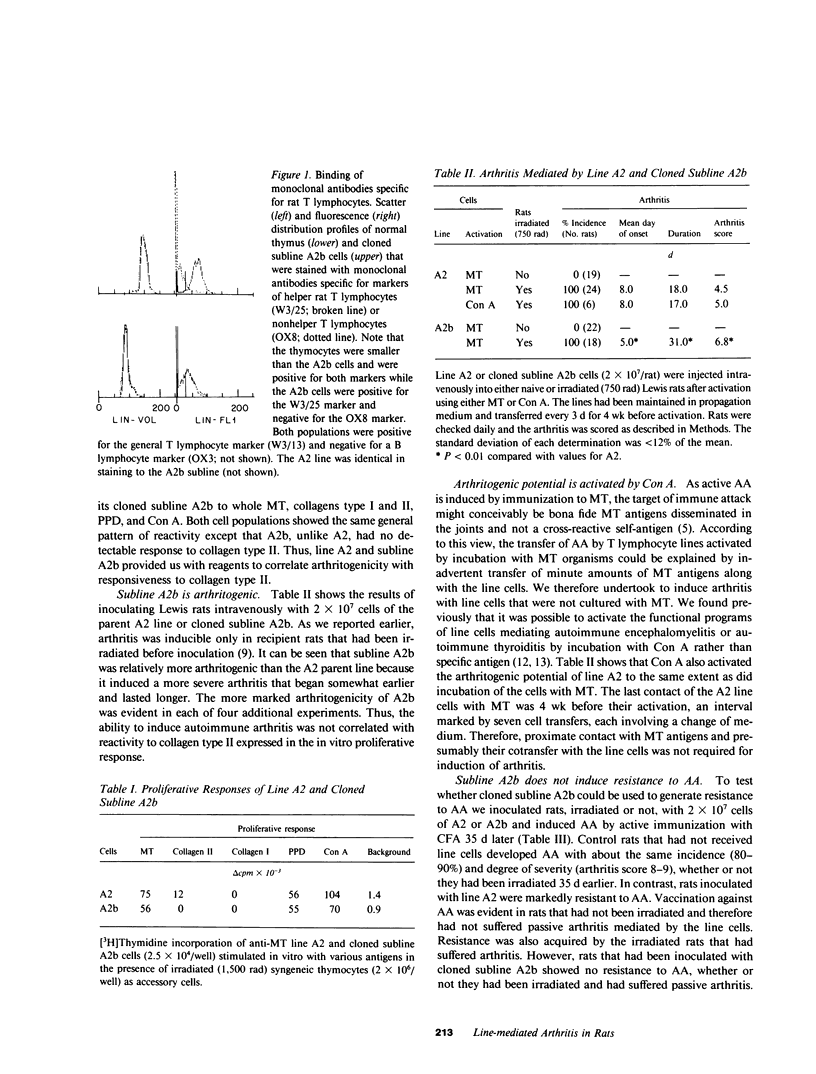
Abstract
We have been studying the pathogenesis of adjuvant arthritis in rats using a long-term cell line of T lymphocytes, the A2 line, which can induce polyarthritis and can also be used to vaccinate rats against adjuvant arthritis. Although line A2 was selected for its proliferative response to mycobacteria, it also responded to collagen type II. To elucidate its role of responsiveness to collagen type II and the relationship between arthritogenicity and vaccination, we cloned A2 and selected a subline A2b. We now report that subline A2b, which bore a marker of helper/delayed hypersensitivity T lymphocytes, was strongly arthritogenic, but could not vaccinate against arthritis. Moreover, A2b showed no response to collagen type II. Therefore, reactivity to collagen type II is not a requisite for arthritogenicity, and mediation of arthritis and vaccination can be distinct properties of different populations of T lymphocytes.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Ben-Nun A., Cohen I. R. Experimental autoimmune encephalomyelitis (EAE) mediated by T cell lines: process of selection of lines and characterization of the cells. J Immunol. 1982 Jul;129(1):303–308. [PubMed] [Google Scholar]
- Ben-Nun A., Wekerle H., Cohen I. R. Vaccination against autoimmune encephalomyelitis with T-lymphocyte line cells reactive against myelin basic protein. Nature. 1981 Jul 2;292(5818):60–61. doi: 10.1038/292060a0. [DOI] [PubMed] [Google Scholar]
- Berry H., Willoughby D. A., Giroud J. P. Evidence for an endogenous antigen in the adjuvant arthritic rat. J Pathol. 1973 Dec;111(4):229–238. doi: 10.1002/path.1711110403. [DOI] [PubMed] [Google Scholar]
- Duksin D., Maoz A., Fuchs S. Differential cytotoxic acitivity of anticollagen serum on rat osteoblasts and fibroblasts in tissue culture. Cell. 1975 May;5(1):83–86. doi: 10.1016/0092-8674(75)90095-1. [DOI] [PubMed] [Google Scholar]
- Holoshitz J., Naparstek Y., Ben-Nun A., Cohen I. R. Lines of T lymphocytes induce or vaccinate against autoimmune arthritis. Science. 1983 Jan 7;219(4580):56–58. doi: 10.1126/science.6336851. [DOI] [PubMed] [Google Scholar]
- Iizuka Y., Chang Y. H. Adjuvant polyarthritis. VII. The role of type II collagen in pathogenesis. Arthritis Rheum. 1982 Nov;25(11):1325–1332. doi: 10.1002/art.1780251108. [DOI] [PubMed] [Google Scholar]
- Naparstek Y., Ben-Nun A., Holoshitz J., Reshef T., Frenkel A., Rosenberg M., Cohen I. R. T lymphocyte lines producing or vaccinating against autoimmune encephalomyelitis (EAE). Functional activation induces peanut agglutinin receptors and accumulation in the brain and thymus of line cells. Eur J Immunol. 1983 May;13(5):418–423. doi: 10.1002/eji.1830130513. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. Development of arthritis, periarthritis and periostitis in rats given adjuvants. Proc Soc Exp Biol Med. 1956 Jan;91(1):95–101. doi: 10.3181/00379727-91-22179. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M. EXPERIMENTAL MODELS IN RHEUMATOID DISEASE. Arthritis Rheum. 1964 Feb;7:80–86. doi: 10.1002/art.1780070111. [DOI] [PubMed] [Google Scholar]
- PEARSON C. M., WOOD F. D. PASSIVE TRANSFER OF ADJUVANT ARTHRITIS BY LYMPH NODE OR SPLEEN CELLS. J Exp Med. 1964 Oct 1;120:547–560. doi: 10.1084/jem.120.4.547. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quagliata F., Phillips-Quagliata J. M. Competence of thoracic duct cells in the transfer of adjuvant disease and delayed hypersensitivity. Evidence that mycobacterial components are required for the successful transfer of the disease. Cell Immunol. 1972 Jan;3(1):78–87. doi: 10.1016/0008-8749(72)90228-6. [DOI] [PubMed] [Google Scholar]
- Stuart J. M., Cremer M. A., Townes A. S., Kang A. H. Type II collagen-induced arthritis in rats. Passive transfer with serum and evidence that IgG anticollagen antibodies can cause arthritis. J Exp Med. 1982 Jan 1;155(1):1–16. doi: 10.1084/jem.155.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Taurog J. D., Sandberg G. P., Mahowald M. L. The cellular basis of adjuvant arthritis. I. Enhancement of cell-mediated passive transfer by concanavalin A and by immunosuppressive pretreatment of the recipient. Cell Immunol. 1983 Feb 1;75(2):271–282. doi: 10.1016/0008-8749(83)90325-8. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., Dynesius R. A., Rocklin R. E., David J. R. Cellular sensitivity to collagen in rheumatoid arthritis. N Engl J Med. 1978 Aug 17;299(7):327–332. doi: 10.1056/NEJM197808172990703. [DOI] [PubMed] [Google Scholar]
- Trentham D. E., McCune W. J., Susman P., David J. R. Autoimmunity to collagen in adjuvant arthritis of rats. J Clin Invest. 1980 Nov;66(5):1109–1117. doi: 10.1172/JCI109940. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trentham D. E., Townes A. S., Kang A. H. Autoimmunity to type II collagen an experimental model of arthritis. J Exp Med. 1977 Sep 1;146(3):857–868. doi: 10.1084/jem.146.3.857. [DOI] [PMC free article] [PubMed] [Google Scholar]



