Abstract
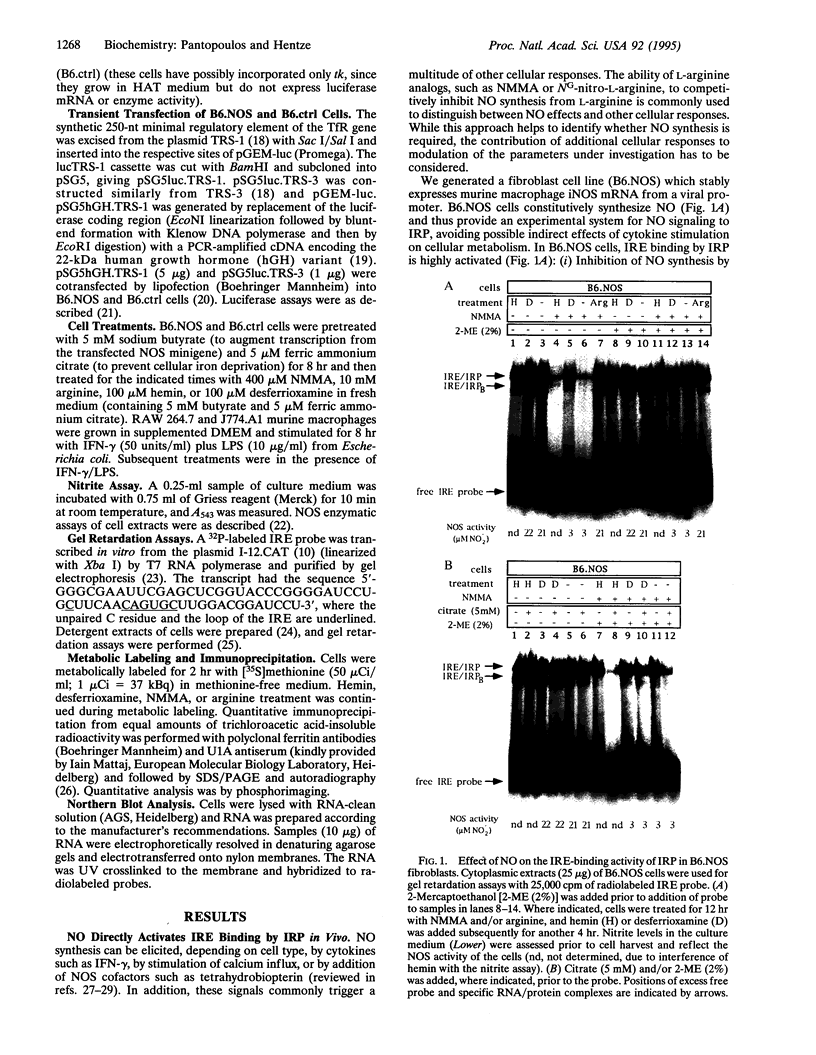
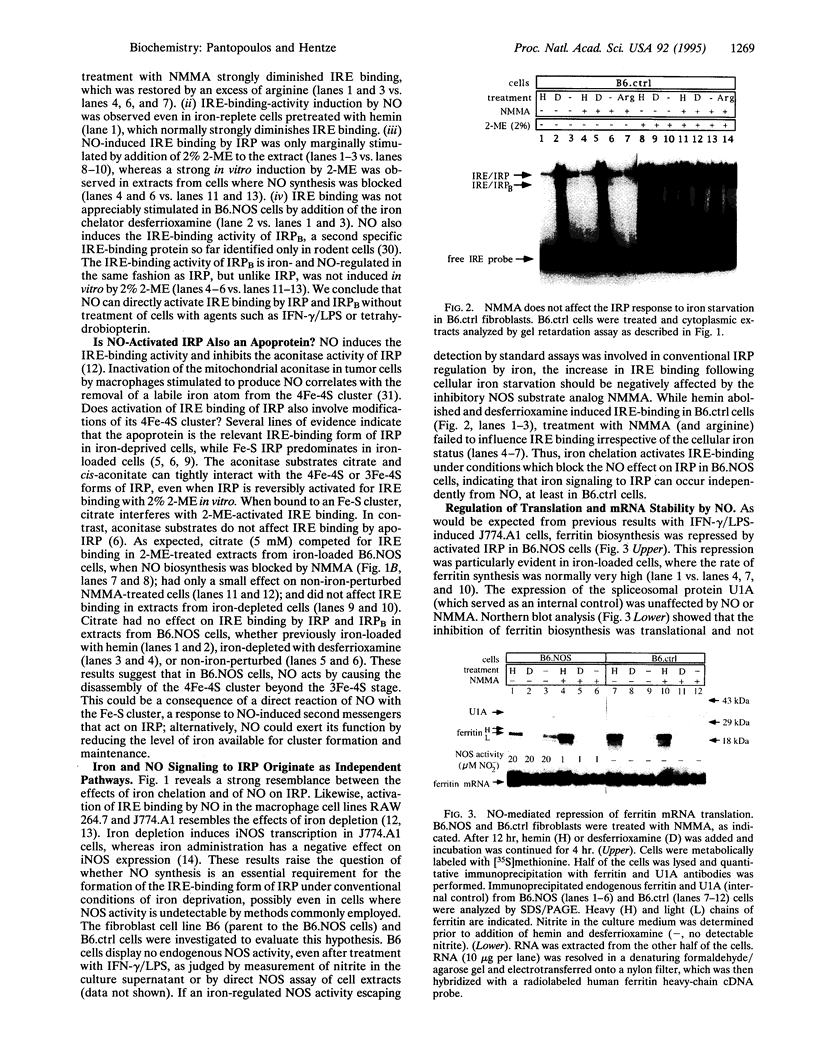
Iron-regulatory protein (IRP) is a master regulator of cellular iron homeostasis. Expression of several genes involved in iron uptake, storage, and utilization is regulated by binding of IRP to iron-responsive elements (IREs), structural motifs within the untranslated regions of their mRNAs. IRP-binding to IREs is controlled by cellular iron availability. Recent work revealed that nitric oxide (NO) can mimic the effect of iron chelation on IRP and on ferritin mRNA translation, whereas the stabilization of transferrin receptor mRNA following NO-mediated IRP activation could not be observed in gamma-interferon/lipopolysaccharide-stimulated murine macrophages. In this study, we establish the function of NO as a signaling molecule to IRP and as a regulator of mRNA translation and stabilization. Fibroblasts with undetectable levels of endogenous NO synthase activity were stably transfected with a cDNA encoding murine macrophage inducible NO synthase. Synthesis of NO activates IRE binding, which in turn represses ferritin mRNA translation and stabilizes transferrin receptor mRNA against targeted degradation. Furthermore, iron starvation and NO release are shown to be independent signals to IRP. The posttranscriptional control of iron metabolism is thus intimately connected with the NO pathways.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Binder R., Horowitz J. A., Basilion J. P., Koeller D. M., Klausner R. D., Harford J. B. Evidence that the pathway of transferrin receptor mRNA degradation involves an endonucleolytic cleavage within the 3' UTR and does not involve poly(A) tail shortening. EMBO J. 1994 Apr 15;13(8):1969–1980. doi: 10.1002/j.1460-2075.1994.tb06466.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgeade M. F., Silbermann F., Kühn L., Testa U., Peschle C., Mémet S., Thang M. N., Besançon F. Post-transcriptional regulation of transferrin receptor mRNA by IFN gamma. Nucleic Acids Res. 1992 Jun 25;20(12):2997–3003. doi: 10.1093/nar/20.12.2997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Di Jeso B., Rao K., Klausner R. D., Harford J. B. Two genetic loci participate in the regulation by iron of the gene for the human transferrin receptor. Proc Natl Acad Sci U S A. 1988 Mar;85(6):1787–1791. doi: 10.1073/pnas.85.6.1787. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey J. L., Koeller D. M., Ramin V. C., Klausner R. D., Harford J. B. Iron regulation of transferrin receptor mRNA levels requires iron-responsive elements and a rapid turnover determinant in the 3' untranslated region of the mRNA. EMBO J. 1989 Dec 1;8(12):3693–3699. doi: 10.1002/j.1460-2075.1989.tb08544.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drapier J. C., Hibbs J. B., Jr Differentiation of murine macrophages to express nonspecific cytotoxicity for tumor cells results in L-arginine-dependent inhibition of mitochondrial iron-sulfur enzymes in the macrophage effector cells. J Immunol. 1988 Apr 15;140(8):2829–2838. [PubMed] [Google Scholar]
- Drapier J. C., Hirling H., Wietzerbin J., Kaldy P., Kühn L. C. Biosynthesis of nitric oxide activates iron regulatory factor in macrophages. EMBO J. 1993 Sep;12(9):3643–3649. doi: 10.1002/j.1460-2075.1993.tb06038.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emery-Goodman A., Hirling H., Scarpellino L., Henderson B., Kühn L. C. Iron regulatory factor expressed from recombinant baculovirus: conversion between the RNA-binding apoprotein and Fe-S cluster containing aconitase. Nucleic Acids Res. 1993 Mar 25;21(6):1457–1461. doi: 10.1093/nar/21.6.1457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Hentze M. W. Iron regulatory protein prevents binding of the 43S translation pre-initiation complex to ferritin and eALAS mRNAs. EMBO J. 1994 Aug 15;13(16):3882–3891. doi: 10.1002/j.1460-2075.1994.tb06699.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gray N. K., Quick S., Goossen B., Constable A., Hirling H., Kühn L. C., Hentze M. W. Recombinant iron-regulatory factor functions as an iron-responsive-element-binding protein, a translational repressor and an aconitase. A functional assay for translational repression and direct demonstration of the iron switch. Eur J Biochem. 1993 Dec 1;218(2):657–667. doi: 10.1111/j.1432-1033.1993.tb18420.x. [DOI] [PubMed] [Google Scholar]
- Green S., Issemann I., Sheer E. A versatile in vivo and in vitro eukaryotic expression vector for protein engineering. Nucleic Acids Res. 1988 Jan 11;16(1):369–369. doi: 10.1093/nar/16.1.369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Haile D. J., Rouault T. A., Harford J. B., Kennedy M. C., Blondin G. A., Beinert H., Klausner R. D. Cellular regulation of the iron-responsive element binding protein: disassembly of the cubane iron-sulfur cluster results in high-affinity RNA binding. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11735–11739. doi: 10.1073/pnas.89.24.11735. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson B. R., Seiser C., Kühn L. C. Characterization of a second RNA-binding protein in rodents with specificity for iron-responsive elements. J Biol Chem. 1993 Dec 25;268(36):27327–27334. [PubMed] [Google Scholar]
- Hentze M. W., Argos P. Homology between IRE-BP, a regulatory RNA-binding protein, aconitase, and isopropylmalate isomerase. Nucleic Acids Res. 1991 Apr 25;19(8):1739–1740. doi: 10.1093/nar/19.8.1739. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hentze M. W., Rouault T. A., Harford J. B., Klausner R. D. Oxidation-reduction and the molecular mechanism of a regulatory RNA-protein interaction. Science. 1989 Apr 21;244(4902):357–359. doi: 10.1126/science.2711187. [DOI] [PubMed] [Google Scholar]
- Kennedy M. C., Mende-Mueller L., Blondin G. A., Beinert H. Purification and characterization of cytosolic aconitase from beef liver and its relationship to the iron-responsive element binding protein. Proc Natl Acad Sci U S A. 1992 Dec 15;89(24):11730–11734. doi: 10.1073/pnas.89.24.11730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Klausner R. D., Rouault T. A., Harford J. B. Regulating the fate of mRNA: the control of cellular iron metabolism. Cell. 1993 Jan 15;72(1):19–28. doi: 10.1016/0092-8674(93)90046-s. [DOI] [PubMed] [Google Scholar]
- Lecomte C. M., Renard A., Martial J. A. A new natural hGH variant--17.5 kd--produced by alternative splicing. An additional consensus sequence which might play a role in branchpoint selection. Nucleic Acids Res. 1987 Aug 25;15(16):6331–6348. doi: 10.1093/nar/15.16.6331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibold E. A., Munro H. N. Cytoplasmic protein binds in vitro to a highly conserved sequence in the 5' untranslated region of ferritin heavy- and light-subunit mRNAs. Proc Natl Acad Sci U S A. 1988 Apr;85(7):2171–2175. doi: 10.1073/pnas.85.7.2171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lowenstein C. J., Snyder S. H. Nitric oxide, a novel biologic messenger. Cell. 1992 Sep 4;70(5):705–707. doi: 10.1016/0092-8674(92)90301-r. [DOI] [PubMed] [Google Scholar]
- Melefors O., Goossen B., Johansson H. E., Stripecke R., Gray N. K., Hentze M. W. Translational control of 5-aminolevulinate synthase mRNA by iron-responsive elements in erythroid cells. J Biol Chem. 1993 Mar 15;268(8):5974–5978. [PubMed] [Google Scholar]
- Melefors O., Hentze M. W. Iron regulatory factor--the conductor of cellular iron regulation. Blood Rev. 1993 Dec;7(4):251–258. doi: 10.1016/0268-960x(93)90012-s. [DOI] [PubMed] [Google Scholar]
- Moncada S., Palmer R. M., Higgs E. A. Nitric oxide: physiology, pathophysiology, and pharmacology. Pharmacol Rev. 1991 Jun;43(2):109–142. [PubMed] [Google Scholar]
- Nathan C. Nitric oxide as a secretory product of mammalian cells. FASEB J. 1992 Sep;6(12):3051–3064. [PubMed] [Google Scholar]
- Pantopoulos K., Weiss G., Hentze M. W. Nitric oxide and the post-transcriptional control of cellular iron traffic. Trends Cell Biol. 1994 Mar;4(3):82–86. doi: 10.1016/0962-8924(94)90179-1. [DOI] [PubMed] [Google Scholar]
- Rouault T. A., Stout C. D., Kaptain S., Harford J. B., Klausner R. D. Structural relationship between an iron-regulated RNA-binding protein (IRE-BP) and aconitase: functional implications. Cell. 1991 Mar 8;64(5):881–883. doi: 10.1016/0092-8674(91)90312-m. [DOI] [PubMed] [Google Scholar]
- Seiser C., Teixeira S., Kühn L. C. Interleukin-2-dependent transcriptional and post-transcriptional regulation of transferrin receptor mRNA. J Biol Chem. 1993 Jun 25;268(18):13074–13080. [PubMed] [Google Scholar]
- Stamatatos L., Leventis R., Zuckermann M. J., Silvius J. R. Interactions of cationic lipid vesicles with negatively charged phospholipid vesicles and biological membranes. Biochemistry. 1988 May 31;27(11):3917–3925. doi: 10.1021/bi00411a005. [DOI] [PubMed] [Google Scholar]
- Weiss G., Goossen B., Doppler W., Fuchs D., Pantopoulos K., Werner-Felmayer G., Wachter H., Hentze M. W. Translational regulation via iron-responsive elements by the nitric oxide/NO-synthase pathway. EMBO J. 1993 Sep;12(9):3651–3657. doi: 10.1002/j.1460-2075.1993.tb06039.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss G., Werner-Felmayer G., Werner E. R., Grünewald K., Wachter H., Hentze M. W. Iron regulates nitric oxide synthase activity by controlling nuclear transcription. J Exp Med. 1994 Sep 1;180(3):969–976. doi: 10.1084/jem.180.3.969. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Werner-Felmayer G., Werner E. R., Fuchs D., Hausen A., Mayer B., Reibnegger G., Weiss G., Wachter H. Ca2+/calmodulin-dependent nitric oxide synthase activity in the human cervix carcinoma cell line ME-180. Biochem J. 1993 Jan 15;289(Pt 2):357–361. doi: 10.1042/bj2890357. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Q. W., Cho H. J., Calaycay J., Mumford R. A., Swiderek K. M., Lee T. D., Ding A., Troso T., Nathan C. Cloning and characterization of inducible nitric oxide synthase from mouse macrophages. Science. 1992 Apr 10;256(5054):225–228. doi: 10.1126/science.1373522. [DOI] [PubMed] [Google Scholar]








