Abstract
Vertebrate heart development is strictly regulated by temporal and spatial expression of growth and transcription factors (TFs). We analyzed nine TFs, selected by in silico analysis of an Nkx2.5 enhancer, for their ability to transactivate the respective enhancer element that drives, specifically, expression of genes in cardiac progenitor cells (CPCs). Mzf1 showed significant activity in reporter assays and bound directly to the Nkx2.5 cardiac enhancer (Nkx2.5 CE) during murine ES cell differentiation. While Mzf1 is established as a hematopoietic TF, its ability to regulate cardiogenesis is completely unknown. Mzf1 expression was significantly enriched in CPCs from in vitro differentiated ES cells and in mouse embryonic hearts. To examine the effect of Mzf1 overexpression on CPC formation, we generated a double transgenic, inducible, tetOMzf1-Nkx2.5 CE eGFP ES line. During in vitro differentiation an early and continuous Mzf1 overexpression inhibited CPC formation and cardiac gene expression. A late Mzf1 overexpression, coincident with a second physiological peak of Mzf1 expression, resulted in enhanced cardiogenesis. These findings implicate a novel, temporal-specific role of Mzf1 in embryonic heart development. Thereby we add another piece of puzzle in understanding the complex mechanisms of vertebrate cardiac development and progenitor cell differentiation. Consequently, this knowledge will be of critical importance to guide efficient cardiac regenerative strategies and to gain further insights into the molecular basis of congenital heart malformations.
Introduction
The understanding of underlying principles in cardiogenesis is crucial to identify pathophysiological mechanisms involved in congenital heart disease and to gain further insights into the molecular basis for a cardiac regenerative therapy [1]–[3]. Vertebrate heart development is strictly regulated by temporal- and spatial-restricted expression of different growth and transcription factors (TFs) [1], [2]. Several cardiac progenitor cell populations, which have been characterized by the expression of different TFs or defined by the activity of specific enhancer elements using transgenic models, are involved in the developmental processes that guide cardiogenesis [3]–[6]. In our study we focused on a murine cardiac progenitor cell (CPC) population defined by the activity of an Nkx2.5 cardiac enhancer (Nkx2.5 CE) element located about 9 kb upstream of the Nkx2.5 start codon [3], [7]. This CPC population has been described to represent the first identifiable heart-forming cell population in the developing mouse embryo [3].
The myeloid zinc finger protein 1 (Mzf1) is a Krüppel class zinc finger TF preferentially expressed in hematopoietic stem cells, myeloid progenitor cells, as well as in differentiated myeloid cells [8]-[10]. Mzf1 is associated with hematopoiesis as transcriptional regulator in committing hematopoietic precursor cells to a myeloid fate, especially for granulopoiesis [8], [11], [12]. Additionally, several reports also suggest a role of Mzf1 in tumorigenesis influencing cell migration and invasion [13]–[16]. Mzf1 has thirteen zinc finger motifs arranged in two different DNA binding domains which recognize the consensus sequences 5′ AGTGGGGA 3′ (zinc fingers 1–4) and 5′ CGGGNGAGGGGGAA 3′ (zinc fingers 5–13) [8], [11]. Mzf1 can act as transcriptional activator or inhibitor in a context dependent manner as shown for a subset of different cell lines [8].
In this study we analyzed nine candidate TFs, selected by in silico analysis of the Nkx2.5 CE, with a known background in embryonic cardiogenesis or hemangiogenesis, for their ability to transactivate the Nkx2.5 CE element [3], [7]. We found, that Mzf1 displayed an impressive activation of Nkx2.5 CE in luciferase reporter assays and we were able to demonstrate specific binding of Mzf1 to the Nkx2.5 CE. In support of a potential role of Mzf1 in cardiac development, we could show that Mzf1 is highly expressed in embryonic CPCs in vivo. To dissect the role of Mzf1 in cardiac differentiation, we generated a doxycyclin inducible Mzf1 overexpressing murine Nkx2.5 CE eGFP ES cell line and examined the differential effects of Mzf1 on CPC formation. Interestingly, Mzf1 was able to either repress or enhance cardiogenesis in a temporal-specific manner as indicated by the frequency of eGFP+ cells and the degree of cardiac gene expression. Thus, our findings support a novel bi-phasic role of Mzf1 during embryonic heart development.
Materials and Methods
Methods are described briefly. Please find a detailed methods section in the online supporting information (Methods S1).
Luciferase Reporter Assays
Cells (HEK 293, H9c2, HL-1 and NFPE) were seeded in 24-well plates and grown to 70–80% confluence. HEK 293 and H9c2 cells are commercially available at ATCC (Manassas, VA). HL-1 cells were a kind gift of Prof. Dr. William Claycomb [17]. NFPE cells were a kind gift of Prof Dr. Karl-Ludwig Laugwitz but are also commercially available at ATCC. Each well of cells was co-transfected with four plasmids: the expression plasmid (pcDNA3.1(−) containing the candidate cDNA; 150 ng), a pCMV β-Gal plasmid (to normalize transfection efficiency, 50 ng), the pBluescript KSII(+) (250 ng, to normalize the quantity of DNA used in each transfection) and a promoterless pGL3 basic reporter plasmid containing the 2.5 kb fragment of the Nkx2.5 CE including the base promoter [3] in front of a luciferase gene (150 ng). In further experiments additional mutant forms of the pGL3-Nkx2.5 CE BP plasmid were used (mutation of the Mzf1 and Mesp1 binding sites). The empty pcDNA3.1 was used as a negative control in all assays. After 48 h cells were lysed, luciferase activity was determined and normalized to β-galactosidase activity. Each transfection experiment was performed in triplicate in at least three independent experiments.
Electromobility Shift Assays (EMSA)
Proteins (Mzf1, Mesp1) were translated in vitro by a TNT T7-coupled reticulocyte lysate system (Promega, Madison, WI). Pairs of complementary Cy5- or Cy3-tagged oligonucleotides were annealed overnight and binding reactions were performed with 5-10 µl of in vitro translated protein (Mzf1, Mesp1; confirmed by Western Blot with specific antibodies) or the same amount of unprogrammed reticulocyte lysate (RL) as a negative control. For competition assays unlabeled specific competitor (same sequence as the Cy5-tagged probes, 10- and 50-fold excess) and mutant competitor (10-fold excess) were added to test for specifity of DNA binding. Three independent experiments were performed.
Chromatin Immunoprecipitation (ChIP) Assays
Cross-linking of day 9 differentiated Nkx2.5 CE eGFP ES cells (a kind gift of Dr. Sean M Wu [3]) was achieved by incubating the cells with 1% formaldehyde for 30 min at RT. For subsequent sonication cell-lysates were diluted and DNA was sheared. Precleared cell-lysates were then incubated with an antibody against Mzf1 or an isotype-matched control and pulled down by protein A/G-Sepharose beads. Immune complexes were washed extensively with buffer (increasing stringency) and eluted by boiling in SDS sample buffer. DNA purification was performed and with the primer sets #1 (forward 5′ TAC CGG CAG AGA CTG AAG TTT 3′, reverse 5′ ATT AGT GTG AAC ACA ACA CTC G 3′ corresponding to -9340 to -9220 of the Nkx2.5 CE, fragment size 121 nt), #2 (forward 5′ AAG CTT GGC GTG TGA CAT TGT 3′, reverse 5′ GAT TGT GAA CCG GTA GGC GG 3′ corresponding to -9123 to -8921 of the Nkx2.5 CE, fragment size 203 nt), #3 (forward 5′ TGA GCG CCG CCG TTT ATG CT 3′, reverse 5′ GAT GGA TCC GAT GGG AGC TG 3′ corresponding to -8360 to -8246 of the Nkx2.5 CE, fragment size 114 nt) and #4 (forward 5′ AAA TCA ATC ACA GCC CCA AGT G 3′, reverse 5′ GTT TAT GGA AAA CTC AAA TAG CAG 3′, corresponding to −8235 to −8048 of the Nkx2.5 CE, fragment size 188 nt) the appearance of specific parts of the Nkx2.5 CE was validated. The precipitation of background DNA was controlled by an amplification with primers against β-Actin (fragment size 97 nt, primer sequence see Table S2). PCRs were performed with equal volumes of Mzf1 chip'd samples and the corresponding IgG control on a Thermo Cycler (Bio-Rad, Munich, Germany) and 35 cycles.
Site Directed Mutagenesis
All mutant forms of the pGL3-Nkx2.5 CE BP plasmid were constructed by long polymerase chain reaction-based techniques using the QuikChange Multi Site-Directed Mutagenesis Kit with PfuTurbo polymerase (Stratagene, La Jolla, CA) and different primers containing the desired mutations (for two putative Mzf1 and two Mesp1 binding sites). After amplification all methylated and hemimethylated DNA was digested with the restriction enzyme DpnI followed by a transformation of the remaining mutated single stranded DNA into XL10 Gold ultracompetent cells. The correct DNA sequence of all constructs was confirmed by DNA sequencing.
Animals
Mice were housed in an accredited facility in compliance with the European Community Directive related to laboratory animal protection (2010/63/EU). All transgenic mouse lines have been described in detail previously. For extraction of embryos or organs mice were first anesthetized with isoflurane (2-chloro-2-(difluoromethoxy)-1,1,1-trifluoro-ethane) and then euthanized by cervical dislocation. Embryos of the Nkx2.5 CE eGFP transgenic mice [3] were collected on E 9.5 from timed matings (a positive mating plug indicates E 0.5). Mouse embryos were used for FACS analysis as described in the respective sections. αMHC-Cre/ROSA26mT/mG transgenic mice [18] were provided for heart extraction for FACS analysis as described in the respective section. All animal experiments, like organ or embryo extractions, were performed in accordance with the European regulations for animal care and handling (2010/63/EU) and were approved by the Regierung von Oberbayern.
Lentiviral transduction of ES cells
We used a previously described doxycyclin inducible lentiviral tet-on expression system [19] (a kind gift of Dr. K. Hochedlinger) modified with an IRES puromycin element. The murine complete Mzf1 cDNA tagged by a flag sequence was subcloned into the modified pLvtetO backbone in front of the IRES element. Lentivirus production by 293 cells was previously described by Gregoire et al. [20]. For transduction the virus containing supernatant was collected after 48 h, filtered and then directly used without further concentration.
A doxycyclin inducible tetOMzf1-Nkx2.5 CE eGFP ES cell line was established by co-transducing Nkx2.5 CE eGFP ES cells with tetOMzf1-IRES-puromycin and rtTA lentiviral particles (approved by the Regierung von Oberbayern, Az. 50-8791-26.384.1776). Antibiotic selection was performed with doxycyclin (dox) and puromycin. About 10 days post-transduction, colonies were individually expanded and scored by qRT-PCR for sufficient expression of Mzf1. Morphology and growing behavior of the transduced cell lines were virtually indistinguishable from untreated murine ES cells.
Flow cytometry
Single cell suspension was prepared from Nkx2.5 CE eGFP and dox-inducible tetOMzf1-Nkx2.5 CE eGFP differentiating ES cells. Nkx2.5 CE eGFP mouse embryos were dissected on E 9.5 and single cell suspension was obtained. Adult cardiomyocytes (CMs) were isolated from six week old αMHC-Cre/ROSA26mT/mG mice. Dead cells were stained with propidium iodide solution for flow cytometry.
RNA Isolation and qRT-PCR
Total RNA was isolated and reverse transcribed into first strand cDNA. Semiquantitative real time PCR (qRT-PCR) was performed using gene-specific primer sets (Table S2) for 40 cycles. ΔCT calculations were performed and each sample was normalized against its β-actin value.
Data Analysis and Statistical Analysis
All assays were at least performed in triplicates. Data are presented as mean values ± standard error (S.E.M.). Statistical differences were evaluated using the unpaired Student's t-test or the Mann-Whitney-U test. Comparison of several groups was done by one way ANOVA or the Kruskal-Wallis test on ranks including appropriate post-hoc tests. A value of p <0.05 was considered to be statistically significant. In all figures statistical significance is indicated as follows: * p <0.05 and ** p <0.01.
Results
An in silico transcription factor (TF) binding site analysis [21] (P-Match [22] http://www.gene-regulation.com/cgi-bin/pub/programs/pmatch/bin/p-match.cgi, PROMO 3.0 http://alggen.lsi.upc.es/recerca/menu_recerca.html, JASPAR database http://jaspar.genereg.net, ConSite http://phylofoot.org/consite, TFSEARCH ver. 1.3 http://www.cbrc.jp/research/db/TFSEARCH.html) of the cardiac specific Nkx2.5 enhancer [7] (−9641 to −7540 bp upstream of the murine Nkx2.5 transcriptional initiation site) and the base promoter [3] (−520 to −24 bp upstream of the ATG of the Nkx2.5 gene) revealed a couple of candidates for potential interaction (Table S1). Nine of these TFs (Gata4 [7], Hand1 [23], Sox17 [24], Klf4 [25], Elk1 [26], Msx1 [27], Mzf1, Brachyury [28], Mesp1 [29]) which are known in the context of embryonic heart development or hemangiogenesis were selected for further analysis.
Mzf1 strongly activates the Nkx2.5 CE using different cell lines
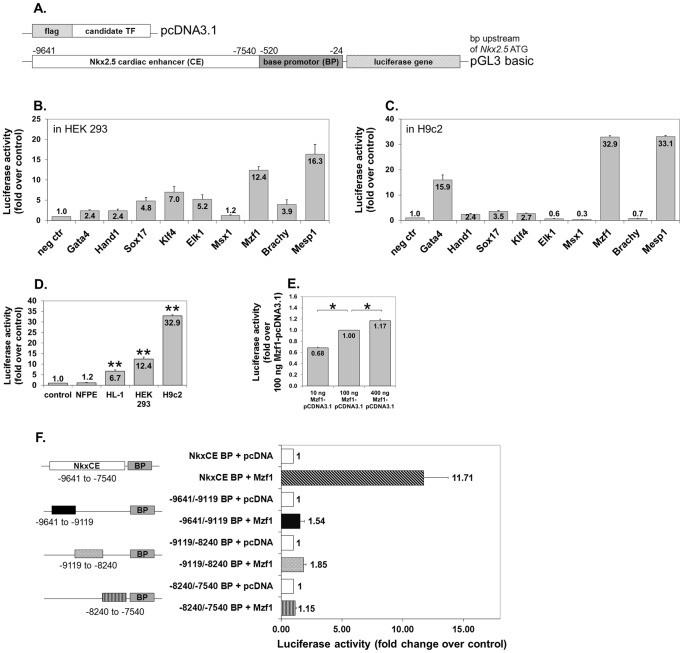
Luciferase reporter assays were performed to evaluate the activation capacity of the candidate TFs on the Nkx2.5 CE. Each TF was inserted into a modified pcDNA3.1 vector and co-transfected with the Nkx2.5 CE luciferase reporter plasmid (Fig. 1A) in HEK 293 cells. A more than 5-fold activation of the luciferase gene was found for Elk1, Klf4, Mesp1 and Mzf1 (Fig. 1B).
Figure 1. TF screening on the Nkx2.5 CE element by luciferase reporter assays.
A. Plasmid constructs for luciferase reporter assays. The empty modified pcDNA3.1 was used as a negative control in all assays. B. TF screening by luciferase assays using HEK 293 cells (human embryonic kidney fibroblasts). Fold change is compared to the negative control (neg ctr) (pcDNA3.1). C. TF screening by luciferase assays using H9c2 cells (rat myoblasts). Fold change is compared to the negative control (neg ctr) (pcDNA3.1). D. Mzf1 activates the Nkx2.5 CE element in atrial HL-1 cells but not in endothelial NFPE cells. Asterisks indicate a significant difference compared to the control (pcDNA3.1); ** = p <0.01. E. Dose dependent effect of Mzf1-pcDNA3.1-DNA on Nkx2.5 CE activation in HEK 293 cells; * = p <0.05. F. Effect of truncating different parts of the Nkx2.5 CE according to Lien and co-workers [7] on luciferase activation by Mzf1 in HEK 293 cells.
To determine if this effect is cell line specific, luciferase assays were also performed in H9c2, a rat myoblastic cell line corroborating a strong (more than 30-fold) transgene activation by Mesp1 and Mzf1 (Fig. 1C). Further luciferase assays using murine atrial HL-1 cells confirmed the induction of luciferase expression by Mzf1 (Fig. 1D) and Mesp1 (Fig. S1A). Interestingly no transactivation of either factor occurred in endothelial NFPE cells (Fig. 1D, Fig. S1A). Luciferase induction occurred in a dose-dependent fashion for both TFs in HEK 293 cells (Fig. 1E, Fig. S1B).
Our results confirmed the findings reported by Bondue et al. [29] regarding the interaction between Mesp1 and the cardiac specific Nkx2.5 CE element. However, we were intrigued by the as yet unknown function of Mzf1 during cardiomyogenesis. Hence, we chose to further explore the role of Mzf1 in this context.
Mzf1 shows activating effects on different parts of the Nkx2.5 CE
According to Lien et al. [7] the identified Nkx2.5 CE consists of activating and non-activating elements regarding the potential to drive a cardiac specific β-galactosidase expression. Corresponding to their description we investigated the potential of Mzf1 and Mesp1 to induce luciferase expression driven by different parts of the Nkx2.5 CE (−9641 to −9119; −9119 to 8240; −8240 to −7540). Luciferase activity was significantly reduced following Mzf1 and Mesp1 treatment when the Nkx2.5 CE was truncated, regardless which part of the Nkx2.5 CE was used for the assay, suggesting the presence of activating binding sites in each portion of the Nkx2.5 CE (Fig. 1F, Fig. S1C). Furthermore, it could be possible that one of the truncated fragments contains a binding site of Mzf1, while the others contain binding sites for essential co-factors.
Mzf1 directly binds to the Nkx2.5 CE
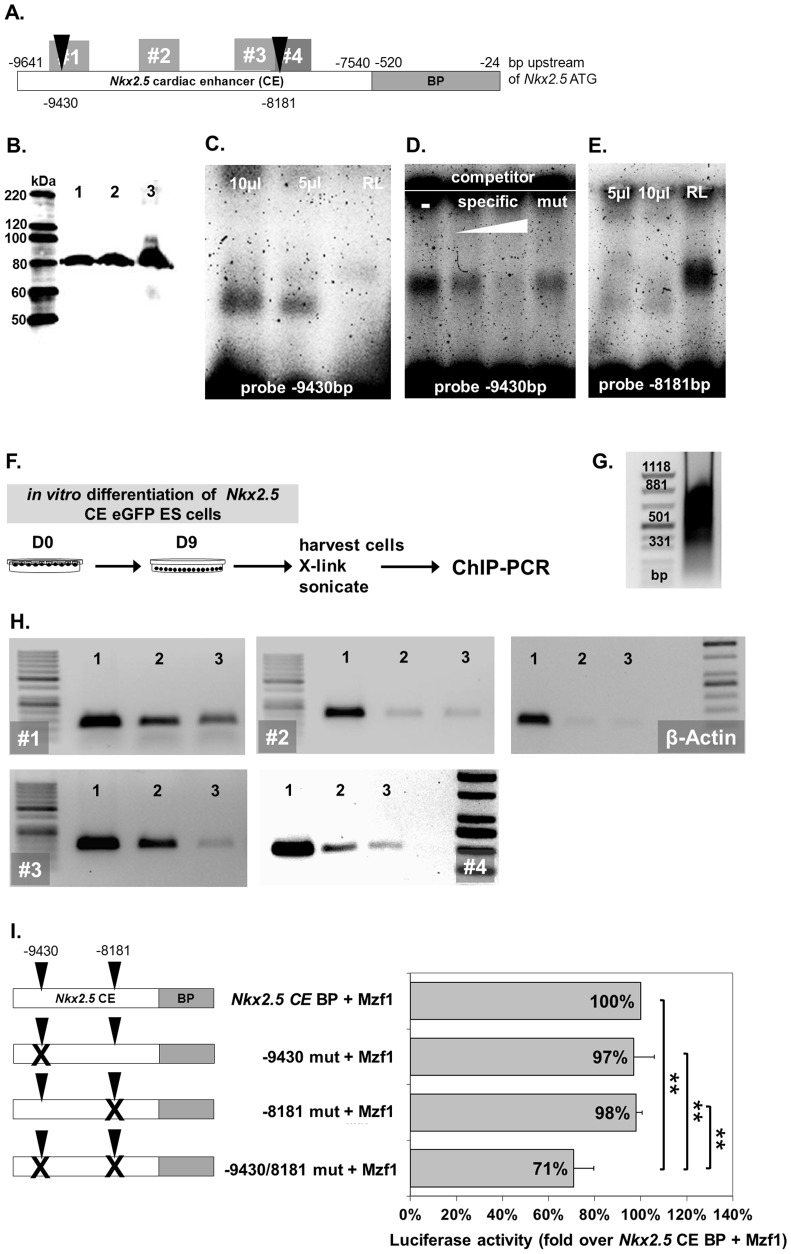
Since in silico analysis of the Nkx2.5 CE revealed between 19 and 92 potential binding sites for Mzf1 (Table S1) distributed all over the Nkx2.5 CE, we decided to focus on two binding motifs similar to the well-known zinc finger motifs already described by other researchers [8], [11].
The binding of Mzf1 to the Nkx2.5 CE could be confirmed by electromobility shift assays (EMSA) using the core Mzf1 binding motif 5′-AGGGGGA-3′ (corresponding to the zinc fingers 5–13, [8], [11]) at position −9430 bp of the Nkx2.5 CE using Cy5-tagged probes and in vitro translated Mzf1 protein (Fig. 2A–C). Competition assays with untagged mutant (10-fold excess) and specific probes (10- and 50-fold excess) were performed to ensure specificity of the binding reaction at position −9430 (Fig. 2D). However, binding to the motif 5′-GTGGGGA-3′ (corresponding to the zinc fingers 1–4, [8], [11]) at position −8181 bp of the Nkx2.5 CE could not be approved by EMSA (Fig. 2E). Direct binding of in vitro translated Mesp1 to the Nkx2.5 CE was also confirmed by EMSA (Fig. S1D–F).
Figure 2. Direct binding of Mzf1 to the Nkx2.5 cardiac enhancer in vitro and in vivo.
A. Locations of analyzed described Mzf1 binding motifs in the Nkx2.5 CE [8], [11] (black triangles) and primer sets #1-#4 for ChIP PCR (grey rectangles with numbers). B. In vitro translated Mzf1 protein from the flag-Mzf1-pcDNA3.1 confirmed by an anti-flag antibody in western-blotting (lane 3). As a control whole cell lysates from 293 cells transfected with the flag-Mzf1-pCDNA3.1 plasmid were used (lane 1 & 2). The predicted molecular weight for Mzf1 is 84 kDa. C. Different amounts of in vitro translated Mzf1 (10µl, 5µl) bound to the Nkx2.5 CE at the binding motif corresponding to zinc fingers 5-13 (black triangle at position -9430 bp) [8], [11] in an electromobility shift assay (EMSA). Unprogrammed reticulocyte lysate (RL) was applied as a control. D. Competition assays with untagged mutant (mut, 10-fold excess) and specific probes (10- and 50-fold excess) were performed to ensure specificity. E. In vitro binding to the motif corresponding to zinc fingers 1-4 (at position −8181 bp) [8], [11] by EMSA could not be confirmed. Different amounts of in vitro translated Mzf1 (10µl, 5µl) were used. Unprogrammed reticulocyte lysate (RL) was applied as a control. F. Experimental set-up for ChIP assays. Chromatin was isolated from day nine differentiated Nkx2.5 CE eGFP ES cells. G. Chromatin was sheared by sonication to obtain fragment sizes between 250 and 1000 bp. H. ChIP-PCR on purified chromatin using a polyclonal anti-Mzf1 and an isotype-matched control antibody. Lane 1: 4% sonicated input chromatin. Lane 2: Chromatin precipitated with the Mzf1 antibody. Lane 3: Chromatin precipitated with an IgG matched control antibody. I. Effect of mutating two Mzf1-binding sites at positions −9430 bp and -8181 bp in the Nkx2.5 CE on luciferase activity by Mzf1 in HEK 293 cells (** = p <0.01).
To further corroborate Mzf1 binding to the Nkx2.5 CE in vivo ChIP assays with a polyclonal anti-Mzf1 antibody were performed on cross-linked murine day nine differentiated Nkx2.5 CE eGFP ES cells followed by PCR analysis (Fig. 2F). Chromatin shearing led to fragment sizes between 250 and 1000 bp (Fig. 2G). Binding of Mzf1 on day nine of in vitro differentiation of murine ES cells could be validated with primer set #3 corresponding to a 114 nt fragment at position −8360 to −8246 of the Nkx2.5 CE (Fig. 2A+H) and primer set #4 corresponding to a 188 nt fragment at position −8235 to −8048 of the Nkx2.5 CE (Fig. 2A+H). Primer set #1 (−9340 to −9220) led to a darker band in the sample precipitated with the anti-Mzf1 antibody compared to the control but the background was very strong for this primer set (Fig. 2H). No binding could be confirmed with primer set #2 (−9123 to −8921) (Fig. 2H). A background control with primers against β-Actin approved that precipitation of unspecific DNA was low (Fig. 2H).
In a next step site directed mutagenesis was performed on the pGL3-Nkx2.5 CE BP plasmid to mutate the analyzed binding sites of Mzf1 (Fig. 2I) and also Mesp1 (Fig. S1G). Subsequent luciferase assays with the Mzf1-pcDNA3.1 could not show a reduced luciferase activity when only one, either at position −9430 bp or −8181 bp, of the binding sites in the Nkx2.5 CE was mutated (Fig. 2I). However, a combined mutation of both binding sites led to a significant reduction of about 30% of luciferase activity (Fig. 2I). For Mesp1 we could confirm the significance of the binding site at position −29 bp by a significant reduction of luciferase activity by 26% (Fig. S1G) when this site was mutated as already indicated by ChiP-assays by Bondue and co-workers [29]. No reduction of luciferase activity could be shown for a mutation of the Mesp1 binding site at position −9138 bp, despite ChiP-assays demonstrated binding of Mesp1 to this site [29].
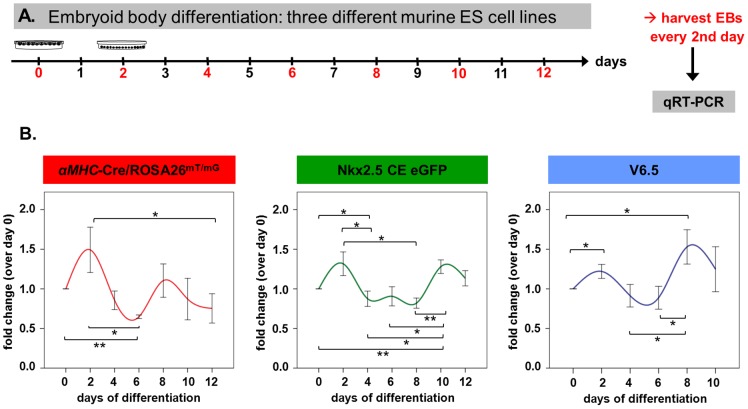
Mzf1 shows biphasic kinetics during in vitro differentiation of murine ES cell lines
Next we analyzed the kinetics of Mzf1 mRNA expression during ES cell in vitro differentiation. Three different murine ES cell lines (V6.5 ES, the transgenic Nkx2.5 CE eGFP ES [3] and the transgenic αMHC-Cre/ROSA26mT/mG ES [18]) were studied every other day starting from day 0 of differentiation (when hanging drops are prepared) for the expression of Mzf1 (for experimental set-up see Fig. 3A). We found a clear biphasic mRNA expression pattern of Mzf1 in all of the three ES cell lines with an early peak around day two and a second peak between day eight and day ten of in vitro differentiation (Fig. 3B).
Figure 3. Biphasic kinetics of Mzf1 expression during in vitro differentiation.
A. Experimental set-up for in vitro differentiation assays of three different murine ES cell lines for the evaluation of time-dependent Mzf1 expression levels. B. Mzf1 expression levels showed a biphasic course during in vitro differentiation of αMHC-Cre/ROSA26mT/mG -, Nkx2.5 CE eGFP - and V6.5 ES cells; * = p <0.05; ** = p <0.01.
Mzf1 gene expression is upregulated in CPCs but not in adult cardiomyocytes
As previously described, luciferase reporter assays indicated an activation of the Nkx2.5 CE element by Mzf1. Additionally, specific binding of Mzf1 to the Nkx2.5 CE element could be confirmed by EMSA and ChIP assays.
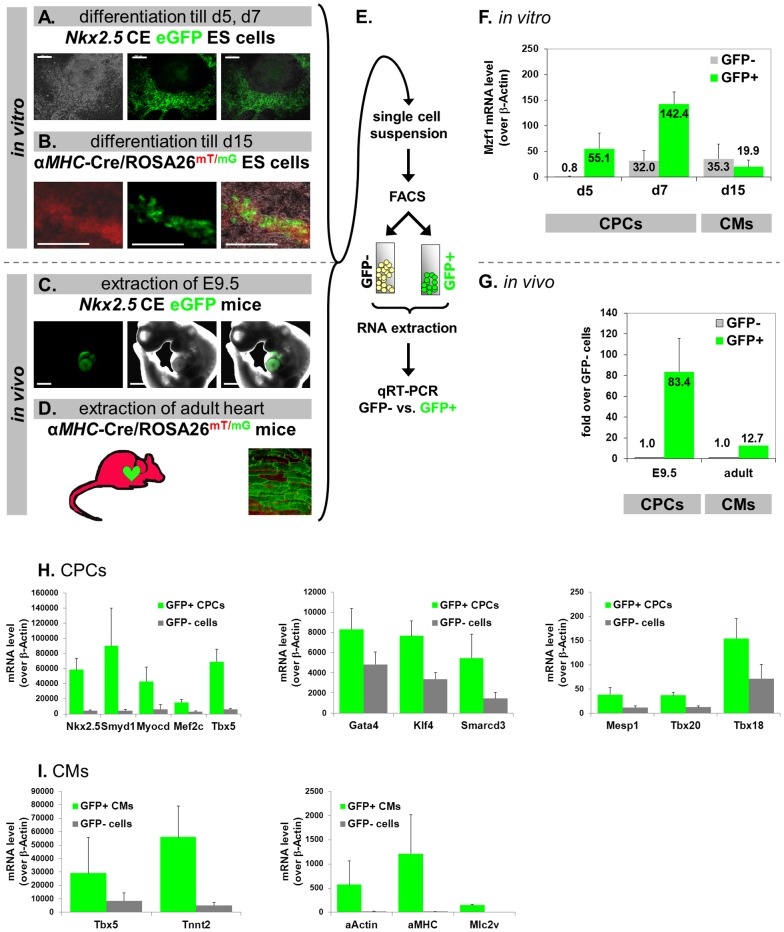
We postulated that if Mzf1 interacts with the Nkx2.5 CE in vivo it should also be differentially expressed within Nkx2.5 CE eGFP positive CPCs (Fig. 4A). To examine this hypothesis, we differentiated Nkx2.5 CE eGFP ES cells for either five or seven days. During in vitro differentiation of this cell line first eGFP positive CPCs usually emerge on day five. EGFP-positive CPCs and eGFP-negative cells were then isolated by fluorescence activated cell sorting (FACS) on day five and seven. Both cell populations were lysed for total RNA extraction and subsequent gene expression analysis by qRT-PCR (Fig. 4E). We observed that CPCs expressed a considerably higher level of Mzf1 than non-CPCs, on day five and seven of in vitro differentiation (Fig. 4F). These isolated eGFP positive CPCs further exhibit high expression levels of typical early cardiac marker genes compared to eGFP negative non-CPCs (Fig. 4H).
Figure 4. Differential expression of Mzf1 in cardiac progenitor cells (CPCs) but not in cardiomyocytes (CMs) and gene expression profiles of in vitro differentiated CPCs and CMs.
Scale bars: 200 µm for all panels, except C: 500 µm. A.-D. Detection of CPCs and mature CMs by activation of eGFP expression. Illustration of transgenic cell lines (A.–B.) and animal models (C.–D.). E. Experimental set-up for isolating eGFP-positive and -negative cell populations by FACS. F. Gene expression analysis after FACS sorting of in vitro differentiated Nkx2.5 CE eGFP ES cells (A.) revealed a considerable up-regulation of Mzf1 in eGFP+ CPCs but not for mature CMs (B.) compared to the respective eGFP− cells. G. Correspondingly, Mzf1 expression was up-regulated in eGFP+ CPCs isolated from E 9.5 embryos (C.) but not to a comparable amount in mature CMs isolated from postnatal (> 3 weeks) hearts (D.) compared to the respective eGFP− cells. H. Nkx2.5 CE eGFP ES cells were differentiated till day 5–7. GFP positive (CPCs) and GFP negative cells were sorted by FACS. Gene expression profiles of typical cardiac developmental marker genes (Nkx2.5, Mef2c, Gata4, Tbx20, etc.) were evaluated by qRT-PCR. I. αMHC-Cre/ROSA26mT/mG ES cells were differentiated till day 15. GFP positive (CMs) and GFP negative cells were sorted by FACS. Gene expression profiles of typical cardiac and sarcomeric marker genes (Tnnt2, αMHC, etc.) were evaluated by qRT-PCR.
To further confirm the role of Mzf1 in Nkx2.5 CE positive CPCs in vivo, time-pregnant transgenic Nkx2.5 CE eGFP mice were dissected on day E 9.5 where eGFP expression and thus Nkx2.5 CE activity peaks during embryonic development [3]. Whole embryos were digested by a collagenase mixture to obtain single cell suspension for accomplishing FACS. As analyzed by qRT-PCR Mzf1 expression in eGFP positive cells, which exclusively correspond to the E 9.5 heart (Fig. 4C) was more than 80-fold upregulated when compared to the level in embryonic eGFP negative cells (Fig. 4G).
To determine the relative expression of Mzf1 in more mature cardiomyocytes, we utilized the αMHC-Cre/ROSA26mT/mG [18] transgenic murine ES cell line for further experiments. Mature cardiomyocytes (CMs) expressing αMHC switch from red to green fluorescence which is induced by Cre-mediated excision of the td-tomato expression cassette (Fig. 4B). GFP positive CMs were isolated by FACS on day 15 of differentiation. In contrast to CPCs the more mature eGFP positive CMs do not show an elevated level of Mzf1 compared to the eGFP negative population (Fig. 4F). A more detailed gene expression profile of isolated CMs including typical cardiac and sarcomeric markers is presented in Fig. 4I.
Furthermore, also isolated eGFP positive CMs from postnatal hearts of the αMHC-Cre/ROSA26mT/mG transgenic mice (> three weeks of age), do not show a similar elevation over non-cardiomyocytes when compared to E 9.5 CPCs (Fig. 4G).
Mzf1 gain-of-function studies modify CPC number during ES differentiation
Next, to directly address the effect of Mzf1 on CPCs and on cardiac differentiation in general, we generated a double-transgenic, doxycyclin (dox) inducible Mzf1 over-expressing murine ES cell line by lentiviral transduction of the transgenic Nkx2.5 CE eGFP ES cell line (tetOMzf1-Nkx2.5 CE eGFP ES). A plasmid driving dox-inducible expression of Mzf1 and puromycin resistance separated by an internal ribosome entry site (IRES) was co-transduced with a plasmid that constitutively expresses a reverse tetracycline transactivator (rtTA) (Fig. 5A, Fig. S2A). Sufficient inducibility of Mzf1 expression was confirmed in three cell lines (clones 42, 44 and 64; Fig. S2B). Furthermore, it was proofed that Mzf1 overexpression decreased steadily after stopping dox supplementation of the medium (Fig. S2C–D). Two days after dox-removal the Mzf1-mRNA-level was more than 50% reduced compared to the starting level. And after four days the Mzf1-mRNA-level was not different from the samples without dox-addition. Morphology (Fig. S2E), pluripotency (Fig. S2F, anti Sox2 immunostaining) and Mzf1-expression (p = 0.242) were comparable between the tetOMzf1-Nkx2.5 CE eGFP ES cell line without dox treatment, and the parent Nkx2.5 CE eGFP ES cell line.
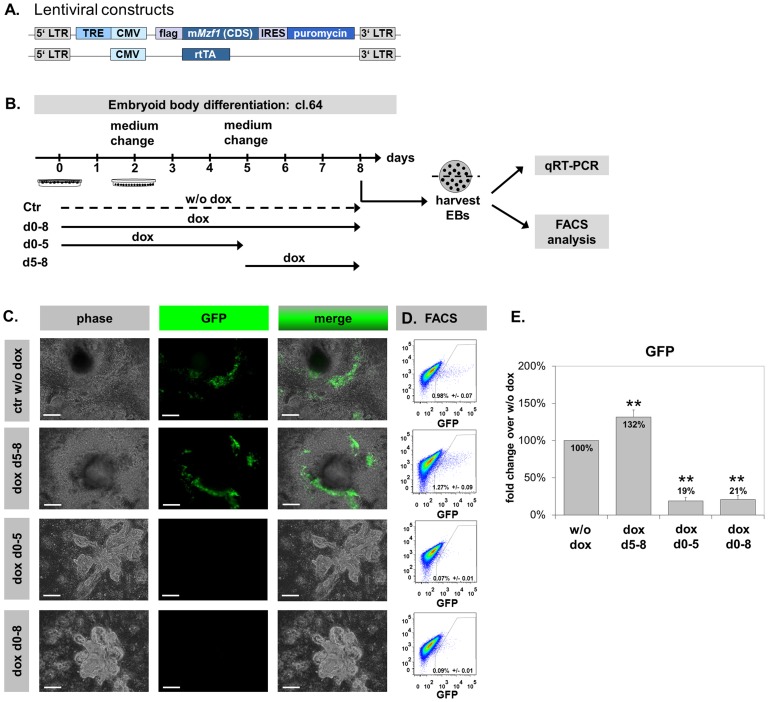
Figure 5. In vitro differentiation of the double-transgenic dox-inducible Mzf1 overexpressing tetOMzf1-Nkx2.5 CE eGFP ES cell line.
Scale bars: 200 µm for all panels. A. Lentiviral constructs for the production of the doxycyclin-inducible tetOMzf1-Nkx2.5 CE eGFP ES line. LTR: long terminal repeats. TRE: tetracyclin responding element. CMV: cytomegalovirus promoter. IRES: internal ribosomal entry site. rtTA: reverse tetracyclin transactivator. B. In vitro differentiation protocols with time-schedules of dox-treatment. C. Morphology of differentiating EBs on day eight. Permanent and day 0–5 dox-treatment led to closely packed globular clusters. In contrast dox-treatment from day 5 showed a normal differentiation pattern comparable to the control w/o dox. D/E. FACS analysis revealed a significant increase in eGFP+ CPCs for dox-treatment from day 5 of differentiation whereas a continuous and day 0-5 dox-treatment resulted in a significant decrease of eGFP+ CPCs compared to control w/o dox; ** = p <0.01.
In vitro differentiation assays of the tetOMzf1-Nkx2.5 CE eGFP ES cell line were performed by the standard hanging drop method [30] to assess the effects of Mzf1 overexpression on CPC number by flow cytometry. ES cells were differentiated for eight days. In line with the physiological, biphasic course of Mzf1-mRNA expression during ES differentiation (Fig. 3B) doxycyclin was added according to different treatment schedules (Fig. 5B). Besides a permanent Mzf1-overexpression by dox-treatment (day 0 - 8), time intervals from zero to five and from five to eight days were analyzed. The tetOMzf1-Nkx2.5 CE eGFP ES cell line differentiated without dox treatment (ctr w/o dox) was used as reference.
The appearance of eGFP positive, beating cells at day five to six of in vitro differentiation was indistinguishable between the control (w/o dox) and the parent murine Nkx2.5 CE eGFP ES cells (Fig. S2G, Video S1, S2).
The comparable amount of dead cells between the different approaches (Fig. S2H) identifiable by propidium iodide staining using FACS analysis indicated that the overexpression of Mzf1 did not influence cell viability during in vitro differentiation.
Cell proliferation was additionally controlled by MTT assays. Whereas tetOMzf1-Nkx2.5 CE eGFP ES cells which grew with dox for 48h were indistinguishable from their untreated counterparts (p = 0.927), ES cells treated with dox for a longer period (five to nine days) proofed significantly more proliferative (p = 0.003) (Fig. S2I).
Furthermore, an efficient Mzf1 overexpression during in vitro differentiation assays was confirmed by qRT-PCR (Fig. S3A) and immunostaining with an anti-flag antibody detecting only exogenous Mzf1 (Fig. S3B). The Mzf1 expression level on day 8 was lower in approaches with permanent dox-treatment than in approaches with late dox-treatment from day 5 during in vitro differentiation (Fig. S3A). This may be due to some self-inhibiting mechanisms within Mzf1 regulation on the mRNA level or due to inactivation of the integrated CMV promoter during in vitro differentiation or a combination of both.
Mzf1 overexpression from day 5 of in vitro differentiation showed no morphological differences and also a regular appearance of beating areas compared to the control w/o dox (Fig. 5C, Video S3). In contrast, permanent overexpression of Mzf1 (day 0–8) and dox treatment from day 0–5 led to severe morphological changes. EBs grew in closely packed, globular clusters while no beating areas could be observed (Fig. 5C).
FACS analysis on day eight of in vitro differentiation revealed about 0.98% ± 0.070 eGFP positive cells (CPCs) in the negative control (w/o dox). Interestingly, an overexpression of Mzf1 from day 5 showed 1.27% ± 0.090 (p = 0.003) eGFP positive cells depicting an increase of nearly 30% compared to the control (w/o dox) and suggesting an enhancement of cardiogenesis. In contrast, permanent Mzf1 overexpression significantly reduced the amount of CPCs to 0.085% ± 0.012 eGFP positive cells (p <0.001) indicating a strong inhibitory effect on cardiogenic differentiation. No significant difference of eGFP positive CPCs could be detected between both protocols with an early overexpression of Mzf1 (day 0–5 and day 0–8) (p = 0.596) (Fig. 5D+E).
Mzf1 modifies cardiac gene expression
Cardiac gene expression was analyzed by qRT-PCR to confirm results obtained from flow cytometry in terms of down- or up-regulation of cardiogenesis, respectively.
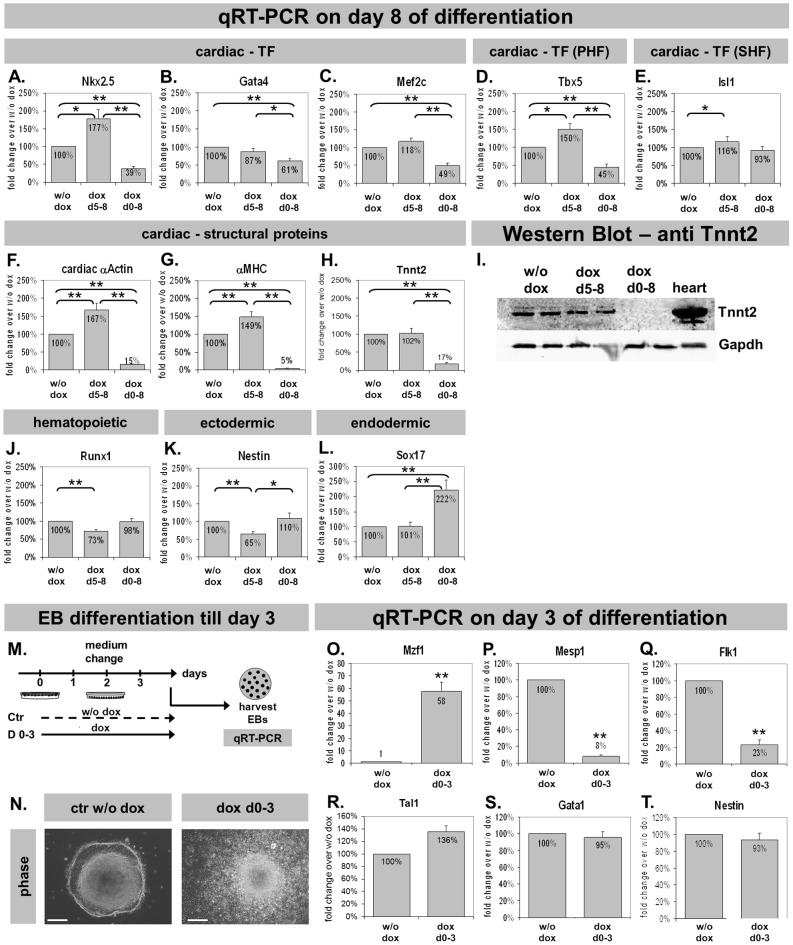
Temporary Mzf1 overexpression from day five led to a significantly up-regulated Nkx2.5 expression compared to the control w/o dox (p = 0.028). In contrast, Nkx2.5 was dramatically down-regulated by a permanent Mzf1 overexpression (p <0.001) (Fig. 6A) confirming regulatory effects of Mzf1 on CPCs. The regulatory effect was also seen for the cardiac TFs Tbx5, Isl1 and Mef2c but not for Gata4 (Fig. 6B–E). Furthermore, cardiac structural genes were significantly repressed by permanent overexpression of Mzf1 whereas a temporary overexpression led to a significant elevation of cardiac structural genes like α-MHC and the pancardiac α-Actin (Fig. 6F-G), but not Troponin T (Tnnt2) (Fig. 6H) (see also western blot, Fig. 6I). The hematopoietic marker Runx1 [31] was down-regulated by dox-stimulated Mzf1 expression from day five (p <0.001) but was not affected by a permanent Mzf1 overexpression (p = 0.371) (Fig. 6J). Ectodermal and endodermal differentiation was assessed by the expression of Nestin and Sox17 [2], [24], respectively. Nestin was down-regulated by Mzf1-overexpression from day 5 (p <0.001) but was unaffected by a continuous overexpression (p = 0.779) (Fig. 6K). Interestingly, Sox17 was unaffected by a late Mzf1 overexpression (p = 0.975) but a permanent Mzf1 overexpression led to a significant increase over the control w/o dox (p <0.001) (Fig. 6L).
Figure 6. Gene expression analysis during in vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells.
* = p<0.05; ** = p <0.01 for all panels. A–H. Expression of selected cardiac genes. PHF: primary heart field. SHF: secondary heart field. I. Protein expression by western-blotting for Tnnt2 and Gapdh. J–L. Expression of selected hematopoietic (J.), ectodermal (K.) and endodermal (L.) genes. M. Experimental set-up for day three in vitro differentiation assays. N. Morphology of differentiating EBs on day three. Dox-treatment for three days increased cell proliferation. Scale bars indicate 200 µm. O–T. Gene expression analysis of Mzf1 (O.), Mesp1 (mesodermal) (P.), Flk1 (cardiovascular progenitor marker) (Q.), Tal1, Gata1 (hematopoietic marker) (R., S.) and Nestin (ectodermal) (T.).
To directly address a cardiac specific inhibition by early Mzf1 overexpression we arranged a different experimental set-up for further in vitro differentiation assays (Fig. 6M). The dox-inducible tetOMzf1-Nkx2.5 CE eGFP ES cell line was differentiated for only three days with or without addition of dox. Figure 6N shows that EBs grew faster under permanent dox-treatment for three days which is in agreement with the increased cell proliferation of tetOMzf1-Nkx2.5 CE eGFP ES cells that grew with dox for more than 48 h (Fig. S2I). On day three EBs were harvested and total RNA was applied to qRT-PCR. First, Mzf1 expression was confirmed by qRT-PCR showing a 58-fold overexpression by dox-treatment compared to untreated control (p <0.001, Fig. 6O). Next, we analyzed marker genes involved in early cardiac development, such as Mesp1, an early cardiac mesoderm marker [32] or Flk1 known as an early marker of cardiovascular commitment [33]. Mesp1 as well as Flk1 were considerably down-regulated by Mzf1 overexpression (p <0.001, Fig. 6P-Q), confirming the already assumed inhibition of cardiogenesis by an early over-expression of Mzf1. Interestingly, Tal1 (also known as Scl), typically expressed in hemangioblasts (progenitor cells of the hematoendothelial lineage, [34]), as well as Gata1, a marker of the hematopoietic lineage [35], and Nestin (ectodermal marker), were not affected by an early Mzf1 over-expression for three days (Fig. 6R-T).
Discussion
The specification and differentiation of pluripotent stem cells in vitro and in vivo is driven by a complex transcriptional regulatory network. Most of the evidence about the TF Mzf1 and its impact on other genes are exclusively based on in vitro luciferase assays and EMSA [8], [36]. Herein we studied, comprehensively, the role of Mzf1 on the frequency of cardiac progenitor cells using an Nkx2.5 cardiac specific enhancer element. We identified for the first time that Mzf1 can activate the Nkx2.5 CE in several cell lines and that Mzf1 binds directly to the Nkx2.5 CE both in vitro and in vivo.
Our diverging results of the Nkx2.5 CE activation by Mzf1 in different cell lines indicates that Mzf1 can act in a cell specific manner as previously implied by Morris and co-workers [8] for hematopoietic (K562, Jurkat) or nonhematopoietic cell lines (NIH 3T3, 293). Interestingly, Mzf1 is able to transactivate the Nkx2.5 CE in muscular and cardiac cell lines such as H9c2 and HL-1 but not in endothelial cell lines such as NFPE cells. This suggests that the mechanism of Mzf1 transcription is dependent on the presence of tissue-specific regulators or differential protein modifications that affect Mzf1 function as postulated previously [8]. Most likely, tissue-specific co-factors are necessary for an appropriate function within a cellular system, (e.g. YY1 acts together with Gata4 in CPCs [20]). Our finding that Mzf1 interacts with the Nkx2.5 CE raises the possibility that the binding of Mzf1 to the Nkx2.5 CE may require the presence of other Nkx2.5 CE-bound TFs [8], [11].
We also found a biphasic pattern of Mzf1 expression during in vitro differentiation of murine ES cell lines potentially indicating a dual mode of action during lineage specification. Other factors like Myf-6 [37] or D-mef2 [38] that influence lineage specification also act in a biphasic manner during embryonic development.
Our hypothesis that Mzf1 plays a role in cardiogenesis via an interaction with the Nkx2.5 CE was further supported by the differential expression of Mzf1 in purified Nkx2.5 CE positive CPCs at days five and seven of differentiation as well as in mouse embryonic hearts at E 9.5 but to a much lower extent in mature adult cardiomyocytes. These results indicate that the main influence of Mzf1 on Nkx2.5 CE labelled CPCs takes place during early cardiomyocyte differentiation but not after terminal differentiation of these cells.
Since Mzf1 appears to regulate gene expression in CPCs, we examined the effect of Mzf1 overexpression using a murine tetOMzf1-Nkx2.5 CE eGFP ES cell line. Flow cytometry results clearly indicated an increased frequency of CPCs induced by an Mzf1 overexpression from day five of in vitro differentiation. In contrast, continuous overexpression of Mzf1 from day 0-8 resulted in significant reduction of CPC formation. We furthermore found evident morphological changes during differentiation under permanent dox-addition. Settled EBs showed globular clusters which were closely packed while no beating areas could be observed. It can be assumed that the permanent Mzf1 overexpression led to a different migration behavior of cells in these EBs since it is well known that Mzf1 plays a role in migration and invasion [13]–[16]. However, Mzf1 overexpression from day 5 exhibited an EB-morphology typical for undirected murine ES-cell differentiations and a regular appearance of beating areas. Based on this observation, we concluded that Mzf1 overexpression can induce cardiac lineage expansion in a temporal-specific fashion.
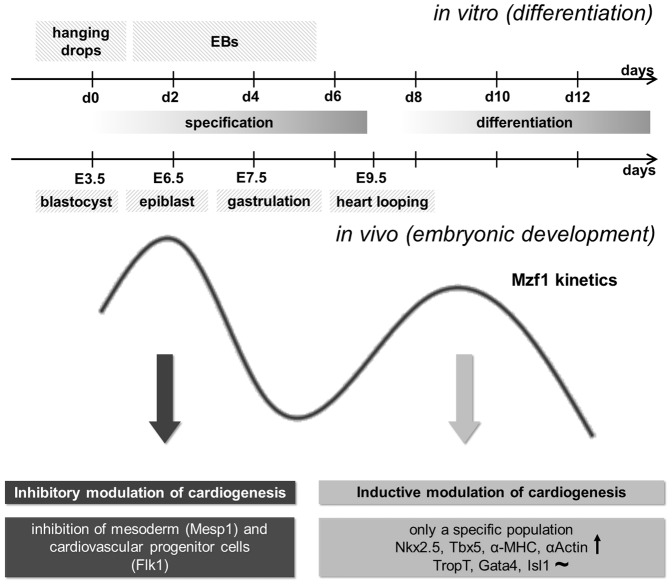
Taken together, our results implicate a role for Mzf1 in the control of cardiac commitment by an interaction with the Nkx2.5 cardiac enhancer. As Mzf1 was significantly enhanced in a CPC population in vitro as well as in embryonic heart tissue and late overexpression of Mzf1 promoted cardiac lineage commitment we propose that Mzf1 may be a novel regulator of embryonic heart development. Figure 7 summarizes the physiological biphasic kinetics of Mzf1 expression. The first peak of Mzf1 up-regulation occurs early during specification of pluripotent cells: Around day two of in vitro differentiation, corresponding with the epiblast stage during murine development on E 6.0 or 6.5. At this time Mzf1 seems to have an inhibitory effect on cardiac lineage commitment as shown by our results (down-regulation of Mesp1). Mzf1 may inhibit the generation of cardiac mesoderm by suppressing Mesp1 and Flk1 expression. Runx1 (hematopoietic) and Nestin (ectodermal) are virtually unaffected by a permanent overexpression of Mzf1. The second physiological peak of Mzf1 expression occurs during differentiation of pluripotent cells around day eight of in vitro differentiation. An overexpression of Mzf1 at the beginning of this peak (from day 5), in parallel with the endogenous upregulation of the Nkx2.5 expression which is initiated at day four of in vitro differentiation and is highly increased at day five to seven [3], results in a moderate stimulation of cardiogenic commitment. Besides Nkx2.5, typical cardiac primary heart field (PHF) genes like Tbx5, sarcomeric genes like αMHC or the pancardiac structural marker cardiac αActin are significantly up-regulated.
Figure 7. Potential mechanistic role of Mzf1 during embryonic development.
The first peak of physiological Mzf1 up-regulation occurs during specification of pluripotent cells, corresponding to the epiblast stage during murine development on E 6.0 or 6.5. At this time Mzf1 seems to have an inhibitory effect on cardiac lineage commitment. The second physiological peak of Mzf1 expression occurs during differentiation of pluripotent cells around day eight of in vitro differentiation. An overexpression of Mzf1 at the beginning of this peak resulted in stimulation of cardiogenesis.
Mzf1 transcriptional regulation mechanisms seem to be tissue-specific as well as stage dependent. The divergent findings of stimulation or repression of specific marker genes by time-dependent Mzf1 overexpression supported earlier suggestions that Mzf1 might be necessary for a normal differentiation program involving a balance between positive and negative regulatory signals [36].
A global deletion of Mzf1 in the mouse did not lead to embryonic lethality nor did the authors mention evident alterations during heart development [10]. It could be speculated that a loss of Mzf1 during development may be compensated by another transcription factor as it is known for Mesp1 and Mesp2 during the early stages of gastrulation [39]. However, we have to assume that the role of Mzf1 in heart development is more stabilizing or modulating than actually stimulating.
In summary, the findings that Mzf1 can simultaneously activate or repress specific genes following time-dependent Mzf1 overexpression support a modulatory role for Mzf1 in normal cardiac development where a proper balance between positive and negative regulatory signals is critical. Further investigation of the role of Mzf1 in cardiac development in vivo may provide novel insights into molecular mechanisms of vertebrate heart development, which are crucial for devising successful cardiac regenerative therapies in the future.
Supporting Information
Luciferase reporter assays and EMSA for Mesp1 on the Nkx2.5 cardiac enhancer element. S1A. Besides in HEK 293 and H9c2 cells Mesp1 activated the Nkx2.5 CE element in atrial HL-1 cells but not in endothelial NFPE cells. Asterisks indicate significance compared to the control (pcDNA3.1); ** = p <0.01. S1B. Dose reduction of Mesp1-pcDNA3.1-DNA significantly decreased luciferase activity in 293 cells; * = p <0.05. S1C. Skipping parts of the Nkx2.5 CE according to Lien and co-workers [7] led to significant reduction of luciferase activation by Mesp1 in HEK 293 cells. S1D. Locations of confirmed Mesp1 binding sites on the Nkx2.5 CE [29] (black triangles). S1E. In vitro translated Mesp1 protein from the flag-Mesp1-pcDNA3.1 confirmed by an anti-flag antibody in western-blotting (lane 2). As a control whole cell lysate from 293 cells transfected with the flag-Mesp1-pCDNA3.1 plasmid was used (lane 1). The predicted molecular weight for Mesp1 is 37 kDa. S1F. In vitro translated Mesp1 (10 µl) bound to an E-Box-motif (black triangle at position -29 bp in the Nkx2.5 CE) [29] in an electromobility shift assay (EMSA). The same amount of unprogrammed reticulocyte lysate (RL) was applied as a control. Competition assays with untagged mutant (mut, 10-fold excess) and specific (10-fold excess) probes were performed to ensure specificity. In vitro binding to another E-Box-motif in the Nkx2.5 CE (at position -9138 bp) [29] could not be confirmed. S1G. Effect of mutating two Mesp1-binding sites at positions -9138 bp and -29 bp in the Nkx2.5 CE BP on luciferase activity by Mesp1 in HEK 293 cells (** = p <0.01).
(TIF)
Generation and verification of a double-transgenic dox-inducible Mzf1 overexpressing Nkx2.5 CE eGFP ES cell line and characterization of the tetOMzf1-Nkx2.5 CE eGFP ES cell line compared to the parent Nkx2.5 CE eGFP ES cells. S2A. Transduction of Nkx2.5 CE eGFP ES cells with Mzf1 and rtTA lentiviruses. Scale bars: 200 µm. S2B. Dox-inducible expression of Mzf1 could be confirmed in three expanded clones (cl. 42, 44 and 64). S2C.+D. Confirmation of sensitivity for dox-inducible Mzf1 expression in clone 64. S2E. The morphology of tetOMzf1-Nkx2.5 CE eGFP ES cells with and w/o dox was undistinguishable from the parent Nkx2.5 CE eGFP ES cells. Scale bars: 200 µm for all panels. S2F. Pluripotency of tetOMzf1-Nkx2.5 CE eGFP ES cells with and w/o dox was evaluated by immunostaining with an anti-Sox2 antibody. As a control the parent Nkx2.5 CE eGFP ES cells were also stained. The negative controls were performed with the secondary antibody only. Scale bars: 200 µm for all panels. S2G. The morphology of differentiated tetOMzf1-Nkx2.5 CE eGFP ES cells w/o dox was comparable to the parent differentiated Nkx2.5 CE eGFP ES cells on day seven of in vitro differentiation. Scale bars: 200 µm for all panels. S2H. A negative effect of permanent or temporary dox-treatment and by this Mzf1 overexpression on differentiating tetOMzf1-Nkx2.5 CE eGFP ES cells was excluded by comparison of the amount of dead cells (evaluated by propidiumiodide staining during flow cytometry) between the different approaches. S2I. Cell proliferation was evaluated by MTT assays in tetOMzf1-Nkx2.5 CE eGFP ES cells treated with dox for different time intervals (control was w/o dox); ** = p <0.01.
(TIF)
Mzf1 upregulation in the tetOMzf1-Nkx2.5 CE eGFP ES cell differentiation assays with different dox-treatment schedules. S3A. Verification of Mzf1 upregulation on day eight of in vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells by qRT-PCR; * = p <0.05; ** = p <0.01. S3B. Co-staining with an anti-flag and an anti-GFP antibody (AB) to detect exogenous overexpression of Mzf1 (red fluorescence) in day 7 differentiated tetOMzf1-Nkx2.5 CE eGFP ES cells and cardiac progenitor cells (green fluorescence). Scale bars: 200 µm for all panels.
(TIF)
Detailed methods section.
(DOCX)
Transcription factor candidates from in silico analysis of the Nx2.5 CE element in alphabetical order. Boldly printed TFs were chosen for further analysis by luciferase reporter assays.
(DOCX)
Sequences of primer-sets for gene expression analysis by qRT-PCR.
(DOCX)
In vitro differentiation of the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50–70 images. Magnification is 100×.
(MPG)
In vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells w/o dox led to eGFP positive beating areas undistinguishable from the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of tetOMzf1-Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation w/o dox. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50-70 images. Magnification is 100×.
(MPG)
In vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells with dox from day 5 led to eGFP positive beating areas undistinguishable from the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of tetOMzf1-Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation with dox from day 5. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50–70 images. Magnification is 100×.
(MPG)
Acknowledgments
We are grateful to Lynette Henkel (Institute for Medical Microbiology, Immunology and Hygiene, Technische Universität München, Munich, Germany) for performing flow cytometry. We also thank Angelika Bernhard and Klaudia Adamczyk for technical assistance (Experimental Surgery, Department of Cardiovascular Surgery, Deutsches Herzzentrum München, Munich, Germany). Thanks to Prof. Dr. Karl-Ludwig Laugwitz (Department of Cardiology, Medical Clinic and Policlinic Rechts der Isar, Munich, Germany) and his team (especially Tatjana Dorn) for the provision of luciferase assay equipment and the kind gift of the NFPE cell line. Great thanks to Prof. Dr. William Claycomb (Department of Biochemistry & Molecular Biology, LSUHSC School of Medicine, New Orleans, LA) for the kind gift of HL-1 cells and to Dr. Konrad Hochedlinger (Harvard University Department of Stem Cell and Regenerative Biology, Massachusetts General Hospital, Boston, MA, USA) for the kind gift of the pLvtetO-plasmid. We are also thankful to Prof. Dr. Agnes Görlach (Experimental Pediatric Cardiology, Department of Pediatric Cardiology, Deutsches Herzzentrum München, Munich, Germany) and her team (especially Florian Riess and Andreas Petry) for the provision of technical equipment for EMSA and ChIP assays.
Data Availability
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.
Funding Statement
This work was supported by the Dr. Rusche Forschungspreis 2011 of the Deutsche Gesellschaft für Thorax-, Herz- und Gefäßchirurgie (http://www.dshf.de/dr_rusche_forschungsprojekt_projekte.php). Grant Support (Doppler et al.): The study was supported by Dr. Rusche Forschungsprojekt (2011) of the DSHF and DGTHG. Marcus-André Deutsch (MAD) is supported by Dr. Rusche Forschungsprojekt (2014) of the DSHF and DGTHG. Rüdiger Lange (RL) is supported by Bayerische Forschungsstiftung (AZ-1012-12). Markus Krane (MK) is supported by Deutsche Stiftung für Herzforschung (F/37/11), Deutsches Zentrum für Herz Kreislauf Forschung (DZHK B 13-050A), Deutsche Forschungsgemeinschaft – Sachmittelantrag (KR3770/7-1), Deutsches Zentrum für Herz Kreislauf Forschung (DZHK B 14-013SE), and Deutsche Forschungsgemeinschaft – Sachmittelantrag (KR3770/9-1). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Srivastava D (2006) Making or breaking the heart: from lineage determination to morphogenesis. Cell 126:1037–1048. [DOI] [PubMed] [Google Scholar]
- 2. Murry CE, Keller G (2008) Differentiation of embryonic stem cells to clinically relevant populations: lessons from embryonic development. Cell 132:661–680. [DOI] [PubMed] [Google Scholar]
- 3. Wu SM, Fujiwara Y, Cibulsky SM, Clapham DE, Lien CL, et al. (2006) Developmental origin of a bipotential myocardial and smooth muscle cell precursor in the mammalian heart. Cell 127:1137–1150. [DOI] [PubMed] [Google Scholar]
- 4. Moretti A, Caron L, Nakano A, Lam JT, Bernshausen A, et al. (2006) Multipotent embryonic isl1+ progenitor cells lead to cardiac, smooth muscle, and endothelial cell diversification. Cell 127:1151–1165. [DOI] [PubMed] [Google Scholar]
- 5. Kattman SJ, Huber TL, Keller GM (2006) Multipotent flk-1+ cardiovascular progenitor cells give rise to the cardiomyocyte, endothelial, and vascular smooth muscle lineages. Dev Cell 11:723–732. [DOI] [PubMed] [Google Scholar]
- 6. Domian IJ, Chiravuri M, van der Meer P, Feinberg AW, Shi X, et al. (2009) Generation of functional ventricular heart muscle from mouse ventricular progenitor cells. Science 326:426–429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lien CL, Wu C, Mercer B, Webb R, Richardson JA, et al. (1999) Control of early cardiac-specific transcription of Nkx2-5 by a GATA-dependent enhancer. Development 126:75–84. [DOI] [PubMed] [Google Scholar]
- 8. Morris JF, Rauscher FJ 3rd, Davis B, Klemsz M, Xu D, et al. (1995) The myeloid zinc finger gene, MZF-1, regulates the CD34 promoter in vitro. Blood 86:3640–3647. [PubMed] [Google Scholar]
- 9. Bavisotto L, Kaushansky K, Lin N, Hromas R (1991) Antisense oligonucleotides from the stage-specific myeloid zinc finger gene MZF-1 inhibit granulopoiesis in vitro. J Exp Med 174:1097–1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Gaboli M, Kotsi PA, Gurrieri C, Cattoretti G, Ronchetti S, et al. (2001) Mzf1 controls cell proliferation and tumorigenesis. Genes Dev 15:1625–1630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Morris JF, Hromas R, Rauscher FJ 3rd (1994) Characterization of the DNA-binding properties of the myeloid zinc finger protein MZF1: two independent DNA-binding domains recognize two DNA consensus sequences with a common G-rich core. Mol Cell Biol 14:1786–1795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Hromas R, Collins SJ, Hickstein D, Raskind W, Deaven LL, et al. (1991) A retinoic acid-responsive human zinc finger gene, MZF-1, preferentially expressed in myeloid cells. J Biol Chem 266:14183–14187. [PubMed] [Google Scholar]
- 13. Mudduluru G, Vajkoczy P, Allgayer H (2010) Myeloid zinc finger 1 induces migration, invasion, and in vivo metastasis through Axl gene expression in solid cancer. Mol Cancer Res 8:159–169. [DOI] [PubMed] [Google Scholar]
- 14. Hsieh YH, Wu TT, Tsai JH, Huang CY, Hsieh YS, et al. (2006) PKCalpha expression regulated by Elk-1 and MZF-1 in human HCC cells. Biochem Biophys Res Commun 339:217–225. [DOI] [PubMed] [Google Scholar]
- 15. Hsieh YH, Wu TT, Huang CY, Hsieh YS, Liu JY (2007) Suppression of tumorigenicity of human hepatocellular carcinoma cells by antisense oligonucleotide MZF-1. Chin J Physiol 50:9–15. [PubMed] [Google Scholar]
- 16. Tsai SJ, Hwang JM, Hsieh SC, Ying TH, Hsieh YH (2012) Overexpression of myeloid zinc finger 1 suppresses matrix metalloproteinase-2 expression and reduces invasiveness of SiHa human cervical cancer cells. Biochem Biophys Res Commun 425:462–467. [DOI] [PubMed] [Google Scholar]
- 17. Claycomb WC, Lanson NA Jr, Stallworth BS, Egeland DB, Delcarpio JB, et al. (1998) HL-1 cells: a cardiac muscle cell line that contracts and retains phenotypic characteristics of the adult cardiomyocyte. Proc Natl Acad Sci U S A 95:2979–2984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Chen JX, Krane M, Deutsch MA, Wang L, Rav-Acha M, et al. (2012) Inefficient reprogramming of fibroblasts into cardiomyocytes using Gata4, Mef2c, and Tbx5. Circ Res 111:50–55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Stadtfeld M, Maherali N, Breault DT, Hochedlinger K (2008) Defining molecular cornerstones during fibroblast to iPS cell reprogramming in mouse. Cell Stem Cell 2:230–240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Gregoire S, Karra R, Passer D, Deutsch MA, Krane M, et al. (2013) Essential and unexpected role of yin yang 1 to promote mesodermal cardiac differentiation. Circ Res 112:900–910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Wasserman WW, Sandelin A (2004) Applied bioinformatics for the identification of regulatory elements. Nat Rev Genet 5:276–287. [DOI] [PubMed] [Google Scholar]
- 22. Chekmenev DS, Haid C, Kel AE (2005) P-Match: transcription factor binding site search by combining patterns and weight matrices. Nucleic Acids Res 33:W432–437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Firulli AB, McFadden DG, Lin Q, Srivastava D, Olson EN (1998) Heart and extra-embryonic mesodermal defects in mouse embryos lacking the bHLH transcription factor Hand1. Nat Genet 18:266–270. [DOI] [PubMed] [Google Scholar]
- 24. Liu Y, Asakura M, Inoue H, Nakamura T, Sano M, et al. (2007) Sox17 is essential for the specification of cardiac mesoderm in embryonic stem cells. Proc Natl Acad Sci U S A 104:3859–3864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Liao X, Haldar SM, Lu Y, Jeyaraj D, Paruchuri K, et al. (2010) Kruppel-like factor 4 regulates pressure-induced cardiac hypertrophy. J Mol Cell Cardiol 49:334–338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Babu GJ, Lalli MJ, Sussman MA, Sadoshima J, Periasamy M (2000) Phosphorylation of elk-1 by MEK/ERK pathway is necessary for c-fos gene activation during cardiac myocyte hypertrophy. J Mol Cell Cardiol 32:1447–1457. [DOI] [PubMed] [Google Scholar]
- 27. Chen YH, Ishii M, Sun J, Sucov HM, Maxson RE Jr (2007) Msx1 and Msx2 regulate survival of secondary heart field precursors and post-migratory proliferation of cardiac neural crest in the outflow tract. Dev Biol 308:421–437. [DOI] [PubMed] [Google Scholar]
- 28. Herrmann BG, Labeit S, Poustka A, King TR, Lehrach H (1990) Cloning of the T gene required in mesoderm formation in the mouse. Nature 343:617–622. [DOI] [PubMed] [Google Scholar]
- 29. Bondue A, Lapouge G, Paulissen C, Semeraro C, Iacovino M, et al. (2008) Mesp1 acts as a master regulator of multipotent cardiovascular progenitor specification. Cell Stem Cell 3:69–84. [DOI] [PubMed] [Google Scholar]
- 30. Huang X, Wu SM (2010) Isolation and functional characterization of pluripotent stem cell-derived cardiac progenitor cells. Curr Protoc Stem Cell Biol Chapter 1: Unit 1F 10 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Van Handel B, Montel-Hagen A, Sasidharan R, Nakano H, Ferrari R, et al. (2012) Scl represses cardiomyogenesis in prospective hemogenic endothelium and endocardium. Cell 150:590–605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Kitajima S, Takagi A, Inoue T, Saga Y (2000) MesP1 and MesP2 are essential for the development of cardiac mesoderm. Development 127:3215–3226. [DOI] [PubMed] [Google Scholar]
- 33. Ema M, Takahashi S, Rossant J (2006) Deletion of the selection cassette, but not cis-acting elements, in targeted Flk1-lacZ allele reveals Flk1 expression in multipotent mesodermal progenitors. Blood 107:111–117. [DOI] [PubMed] [Google Scholar]
- 34. Ismailoglu I, Yeamans G, Daley GQ, Perlingeiro RC, Kyba M (2008) Mesodermal patterning activity of SCL. Exp Hematol 36:1593–1603. [DOI] [PubMed] [Google Scholar]
- 35. Caprioli A, Koyano-Nakagawa N, Iacovino M, Shi X, Ferdous A, et al. (2011) Nkx2-5 represses Gata1 gene expression and modulates the cellular fate of cardiac progenitors during embryogenesis. Circulation 123:1633–1641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Perrotti D, Melotti P, Skorski T, Casella I, Peschle C, et al. (1995) Overexpression of the zinc finger protein MZF1 inhibits hematopoietic development from embryonic stem cells: correlation with negative regulation of CD34 and c-myb promoter activity. Mol Cell Biol 15:6075–6087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Bober E, Lyons GE, Braun T, Cossu G, Buckingham M, et al. (1991) The muscle regulatory gene, Myf-6, has a biphasic pattern of expression during early mouse development. J Cell Biol 113:1255–1265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Nguyen HT, Bodmer R, Abmayr SM, McDermott JC, Spoerel NA (1994) D-mef2: a Drosophila mesoderm-specific MADS box-containing gene with a biphasic expression profile during embryogenesis. Proc Natl Acad Sci U S A 91:7520–7524. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Bondue A, Blanpain C (2010) Mesp1: a key regulator of cardiovascular lineage commitment. Circ Res 107:1414–1427. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Luciferase reporter assays and EMSA for Mesp1 on the Nkx2.5 cardiac enhancer element. S1A. Besides in HEK 293 and H9c2 cells Mesp1 activated the Nkx2.5 CE element in atrial HL-1 cells but not in endothelial NFPE cells. Asterisks indicate significance compared to the control (pcDNA3.1); ** = p <0.01. S1B. Dose reduction of Mesp1-pcDNA3.1-DNA significantly decreased luciferase activity in 293 cells; * = p <0.05. S1C. Skipping parts of the Nkx2.5 CE according to Lien and co-workers [7] led to significant reduction of luciferase activation by Mesp1 in HEK 293 cells. S1D. Locations of confirmed Mesp1 binding sites on the Nkx2.5 CE [29] (black triangles). S1E. In vitro translated Mesp1 protein from the flag-Mesp1-pcDNA3.1 confirmed by an anti-flag antibody in western-blotting (lane 2). As a control whole cell lysate from 293 cells transfected with the flag-Mesp1-pCDNA3.1 plasmid was used (lane 1). The predicted molecular weight for Mesp1 is 37 kDa. S1F. In vitro translated Mesp1 (10 µl) bound to an E-Box-motif (black triangle at position -29 bp in the Nkx2.5 CE) [29] in an electromobility shift assay (EMSA). The same amount of unprogrammed reticulocyte lysate (RL) was applied as a control. Competition assays with untagged mutant (mut, 10-fold excess) and specific (10-fold excess) probes were performed to ensure specificity. In vitro binding to another E-Box-motif in the Nkx2.5 CE (at position -9138 bp) [29] could not be confirmed. S1G. Effect of mutating two Mesp1-binding sites at positions -9138 bp and -29 bp in the Nkx2.5 CE BP on luciferase activity by Mesp1 in HEK 293 cells (** = p <0.01).
(TIF)
Generation and verification of a double-transgenic dox-inducible Mzf1 overexpressing Nkx2.5 CE eGFP ES cell line and characterization of the tetOMzf1-Nkx2.5 CE eGFP ES cell line compared to the parent Nkx2.5 CE eGFP ES cells. S2A. Transduction of Nkx2.5 CE eGFP ES cells with Mzf1 and rtTA lentiviruses. Scale bars: 200 µm. S2B. Dox-inducible expression of Mzf1 could be confirmed in three expanded clones (cl. 42, 44 and 64). S2C.+D. Confirmation of sensitivity for dox-inducible Mzf1 expression in clone 64. S2E. The morphology of tetOMzf1-Nkx2.5 CE eGFP ES cells with and w/o dox was undistinguishable from the parent Nkx2.5 CE eGFP ES cells. Scale bars: 200 µm for all panels. S2F. Pluripotency of tetOMzf1-Nkx2.5 CE eGFP ES cells with and w/o dox was evaluated by immunostaining with an anti-Sox2 antibody. As a control the parent Nkx2.5 CE eGFP ES cells were also stained. The negative controls were performed with the secondary antibody only. Scale bars: 200 µm for all panels. S2G. The morphology of differentiated tetOMzf1-Nkx2.5 CE eGFP ES cells w/o dox was comparable to the parent differentiated Nkx2.5 CE eGFP ES cells on day seven of in vitro differentiation. Scale bars: 200 µm for all panels. S2H. A negative effect of permanent or temporary dox-treatment and by this Mzf1 overexpression on differentiating tetOMzf1-Nkx2.5 CE eGFP ES cells was excluded by comparison of the amount of dead cells (evaluated by propidiumiodide staining during flow cytometry) between the different approaches. S2I. Cell proliferation was evaluated by MTT assays in tetOMzf1-Nkx2.5 CE eGFP ES cells treated with dox for different time intervals (control was w/o dox); ** = p <0.01.
(TIF)
Mzf1 upregulation in the tetOMzf1-Nkx2.5 CE eGFP ES cell differentiation assays with different dox-treatment schedules. S3A. Verification of Mzf1 upregulation on day eight of in vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells by qRT-PCR; * = p <0.05; ** = p <0.01. S3B. Co-staining with an anti-flag and an anti-GFP antibody (AB) to detect exogenous overexpression of Mzf1 (red fluorescence) in day 7 differentiated tetOMzf1-Nkx2.5 CE eGFP ES cells and cardiac progenitor cells (green fluorescence). Scale bars: 200 µm for all panels.
(TIF)
Detailed methods section.
(DOCX)
Transcription factor candidates from in silico analysis of the Nx2.5 CE element in alphabetical order. Boldly printed TFs were chosen for further analysis by luciferase reporter assays.
(DOCX)
Sequences of primer-sets for gene expression analysis by qRT-PCR.
(DOCX)
In vitro differentiation of the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50–70 images. Magnification is 100×.
(MPG)
In vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells w/o dox led to eGFP positive beating areas undistinguishable from the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of tetOMzf1-Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation w/o dox. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50-70 images. Magnification is 100×.
(MPG)
In vitro differentiation of tetOMzf1-Nkx2.5 CE eGFP ES cells with dox from day 5 led to eGFP positive beating areas undistinguishable from the parent Nkx2.5 CE eGFP ES cell line. Time-lapse imaging of tetOMzf1-Nkx2.5 CE eGFP EBs on day eight of in vitro differentiation with dox from day 5. The video is not real-time but assembled from single pictures photographed in a time series. Therefore beating frequency is an artifact of exposure time. The video displays 50–70 images. Magnification is 100×.
(MPG)
Data Availability Statement
The authors confirm that all data underlying the findings are fully available without restriction. All relevant data are within the paper and its Supporting Information files.