Abstract
Object
Resected brain metastases have a high rate of local recurrence without adjuvant therapy. Adjuvant whole brain radiotherapy (WBRT) remains the standard of care with the rate of local control >90%. However, WBRT is delivered over 10–15 days, which can delay other therapy and is associated with acute and long-term toxicities. Intra-operative permanent Cesium-131 (Cs-131) implants can be performed at the time of surgery, thereby avoiding any additional therapy. We evaluate the safety, feasibility and efficacy of a novel treatment approach of brain metastases with a permanent intra-operative Cs-131 brachytherapy.
Methods
After IRB approval, 24 patients with a newly diagnosed metastasis to the brain (n=24) were accrued on a prospective protocol between 2010 and 2012. There were 10 frontal, 7 parietal, 4 cerebellar, 2 occipital, and 1 temporal metastases. Histology included lung (16), breast (2), kidney (2), melanoma (2), colon (1), and cervix (1). Cs-131 stranded seeds were placed as a permanent volume implant. Prescription dose was 80Gy at 5mm depth from the resection cavity surface. Distant metastases were treated with stereotactic radiosurgery (SRS) or WBRT, depending on the number of lesions. Primary end point was resection cavity freedom from progression (FFP). Secondary end points included distant metastases FFP, median survival, overall survival (OS), and toxicity.
Results
Median follow-up was 19.3 months (range, 12.89 – 29.57 months). Median age was 65 years (range, 45–84 years). Median volume of resected tumor was 10.31 cc (range, 1.77 - 87.11 cc). Median number of seeds employed was 12 (range, 4–35) with median activity per seed of 3.82 mCi (range, 3.31–4.83 mCi) and total activity of 46.91 mCi (range, 15.31–130.70 mCi). Local recurrence FFP was 100%. There was 1 adjacent leptomeningeal recurrence, resulting in a 1-year regional FFP of 93.8% (95% CI = 63.2%, 99.1%). Distant metastasis FFP was 48.4% (95% CI = 26.3%, 67.4%). Median OS was 9.9 months (95% CI = 4.8 months, upper limit not estimated) and 1-year OS was 50.0% (95% CI = 29.1%, 67.8%). Complications included cerebrospinal fluid leak (1), seizure (1), infection (1). There was no radiation necrosis.
Conclusions
Cs-131 post-resection permanent brachytherapy implants resulted in no local recurrences and no radiation necrosis. This treatment approach was safe, well tolerated, and convenient for patients, resulting in a short radiation treatment course, high response rates, and minimal toxicity. These results merit further study with a multicenter trial.
Keywords: cesium-131 (Cs-131), brachytherapy, metastases, radiation, radiotherapy
Introduction
Brain metastases are the most common intracranial tumors, occurring in up to 40% of cancer patients with a rising annual incidence.8,47 It has been recently reported the brain metastases account for ~60% of solid metastases arising primarily from lung, breast, kidney, colon and skin melanoma causing major morbidity and mortality.34,53 In the last decade the incidence of brain metastases has been rising, attributed to the increased length of survival from cancer patients.58
Without treatment, prognosis is dismal with survival of only 1–2 months. Survival can be extended with WBRT to 3–6 months, and with either surgery and adjuvant SRS or surgery followed by adjuvant WBRT to 11 months.19,20,29,46 Although WBRT is effective at preventing local recurrence and controlling distant disease, it has been associated with acute detriments in quality-of-life (QoL) measures10,32 and deterioration in neurocognitive abilities.9,11,14,43 In addition, WBRT offers no overall survival benefit compared with local therapy.2,45,54 For these reasons attention has turned to the option of aggressive local therapy for oligometastatic disease, withholding the addition of salvage WBRT for disease recurrence.
There are a variety of focal post-resection treatment strategies that are available in this setting. Among such options are post-op SRS4,15,18,23-25,27,28,30,31,35,39,48,50,55 and intra-operative application of either permanent low-dose7,12,22,52 or temporary high-dose5,40,44,51,61 radio-isotopes (generally Iodine-125 (I-125)) into the surgical cavity. Post-op SRS is the more commonly used of these treatment modalities due to its wider availability. Brachytherapy has historically utilized the I-125 radio-isotope for both permanent and temporary seeds implants. Although I-125 has been shown to confer local control comparable to that of post-op SRS and WBRT,5,7,12,22,40,44,51,52,61 the rates of radiation necrosis have been critiqued. Cs-131 is a novel radio-isotope which confers both physical and radio-biological advantages when compared to both post-op SRS and I-125 brachytherapy. In this present study we prospectively evaluated the safety, feasibility and efficacy of a novel treatment approach of brain metastases with a permanent intra-operative Cs-131 brachytherapy.
Methods
Patient Selection
Between 2010 and 2012, patients undergoing surgery for newly diagnosed brain metastatses, in whom surgery was deemed appropriate, were accrued to an IRB approved prospective trial and signed an informed consent. In general, selection criteria included a metastatic tumor in which surgery was indicated either to relieve mass effect, reduce symptoms, for diagnostic purposes or based on size > 2.5 cm. Patients had to have ECOG/Zubrod performance status 0, 1, or 2 and expected survival ≥6months. Exclusion criteria included: the tumor’s proximity to the chiasm, brainstem (increasing the risk of radiation treatment), small cell cancer histology metastatic to the brain, pregnancy or unwillingness to practice a form of birth control (i.e. abstinence, oral contraceptives, etc.) and previous history of radiotherapy.
Treatment Technique
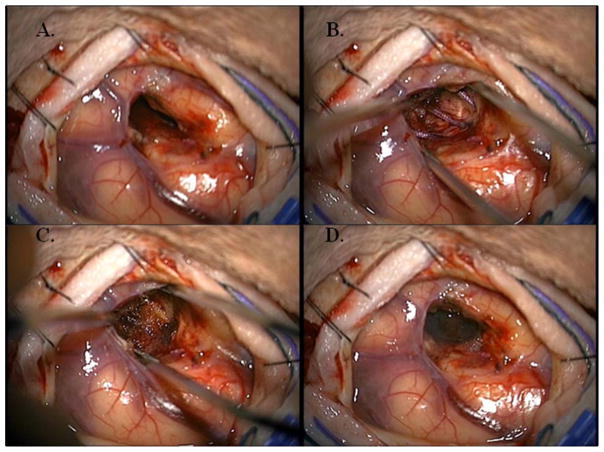
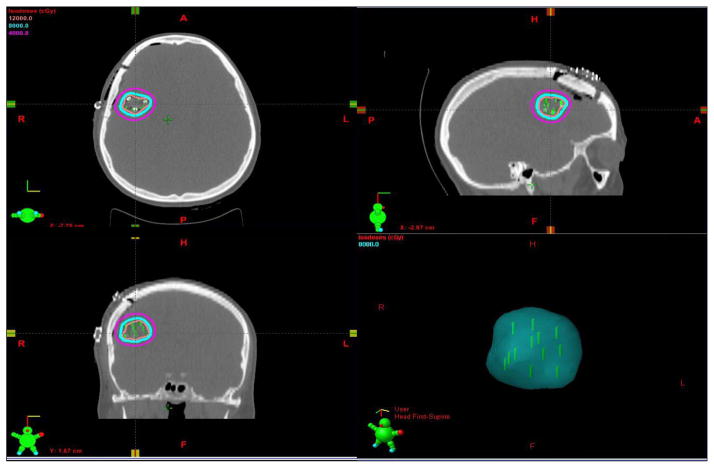
Patients underwent maximally safe neurosurgical resection of lesions. The extent of resection and whether surgery was en bloc or piecemeal was noted intra-operatively and from post-operative MRI scans within 48 hours of surgery. During the time of surgical resection the size of resected tumor (maximum diameter and volume), location (supratentorial versus infratentorial), pial-based versus non-pial-based was noted. At the time of resection, Cs-131 stranded seeds (IsoRay, Richland, WA) with an activity of 3–5 mCi were implanted with a planned dose of 80 Gy to a 5 mm depth from the surface of the resection cavity. The implant was pre-calculated based on pre-operative data of tumor size and our institutional physics nomogram and adjusted real time for the resulting intracavitary volume of the resected metastasis (Figure 1A). The 10cm Cs-131 suture-stranded seeds (0.5cm inter-seed spacing) were delivered in strings of 10 seeds per string and subsequently cut into smaller lengths as per the nomogram and placed as a permanent volume implant along the cavity in a tangential pattern to maintain a 7–10mm spacing between seeds. As a result, the cavity is lined like “barrel staves” or “parallel tracks” (Figure 1B). The seeds are then covered with Surgicel (Ethicon) to prevent seed migration and alteration of dosimetry (Figure 1C) and Tisseel (Baxter) is used to line the cavity to limit cavity shrinkage and further prevent seed dislodgement (Figure 1D). Within 24–48 hours post-implant, the patient underwent a post-implant CT scan to determine dose distribution (Figure 2).
Figure 1.
Resection cavity throughout the implant procedure. A: Empty resection cavity B: Resection Cavity lined like “barrel staves” or “parallel tracks” with Cs-131 seeds C: Cs-131 seeds covered with Surgical (Ethicon) D: Cs-131 seeds covered with Tisseel (Baxter).
Figure 2.
CT scan of Cs-131 brachytherapy seeds in the postoperative resection cavity. A: Axial plane B: Sagittal plane. C: Coronal plane. D: 3-D Radiation cloud from the 80 Gy Isodose Line.
Follow Up
Follow-up exams included MRI every 2 months. At the time of disease progression the metastases were treated with SRS or WBRT, depending on the number of lesions.
Endpoints and Statistical Methods
Descriptive statistics (including mean, standard deviation, median, range, frequency, percent) were calculated to characterize the study cohort. The primary endpoints of the trial were local resection cavity FFP, regional FFP, distant FFP. Secondary end points included median survival, overall survival (OS), and toxicity. The treatment response was rated based upon follow-up MRI brain scans as compared to the prior MRI brain scans. Local FFP was defined as the absence of new nodular contrast enhancement ≤5mm from the resection cavity. Regional failure was defined as new or increased contrast enhancement, > 5mm from the resection cavity. Distant failure was defined as new or increased contrast enhancement elsewhere in the brain. All survival endpoints were defined as the time from the date of resection and implantation of the Cs-131 brachytherapy seeds until either 1) the date of local recurrence (for local FFP), 2) the date of regional recurrence (for regional FFP), 3) the date of new metastasis (for distant FFP, or 4) the date of death (for OS). Patients without these events were censored at their date of last follow-up. Kaplan-Meier survival analysis was performed to generate survival curves for the above defined survival outcomes. Median and one-year local FFP, regional FFP, distant FFP, and OS were estimated, as appropriate, and 95% confidence intervals were calculated to assess the precision of the obtained survival estimates. The Spearman-rank correlation coefficient was used to evaluate the correlation between Cs-131 brachytherapy seed characteristics of interest. All p-values are two-sided with statistical significance evaluated at the 0.05 alpha level. All analyses were performed using SPSS version 21.0 (SPSS Inc., Chicago, IL) and STATA Version 12.0 (StataCorp, College Station, TX).
Results
Patient Characteristics
Patient characteristics are summarized in Table 1. There were 14 females and 10 males with a median age of 65 (range 45–84). The brain metastases were located in the frontal (10), parietal (7), cerebellar (4), occipital (2), and temporal (1) regions. The histology from the metastases was lung (16), breast (2), kidney (2), melanoma (2), colon (1), and cervix (1).
TABLE 1.
Summary of characteristics in 24 patients with brain metastases
| Variable | No. (%) |
|---|---|
| sex | |
| male | 10 (41.67) |
| female | 14 (58.33) |
| age in yrs | |
| range | 45–84 |
| median | 65 |
| no. of tumors | |
| 1 | 15 (62.5) |
| 2 | 4 (16.67) |
| 3 | 3 (12.5) |
| >3 | 2 (8.33) |
| prior RT | |
| none | 21 (87.5) |
| SRS | 3* (12.5) |
| tumor location | |
| frontal | 10 (41.67) |
| parietal | 7 (21.97) |
| cerebellar | 4 (16.67) |
| occipital | 2 (8.33) |
| temporal | 1 (4.17) |
| tumor histology | |
| lung cancer | 16 (66.67) |
| breast cancer | 2 (8.33) |
| kidney cancer | 2 (8.33) |
| melanoma | 2 (8.33) |
| colon cancer | 1 (4.17) |
| cervix cancer | 1 (4.17) |
One patient had both SRS and WBRT.
Treatment Parameters
Details from the surgical resection on Cs-131 implant are shown in Table 2. Among the 24 patients who underwent resection and Cs-131 brachytherapy implant, all patients achieved gross total resection (defined as resection of contrast enhancing disease). Based on the pre-operative MRI the median volume of the resected tumor was 10.31 cc (range, 1.77–87.11 cc). Based on intra-operative measurements, the median volume of the cavity after tumor resection was 3.13 cc (range, 1–17 cc) indicating a 69.6% decrease in cavity volume before the seeds were placed. The median number of seeds employed was 12 (range, 4–35) with median activity per seed of 3.82 mCi (range, 3.31–4.83 mCi) and total activity of 46.91 mCi (range, 15.31–130.70 mCi).
TABLE 2.
Summary of treatment details in 24 patients treated with resection and 131Cs brachytherapy*
| Variable | Value |
|---|---|
| extent of resection | |
| GTR | 24 (100) |
| STR | 0 (0) |
| preop tumor vol based on MRI (cc) | |
| median | 10.31 |
| range | 1.77–87.11 |
| intraop cavity vol (cc) | |
| median | 3.13 |
| range | 1–17 |
| no. of seeds placed | |
| median | 12 |
| range | 4–35 |
| seed activity (U) | |
| median | 2.44 |
| range | 2.11–3.08 |
| total activity (U) | |
| median | 29.9 |
| range | 9.76–83.3 |
| activity per seed (mCi) | |
| median | 3.82 |
| range | 3.31–4.83 |
| total seed activity (mCi) | |
| median | 46.91 |
| range | 15.31–130.7 |
GTR = gross-total resection; STR = subtotal resection.
Survival
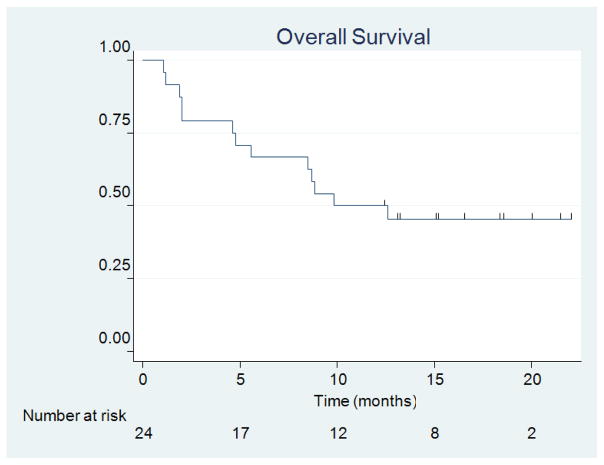
At the time of analysis there were 11 patients still alive and 13 deaths with a median follow-up of 19.3 months (range, 12.89–29.57 months). Among the 11 patients who are still alive, 8 had a primary originating from the lung, 2 from the breast, and 1 from the colon. One of these patients was previously treated with SRS for a brain metastasis in a different area and then implanted by the Cs-131 brachytherapy to a second lesion. Eight of these patients had only one lesion, two patients had two lesions, and two patients had three lesions. For the 13 patients who died, there were 8 with a primary originating from the lung, 2 from the kidney, 2 from melanoma, and 1 from the cervix. These patients included 2 who had been previously treated with SRS to a different area of the brain. Seven of these patients had one lesion, three patients had two lesions, one patient had three lesions, and two patients had >3 lesions. The median OS was 9.9 months (95% CI = 4.8 months, upper limit not estimated) (Figure 3). The 1-year OS was 50.0% (95% CI = 29.1%, 67.8%).
Figure 3.
Overall Survival.
Freedom From Progression
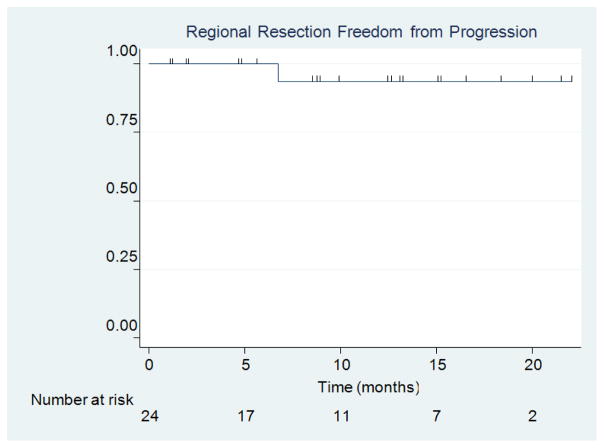
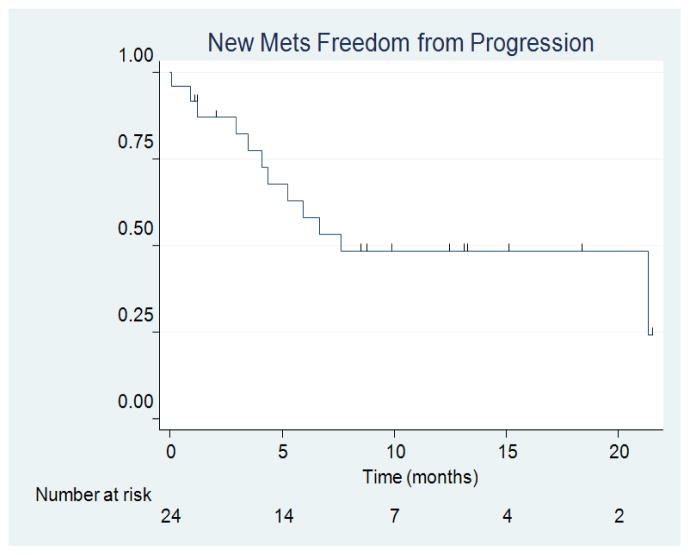
There were zero cases of local recurrence within 5 mm of the resection cavity (Figure 4). This yielded a local recurrence FFP of 100%. There was one case of regional recurrence which yielded a 1-year regional resection cavity FFP of 93.8% (95% CI = 63.2%, 99.1%) (Figure 5). This case of regional recurrence was evident 7 months post-implant and was leptominengeal in origin (Figure 6). This patient was subsequently treated with SRS and is still alive at the time of analysis. There were 12 cases of distant metastases, which yielded a median distant metastases FFP of 7.6 months (95% CI = 4.1 months, upper limit not estimated) and a 1-year distant metastases FFP of 48.4% (95% CI = 26.3%, 67.4%) (Figure 7). Distant progression was treated with either WBRT (1 patient) or SRS (8 patients).
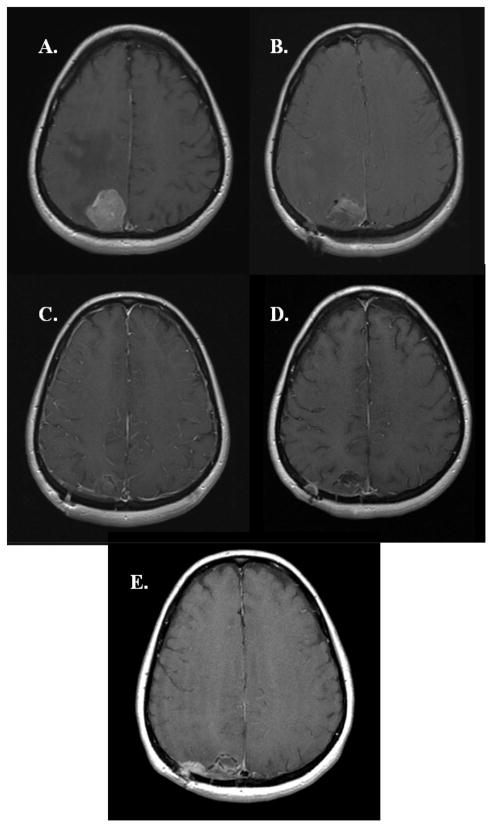
Figure 4.
MRI series of local FFP. A: Pre-op B: Post-op C: 1 Month Post-op D: 2 Months Post-op E: 4 Months Post-op F: 6 Months Post-op G: 11 Months Post-op H: 13 Months Post-op.
Figure 5.
Regional Resection FFP.
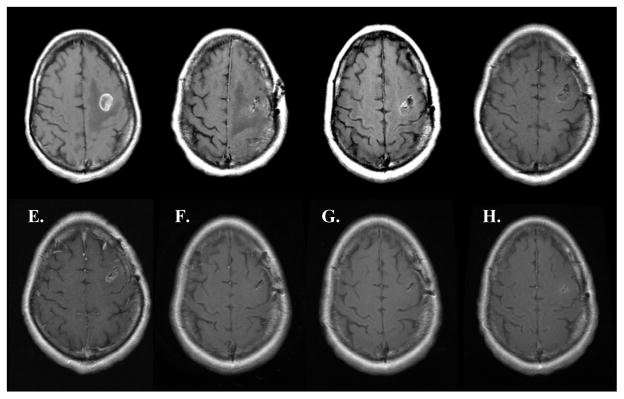
Figure 6.
MRI series of regional recurrence. A: Pre-op B: Post-op C: 1 Month Post-op D: 4 Months Post-op E: 7 Months Post-op.
Figure 7.
New Metastases FFP.
Complications
Post-operatively, the patients were treated with 4mg of dexamethasone twice a day for 2 weeks. There were no instances of radiation necrosis. There was one instance of a dural tear which required re-operation at 1.2 months post-implant. Additional complications included one case of infection and one case of seizure.
Discussion
Surgical resection for the treatment of brain metastases has been utilized to establish a histologic diagnosis, provide rapid relief of symptoms resulting from the mass effect of a large tumor, and to improve local control. Unfortunately, tumor recurrence with surgery alone has been shown to be as high as 46%.45 Rates of recurrence correlate with factors such as tumor size, location, histology, and en bloc resection. With the addition of radiation therapy in the post-operative setting, classically in the form of WBRT, the rates of recurrence can be reduced to 10–20% but at the expense of the QoL and neurocognitive deficits.9-11,14,32,43,46 For this reason, attention has been turned to the addition of focal radiation to the resection bed in an effort to reduce the incidence of local failure.
The use of post-operative SRS to the resected surgical cavity has been increasing with a number of recent publications appearing in the literature and several institutions adopting this technique as a new standard of care. 4,15,18,23-25,27,28,30,31,35,39,48,50,55 While the Phase III trial from the North Central Cancer Treatment Group (N107C) comparing post-operative SRS to WBRT in the post-op setting for brain metastases in progress, the results of phase I and phase II trials demonstrated that local control of the resection cavity to be similar to WBRT, ranging from 73–94%with an incidence of radiation necrosis ranging from 0–10%.4,15,18,23-25,27,28,30,31,35,39,48,50,55 Intracranial distant failure was reported in 44–65% at 1 year, and death due to neurologic causes in ~25%.4,15,18,23-25,27,28,30,31,35,39,48,50,55 The typical time frame for the delivery of post-operative SRS is between 2–6 weeks following the surgical resection. This buffer time is needed in order to allow adequate wound healing following the surgery and to allow the cavity to shrink to a smaller, stable size in preparation for radiosurgery targeting. Nevertheless, the majority of the dose is administered to an empty cavity. Likewise, the delay in treatment may be disadvantageous as radiographically evident repopulation of tumor cells has been shown to occur in this time period.56 The ideal target for SRS is a small round cavity. In patients with tumor cavities of irregular shape or larger size (> 3 cm) this presents a challenge in developing of a treatment plan with a high degree of conformality. Indeed, it has been shown that larger tumor cavities treated with post-operative SRS have poor local control resulting from less conformal treatment plans.1,16,37 Furthermore, the volume of irradiated tissue is a clear predictor of symptomatic radiation necrosis in patients irradiated with SRS.6,41 For this reason, some centers suggest that larger tumor beds be treated with hypofractionated stereotactic radiotherapy (HSRT) .1,17,33,38,42,57
Intra-operative interstitial brachytherapy has several theoretical and practical advantages over SRS for improving local control of resected brain metastases. Brachytherapy allows a high dose of radiation to be given to a localized area with a very steep dose fall-off, thus covering the tumor bed but sparing normal brain tissue beyond the vicinity of the tumor bed. The dose is delivered maximally to the area of microscopic tumor cells and not an empty cavity. Prior studies have shown local control of the resection cavity to be between 80–95%.5,7,12,22,40,44,51,52,61 For this reason, the conformality index of interstitial brachytherapy is higher than that of post-operative SRS, a factor which has been shown to cause failure of post-operative SRS.35,39 Having a very steep dose fall-off is a feature makes brachytherapy a rather attractive option as it may avoid radiation necrosis of already compromised brain .In addition, brachytherapy delivers the entire treatment (surgery plus radiation) in one procedure, which is more convenient for the patient and may increase patient satisfaction. The ability to deliver all the treatment in one sitting is particularly appealing for patients who live at a far distance from a medical center, for whom travel may be prohibitively expensive and/or time-consuming, particularly in a weakened state. Hence, compliance may be increased. Brachytherapy is also more cost effective compared with WBRT and SRS.59 Further, there is a radiobiological advantage to administering immediate radiotherapy so as to preclude cancer cell repopulation which typically occurs at ~4 weeks. Lastly, in contrast with postoperative SRS, which generally requires application of a metal stereotactic frame affixed with screws to a patient skull, brachytherapy requires no frame or a special fixation as it is performed at the time of surgery.
There are also radiobiological advantages to using brachytherapy. Continuous dose rate radiation of brachytherapy at 0.3–3.5 Gy/h inhibits mitosis and causes proliferating tumor cells to accumulate in G2, a radiosensitive phase of the cell cycle.21 There is less radioresistance of hypoxic cells treated with brachytherapy secondary to impaired repair of sublethal damage under hypoxic conditions36 and the opportunity for hypoxic cells to become re-oxygenated during the treatment.21
Criticisms of brachytherapy have focused on the high rates of radiation necrosis reported in some series. Early series involved a stereotactic biopsy followed by permanent high-dose implants22 or were performed for recurrent lesions refractory to external WBRT5,52 or concurrent with WBRT.61 The use of brachytherapy for local control of newly resected metastases without WBRT has been reported more recently. In these series, radiation necrosis has been more common using high-dose temporary brachytherapy, such as the Gliasite balloon, which reported a 23% rate of radiation necrosis.51 In the continuous low-dose permanent brachytherapy setting, 0% rates of radiation necrosis were shown by Bogart et al., who used seeds with activity 0.32–0.45 mCi and a cumulative dose of 80–160 Gy using a median of 13 seeds7,49 but achieved a local control of only 80%. On the other hand, Huang et al, reported a 26% rate of radiation necrosis using a median of 43 I-125 seeds, with a median activity of 0.79 mCi and median dose 800 Gy to the surface (200Gy to a depth of 1 cm) with a reported local control of 92%.22 These data indicate that a lower seed activity coupled with a lower prescription dose will decrease the rate of radiation necrosis with only a minimal impact on local control.
We carefully took into account the aforementioned information while designing our trial with Cs-131 so as to minimize the incidence of radiation necrosis. The lowered seed activity of Cs-131 and a dose prescription in our study did not only achieve a high rate of local control but resulted in no incidence of radiation necrosis. The rationale behind employing Cs-131instead of I-125 lies in several physical and radiobiological advantages of the former. Whereas I-125 has a dose rate of 0.069Gy/h, Cs-131 has a higher dose rate of 0.342 Gy/h. In essence, this means that after the implant with Cs-131, 90% the dose is absorbed by 33 days, in contrast with 32% of the dose absorption that occurs with I-125. This short t 1/2 life of 9.69 days (compared with 59.4 days for I-125) ensures shorter average life of the radioactive seed, which not only ensures increased safety to the family and treating physicians but also provides an early possibility of initiating adjuvant systemic therapy after only 1 month of implantation. In this current series there was one patient who required re-operation for a dural tear at 1.2 months post-implant. Due to the short t 1/2 of Cs-131 there was no risk of exposure to the surgical team at that time point. The high mean energy of Cs-131 of 29 keV allows fewer radioactive seeds to be implanted per given volume. Dosimetric studies comparing various isotopes in prostate cancer have shown superiority of Cs-131 to I-125 and Palladium-103.60
Another reason for our success may be a higher rate of gross total resection of the tumors or more careful, conformal placement of the seeds to prevent areas of inadequate dosing. Clearly, careful attention to technical details will increase the success of any treatment. Complicating the use of interstitial brachytherapy is the gradual shrinkage of the resection cavity, which is a poorly understood process that progressively moves the seeds closer together over time.3,13,26,60 However, cavity shrinkage would likely result in pockets of higher dose delivery and higher rates of radiation necrosis, which we did not observe. We undertook several measures to decrease the degree of cavity shrinkage once the seeds were placed. The seeds were not placed as individual seeds but were attached by strings with tensile strength. These strings lined the cavity like barrel stave maintaining a certain amount of outward pressure on the cavity to keep it from collapsing. Likewise, fibrin glue was placed over the seeds not only to keep them from moving but to create additional outward pressure on the cavity to prevent cavity shrinkage. Since the majority of the mass effect of the tumor bulk was relieved after the initial surgery, indicated by the 69.6% shrinkage in cavity volume prior to seed placement, the maintenance of a smaller residual volume during the treatment period did not compromise the surgical goal of relieving mass effect.
Limitations
In this analysis, we report results of the initial 24 patients. More substantial numbers of patients from other institutions treated in such a manner will be required to make more definitive conclusions. Likewise, randomized comparisons between brachytherapy and SRS are indicated. The details of the kinetics and dynamics of the size and shape of the resection cavity and its changes over time will be required for more precise treatment planning and these studies are ongoing. Finally, formal objective measures of QoL and cognitive processing as well as cost will help in comparing Cs-131 brachytherapy with other treatment options.
Conclusions
This is the first prospective report of patients with newly diagnosed metastases treated with maximally safe neurosurgical resection and intra-operative application of Cs-131. To date, this method of brachytherapy, based on our institutional nomogram and surgical technique, has rendered excellent local control, proved to be safe and efficacious. A multi-center trial will soon be underway to evaluate this novel radioisotope as a promising treatment modality in the treatment of patients with brain metastases requiring neurosurgical intervention.
TABLE 3.
Treatment details by patient
| Case No. | Primary Tumor Histology | Prior SRS to Lesion Elsewhere in Brain | Prior WBRT | Subsequent SRS to a Different Lesion | Site of Recurrence (Local, Regional, Distant) | No. of Regional or Distant Lesions | Salvage SRS | Salvage WBRT | Deceased |
|---|---|---|---|---|---|---|---|---|---|
| 1 | cervix | — | - | - | distant | >3 | - | - | yes |
| 2 | lung | - | - | - | distant | >3 | - | yes | yes |
| 3 | lung | - | - | yes | - | - | - | - | yes |
| 4 | melanoma | yes | - | - | distant | 2 | - | - | yes |
| 5 | lung | yes | - | - | - | - | - | - | - |
| 6 | lung | - | - | yes | distant | 1 | - | - | - |
| 7 | colon | - | - | - | - | - | - | - | - |
| 8 | lung | yes | yes | - | - | - | - | - | yes |
| 9 | breast | - | - | - | distant | 3 | yes | - | - |
| 10 | lung | - | - | - | regional, distant | 2 | yes | - | - |
| 11 | lung | - | - | yes | - | - | - | - | - |
| 12 | lung | - | - | - | - | - | - | - | yes |
| 13 | breast | - | - | yes | distant | 3 | - | - | - |
| 14 | melanoma | - | - | - | distant | >3 | - | - | yes |
| 15 | lung | - | - | - | distant | 1 | yes | - | - |
| 16 | lung | - | - | - | - | - | - | - | - |
| 17 | kidney | - | - | - | distant | >3 | - | - | yes |
| 18 | lung | - | - | - | - | - | - | - | - |
| 19 | lung | - | - | - | - | - | - | - | yes |
| 20 | lung | - | - | yes | - | - | - | - | yes |
| 21 | kidney | - | - | - | - | - | - | - | yes |
| 22 | lung | - | - | - | - | - | - | - | - |
| 23 | lung | - | - | - | distant | 1 | - | - | yes |
| 24 | lung | - | - | - | distant | >3 | - | - | yes |
Acknowledgments
Dr. A.Gabriella Wernicke was supported by the NIH KL2 grant 3KL2RR024997 Dr. Paul Christos was partially supported by the following grant: Clinical Translational Science Center (CTSC) (UL1-TR000457-06
Abbreviations
- WBRT
Whole brain radiotherapy
- Cs-131
Cesium-131
- SRS
stereotactic radiosurgery
- FFP
freedom from progression
- OS
overall survival
- Qol
quality of life
- I-125
Iodine-125
- HSRT
hypofractionated stereotactic radiotherapy
Footnotes
Portions of this work were presented as an oral presentation at ASTRO’s 54th Annual Meeting, Boston, MA, October 28–31, 2012
Disclosure:
All the authors declare that they have no personal financial or institutional conflict of interest in any of the materials discussed in this article.
References
- 1.Aoyama H, Shirato H, Onimaru R, Kagei K, Ikeda J, Ishii N, et al. Hypofractionated stereotactic radiotherapy alone without whole-brain irradiation for patients with solitary and oligo brain metastasis using noninvasive fixation of the skull. Int J Radiat Oncol Biol Phys. 2003;56:793–800. doi: 10.1016/s0360-3016(03)00014-2. [DOI] [PubMed] [Google Scholar]
- 2.Aoyama H, Shirato H, Tago M, Nakagawa K, Toyoda T, Hatano K, et al. Stereotactic radiosurgery plus whole-brain radiation therapy vs stereotactic radiosurgery alone for treatment of brain metastases: a randomized controlled trial. JAMA. 2006;295:2483–2491. doi: 10.1001/jama.295.21.2483. [DOI] [PubMed] [Google Scholar]
- 3.Atalar B, Choi CY, Harsh GR, 4th, Chang SD, Gibbs IC, Adler JR, et al. Cavity volume dynamics after resection of brain metastases and timing of postresection cavity stereotactic radiosurgery. Neurosurgery. 2013;72:180–185. doi: 10.1227/NEU.0b013e31827b99f3. [DOI] [PubMed] [Google Scholar]
- 4.Beal K, Chan K, Chan T, Yamada Y, Narayana A, Lymberis S, et al. A Phase II Prospective Trial of Stereotactic Radiosurgery Boost Following Surgical Resection for Brain Metastases. Int J Radiat Oncol Biol Phys. 2009;75:S126–S127. doi: 10.1016/j.ijrobp.2013.09.051. (Abstract) [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bernstein M, Cabantog A, Laperriere N, Leung P, Thomason C. Brachytherapy for recurrent single brain metastasis. Can J Neurol Sci. 1995;22:13–16. doi: 10.1017/s0317167100040439. [DOI] [PubMed] [Google Scholar]
- 6.Blonigen BJ, Steinmetz RD, Levin L, Lamba MA, Warnick RE, Breneman JC. Irradiated volume as a predictor of brain radionecrosis after linear accelerator stereotactic radiosurgery. Int J Radiat Oncol Biol Phys. 2010;77:996–1001. doi: 10.1016/j.ijrobp.2009.06.006. [DOI] [PubMed] [Google Scholar]
- 7.Bogart JA, Ungureanu C, Shihadeh E, Chung TC, King GA, Ryu S, et al. Resection and permanent I-125 brachytherapy without whole brain irradiation for solitary brain metastasis from non-small cell lung carcinoma. J Neurooncol. 1999;44:53–57. doi: 10.1023/a:1006285304892. [DOI] [PubMed] [Google Scholar]
- 8.Bradley KA, Mehta MP. Management of brain metastases. Semin Oncol. 2004;31:693–701. doi: 10.1053/j.seminoncol.2004.07.012. [DOI] [PubMed] [Google Scholar]
- 9.Chang EL, Wefel JS, Hess KR, Allen PK, Lang FF, Kornguth DG, et al. Neurocognition in patients with brain metastases treated with radiosurgery or radiosurgery plus whole-brain irradiation: a randomised controlled trial. Lancet Oncol. 2009;10:1037–1044. doi: 10.1016/S1470-2045(09)70263-3. [DOI] [PubMed] [Google Scholar]
- 10.Chow E, Davis L, Holden L, Tsao M, Danjoux C. Prospective assessment of patient-rated symptoms following whole brain radiotherapy for brain metastases. J Pain Symptom Manage. 2005;30:18–23. doi: 10.1016/j.jpainsymman.2005.02.009. [DOI] [PubMed] [Google Scholar]
- 11.Crossen JR, Garwood D, Glatstein E, Neuwelt EA. Neurobehavioral sequelae of cranial irradiation in adults: a review of radiation-induced encephalopathy. J Clin Oncol. 1994;12:627–642. doi: 10.1200/JCO.1994.12.3.627. [DOI] [PubMed] [Google Scholar]
- 12.Dagnew E, Kanski J, McDermott MW, Sneed PK, McPherson C, Breneman JC, et al. Management of newly diagnosed single brain metastasis using resection and permanent iodine-125 seeds without initial whole-brain radiotherapy: a two institution experience. Neurosurg Focus. 2007;22:E3. doi: 10.3171/foc.2007.22.3.4. [DOI] [PubMed] [Google Scholar]
- 13.Dale RG, Jones B, Coles IP. Effect of tumour shrinkage on the biological effectiveness of permanent brachytherapy implants. Br J Radiol. 1994;67:639–645. doi: 10.1259/0007-1285-67-799-639. [DOI] [PubMed] [Google Scholar]
- 14.DeAngelis LM, Delattre JY, Posner JB. Radiation-induced dementia in patients cured of brain metastases. Neurology. 1989;39:789–796. doi: 10.1212/wnl.39.6.789. [DOI] [PubMed] [Google Scholar]
- 15.Do L, Pezner R, Radany E, Liu A, Staud C, Badie B. Resection followed by stereotactic radiosurgery to resection cavity for intracranial metastases. Int J Radiat Oncol Biol Phys. 2009;73:486–491. doi: 10.1016/j.ijrobp.2008.04.070. [DOI] [PubMed] [Google Scholar]
- 16.Elaimy AL, Mackay AR, Lamoreaux WT, Fairbanks RK, Demakas JJ, Cooke BS, et al. Clinical outcomes of stereotactic radiosurgery in the treatment of patients with metastatic brain tumors. World Neurosurg. 2011;75:673–683. doi: 10.1016/j.wneu.2010.12.006. [DOI] [PubMed] [Google Scholar]
- 17.Ernst-Stecken A, Ganslandt O, Lambrecht U, Sauer R, Grabenbauer G. Phase II trial of hypofractionated stereotactic radiotherapy for brain metastases: results and toxicity. Radiother Oncol. 2006;81:18–24. doi: 10.1016/j.radonc.2006.08.024. [DOI] [PubMed] [Google Scholar]
- 18.Gans JH, Raper DM, Shah AH, Bregy A, Heros D, Lally BE, et al. The role of radiosurgery to the tumor bed after resection of brain metastases. Neurosurgery. 2013;72:317–325. doi: 10.1227/NEU.0b013e31827fcd60. [DOI] [PubMed] [Google Scholar]
- 19.Gaspar L, Scott C, Rotman M, Asbell S, Phillips T, Wasserman T, et al. Recursive partitioning analysis (RPA) of prognostic factors in three Radiation Therapy Oncology Group (RTOG) brain metastases trials. Int J Radiat Oncol Biol Phys. 1997;37:745–751. doi: 10.1016/s0360-3016(96)00619-0. [DOI] [PubMed] [Google Scholar]
- 20.Gaspar LE, Scott C, Murray K, Curran W. Validation of the RTOG recursive partitioning analysis (RPA) classification for brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:1001–1006. doi: 10.1016/s0360-3016(00)00547-2. [DOI] [PubMed] [Google Scholar]
- 21.Hall EJ. Radiobiology for the radiologist. Philadelphia, PA: Lippincott; 2011. pp. 86–101. [Google Scholar]
- 22.Huang K, Sneed PK, Kunwar S, Kragten A, Larson DA, Berger MS, et al. Surgical resection and permanent iodine-125 brachytherapy for brain metastases. J Neurooncol. 2009;91:83–93. doi: 10.1007/s11060-008-9686-2. [DOI] [PubMed] [Google Scholar]
- 23.Hwang SW, Abozed MM, Hale A, Eisenberg RL, Dvorak T, Yao K, et al. Adjuvant Gamma Knife radiosurgery following surgical resection of brain metastases: a 9-year retrospective cohort study. J Neurooncol. 2010;98:77–82. doi: 10.1007/s11060-009-0051-x. [DOI] [PubMed] [Google Scholar]
- 24.Iwai Y, Yamanaka K, Yasui T. Boost radiosurgery for treatment of brain metastases after surgical resections. Surg Neurol. 2008;69:181–186. doi: 10.1016/j.surneu.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 25.Jagannathan J, Yen CP, Ray DK, Schlesinger D, Oskouian RJ, Pouratian N, et al. Gamma Knife radiosurgery to the surgical cavity following resection of brain metastases. J Neurosurg. 2009;111:431–438. doi: 10.3171/2008.11.JNS08818. [DOI] [PubMed] [Google Scholar]
- 26.Jarvis LA, Simmons NE, Bellerive M, Erkmen K, Eskey CJ, Gladstone DJ, et al. Tumor bed dynamics after surgical resection of brain metastases: implications for postoperative radiosurgery. Int J Radiat Oncol Biol Phys. 2012;84:943–948. doi: 10.1016/j.ijrobp.2012.01.067. [DOI] [PubMed] [Google Scholar]
- 27.Jensen CA, Chan MD, McCoy TP, Bourland JD, Deguzman AF, Ellis TL, et al. Cavity-directed radiosurgery as adjuvant therapy after resection of a brain metastasis. J Neurosurg. 2011;114:1585–1591. doi: 10.3171/2010.11.JNS10939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kalani MY, Filippidis AS, Kalani MA, Sanai N, Brachman D, McBride HL, et al. Gamma Knife surgery combined with resection for treatment of a single brain metastasis: preliminary results. J Neurosurg. 2010;113:S90–S96. doi: 10.3171/2010.8.GKS101067. [DOI] [PubMed] [Google Scholar]
- 29.Kalkanis SN, Kondziolka D, Gaspar LE, Burri SH, Asher AL, Cobbs CS, et al. The role of surgical resection in the management of newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:33–43. doi: 10.1007/s11060-009-0061-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Karlovits BJ, Quigley MR, Karlovits SM, Miller L, Johnson M, Gayou O, et al. Stereotactic radiosurgery boost to the resection bed for oligometastatic brain disease: challenging the tradition of adjuvant whole-brain radiotherapy. Neurosurg Foc. 2009;27:E7. doi: 10.3171/2009.9.FOCUS09191. [DOI] [PubMed] [Google Scholar]
- 31.Kelly PJ, Lin YB, Yu AY, Alexander BM, Hacker F, Marcus KJ, et al. Stereotactic irradiation of the postoperative resection cavity for brain metastasis: a frameless linear accelerator-based case series and review of the technique. Int J Radiat Oncol Biol Phys. 2012;82:95–101. doi: 10.1016/j.ijrobp.2010.10.043. [DOI] [PubMed] [Google Scholar]
- 32.Kondziolka D, Niranjan A, Flickinger JC, Lunsford LD. Radiosurgery with or without whole-brain radiotherapy for brain metastases: the patients' perspective regarding complications. Am J Clin Oncol. 2005;28:173–179. doi: 10.1097/01.coc.0000143016.15783.5b. [DOI] [PubMed] [Google Scholar]
- 33.Kwon AK, Dibiase SJ, Wang B, Hughes SL, Milcarek B, Zhu Y. Hypofractionated stereotactic radiotherapy for the treatment of brain metastases. Cancer. 2009;115:890–898. doi: 10.1002/cncr.24082. [DOI] [PubMed] [Google Scholar]
- 34.Le Chevalier T, Smith FP, Caille P, Constans JP, Rouesse JG. Sites of primary malignancies in patients presenting with cerebral metastases. A review of 120 cases. Cancer. 1985;56:880–882. doi: 10.1002/1097-0142(19850815)56:4<880::aid-cncr2820560430>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 35.Limbrick DD, Jr, Lusis EA, Chicoine MR, Rich KM, Dacey RG, Dowling JL, et al. Combined surgical resection and stereotactic radiosurgery for treatment of cerebral metastases. Surg Neurol. 2009;71:280–288. doi: 10.1016/j.surneu.2007.12.019. [DOI] [PubMed] [Google Scholar]
- 36.Ling CC, Spiro IJ, Mitchell J, Stickler R. The variation off OER with dose rate. Int J Radiat Oncol Biol Phys. 1985;11:1367–1373. doi: 10.1016/0360-3016(85)90253-6. [DOI] [PubMed] [Google Scholar]
- 37.Linskey ME, Andrews DW, Asher AL, Burri SH, Kondziolka D, Robinson PD, et al. The role of stereotactic radiosurgery in the management of patients with newly diagnosed brain metastases: a systematic review and evidence-based clinical practice guideline. J Neurooncol. 2010;96:45–68. doi: 10.1007/s11060-009-0073-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Manning MA, Cardinale RM, Benedict SH, Kavanagh BD, Zwicker RD, et al. Hypofractionated stereotactic radiotherapy as an alternative to radiosurgery for the treatment of patients with brain metastases. Int J Radiat Oncol Biol Phys. 2000;47:603–608. doi: 10.1016/s0360-3016(00)00475-2. [DOI] [PubMed] [Google Scholar]
- 39.Mathieu D, Kondziolka D, Flickinger JC, Fortin D, Kenny B, Michaud K, et al. Tumor bed radiosurgery after resection of cerebral metastases. Neurosurgery. 2008;62:817–823. doi: 10.1227/01.neu.0000316899.55501.8b. [DOI] [PubMed] [Google Scholar]
- 40.McDermott MW, Cosgrove GR, Larson DA, Sneed PK, Gutin PH. Interstitial brachytherapy for intracranial metastases. Neurosurg Clin N Am. 1996;7:485–495. [PubMed] [Google Scholar]
- 41.Minniti G, Clarke E, Lanzetta G, Osti MF, Trasimeni G, Bozzao A, et al. Stereotactic radiosurgery for brain metastases: analysis of outcome and risk of brain radionecrosis. Radiat Oncol. 2011;6:48. doi: 10.1186/1748-717X-6-48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Narayana A, Chang J, Yenice K, Chan K, Lymberis S, Brennan C, et al. Hypofractionated stereotactic radiotherapy using intensity-modulated radiotherapy in patients with one or two brain metastases. Stereotact Funct Neurosurg. 2007;85:82–87. doi: 10.1159/000097923. [DOI] [PubMed] [Google Scholar]
- 43.Nieder C, Schwerdtfeger K, Steudel WI, Schnabel K. Patterns of relapse and late toxicity after resection and whole-brain radiotherapy for solitary brain metastases. Strahlenther Onkol. 1998;174:275–278. doi: 10.1007/BF03038721. [DOI] [PubMed] [Google Scholar]
- 44.Ostertag CB, Kreth FW. Interstitial iodine-125 radiosurgery for cerebral metastases. Br J Neurosurg. 1995;9:593–603. doi: 10.1080/02688699550040873. [DOI] [PubMed] [Google Scholar]
- 45.Patchell RA, Tibbs PA, Regine WF, Dempsey RJ, Mohiuddin M, Kryscio RJ, et al. Postoperative radiotherapy in the treatment of single metastases to the brain: a randomized trial. JAMA. 1998;280:1485–1489. doi: 10.1001/jama.280.17.1485. [DOI] [PubMed] [Google Scholar]
- 46.Patchell RA, Tibbs PA, Walsh JW, Dempsey RJ, Maruyama Y, Kryscio RJ, et al. A randomized trial of surgery in the treatment of single metastases to the brain. N Engl J Med. 1990;322:494–500. doi: 10.1056/NEJM199002223220802. [DOI] [PubMed] [Google Scholar]
- 47.Patchell RA. The management of brain metastases. Cancer Treat Rev. 2003;29:533–540. doi: 10.1016/s0305-7372(03)00105-1. [DOI] [PubMed] [Google Scholar]
- 48.Pieper D, Suen AW, Grills IS, Nandalur S, Mohammed N, Mitchell C, et al. Gamma Knife Stereotactic Radiosurgery for Resected Brain Metastases. Int J Radiat Oncol Biol Phys. 2008;72:S227–S228. [Google Scholar]
- 49.Prasad SC, Bassano DA, Fear PL, King GA. Dosimetry of I-125 seeds implanted on the surface of a cavity. Med Dosimetry. 1990;15:217–219. doi: 10.1016/0958-3947(90)90011-6. [DOI] [PubMed] [Google Scholar]
- 50.Quigley MR, Fuhrer R, Karlovits SM, Karlovits BJ, Johnson M. Single session stereotactic radiosurgery boost to the post-operative site in lieu of whole brain radiation in metastatic brain disease. J Neurooncol. 2008;87:327–332. doi: 10.1007/s11060-007-9515-z. [DOI] [PubMed] [Google Scholar]
- 51.Rogers LR, Rock JP, Sills AK, Vogelbaum MA, Suh JH, Ellis TL, et al. Results of a phase II trial of the GliaSite radiation therapy system for the treatment of newly diagnosed, resected single brain metastases. J Neurosurg. 2006;105:375–384. doi: 10.3171/jns.2006.105.3.375. [DOI] [PubMed] [Google Scholar]
- 52.Schulder M, Black PM, Shrieve DC, Alexander E, 3rd, Loeffler JS. Permanent low-activity iodine-125 implants for cerebral metastases. J Neurooncol. 1997;33:213–221. doi: 10.1023/a:1005798027813. [DOI] [PubMed] [Google Scholar]
- 53.Sheline GE, Brady LW. Radiation therapy for brain metastases. J Neurooncol. 1987;4:219–225. doi: 10.1007/BF00150613. [DOI] [PubMed] [Google Scholar]
- 54.Sneed PK, Suh JH, Goetsch SJ, Sanghavi SN, Chappell R, Buatti JM, et al. A multi-institutional review of radiosurgery alone vs. radiosurgery with whole brain radiotherapy as the initial management of brain metastases. Int J Radiat Oncol Biol Phys. 2002;53:519–526. doi: 10.1016/s0360-3016(02)02770-0. [DOI] [PubMed] [Google Scholar]
- 55.Soltys SG, Adler JR, Lipani JD, Jackson PS, Choi CY, Puataweepong P, et al. Stereotactic radiosurgery of the postoperative resection cavity for brain metastases. Int J Radiat Oncol Biol Phys. 2008;70:187–193. doi: 10.1016/j.ijrobp.2007.06.068. [DOI] [PubMed] [Google Scholar]
- 56.Suwinski R, Sowa A, Rutkowski T, Wydmanski J, Tarnawski R, Maciejewski B. Time factor in postoperative radiotherapy: a multivariate locoregional control analysis in 868 patients. Int J Radiat Oncol Biol Phys. 2003;56:399–412. doi: 10.1016/s0360-3016(02)04469-3. [DOI] [PubMed] [Google Scholar]
- 57.Wang CC, Floyd SR, Chang CH, Warnke PC, Chio CC, Kasper EM, et al. Cyberknife hypofractionated stereotactic radiosurgery (HSRS) of resection cavity after excision of large cerebral metastasis: efficacy and safety of an 800 cGy × 3 daily fractions regimen. J Neurooncol. 2012;106:601–610. doi: 10.1007/s11060-011-0697-z. [DOI] [PubMed] [Google Scholar]
- 58.Weil RJ. Does trastuzumab increase the risk of isolated central nervous system metastases in patients with breast cancer? Nat Clin Pract Oncol. 2006;3:236–237. doi: 10.1038/ncponc0487. [DOI] [PubMed] [Google Scholar]
- 59.Wernicke AG, Chao KS, Nori D, Parashar B, Yondorf M, Boockvar JA, et al. The Cost-effectiveness of Surgical Resection Plus Cesium-131 (Cs-131) Brachytherapy versus Stereotactic Radiosurgery versus Surgery+Whole Brain Radiotherapy (WBRT) versus WBRT in the Treatment of Metastatic Brain Tumors. Neuro Oncol. 2012;14(S6):vi139. (Abstract) [Google Scholar]
- 60.Yang R, Wang J, Zhang H. Dosimetric study of CS-131, I-125 and Pd-103 seeds for permanent prostate brachytherapy. Cancer Biother Radiopharm. 2009;24:701–705. doi: 10.1089/cbr.2009.0648. [DOI] [PubMed] [Google Scholar]
- 61.Zamorano L, Yakar D, Dujovny M, Sheehan M, Kim J. Permanent iodine-125 implant and external beam radiation therapy for the treatment of malignant brain tumors. Stereotact Funct Neurosurg. 1992;59:183–192. doi: 10.1159/000098940. [DOI] [PubMed] [Google Scholar]