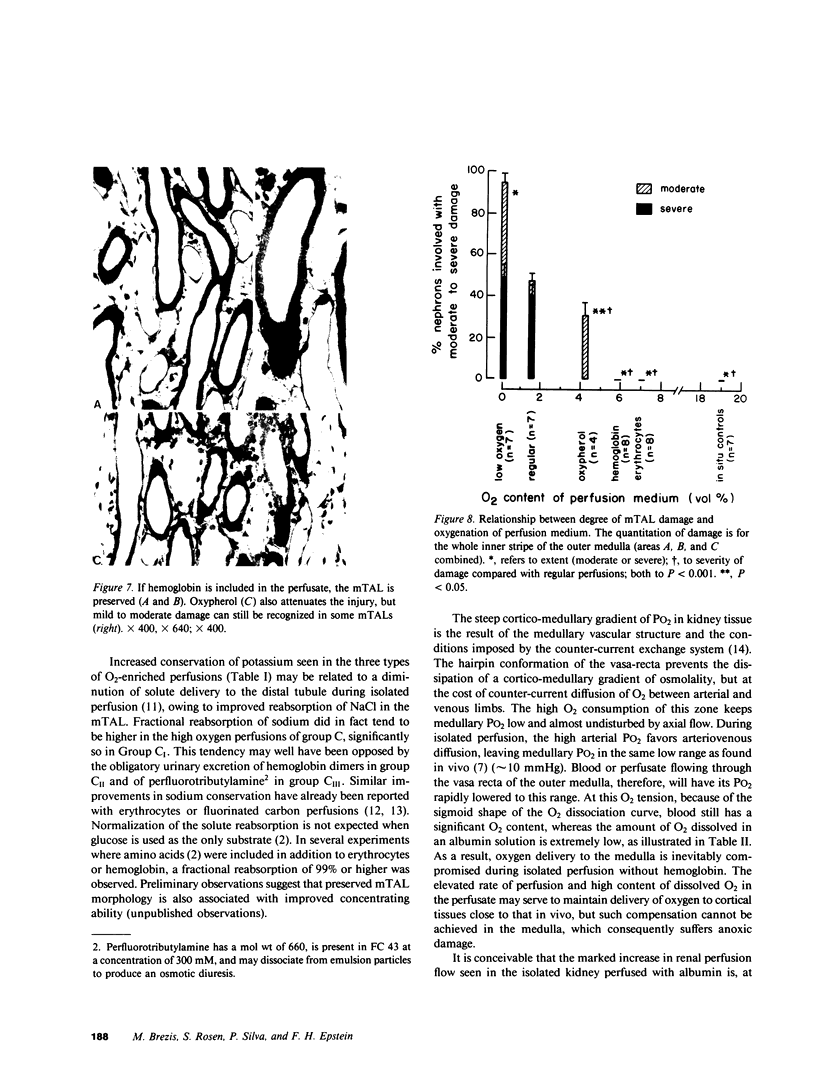
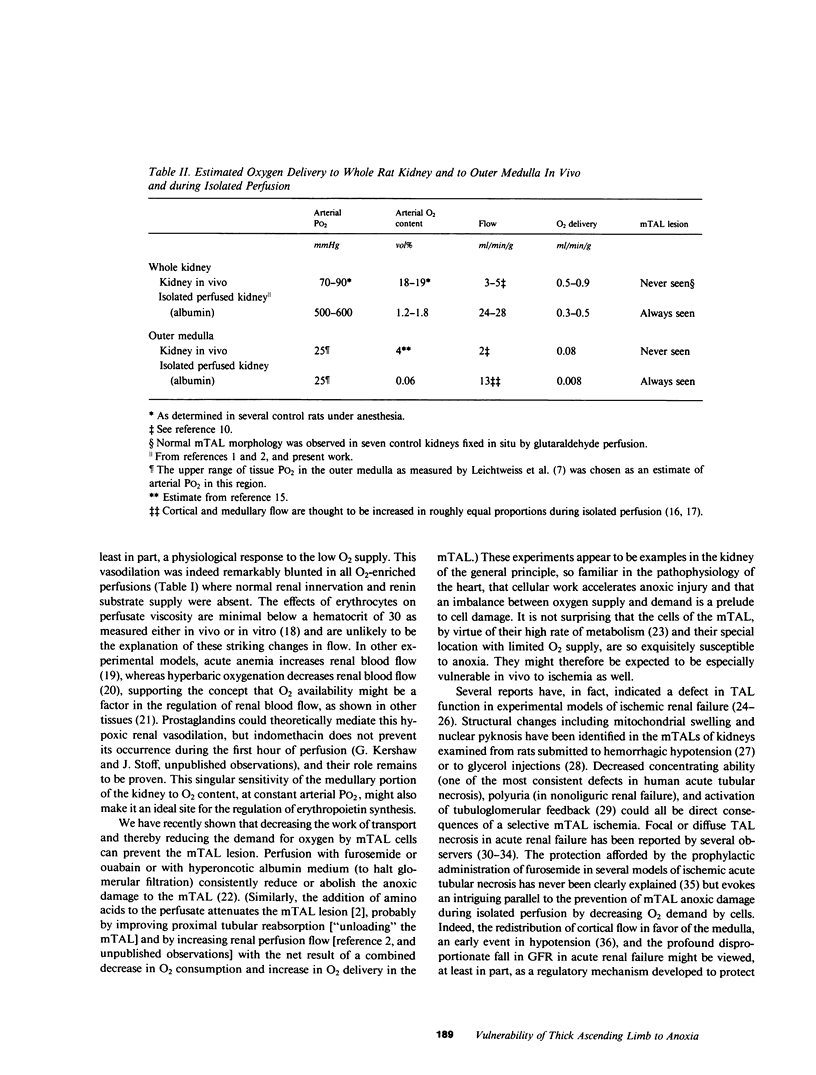
A specific anatomical lesion sharply localized to the cells of the medullary thick ascending limbs (mTAL) and characterized by mitochondrial swelling progressing to nuclear pyknosis and cell death is elicited reproducibly in isolated rat kidneys perfused for 15 or 90 min with cell-free albumin-Ringer's medium gassed with 5% CO2, 95% O2 (O2 content, 1.5 vol/100 ml). The lesion, involving about half of mTALs, appears first in mTALs removed from vascular bundles and near the inner medulla, areas most likely to be anoxic. Hypoxic perfusion (O2 content 0.12 vol/100 ml) exaggerates the lesion, wiping out gradations of damage and extending it to all mTALs. O2-enriched perfusions using rat erythrocytes (O2 content 7.1 vol/100 ml) completely eliminates the lesion (unless gassed with carbon monoxide). Similarly, supplementation of the perfusion medium with a purified hemoglobin (O2 content 5.8 vol/100 ml) prevents mTAL injury. Perfusion with a fluorinated hydrocarbon blood substitute, Oxypherol (O2 content 4.3 vol/100 ml) also attenuates the lesion. These findings suggest that the mTAL is exquisitely susceptible to anoxic damage because of low O2 supply imposed by the medullary vascular system and the high rate of metabolism mandated by active reabsorption of sodium chloride. The vulnerability of the mTAL to anoxic injury could play a key role in the pathogenesis of ischemic renal injury.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Alcorn D., Emslie K. R., Ross B. D., Ryan G. B., Tange J. D. Selective distal nephron damage during isolated kidney perfusion. Kidney Int. 1981 May;19(5):638–647. doi: 10.1038/ki.1981.63. [DOI] [PubMed] [Google Scholar]
- Anderson R. J., Gordon J. A., Kim J., Peterson L. M., Gross P. A. Renal concentration defect following nonoliguric acute renal failure in the rat. Kidney Int. 1982 Apr;21(4):583–591. doi: 10.1038/ki.1982.65. [DOI] [PubMed] [Google Scholar]
- Balaban R. S., Sylvia A. L. Spectrophotometric monitoring of O2 delivery to the exposed rat kidney. Am J Physiol. 1981 Sep;241(3):F257–F262. doi: 10.1152/ajprenal.1981.241.3.F257. [DOI] [PubMed] [Google Scholar]
- Cohen J. J. Is the function of the renal papilla coupled exclusively to an anaerobic pattern of metabolism? Am J Physiol. 1979 May;236(5):F423–F433. doi: 10.1152/ajprenal.1979.236.5.F423. [DOI] [PubMed] [Google Scholar]
- De Mello G., Maack T. Nephron function of the isolated perfused rat kidney. Am J Physiol. 1976 Dec;231(6):1699–1707. doi: 10.1152/ajplegacy.1976.231.6.1699. [DOI] [PubMed] [Google Scholar]
- Eknoyan G., Qunibi W. Y., Grissom R. T., Tuma S. N., Ayus J. C. Renal papillary necrosis: an update. Medicine (Baltimore) 1982 Mar;61(2):55–73. doi: 10.1097/00005792-198203000-00001. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Balaban R. S., Ross B. D. Redox state of cytochrome aa3 in isolated perfused rat kidney. Am J Physiol. 1982 Oct;243(4):F356–F363. doi: 10.1152/ajprenal.1982.243.4.F356. [DOI] [PubMed] [Google Scholar]
- Epstein F. H., Brosnan J. T., Tange J. D., Ross B. D. Improved function with amino acids in the isolated perfused kidney. Am J Physiol. 1982 Sep;243(3):F284–F292. doi: 10.1152/ajprenal.1982.243.3.F284. [DOI] [PubMed] [Google Scholar]
- Eveloff J., Bayerdörffer E., Silva P., Kinne R. Sodium-chloride transport in the thick ascending limb of Henle's loop. Oxygen consumption studies in isolated cells. Pflugers Arch. 1981 Mar;389(3):263–270. doi: 10.1007/BF00584788. [DOI] [PubMed] [Google Scholar]
- Franke H., Mályusz M., Runge D. Improved sodium and PAH transport in the isolated fluorocarbon-perfused rat kidney. Nephron. 1978;22(4-6):423–431. doi: 10.1159/000181485. [DOI] [PubMed] [Google Scholar]
- Grupp I., Grupp G., Holmes J. C., Fowler N. O. Regional blood flow in anemia. J Appl Physiol. 1972 Oct;33(4):456–461. doi: 10.1152/jappl.1972.33.4.456. [DOI] [PubMed] [Google Scholar]
- Hanley M. J. Isolated nephron segments in a rabbit model of ischemic acute renal failure. Am J Physiol. 1980 Jul;239(1):F17–F23. doi: 10.1152/ajprenal.1980.239.1.F17. [DOI] [PubMed] [Google Scholar]
- Kramer H. J., Schürmann J., Wassermann C., Düsing R. Prostaglandin-independent protection by furosemide from oliguric ischemic renal failure in conscious rats. Kidney Int. 1980 Apr;17(4):455–464. doi: 10.1038/ki.1980.53. [DOI] [PubMed] [Google Scholar]
- Kreisberg J. I., Bulger R. E., Trump B. F., Nagle R. B. Effects of transient hypotension on the structure and function of rat kidney. Virchows Arch B Cell Pathol. 1976 Nov 2;22(2):121–133. doi: 10.1007/BF02889210. [DOI] [PubMed] [Google Scholar]
- Leichtweiss H. P., Lübbers D. W., Weiss C., Baumgärtl H., Reschke W. The oxygen supply of the rat kidney: measurements of int4arenal pO2. Pflugers Arch. 1969 Jun 19;309(4):328–349. doi: 10.1007/BF00587756. [DOI] [PubMed] [Google Scholar]
- Mason J., Gutsche H. U., Moore L., Müller-Suur R. The early phase of experimental acute renal failure. IV. The diluting ability of the short loops of Henle. Pflugers Arch. 1979 Feb 14;379(1):11–18. doi: 10.1007/BF00622899. [DOI] [PubMed] [Google Scholar]
- Norman J. N., Shearer J. R., Napper A. J., Robertson I. M., Smith G. Action of oxygen on the renal circulation. Am J Physiol. 1974 Sep;227(3):740–744. doi: 10.1152/ajplegacy.1974.227.3.740. [DOI] [PubMed] [Google Scholar]
- OLIVER J., MacDOWELL M., TRACY A. The pathogenesis of acute renal failure associated with traumatic and toxic injury; renal ischemia, nephrotoxic damage and the ischemic episode. J Clin Invest. 1951 Dec;30(121):1307–1439. doi: 10.1172/JCI102550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross B. D., Epstein F. H., Leaf A. Sodium reabsorption in the perfused rat kidney. Am J Physiol. 1973 Nov;225(5):1165–1171. doi: 10.1152/ajplegacy.1973.225.5.1165. [DOI] [PubMed] [Google Scholar]
- Ross B. D. The isolated perfused rat kidney. Clin Sci Mol Med Suppl. 1978 Dec;55(6):513–521. doi: 10.1042/cs0550513. [DOI] [PubMed] [Google Scholar]
- SEVITT S. Distal tubular necrosis with little or no oliguria. J Clin Pathol. 1956 Feb;9(1):12–30. doi: 10.1136/jcp.9.1.12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- SEVITT S. Pathogenesis of traumatic uraemia: a revised concept. Lancet. 1959 Aug 22;2(7095):135–141. doi: 10.1016/s0140-6736(59)90557-4. [DOI] [PubMed] [Google Scholar]
- Sparks H. V., Jr, Belloni F. L. The peripheral circulation: local regulation. Annu Rev Physiol. 1978;40:67–92. doi: 10.1146/annurev.ph.40.030178.000435. [DOI] [PubMed] [Google Scholar]
- Suzuki T., Mostofi F. K. Electron microscopic studies of acute tubular necrosis. Early changes in lower tubules of rat kidney after subcutaneous injection of glycerin. Lab Invest. 1970 Jul;23(1):15–28. [PubMed] [Google Scholar]
- Swanson J. W., Besarab A., Pomerantz P. P., DeGuzman A. Effect of erythrocytes and globulin on renal functions of the isolated rat kidney. Am J Physiol. 1981 Aug;241(2):F139–F150. doi: 10.1152/ajprenal.1981.241.2.F139. [DOI] [PubMed] [Google Scholar]
- Thurau K., Boylan J. W. Acute renal success. The unexpected logic of oliguria in acute renal failure. Am J Med. 1976 Sep;61(3):308–315. doi: 10.1016/0002-9343(76)90365-x. [DOI] [PubMed] [Google Scholar]
- Vatner S. F. Effects of hemorrhage on regional blood flow distribution in dogs and primates. J Clin Invest. 1974 Aug;54(2):225–235. doi: 10.1172/JCI107757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Whittaker S. R., Winton F. R. The apparent viscosity of blood flowing in the isolated hindlimb of the dog, and its variation with corpuscular concentration. J Physiol. 1933 Jul 10;78(4):339–369. doi: 10.1113/jphysiol.1933.sp003009. [DOI] [PMC free article] [PubMed] [Google Scholar]