Abstract
BACKGROUND
Many patients with primary hyperparathyroidism (PHPT) present with less severe biochemical parameters. The purpose of this study was to compare the presentation, operative findings, and outcomes of these patients with “mild” PHPT to patients with “overt” disease.
METHODS
A retrospective review of a prospectively collected parathyroid database was performed to identify cases of PHPT undergoing an initial neck operation. Patients were classified as mild when either the preoperative calcium or PTH was within the normal limits. Comparisons were made with the student’s t-test, Chi-squared test, or Wilcox on rank-sum test where appropriate. Kaplan-Meier estimates were plotted for disease-free survival and compared by the log-rank test.
RESULTS
Of the 1,429 patients who met inclusion criteria, 1,049 were classified as overt and 388 (27.1%) were mild. Within the mild group, 122 (31.4%) presented with normocalcemic PHPT and 266 (68.6%) had a normal PTH. The two groups had similar demographics and renal function. Interestingly, the mild group had more than double the rate of kidney stones (3.1% vs. 1.3%, p = 0.02). The mild group was less likely to localize on sestamibi scan (62.4% vs. 78.7%, p<0.01). Intraoperatively, more mild patients exhibited multigland disease (34.3% vs. 14.1%, p<0.01). When examining intraoperative PTH (IoPTH) kinetics where single adenomas were excised, the IoPTH fell at a rate of 6.9 pg/min in mild patients compared to 11.5 pg/min in the overt group (p<0.01). Accordingly, 62.2% of patients in the overt group and 53.3% in the mild group were cured at five minutes post-excision (p<0.01). There was no difference in the rates of persistence or recurrence between the groups, and disease-free survival estimates were identical (p = 0.27).
CONCLUSIONS
Patients with mild PHPT were more likely to have multigland disease and a slower decline in IoPTH, but these patients can be successfully treated with surgery.
Introduction
Primary hyperparathyroidism (PHPT) is the most common cause of hypercalcemia, diagnosed in over 100,000 patients annually in the U.S (1). Elevated serum calcium and parathyroid hormone (PTH) levels make determine the biochemical diagnosis, and the disease classically manifests with “stones, bones, and psychic moans” due to the disruption of bone and mineral metabolism (2). With the advent of more sensitive and rapid immunoassays for PTH, many more patients with dysregulated PTH secretion are discovered as part of metabolic assessments for skeletal health (3, 4). In addition, improved pre- and intraoperative localization techniques and intraoperative PTH (IoPTH) monitoring enabled surgeons to offer patients a focused, or minimally invasive, parathyroidectomy (5–7). These factors have shifted the classic presentation toward more non-specific presenting symptoms and milder biochemical forms of the disease.
In 1990, the National Institutes of Health consensus conference on symptomatic primary hyperparathyroidism developed criteria by which asymptomatic patients should be referred for parathyroidectomy (8). Although an international task force modified these guidelines in 2002 and again in 2008 (9, 10), the recommendations still require overt biochemical evidence of disease, or obvious manifestations such as kidney stones or osteoporosis as pre-requisites for surgical treatment (9). Many patients do not meet these stringent criteria and fall into the category of “mild” disease – either normocalcemic hyperparathyroidism (HPT) or hypercalcemia with an inappropriately normal PTH level (11–13).
Many surgeons feel such patients can still benefit from parathyroidectomy since the removal of one or more abnormal parathyroid glands has been shown to improve quality of life (1, 4, 13, 14). Furthermore, the same IoPTH criterion can successfully predict cure in patients with mild disease as in overt hyperparathyroidism (15). Emerging evidence from large datasets indicate that even mild hypercalcemia impacts cardiovascular morbidity and mortality (16, 17).
To date, studies of mild primary hyperparathyroidism in the U.S. have included relatively few patients and have not consistently reported outcomes like recurrence (11, 13, 15, 18–27). The purpose of this study was to characterize patterns of disease presentation, intraoperative PTH kinetics, and postoperative outcomes in patients with mild hyperparathyroidism.
Methods
After obtaining approval from University of Wisconsin’s Institutional Review Board, we conducted a retrospective review of a prospectively collected parathyroid database. Patients with PHPT undergoing an initial neck operation between 2001 and 2012 were identified. Those patients with familial, secondary, or tertiary hyperparathyroidism were excluded. Also excluded were patients undergoing re-operative parathyroid surgery or concomitant thyroid operations. Patients were classified as having “mild” disease if preoperative labs demonstrated either the calcium or PTH was within the normal limits (calcium ≤10.2 mg/dL, or PTH ≤ 72 pg/mL). “Overt” disease meant that both preoperative calcium and PTH levels were elevated. At our institution, patients with a biochemical diagnosis of hyperparathyroidism are referred for surgical consultation regardless of symptoms or specific laboratory criteria since we believe in the benefits of parathyroidectomy for prevention of complications and long-term quality of life. The decision to operate is made in a multidisciplinary fashion with consideration of each patient’s operative risk, benefits, and preference.
At the University of Wisconsin, all patients with biochemical evidence of PHPT are evaluated with sestamibi scans and selected patients also undergo ultrasonography. Whenever any localization study is positive, patients are offered a minimally invasive parathyroidectomy (unilateral exploration). As previously described, radio guidance and IoPTH guide the extent of operation and trigger conversion to bilateral exploration if additional enlarged glands are detected or IoPTH levels do not fall appropriately (5, 7). The IoPTH criteria used requires a 50% decline in PTH from the peak level measured at 5, 10, or 15 minutes post-excision (7, 28).
Pre-operative patient characteristics, lab values, and intraoperative findings were tabulated. Outcomes were determined by laboratory values measured postoperatively. Persistent hyperparathyroidism occurred when calcium was elevated (≥10.2 mg/dL) within six months postoperatively, and recurrence was defined as elevated calcium beyond six months postoperatively. Persistence and recurrence were further evaluated with repeat calcium, PTH, and Vitamin D levels to confirm the etiology was PHPT.
Comparisons between groups were made with the student’s t-test, Chi-squared test, or Wilcox on rank-sum test where appropriate. The rate of decline in IoPTH (slope) was calculated with linear regression. Outcomes were analyzed using disease-free survival analysis. Kaplan-Meier estimates were plotted for disease-free survival and compared by the log-rank test. A p- value less than 0.05 was considered significant. All statistical analyses were performed using STATA v. 12.1 (StatCorp, College Station, TX)
Results
Classification and Preoperative Characteristics
There were 1,429 patients with primary hyperparathyroidism who met inclusion criteria for this study. Of these, 388 (27.1%) had mild disease and 1,049 had overt PHPT. Within the mild category, 122 (31.4%) presented with normocalcemic PHPT and 266 (68.6%) had an inappropriately normal PTH.
In comparing the mild to the overt group, there were a similar proportion of males to females, and no differences in mean age (Table 1). More than double the percentage of patients with mild disease presented with a history of kidney stones compared to the overt group (3.1% vs. 1.25%, p = 0.02, Table 1). Slightly more patients with mild disease complained of fatigue compared to those with overt disease. Not all patients had preoperative bone mineral density scans (n = 706), but of the patients with bone mineral density data available, there were no differences in the proportion of patients with bone disease (osteopenia or osteoporosis) between the two groups. On physical exam, the group with overt disease had a slightly higher BMI. Notably, higher systolic and diastolic blood pressures were measured in the overt group compared to patients with mild disease. For example, the mean systolic blood pressure in patients with overt disease was 133 ± 1 mmHg compared to 129 ± 1 mmHg in the mild group (p < 0.01, Table 1).
Table 1.
Preoperative Features
| Variable | Mild (n = 388) | Overt (n = 1049) | p value |
|---|---|---|---|
| DEMOGRAPHICS | |||
| Age | 60±1 | 61± 0 | 0.24 |
| Female gender | 309 (79.8) | 796 (77.3) | 0.30 |
| SYMPTOMS | |||
| Kidney stones | 12 (3.1) | 13 (1.3) | 0.02 |
| Fatigue | 46 (11.9) | 84 (8.1) | 0.03 |
| Bone disease+ | 119 (40.1) | 176 (43.0) | 0.49 |
| EXAM | |||
| BMI (kg/m2) | 30.3± 0.4 | 31.6± 0.3 | 0.02 |
| Systolic BP (mmHg) | 129±1 | 133±1 | <0.01 |
| Diastolic BP (mmHg) | 75±1 | 78±1 | 0.01 |
| LABS | |||
| Ca (mg/dL) | 10.6 ± 0.0 | 11.2 ± 0.0 | <0.01 |
| Phos (mg/dL) | 3.2 ± 0.1 | 2.8± 0.1 | <0.01 |
| PTH (pg/mL) | 75 ± 2 | 145 ± 4 | <0.01 |
| Urinary Ca (mg/dL) | 297 ± 10 | 335 ±9 | <0.01 |
| Alk Phos (U/L) | 89±3 | 101 ±2 | <0.01 |
| Vit D (ng/mL) | 35±1 | 30±1 | <0.01 |
| Creatinine (mg/dL) | 0.99± 0.0 | 1.04 ± 0.0 | 0.15 |
| GFR (mL/min) | 90± 2 | 89± 1 | 0.63 |
| IMAGING | |||
| + Sestamibi* | 242 (62.4) | 826 (78.6) | <0.01 |
| BMD worst T score# | −1.9 ± 0.1 | −1.8 ± 0.2 | 0.67 |
Data are represented as the mean ± standard error of the mean for continuous variables or as the number in each category with the percentage in parentheses for categorical variables
Bone disease indicates either osteopenia or osteoporosis measured by bone mineral density scan
Indicates a sestamibi scan that localizes a suspected parathyroid adenoma
The lowest T score from any site on preoperative bone mineral density scan
BMI = body mass index; Kg = kilograms; m2 = meters squared; BP = blood pressure; Ca = calcium; Phos = phosphorous; PTH = parathyroid hormone; Urinary Ca = 24 hour urinary calcium; Alk Phos = alkaline phosphatase; Vit D = 25-hydroxyvitamin D; mg = milligrams; dL = deciliter; GFR = glomerular filtration rate; mL = milliliters; min = minute; BMD = bone mineral density
As expected, the mild group had significantly lower calcium and PTH levels compared to the overt group while phosphorous levels were significantly lower in the overt group (Table 1). The mild group had significantly greater levels of total vitamin D compared to the overt group (35±1 ng/mL vs. 30±1 ng/mL, p<0.01, Table 1). Importantly, the two groups did not differ in terms of renal function (Table 1).
Significantly more patients with overt disease had a localizing preoperative sestamibi scan compared to those with mild disease (78.6% vs. 62.4%, p<0.01, Table 1). The two groups had equivalent bone mineral density T scores in the osteopenic range.
Intraoperative Findings
More than one-third (34.6%) of patients with mild hyperparathyroidism were found to have multigland disease in the operating room, and this was a significantly greater proportion compared to patients with overt disease (14.1%, p<0.01, Table 2).
Table 2.
Intraoperative Features
| Variable | Mild (n = 388) | Overt (n = 1049) | p value |
|---|---|---|---|
| Multigland disease | 133 (34.6) | 147 (14.1) | <0.01 |
| Slope IoPTH* (pg/min) | −6.9± 0.8 | −11.6± 0.6 | <0.01 |
| Cured at 5 minutes | 207 (53.4) | 647 (62.2) | <0.01 |
| Median time to cure | 10 | 5 | <0.01 |
| Gamma probe counts# (% of background) | 67±3 | 96 ±2 | <0.01 |
| Gland weight (mg)# | 365± 18 | 879 ± 46 | <0.01 |
Data are represented as the mean ± standard error of the mean for continuous variables or as the number in each category with the percentage in parentheses for categorical variables
Slopes were compared only for cases where a single adenoma was resected
The gamma probe count and weight of the largest gland is reported for cases of multigland disease
pg = picograms; min = minute; mg = milligrams
The IoPTH kinetics were also significantly different between the two groups. When examining IoPTH kinetics where single adenomas were excised, the IoPTH fell at a rate of 6.9± 0.8 pg/min in mild patients compared to 11.6± 0.6 pg/min in the overt group (Table 2, Figure 1, p<0.01). For this reason, operations for mild PHPT took longer than for overt PHPT. The median time to achieve cure in the mild group was 10 minutes while it was five minutes in the overt group (Table 2). Significantly fewer patients with mild PHPT met criteria for cure (50% decline in IoPTH) at five minutes post-excision compared to patients with overt PHPT (53.4% vs. 62.2%, p<0.01, Table 2).
Figure 1. IoPTH Slopes.
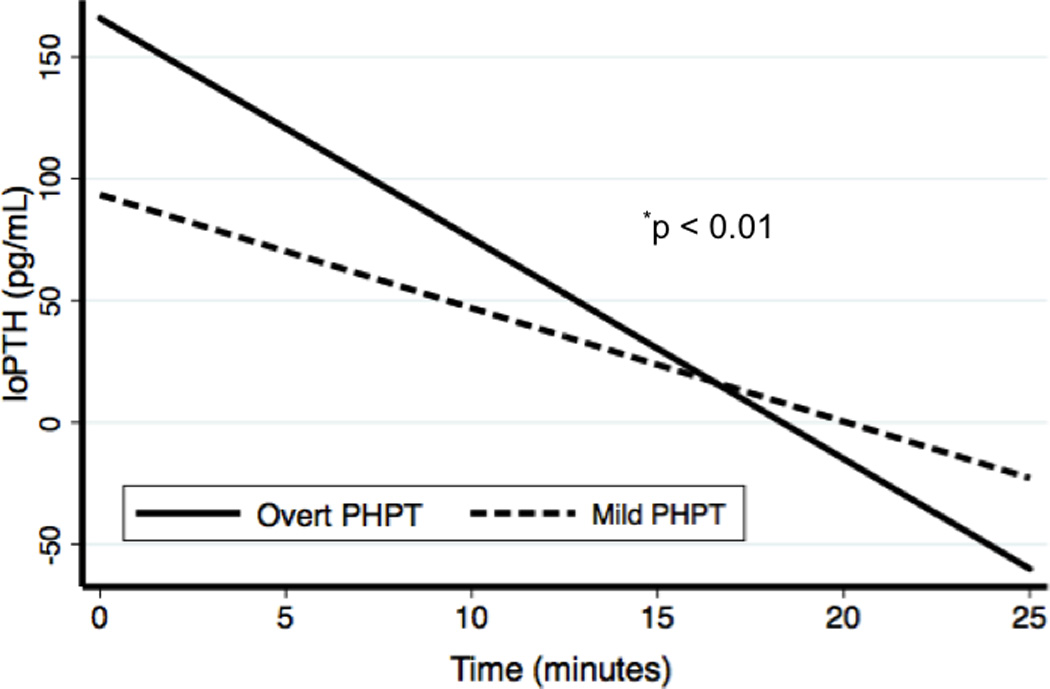
Linear regression was used to fit lines to the intraoperative PTH (IoPTH) values, and patients with mild PHPT were compared to patients with overt PHPT.
At the University of Wisconsin, all parathyroid operations are performed using the radioguided probe as previously described (5, 29, 30). As expected, patients with mild disease had glands with significantly lower gamma probe counts compared to patients with overt PHPT (Table 2). Similarly, the weight of the largest gland excised from patients with mild disease was less than the weight of the largest gland excised from patients with overt disease (365 ± 18 mg vs. 879 ± 46 mg, p<0.01, Table 2).
Outcomes
Follow-up time extended to a maximum of 10 years for the earliest patients in this series. Median follow-up time was 9.0 months (interquartile range, 19.5 months), and did not differ between the two groups. In the total cohort, 71% of patients had follow-up time exceeding 6 months. There were no differences in rates of recurrent or persistent PHPT when comparing patients with mild and overt PHPT (Table 3). Of the six recurrences in the mild group, four were in patients with normocalcemic hyperparathyroidism and two were in hypercalcemic patients an inappropriately normal PTH. The one case of persistent disease within the mild group was a patient with hypercalcemia and an inappropriately normal PTH. To further evaluate patterns of recurrence accounting for follow-up time, Kaplan-Meier disease-free survival estimates were also plotted. Disease-free survival estimates were equivalent (p = 0.27) when comparing the mild to the overt group (Figure 2).
Table 3.
Outcomes
| Variable | Mild (n = 388) | Overt (n = 1049) | p value |
|---|---|---|---|
| Persistent Disease (%) | 1 (0.26) | 6 (0.57) | 0.21 |
| Recurrent Disease (%) | 6 (1.55) | 26 (2.48) | 0.32 |
Data are represented as the number in each category with the percentage in parentheses
Figure 2. Disease-Free Survival.
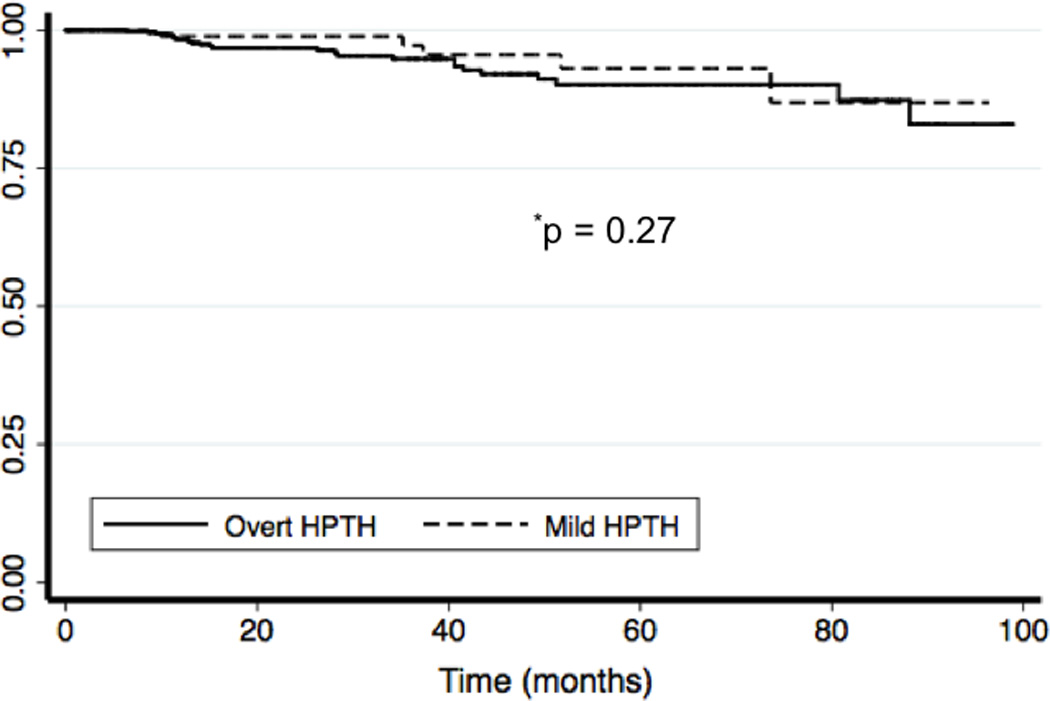
Kaplan-Meier disease-free survival curves are plotted for both patients with overt and mild PHPT.
Discussion
Here we present preoperative, intraoperative, and outcome details for 388 patients with mild PHPT. In contrast to earlier surgical series of patients with mild disease, a greater percentage of patients presented with mild PHPT. Previous studies report the rate of mild disease among patients undergoing parathyroidectomy to range from 5 to 14% (11, 15, 22, 31). In contrast, 27% of patients in this study presented with mild PHPT. This increased proportion of patients with mild disease likely reflects a more aggressive approach to this disease at our institution and a broader definition of mild PHPT, including both patients with normocalcemic hyperparathyroidism and hypercalcemic patients with an inappropriately normal PTH. Given these parameters, this is one of the largest operative series to date that reports the unique features of mild PHPT, revealing how this entity differs from “overt” PHPT and thereby improving surgeons’ expectations for what they may find intraoperatively.
One of the key intraoperative findings is the increased proportion of multigland disease among patients with mild PHPT. Nearly 35% of patients with mild PHPT had multigland disease, which was more than double the rate of multigland disease in patients with overt PHPT (Table 2). Carneiro-Pla et al. found a very similar proportion of patients with mild PHPT and multigland disease (31). In contrast, Wallace and colleagues found that 74% of their patients had a single adenoma, but this study only considered hypercalcemic patients with an inappropriately normal PTH (11). Increased multigland disease in the setting of mild disease makes intuitive sense given the abundance of scoring systems or algorithms indicating that patients with more severe biochemical indices (higher PTH and/or calcium) are more likely to have a single adenoma (32–34). This means a surgeon caring for a patient with mild PHPT should suspect multigland disease, and either treat this patient with a bilateral exploration or have a low threshold for converting from an MIP to a bilateral exploration depending on intraoperative findings and IoPTH results.
The surgeon’s expectations for operative time and IoPTH must also be altered for patients with mild disease. These patients display a much slower decline in IoPTH. This IoPTH slope was almost half that of patients with overt disease (Table 2). Accordingly, fewer patients with mild disease met criteria for cure at five minutes post-excision. A previous study from our institution examined hypercalcemic patients with an inappropriately normal PTH and found that some patients require up to 20 minutes post-excision before criteria for cure are met (15). Nonetheless, the standard IoPTH criteria (50% drop in IoPTH) still apply to this subset of patients, even if the PTH is within normal limits (15). Moreover, while mild HPT patients take longer to reach biochemical cure determined by IoPTH, rates of persistence and recurrence were equivalent and very low in both groups, so the standard IoPTH protocol still appies. These results have been substantiated in this larger series. The surgeon should therefore plan for longer operative times, due to a slower decline in IoPTH or the need for further exploration with multigland disease as discussed above. Future research will focus on alternative methods to distinguish single gland from multigland disease more quickly.
It is important to note that mild biochemical PHPT does not necessarily indicate a lack of symptoms (35). The proportion of patients with kidney stones in the mild group was more than double that of the overt group (Table 1). Furthermore, significantly more patients in the mild group complained of fatigue compared to the overt group (Table 1). Resolution of non-specific symptoms like fatigue may contribute to the improved quality of life observed by investigators who have studied patients undergoing parathyroidectomy for mild disease (13, 23). Despite more severe biochemical parameters of disease, the overt group had equivalent bone mineral density t-scores to the mild group, although both cohorts had mean t-scores consistent with osteopenia (Table 1). Several other series of patients with mild PHPT also demonstrated that patients with mild PHPT do have significant bone mineral density loss (3, 19, 23).
Wallace and colleagues suggest that a subset of patients exhibit a lower PTH set point that explains overt or “typical” features of PHPT in the setting of hypercalcemia with normal PTH levels (11). Others have cited fluctuating or pulsatile PTH secretion, target tissue resistance to PTH, PTH-related peptide, variability in sample storage and measurement, circulating inhibitors to PTH, and post-translational modification of the PTH molecule as explanations for this phenomenon (11, 24, 27, 36). Potential explanations for normocalcemic hyperparathyroidism include low serum binding protein concentrations, target tissue resistance to PTH, and vitamin D deficiency (19, 37, 38). Both forms of mild PHPT have been described as an earlier form of overt disease (3, 18).
Rates of both persistence and recurrence were similar when comparing outcomes for the mild and overt groups (Table 3, Figure 1). Goasguen et al. reported that mild disease was associated with smaller adenomas (micro adenomas) that made locating these glands more difficult, and eight percent of their patients with micro adenomas had persistent disease (22). In our series, the glands excised from patients with mild disease were significantly smaller compared to those with overt PHPT (Table 2), but rates of persistence and recurrence were extremely low and equivalent between the two groups (Table 3).
This study is limited by its retrospective nature. Since this study presents a surgical cohort, there may be selection bias, and therefore the results will not necessarily represent all patients with mild PHPT. Symptoms and bone disease could be worse in those referred for parathyroidectomy. However, the referring endocrinologists at our institution recommend surgery rather than observation in most patients with PHPT regardless of specific symptoms or laboratory cutoffs. As a multidisciplinary group, we believe in early intervention for patients with mild disease to prevent complications and improve quality of life. However, many patients are referred from a bone health clinic at our institution. Finally, although greater than 70% of our patients have more than six months follow-up, an even longer follow-up period may reveal more recurrences. As our group has previously shown, many recurrences are detected beyond five years follow-up, and this may represent a metachronous presentation of multigland disease (7). Therefore, given the greater proportion of multigland disease among patients with mild PHPT, longer follow-up may reveal more recurrences among the mild cohort.
Despite these limitations, this large series of patients with mild PHPT reveals important points for the parathyroid surgeon: these patients are more likely to have multigland disease and a slower decline in IoPTH. Kidney stones and bone disease are still associated with mild PHPT, supporting the notion that parathyroidectomy benefits patients who do not meet consensus criteria for surgery. Finally, these patients can be treated successfully as evidenced by low rates of persistence and recurrence.
Acknowledgements
This work was supported by NIH T32 CA009614-23 to DF Schneider and NIH T32 CA090217 to JF Burke.
References
- 1.Coker LH, Rorie K, Cantley L, Kirkland K, Stump D, Burbank N, et al. Primary hyperparathyroidism, cognition, and health-related quality of life. Ann Surg. 2005;242(5):642–650. doi: 10.1097/01.sla.0000186337.83407.ec. PMCID: 1409861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Ghandur-Mnaymneh L, Cassady J, Hajianpour MA, et al. The parathyroid glands in health and disease. Am J Pathol. 1986:125. [PMC free article] [PubMed] [Google Scholar]
- 3.Lowe H, McMahon DJ, Rubin MR, Bilezikian JP, Silverberg SJ. Normocalcemic primary hyperparathyroidism: further characterization of a new clinical phenotype. J Clin Endocrinol Metab. 2007;92(8):3001–3005. doi: 10.1210/jc.2006-2802. [DOI] [PubMed] [Google Scholar]
- 4.Adler JT, Sippel RS, Chen H. New trends in parathyroid surgery. Curr Probl Surg. 2010;47(12):958–1017. doi: 10.1067/j.cpsurg.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 5.Chen H. Radioguided Parathyroid surgery. Adv Surg. 2004;38:377–392. [PubMed] [Google Scholar]
- 6.Udelsman R, Donovan PI. Open minimally invasive parathyroid surgery. World J Surg. 2004;28(12):1224–1226. doi: 10.1007/s00268-004-7600-4. [DOI] [PubMed] [Google Scholar]
- 7.Schneider DF, Mazeh H, Sippel RS, Chen H. Is minimally invasive parathyroidectomy associated with higher recurrence compared to bilateral exploration? Surgery. 2012 doi: 10.1016/j.surg.2012.08.022. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Diagnosis and management of asymptomatic primary hyperparathyroidism. NIH Consensus Statement. 1990 Oct 29–31;:1–18. [Google Scholar]
- 9.Silverberg SJ, Lewiecki EM, Mosekilde L, Peacock M, Rubin MR. Presentation of asymptomatic primary hyperparathyroidism: proceedings of the third international workshop. J Clin Endocrinol Metab. 2009;94(2):351–365. doi: 10.1210/jc.2008-1760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bilezikian JP, Khan AA, Potts JT., Jr Guidelines for the management of asymptomatic primary hyperparathyroidism: summary statement from the third international workshop. J Clin Endocrinol Metab. 2009;94(2):335–339. doi: 10.1210/jc.2008-1763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Wallace LB, Parikh RT, Ross LV, Mazzaglia PJ, Foley C, Shin JJ, et al. The phenotype of primary hyperparathyroidism with normal parathyroid hormone levels: how low can parathyroid hormone go? Surgery. 2011;150(6):1102–1112. doi: 10.1016/j.surg.2011.09.011. [DOI] [PubMed] [Google Scholar]
- 12.Miller BS, England BG, Nehs M, Burney RE, Doherty GM, Gauger PG. Interpretation of intraoperative parathyroid hormone monitoring in patients with baseline parathyroid hormone levels of <100 pg/mL. Surgery. 2006;140(6):883–889. doi: 10.1016/j.surg.2006.07.016. discussion 9–90. [DOI] [PubMed] [Google Scholar]
- 13.Adler JT, Sippel RS, Schaefer S, Chen H. Surgery improves quality of life in patients with "mild" hyperparathyroidism. Am J Surg. 2009;197(3):284–290. doi: 10.1016/j.amjsurg.2008.09.009. [DOI] [PubMed] [Google Scholar]
- 14.Caron NR, Pasieka JL. What symptom improvement can be expected after operation for primary hyperparathyroidism? World J Surg. 2009;33(11):2244–2255. doi: 10.1007/s00268-009-9987-4. [DOI] [PubMed] [Google Scholar]
- 15.Alhefdhi A, Pinchot SN, Davis R, Sippel RS, Chen H. The necessity and reliability of intraoperative parathyroid hormone (PTH) testing in patients with mild hyperparathyroidism and PTH levels in the normal range. World J Surg. 2011;35(9):2006–2009. doi: 10.1007/s00268-011-1179-3. [DOI] [PubMed] [Google Scholar]
- 16.Lundgren E, Lind L, Palmer M, Jakobsson S, Ljunghall S, Rastad J. Increased cardiovascular mortality and normalized serum calcium in patients with mild hypercalcemia followed up for 25 years. Surgery. 2001;130(6):978–985. doi: 10.1067/msy.2001.118377. [DOI] [PubMed] [Google Scholar]
- 17.Persson A, Bollerslev J, Rosen T, Mollerup CL, Franco C, Isaksen GA, et al. Effect of surgery on cardiac structure and function in mild primary hyperparathyroidism. Clin Endocrinol (Oxf) 2011;74(2):174–180. doi: 10.1111/j.1365-2265.2010.03909.x. [DOI] [PubMed] [Google Scholar]
- 18.Bergenfelz A, Lindblom P, Lindergard B, Valdemarsson S, Westerdahl J. Preoperative normal level of parathyroid hormone signifies an early and mild form of primary hyperparathyroidism. World J Surg. 2003;27(4):481–485. doi: 10.1007/s00268-002-6649-1. [DOI] [PubMed] [Google Scholar]
- 19.Monchik JM, Gorgun E. Normocalcemic hyperparathyroidism in patients with osteoporosis. Surgery. 2004;136(6):1242–1246. doi: 10.1016/j.surg.2004.06.052. [DOI] [PubMed] [Google Scholar]
- 20.Siperstein AE, Shen W, Chan AK, Duh QY, Clark OH. Normocalcemic hyperparathyroidism. Biochemical and symptom profiles before and after surgery. Arch Surg. 1992;127(10):1157–1156. doi: 10.1001/archsurg.1992.01420100015003. discussion 61–3. [DOI] [PubMed] [Google Scholar]
- 21.Carneiro-Pla DM, Irvin GL, 3rd, Chen H. Consequences of parathyroidectomy in patients with "mild" sporadic primary hyperparathyroidism. Surgery. 2007;142(6):795–799. doi: 10.1016/j.surg.2007.07.023. discussion 9 e1-2. [DOI] [PubMed] [Google Scholar]
- 22.Goasguen N, Chirica M, Roger N, Munoz-Bongrand N, Zohar S, Noullet S, et al. Primary hyperparathyroidism from parathyroid micro adenoma: specific features and implications for a surgical strategy in the era of minimally invasive parathyroidectomy. J Am Coll Surg. 2010;210(4):456–462. doi: 10.1016/j.jamcollsurg.2009.12.017. [DOI] [PubMed] [Google Scholar]
- 23.Ambrogini E, Cetani F, Cianferotti L, Vignali E, Banti C, Viccica G, et al. Surgery or surveillance for mild asymptomatic primary hyperparathyroidism: a prospective, randomized clinical trial. J Clin Endocrinol Metab. 2007;92(8):3114–3121. doi: 10.1210/jc.2007-0219. [DOI] [PubMed] [Google Scholar]
- 24.Hollenberg AN, Arnold A. Hypercalcemia with low-normal serum intact PTH: a novel presentation of primary hyperparathyroidism. Am J Med. 1991;91(5):547–548. doi: 10.1016/0002-9343(91)90193-2. [DOI] [PubMed] [Google Scholar]
- 25.Glendenning P, Gutteridge DH, Retallack RW, Stuckey BG, Kermode DG, Kent GN. High prevalence of normal total calcium and intact PTH in 60 patients with proven primary hyperparathyroidism: a challenge to current diagnostic criteria. Aust N Z J Med. 1998;28(2):173–178. [PubMed] [Google Scholar]
- 26.Perez JB, Pazianos AG. Unusual presentation of primary hyperparathyroidism with osteoporosis, hypercalcemia, and normal parathyroid hormone level. South Med J. 2001;94(3):339–341. [PubMed] [Google Scholar]
- 27.Lafferty FW, Hamlin CR, Corrado KR, Arnold A, Shuck JM. Primary hyperparathyroidism with a low-normal, atypical serum parathyroid hormone as shown by discordant immunoassay curves. J Clin Endocrinol Metab. 2006;91(10):3826–3829. doi: 10.1210/jc.2006-0273. [DOI] [PubMed] [Google Scholar]
- 28.Cook MR, Pitt SC, Schaefer S, Sippel R, Chen H. A rising ioPTH level immediately after parathyroid resection: are additional hyper functioning glands always present? An application of the Wisconsin Criteria. Ann Surg. 2010;251(6):1127–1130. doi: 10.1097/SLA.0b013e3181d3d264. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Chen H, Mack E, Starling JR. Radio guided parathyroidectomy is equally effective for both adenomatous and hyperplastic glands. Ann Surg. 2003;238(3):332–337. doi: 10.1097/01.sla.0000086546.68794.9a. discussion 7–8. PMCID: 1422704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Chen H, Sippel RS, Schaefer S. The effectiveness of radio guided parathyroidectomy in patients with negative technetium tc 99m-sestamibi scans. Arch Surg. 2009;144(7):643–648. doi: 10.1001/archsurg.2009.104. [DOI] [PubMed] [Google Scholar]
- 31.Carneiro-Pla DM, Solorzano CC, Irvin GL., 3rd Consequences of targeted parathyroidectomy guided by localization studies without intraoperative parathyroid hormone monitoring. J Am Coll Surg. 2006;202(5):715–722. doi: 10.1016/j.jamcollsurg.2006.02.003. [DOI] [PubMed] [Google Scholar]
- 32.Kebebew E, Hwang J, Reiff E, Duh QY, Clark OH. Predictors of single-gland vs multigland parathyroid disease in primary hyperparathyroidism: a simple and accurate scoring model. Arch Surg. 2006;2006:777–782. doi: 10.1001/archsurg.141.8.777. [DOI] [PubMed] [Google Scholar]
- 33.Mazeh H, Chen H, Leverson G, Sippel RS. Creation of a "Wisconsin Index" Nomogram to Predict the Likelihood of Additional Hyper functioning Parathyroid Glands During Parathyroidectomy. Ann Surg. 2012 doi: 10.1097/SLA.0b013e31825ffbe1. [DOI] [PubMed] [Google Scholar]
- 34.Clerici T, Brandle M, Lange J, Doherty GM, Gauger PG. Impact of intraoperative parathyroid hormone monitoring on the prediction of multiglandular parathyroid disease. World J Surg. 2004;28(2):187–192. doi: 10.1007/s00268-003-7255-6. [DOI] [PubMed] [Google Scholar]
- 35.Bargren AE, Repplinger D, Chen H, Sippel RS. Can biochemical abnormalities predict symptomatology in patients with primary hyperparathyroidism? J Am Coll Surg. 2011;213(3):410–414. doi: 10.1016/j.jamcollsurg.2011.06.401. [DOI] [PubMed] [Google Scholar]
- 36.Gurrado A, Marzullo A, Lissidini G, Lippolis A, Rubini D, Lastilla G, et al. Substernal oxyphil parathyroid adenoma producing PTHrP with hypercalcemia and normal PTH level. World J Surg Oncol. 2008;6:24. doi: 10.1186/1477-7819-6-24. PMCID: 2279131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Norman J, Goodman A, Politz D. Calcium, parathyroid hormone, and vitamin D in patients with primary hyperparathyroidism: normograms developed from 10,000 cases. Endocr Pract. 2011;17(3):384–394. doi: 10.4158/EP09346.OR. [DOI] [PubMed] [Google Scholar]
- 38.Maruani G, Hertig A, Paillard M, Houillier P. Normocalcemic primary hyperparathyroidism: evidence for a generalized target-tissue resistance to parathyroid hormone. J Clin Endocrinol Metab. 2003;88(10):4641–4648. doi: 10.1210/jc.2002-021404. [DOI] [PubMed] [Google Scholar]


