Abstract
BACKGROUND
The Gynecologic Oncology Group conducted this phase II trial to estimate the anti-tumor activity of bevacizumab and to determine the nature and degree of toxicity in patients with recurrent sex cord-stromal tumors of the ovary.
METHODS
A prospective, multi-institutional cooperative group trial was performed in women with recurrent measurable ovarian stromal tumor. Patients were allowed to have unlimited prior therapy, excluding bevacizumab. Bevacizumab 15 mg/kg was administered intravenously on day one of every 21 day cycle until disease progression or adverse effects prohibited further treatment. The primary endpoint was response rate (RR). Inhibin A and B levels were measured prior to each cycle, and the values were examined in relation to response and progression.
RESULTS
Thirty-six patients were enrolled, all of whom were eligible and evaluable. Patients received a median of nine cycles of treatment (range, 2-37 cycles). Six patients (16.7%) had partial responses (90% CI: 7.5%, 30.3%), 28 patients (77.8%) had stable disease, and two patients (5.6%) had increasing disease. This met the criterion for declaring the regimen active. The median PFS was 9.3 months. The median OS has not been reached. Two grade 4 toxicities occurred: hypertension and proteinuria; the most common grade 3 toxicities were hypertension (n=5) and pain (n=5). Inhibin A and B values were lower in patients who responded to treatment.
CONCLUSIONS
Bevacizumab has activity in the treatment of recurrent sex cord-stromal tumors of the ovary; toxicity is acceptable. Further investigation is warranted.
Keywords: Stromal ovarian tumors, bevacizumab
INTRODUCTION
Sex cord-stromal tumors (SCSTs) of the ovary are rare neoplasms, which account for less than 5% of all ovarian malignancies.1-3 This group of ovarian stromal tumors include the granulosa cell tumor, granulosa cell–theca cell tumor, Sertoli-Leydig cell tumor (androblastoma), steroid (lipid) cell tumor, gynandroblastoma, unclassified sex cord-stromal tumor, and sex cord tumor with annular tubules. SCSTs tend to have an indolent course, compared to epithelial ovarian cancer, and can recur decades after their initial diagnosis and treatment. Unfortunately, these tumors are particularly resistant to chemotherapy, and treatment of recurrent disease is difficult, even with the use of surgery, radiotherapy, chemotherapy, and hormonal agents.4-6
The investigation of novel therapeutic approaches is warranted based on the limited efficacy of these standard treatment options. The tumor biology of SCSTs suggests the importance of angiogenesis in tumor development and progression. These tumors are known to be vascular, and can present with hemoperitoneum in up to 30% of patients.5 Lymph node metastases are extremely rare yet distant metastasis is common, further suggesting that hematogenous spread predominates and that angiogenesis may play an important role in these tumors.7-8 A recent case series of eight patients treated at a single institution suggested that bevacizumab is active in the setting of recurrent disease, but this included multiple regimens with and without cytotoxic therapy and was retrospective in nature. This report also suggested that VEGF overexpression and microvessel density were associated with worse outcome and a more aggressive phenotype, though the sample size was too small to calculate statistical significance.8 Together, these observations suggest that antiangiogenic agents, such as bevacizumab, may have a role in treating women with SCSTs. However, the body of literature surrounding these characteristics is limited, and a prospective study of any antiangiogenic agent in SCSTs has not been previously performed.
Since SCSTs are uncommon, clinical trials exploring therapeutic options have been limited. Performing these trials in a cooperative group setting such as the Gynecologic Oncology Group (GOG) allows for rapid accrual and evaluation. The primary objective of our phase II study was to estimate the anti-tumor activity of bevacizumab by assessing the frequency of objective response in patients with recurrent sex cord-stromal tumors of the ovary with measurable disease. The secondary objectives were to examine the overall survival (OS) and progression-free survival (PFS), and to determine the nature and degree of toxicity in these patients. A translational research objective was to determine the association of inhibin A and inhibin B with response to treatment.
MATERIALS AND METHODS
Eligibility Criteria
Eligible patients had a histologically confirmed diagnosis of recurrent ovarian stromal tumor, including granulosa cell tumor, granulosa cell-theca cell tumor, Sertoli-Leydig cell tumor (androblastoma), steroid (lipid) cell tumor, gynandroblastoma, unclassified sex cord-stromal tumor, or sex cord tumor with annular tubules. Since some patients had experienced multiple episodes of recurrent disease prior to enrolling in this protocol, any histologic confirmation of recurrent SCST was considered sufficient for enrollment, and a repeat biopsy was not mandated. Central pathologic review confirmed the diagnosis of ovarian SCST. Patients with measurable disease according to Response Evaluation Criteria in Solid Tumors (RECIST) criteria, Version 1.1, defined as at least one lesion that could be accurately measured in at least one dimension (longest dimension to be recorded), with each target lesion ≥20 mm when measured by conventional techniques, including palpation, plain radiograph, computerized tomography, or magnetic resonance imaging, or ≥10 mm when measured by spiral CT were eligible. Other eligibility criteria included a GOG performance status of 0, 1, or 2; adequate hematologic, renal, and hepatic function; and patients of childbearing potential with a negative pregnancy test must have agreed to practice an effective means of birth control. There were no restrictions on prior therapy, but patients could not have previously received bevacizumab.
Study Design
This GOG prospective, multi-institutional phase II trial was designed to estimate the anti-tumor activity of bevacizumab by assessing the frequency of objective response, to examine the OS and PFS, and to determine the nature and degree of toxicity in these patients. Patients were to receive bevacizumab intravenously at a dose of 15 mg/kg every 21 days until disease progression or prohibitive toxicity. Bevacizumab was provided by the manufacturer, free of charge to the patient. Each 21 day period was considered 1 cycle. Serum inhibin A and B levels were measured every cycle of treatment. The study received local institutional review board approval at participating institutions, and all patients gave written informed consent according to institutional and federal guidelines before treatment.
Treatment Plan and Dose Modification
Toxicity was monitored with history, physical examination, and laboratory assessment before each treatment cycle, with adverse events defined and graded according to National Cancer Institute Common Toxicity Criteria version 3.0. Guidelines were established for dose delay and dose reduction for hematologic and non hematologic toxicity.
Patient Assessment
Activity of bevacizumab was assessed according to RECIST, either by clinical evaluation, computed tomography or magnetic resonance imaging at baseline, and before every other cycle for the measurement of target lesions, the classification of clinical response, and the determination of disease progression. Therapy was discontinued for disease progression, unacceptable toxicity, receipt of other anticancer therapy, or voluntary withdrawal.
Statistical Analysis
The primary objective of the study evaluated the efficacy of the study agent as assessed by objective tumor responses. Tumor response rate (RR) was used as the primary endpoint, rather than survival or PFS, for two reasons. First, there are limited prior data serving as a reference for PFS and OS in this disease setting, so these endpoints cannot be used to define activity of the agent. Secondly, an objective response is one meaningful way to assess antitumor activity. By setting a low threshold for tumor response (5%), this enables us to have reasonable probability (alpha of 10%) of ruling out truly inactive agents for further investigation.
The targeted accrual for the first stage was 19 patients but was allowed to deviate for administrative purposes. With 24 eligible and evaluable patients, more than one patient responding was required to go to second stage. The cumulative targeted accrual for the second stage was 34 but was allowed to deviate. With 36 eligible and evaluable patients, more than three patients with responses were required to declare the agent sufficiently active to warrant further evaluation.
The design aimed to limit the expected probabilities of type I and II errors to no more than 10% under the assumed accrual ranges of 15 to 22 (stage 1) and 30 to 37 (cumulatively after stage 2). Using the method of Chen and Ng9, if the true RR was 5%, the study design limited the average probability (across the accrual range) of incorrectly designating the treatment as active to 10%. On the other hand, if the true RR was 20%, then the average probability of correctly classifying the treatment as active was 90%.
Kaplan-Meier estimates were used for PFS and OS. Exact confidence intervals (CIs) were used for binary parameters. The primary endpoint was RR (RR), evaluated in a two-stage design (with power of 0.90 for a RR of 20% and with alpha of 0.10 for RR of 5%). The reverse Kaplan-Meier method was used to calculate median follow-up.10
Inhibin A and inhibin B values were measured every cycle of treatment. A small number of inhibin A and inhibin B results were determined to be less than the cutoff; in these cases, the cutoff value was used for calculation. A number of inhibin B values were determined to be above the cutoff; in these cases, the cutoff value was used for analysis. Results were similar when these values were excluded from the analysis and when 0.5 times the lower cutoff and 1.5 times the upper cutoff were used. Analyses of timepoint-specific values were done using medians and rank-based (nonparametric) methods. Medians of the 25th and 75th percentiles were used for summary statistics, and Wilcoxon rank sum tests were used to compare groups. Summary statistics and comparison of inhibin A and inhibin B values between responders and nonresponders are presented for only the first 4 cycles, because the number of patients with available data decreased from 32 patients with available data in cycle 1 to 22 patients with available data in cycle 5. Joint models of both longitudinal inhibin values and progression-free survival (PFS) were also random effects models to model the inhibin values over time and proportional hazards models. Natural log inhibin values were used in these joint models.11
RESULTS
Patient characteristics
From September 2008 through January 2011, 36 patients were enrolled, all of whom were eligible and evaluable. Patient characteristics of the study group are listed in Table 1. The GOG performance status was zero in 30 patients (83.3%) and one in six patients (16.7%). Of the 36 patients, 32 patients had a granulosa cell tumor (88.9%) and four patients had an unclassified sex cord-stromal tumor (11.1%). When the referring pathology diagnosis was compared with central pathology review, 33 of 36 cases (91.7%) were concordant. All 3 patients for whom a different diagnosis was rendered by central pathology review were ultimately determined to have SCST, unclassified; these patients had initial diagnoses of granulosa cell tumor, Sertoli-Leydig cell tumor, and unknown, respectively. Of the 36 patients, 33 patients (91.7%) had received prior chemotherapy, with a median of two prior regimens. Six patients had previously received radiotherapy (16.7%). None had received prior immunotherapy.
Table 1.
Patient characteristics
| Characteristics | Number | % |
|---|---|---|
| Age | ||
| 30-39 | 1 | 2.8 |
| 40-49 | 8 | 22.2 |
| 50-59 | 12 | 33.3 |
| 60-69 | 11 | 30.6 |
| 70-79 | 4 | 11.1 |
| Race | ||
| Unspecified | 2 | 5.6 |
| Asian | 1 | 2.8 |
| African-American | 3 | 8.3 |
| White | 30 | 83.3 |
| Ethnicity | ||
| Hispanic | 2 | 5.6 |
| Non-Hispanic | 30 | 83.3 |
| Unspecified | 4 | 11.1 |
| Prior Chemotherapy | ||
| 0 | 3 | 8.3 |
| 1 | 12 | 33.3 |
| 2 | 14 | 38.9 |
| 3 | 4 | 11.1 |
| 4 | 2 | 5.6 |
| 6 | 1 | 2.8 |
Treatment Administration
A total of 491 cycles of bevacizumab were administered, with a median of nine cycles of treatment per patient (range, 2-50 cycles, with the latter patient still on study).
Activity of bevacizumab
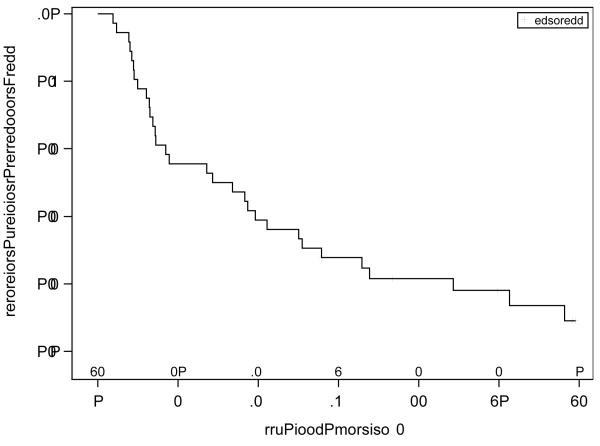
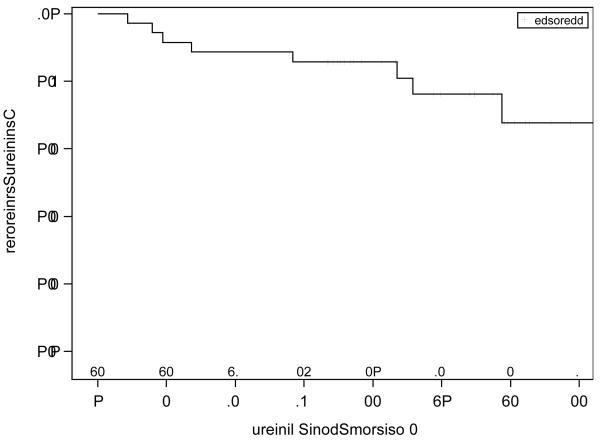
The activity of bevacizumab was analyzed in the 36 enrolled patients. Six patients had a partial response (16.7%; 90% CI, 7.5 to 30.3%), 28 patients had confirmed stable disease (77.8%), and two patients had increasing disease (5.6%). This RR met the criteria to declare the agent active. For the six patients who had partial responses, the median (25th - 75th percentiles) time to response was 8.2 (1.5 – 14.4) months, and the duration of response was 18.6 (17.9 – 27.2) months. The median PFS was 9.3 months, and the median OS was not reached with a median followup of 32.5 months. The graphs of the Kaplan-Meier estimates of PFS and OS for the study population are shown in Figures 1 and 2.
Figure 1.
Kaplan-Meier estimates of PFS
Figure 2.
Kaplan-Meier estimates of OS
As of December 2012, one patient remains on study after 50 cycles and with a partial response. Five patients remain alive without progression, 23 patients remain alive with progression, and eight patients have died: five due to disease, one due to treatment and disease, and two whose cause of death is pending.
Toxicity
As shown in Table 3, the safety of bevacizumab in all 36 patients was analyzed descriptively; events listed had been reported as at least possibly related to study drug. Grade 3 adverse events at least possibly related to bevacizumab included hypertension (n = 5), pain (n = 5), and dyspnea, dyspnea+hypoxia, leukopenia, anemia, hypophosphatemia, proteinuria, coagulation, tinnitus, constipation and distension, fatigue, genitourinary/renal, hematoma, and syncope (n =1 each). Two grade 4 toxicities were observed: hypertension and proteinuria. The cardiac events were all related to hypertension, and the gastrointestinal event was an incarcerated hernia requiring emergent surgery. There were no gastrointestinal perforations. Four (11%) of the 36 patients came off study due to toxicity.
Table 3.
Summary Statistics of Inhibin A – Overall and by Responder Status
| All | Complete or Partial Response | p-value† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-responder | Responder | ||||||||||||
| n |
Median
(pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
N | Median (pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
n | Median (pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
||
| Cycle 1 |
31 |
12 |
4 |
58 |
26 |
15 |
4 |
80 |
5 |
6 |
2 |
10 |
|
| .100 | |||||||||||||
| 2 | 32 | 12 | 4 | 81 | 28 | 30 | 6 | 104 | 4 | 2 | 1 | 4 | .020 |
| 3 | 31 | 11 | 4 | 85 | 26 | 32 | 7 | 125 | 5 | 2 | 2 | 4 | .011 |
| 4 | 32 | 9 | 3 | 92 | 27 | 34 | 5 | 141 | 5 | 2 | 1 | 2 | .008 |
p-value for difference between responders and non-responders (exact Wilcoxon rank sum test).
Inhibin A and Inhibin B
The median inhibin A value was 12 pg/mL at cycle 1 (baseline) and decreased slightly across the following three cycles to 12, 11, and 9 pg/mL. Inhibin A values were lower (although not statistically significantly, p=0.100) at baseline in responders versus non-responders (medians of 6 pg/mL versus 15 pg/mL), and values in cycles 2, 3, and 4 were statistically significantly lower in patients that responded to treatment (median value of 2 pg/mL) than in those who did not respond to treatment (median values of 30, 32, and 34 pg/mL in cycles 2, 3, and 4, respectively) (Table 3). The median inhibin B value was 217 pg/mL at cycle 1 (baseline) and decreased after treatment to 168, 169, and 169 pg/mL in cycles 2, 3, and 4, respectively. Inhibin B values were lower (although not statistically significantly, p=0.100) at baseline in responders versus non-responders (medians of 61 pg/mL versus 308 pg/mL), and values in cycles 2, 3, and 4 were statistically significantly lower in patients that responded to treatment than in those who did not respond to treatment, and this was significant in cycles 2, 3, and 4 (Table 4). Results from the joint models of inhibin and PFS showed a nonstatistically signficant increased hazard of progression or death with increasing levels of inhibin A or inhibin B. For inhibin A, a one unit increase in the log inhibin A levels was associated with a hazard rate [HR] (95% CI) of 1.15 (0.96, 1.38), p=0.129. For inhibin B, a one unit increase in the log inhibin B levels was associated with a HR (95% CI) of 1.09 (0.89, 1.33), p=0.424.
Table 4.
Summary Statistics of Inhibin B - Overall and by Responder Status
| All | Complete or Partial Response | p-value† | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Non-responder | Responder | ||||||||||||
| n |
Median
(pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
N | Median (pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
n | Median (pg/mL) |
25th %ile (pg/m L) |
75th %ile (pg/m L) |
||
| Cycle |
32 |
217 |
63 |
492 |
27 |
308 |
91 |
542 |
5 |
61 |
48 |
141 |
|
| 1 | .100 | ||||||||||||
| 2 | 36 | 168 | 35 | 778 | 30 | 236 | 71 | 959 | 6 | 22 | 5 | 55 | .005 |
| 3 | 33 | 169 | 40 | 833 | 27 | 274 | 122 | 1500 | 6 | 36 | 15 | 69 | .008 |
| 4 | 33 | 168 | 62 | 818 | 27 | 274 | 102 | 1500 | 6 | 57 | 15 | 97 | .007 |
p-value for difference between responders and non-responders (exact Wilcoxon rank sum test).
DISCUSSION
Bevacizumab administered as a single agent appears to be active and have acceptable toxicity in women with recurrent sex cord-stromal tumors of the ovary. This represents a significant advance in women with this rare disease in whom effective treatment options are quite limited and disease is often chemorefractory. Previously, the only options for systemic therapy included BEP, paclitaxel and carboplatin, and hormonal therapy4-5, 12-13. The ability to use bevacizumab as an effective means of controlling tumor progression is a meaningful addition to the short list of therapeutics available for these patients.
Pre-treatment inhibin A and inhibin B values were lower in patients who responded to therapy than in those who did not respond to therapy, although the differences were not statistically signficant (p=0.100). These values were found to be significantly lower postbaseline in patients who responded to bevacizumab than in patients who did not respond. These data suggest that further investigation into the inhibins’ potential prognostic and predictive relevance is warranted. Patients with high inhibin levels may have a more aggressive tumor type that will not respond to bevacizumab or other treatments, while patients with lower levels may be good candidates for certain treatments, as seen in this study. The conclusions drawn from these pilot data, however, need to be carefully considered since this is a small study, and further investigation is required before this is used as a guideline. It is also interesting that the trend of inhibin A and B levels followed the pattern of disease response, though this did not reach statistical significance. The hazard level associated with progression or death was higher with increasing inhibin A than increasing inhibin B, but neither trend reached significance; therefore this does not suggest that inhibin A is a better marker than inhibin B. It may be that the high percentage of stable disease in this study precluded the changing levels of inhibin A and B reaching significance. As has been suggested, inhibin A and B may represent effective markers for following progression or response of disease on therapy, like CA-125 in epithelial ovarian cancer, but further evaluation as to the accuracy of correlation needs to be done.14-15 The use of high inhibin levels to triage patients away from bevacizumab, however, is a new concept that bears further investigation.
The histology of most of these patients was granulosa cell tumor, which reflects the relative frequency of this histologic subtype. While one must be careful in generalizing the results of this report to all patients with stromal ovarian tumors, it is likely that the results of this trial do apply to other histologic subtypes of stromal ovarian tumors, based on historical findings of similar responses among patients with various subtypes of stromal tumors3-4, 6, 12-13.
In this study, tumor RR was used as the primary endpoint, rather than survival or PFS, for several reasons. RR represents a clinically meaningful decision criterion for determining whether the therapy was effective or ineffective when deciding on continuation into the second stage of the study and when deciding on the success of the overall study. There are limited prior data in this patient population, which precluded the use of PFS or OS to judge the activity of the agent in this disease setting. Instead, objective response was used, and a relatively low threshold for tumor response (5%) enabled us to have reasonable probability (alpha of 10%) of ruling out truly inactive agents for further investigation. A prior GOG trial in previously treated patients with stromal tumors evaluated bleomycin, etoposide and cisplatin.12 The observed RR in that trial was 37.5% (9/24). However, that study required patients to be chemo naíve, whereas the current study allows patients to have any number of prior chemo regimens. It is important to note that no biologic agent has been prospectively studied in patients with SCSTs before this trial. Therefore, the RR was chosen as the primary endpoint, as it is the more clinically useful parameter to gauge activity of bevacizumab in this specific disease setting.
Since the rationale for this study was to identify an antivascular agent for future combination with cytotoxic chemotherapy and not simply utility as monotherapy, the criteria to declare this agent interesting based on an objective RR was set lower than for identifying an agent with monotherapy activity. Therefore, the null hypothesis was set at 5%; if bevacizumab yielded a RR of 5% or less, it would not be of interest to evaluate further in combination with other agents. Therefore, although the RR is less than the conventionally accepted rate of 20% to declare an agent active, bevacizumab met the conditions of this trial to declare it as an active agent.
A substantial percentage of patients experienced stable disease. To determine stable disease in this clinical trial required imaging documenting stable disease followed by confirmed imaging after two additional courses. Since courses were three weeks apart, this implies at least 12 weeks of stable disease. The possibility exists that the indolent nature of this disease might lead to a suggestion that the drug was responsible for stable disease when in fact tumor biology was responsible for the large percentage of patients who experienced stable disease. In the absence of a randomized trial comparing therapy with a placebo arm, however, this is impossible to determine with certainty. The eventual progression or death from disease of 29 patients, and the median PFS of 9.3 months, however, suggests that this disease does grow and not simply remain dormant, leading to our opinion that the substantial rate of stable disease in this trial truly represents drug effect. Previous reports on “active” agents for stromal ovarian tumors report a PFS of 14-19.6 months in patients with recurrent, measurable disease4, 12-13 The long median time to progression in those patients with stable disease, as well as a PFS which is comparable to that described in the published literature for “active” compounds for stromal ovarian tumors4, 12-13 leads to the conclusion that the response rate, PFS, and time to progression do represent real drug effects rather than indolent tumor behavior.
The toxicity profile described in this study is typical for bevacizumab, and represents findings that would be expected. Bevacizumab appears to be tolerable in patients with recurrent SCSTs.
To conduct meaningful clinical trials in rare tumors outside of a cooperative group setting is difficult. The short accrual time required to complete this trial highlights the feasibility of efficiently completing clinical trials in patients with rare tumors in a cooperative group setting. Furthermore, the finding of activity in this tumor that has historically been relatively chemoresistant underscores the importance of allocating resources to perform cooperative group trials in rare tumors, as this substantially moves the field forward. An additional strength of running trials in the cooperative group setting is the ability to assemble historical data sets against which future trial results can be judged; this trial is the first step in doing so for SCSTs of the ovary.
The results of this clinical trial indicate that bevacizumab has activity in the treatment of recurrent SCSTs of the ovary, and that toxicity is acceptable in this treatment setting. Inhibin A and inhibin B appear to be associated with response to therapy. Further investigation is warranted, and combination therapy with a cytotoxic, targeted, or hormonal agent may be an upcoming investigation in this cooperative group setting. In addition, a randomized phase II study comparing paclitaxel and carboplatin with bleomycin, etoposide, and cisplatin in patients with newly diagnosed advanced and recurrent chemo naive stromal ovarian tumors is currently underway within this cooperative group, further refining optimal therapy for patients with these rare tumors.
Table 2.
Adverse Events by Number of Episodes
| AE Category | 0 | 1 | 2 | 3 | 4 | 5 | Total |
|---|---|---|---|---|---|---|---|
| Leukopenia | 32 | 2 | 1 | 1 | 0 | 0 | 36 |
| Thrombocytopenia | 28 | 8 | 0 | 0 | 0 | 0 | 36 |
| Anemia | 21 | 7 | 7 | 1 | 0 | 0 | 36 |
| Other Hematologic | 33 | 2 | 1 | 0 | 0 | 0 | 36 |
| Allergy/Immunology | 23 | 11 | 2 | 0 | 0 | 0 | 36 |
| Auditory/Ear | 29 | 0 | 6 | 1 | 0 | 0 | 36 |
| Cardiac | 15 | 6 | 9 | 5 | 1 | 0 | 36 |
| Coagulation | 35 | 0 | 0 | 1 | 0 | 0 | 36 |
| Constitutional | 12 | 10 | 13 | 1 | 0 | 0 | 36 |
| Dermatologic | 17 | 14 | 4 | 1 | 0 | 0 | 36 |
| Endocrine | 33 | 2 | 1 | 0 | 0 | 0 | 36 |
| Gastrointestinal | 6 | 10 | 15 | 5 | 0 | 0 | 36 |
| Genitourinary/Renal | 29 | 5 | 1 | 1 | 0 | 0 | 36 |
| Hemorrhage | 17 | 18 | 0 | 1 | 0 | 0 | 36 |
| Infection | 23 | 0 | 11 | 2 | 0 | 0 | 36 |
| Lymphatics | 31 | 4 | 1 | 0 | 0 | 0 | 36 |
| Metabolic | 13 | 10 | 8 | 3 | 2 | 0 | 36 |
| Musculoskeletal | 30 | 5 | 1 | 0 | 0 | 0 | 36 |
| Neurosensory | 21 | 13 | 1 | 1 | 0 | 0 | 36 |
| Other Neurological | 22 | 12 | 1 | 1 | 0 | 0 | 36 |
| Ocular/Visual | 30 | 6 | 0 | 0 | 0 | 0 | 36 |
| Pain | 1 | 11 | 16 | 8 | 0 | 0 | 36 |
| Pulmonary | 12 | 20 | 1 | 3 | 0 | 0 | 36 |
| Sexual/Reproductive | 32 | 4 | 0 | 0 | 0 | 0 | 36 |
| Syndromes | 35 | 1 | 0 | 0 | 0 | 0 | 36 |
| Vascular | 35 | 0 | 1 | 0 | 0 | 0 | 36 |
| Death, Not CTC coded | 35 | 0 | 0 | 0 | 0 | 1 | 36 |
ACKNOWLEDGEMENTS
This study was supported by National Cancer Institute grants to the Gynecologic Oncology Group Administrative Office (CA 27469) and the Gynecologic Oncology Group Statistical Office (CA 37517).
The following Gynecologic Oncology Group member institutions participated in the primary treatment studies: The University of Texas M.D. Anderson Cancer Center, Memorial Sloan-Kettering Cancer Center, Northwestern University, University of North Carolina School of Medicine, University of Chicago, University of Iowa, Central Connecticut, The Cleveland Clinic Foundation, Fox Chase Cancer Center, University of Mississippi Medical Center, University of Oklahoma, Columbus Cancer Council, and University of Minnesota.
ROLE OF THE FUNDING SOURCE
The study sponsor (Genentech) supplied drug for the study but did not participate in the design of the trial. They did not participate in the collection, analysis, or interpretation of the data, nor in the writing of the report or the decision to submit the paper for publication. They were given the courtesy of receiving a draft manuscript prior to submission. The corresponding author had full access to all the data in the study and had final responsibility for the decision to submit for publication.
REFERENCES
- 1.Björkjolm E, Silfverswärd C. Prognostic factors in granulosa-cell tumors. Gynecol Oncol. 1981;11:261–74. doi: 10.1016/0090-8258(81)90040-8. [DOI] [PubMed] [Google Scholar]
- 2.Evans AT, 3rd, Gaffey TA, Malkasian GD, Jr., et al. Clinicopathologic review of 118 granulosa and 82 theca cell tumors. Obstet Gynecol. 1980;55:231–8. [PubMed] [Google Scholar]
- 3.Fox H, Agrawal K, Langley FA. A clinicopathologic study of 92 cases of granulosa cell tumor of the ovary with special reference to the factors influencing prognosis. Cancer. 1975;35:231–41. doi: 10.1002/1097-0142(197501)35:1<231::aid-cncr2820350128>3.0.co;2-o. [DOI] [PubMed] [Google Scholar]
- 4.Brown J, Shvartsman HS, Deavers MT, et al. The activity of taxanes in the treatment of sex cord-stromal ovarian tumors. J Clin Oncol. 2004;22:3517–23. doi: 10.1200/JCO.2004.12.074. [DOI] [PubMed] [Google Scholar]
- 5.Modesitt SC, Brown J. Germ cell and sex cord-stromal ovarian cancers. In: Karlan BY, Bristow RE, Li AJ, editors. Gynecologic Oncology: Clinical Practice and Surgical Atlas. McGraw-Hill; New York: 2012. [Google Scholar]
- 6.Brown J, Sood AK, Deavers MT, et al. Patterns of metastasis in sex cord-stromal tumors of the ovary: Can routine staging lymphadenectomy be omitted? Gynecol Oncol. 2009;113:86–90. doi: 10.1016/j.ygyno.2008.12.007. [DOI] [PubMed] [Google Scholar]
- 7.Abu-Rustum N, Restivo A, Ivy J, et al. Retroperitoneal nodal metastasis in primary and recurrent granulosa cell tumors of the ovary. Gynecol Oncol. 2006;103:31–4. doi: 10.1016/j.ygyno.2006.01.050. [DOI] [PubMed] [Google Scholar]
- 8.Tao X, Sood AK, Deavers MT, et al. Anti-angiogenesis therapy with bevacizumab for patients with ovarian granulosa cell tumors. Gynecol Oncol. 2009;114:431–6. doi: 10.1016/j.ygyno.2009.04.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Chen TT, Ng T. Optimal flexible designs in phase II clinical trials. Stat Med. 1998;17:2301–12. doi: 10.1002/(sici)1097-0258(19981030)17:20<2301::aid-sim927>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 10.Schemper M, Smith TL. A note on quantifying follow-up in studies of failure time. Controlled Clinical Trials. 1996;17:343–346. doi: 10.1016/0197-2456(96)00075-x. [DOI] [PubMed] [Google Scholar]
- 11.Rizopoulos D. Joint Models for Longitudinal and Time-to-Event Data with Applications in R. Chapman & Hall/CRC; Boca Raton: 2012. [Google Scholar]
- 12.Homesley HD, Bundy BN, Hurteau JA, et al. Bleomycin, etoposide and cisplatin combination therapy of ovarian granulosa cell tumors and other stromal malignancies: a Gynecologic Oncology Group Study. Gynecol Oncol. 1999;72:131–7. doi: 10.1006/gyno.1998.5304. [DOI] [PubMed] [Google Scholar]
- 13.Gershenson DM, Morris M, Burke TW, Levenback C, Matthews CM, Wharton JT. Treatment of poor-prognosis sex cord-stromal tumors of the ovary with the combination of bleomycin, etoposide, and cisplatin. Obstet Gynecol. 1996;87:527. doi: 10.1016/0029-7844(95)00491-2. [DOI] [PubMed] [Google Scholar]
- 14.Lyubimova NV, Beyshembaev AM, Kushlinskiy DN, et al. Granulosa cell tumors of the ovary and inhibin B. Bull Exp Biol Med. 2011;150:635–8. doi: 10.1007/s10517-011-1209-z. [DOI] [PubMed] [Google Scholar]
- 15.Geerts I, Vergote I, Neven P, et al. The role of inhibins B and antimullerian hormone for diagnosis and follow-up of granulosa cell tumors. Int J Gynecol Cancer. 2009;19:847–55. doi: 10.1111/IGC.0b013e3181a702d1. [DOI] [PubMed] [Google Scholar]