Abstract
Specialty pharmaceuticals include most injectable and biologic agents used to treat complex conditions such as rheumatoid arthritis, multiple sclerosis, and cancer. We analyzed trends in specialty drug spending among Medicare beneficiaries ages sixty-five and older using 2007–11 pharmacy claims data from a 20 percent sample of Medicare beneficiaries. Annual specialty drug spending per beneficiary who used specialty drugs increased considerably during the study period, from $2,641 to $8,976. However, specialty drugs accounted for only 6.7 percent of total drug spending per beneficiary in 2007 and 9.1 percent in 2011. Moreover, in 2011 cost-sharing reductions under the Affordable Care Act significantly reduced specialty drug users’ out-of-pocket burden, which decreased 26 percent from 2010. Oral cancer agents accounted for a significant proportion of the increase in specialty drug spending among the study population. This suggests that the migration of specialty drug coverage from Medicare’s Part B medical benefit to the Part D pharmacy benefit because of new treatment options may play an important role in specialty pharmacy trends. This shift is likely to continue as pharmaceutical innovations enable more specialty therapeutics to be self-administered and to be covered under the pharmacy instead of the medical benefit.
Specialty pharmaceuticals include most injectable and biologic agents used to treat complex conditions such as rheumatoid arthritis, multiple sclerosis, and cancer. Recent advances in this class of drugs have resulted in considerable attention being focused on their very high prices and concerns about their contribution to unsustainable growth in health care spending.[1,2]
For example, Sovaldi, a recently approved drug for hepatitis C, offers significantly improved clinical outcomes and reduced side effects, compared to existing interferon-based treatments and/or liver transplant. However, Sovaldi’s high price (up to $1,000 per day for a twelve-week course of treatment, or $84,000 in all) has intensified interest in specialty drugs among the media and policy makers. The drug’s cost has been a primary concern,[3,4] but some articles have focused on its value in terms of improved results and the importance of encouraging future pharmaceutical innovations.[5,6] This dichotomy highlights the inherent trade-off associated with specialty drugs: They offer substantial benefits for a select group of patients, but they have very high prices.[7]
Sovaldi is not an isolated example but is part of a broader trend of increasing spending on specialty pharmaceuticals—spending whose growth has outpaced that of other drugs in recent years.[8,9] This has intensified health plans’ interest in monitoring and containing the drugs’ use.[10] Moreover, these rising drug prices, coupled with high levels of cost sharing, raise concerns related to access and financial burden for patients who could benefit from these improved treatment options. A substantial proportion of specialty drug users are Medicare beneficiaries. The Medicare Prescription Drug Program (Medicare Part D) defines specialty drugs as those with a negotiated monthly price of more than $600[11] and allows plans to place these products on a specialty tier. This means that beneficiaries typically face a coinsurance rate of 25 percent or 33 percent of the price of these drugs, as opposed to the commonly used flat copayments for generic drugs and for preferred or nonpreferred brand-name drugs.
Provisions in the Affordable Care Act (ACA) may have important implications for Medicare beneficiaries’ use of specialty drugs and out-of-pocket burden. Beginning in 2011 the ACA enhanced prescription drug coverage for Medicare beneficiaries who were in the coverage gap (the so-called doughnut hole), resulting in 50 percent discounts for brand-name drugs. In addition to these manufacturer-financed discounts, additional plan-financed discounts that reduced patients’ cost sharing in the doughnut hole took effect for generic and brand-name drugs in 2011 and 2013, respectively.
These discounts will grow over time through 2020, at which point beneficiaries’ cost sharing in the doughnut hole for all drugs will become subject to 75 percent discounts, indefinitely. Because of the high cost of specialty drugs, the majority of Medicare beneficiaries taking them reach the doughnut hole in any given year. [12]
The ACA cost-sharing reductions will likely have a significant impact in terms of reducing out-of-pocket burden for Medicare beneficiaries taking high-cost medications for the treatment of cancer, rheumatoid arthritis, multiple sclerosis, and other complex chronic conditions.
In this article we highlight recent trends in specialty drug spending and out-of-pocket burden among elderly Medicare beneficiaries (those ages sixty-five and older) enrolled in stand-alone prescription drug plans and Medicare Advantage plans that have prescription drug coverage.[13] We used data from a nationally representative sample of Medicare beneficiaries to evaluate these trends for the period 2007–11. Our analyses evaluate the growth in the contribution of specialty drug spending to overall pharmacy spending and highlight the significant reduction in out-of-pocket burden among specialty drug users as a result of the ACA-based reforms. Additionally, we assess the contribution of various drug classes to overall growth in specialty drug spending.
Study Data And Methods
Data
We evaluated pharmacy claims data from a 20 percent random sample of Medicare beneficiaries. The data included pharmacy claims for 2007–11 for elderly Medicare beneficiaries enrolled in standalone prescription drug plans or in Medicare Advantage plans. For each year, we restricted our sample to elderly beneficiaries[14] who were enrolled in the same plan for the entire year and whose eligibility for extra help with premiums and cost sharing in the form of low-income subsidies did not change during the year.
The number of beneficiaries in our sample increased from about 3.5 million in 2007 to about 4.3 million in 2011. The beneficiaries accounted for $7.6 billion and $10.2 billion in total pharmacy spending in 2007 and 2011, respectively. Consistent with national trends, the percentage of beneficiaries enrolled in Medicare Advantage plans increased from 33 percent to 40 percent during the study period. The proportion of beneficiaries who received low-income subsidies in our sample decreased slightly, from 27 percent to 24 percent.[15] The online Appendix includes additional information about our methodology and a discussion of differences across plan types.[16]
Annual Spending Trends
Our first goal was to analyze trends in specialty pharmacy spending during the study period. To understand the relative contribution of specialty drugs to overall drug spending, we computed per beneficiary measures of specialty and total drug spending in the pharmacy benefit for all enrollees in all plan types in each year. To do so, we summed all expenditures for specialty drugs and for all drugs, respectively, and divided by total enrollment in all plan types.
Medicare rules allow drugs with a monthly negotiated price of more than $600 to be placed on a plan’s specialty tier, as noted above. However, there is wide variation across plans in terms of which drugs are actually placed on that tier.[17] Thus, for the purposes of this study, we categorized drugs as specialty or nonspecialty according to whether or not their National Drug Code appeared on a specialty drug claim for any enrollee in any prescription drug plan or Medicare Advantage plan in a given year. We took this approach, instead of defining specialty drugs as those placed on the specialty tier in the beneficiary’s plan, to avoid potential bias from beneficiaries selecting drug plans based on more generous coverage of specialty products that they are likely to use.
We computed annual specialty pharmacy spending per beneficiary among beneficiaries taking specialty drugs by dividing total specialty pharmacy expenditures by the number of beneficiaries who had a specialty pharmacy claim. We then calculated total annual out-of-pocket spending per beneficiary for specialty drug users and nonusers by summing all out-of-pocket expenditures for each patient group and dividing the result by the number of beneficiaries in that group. We excluded from this analysis beneficiaries who received a low-income subsidy because they have little or no cost sharing. We measured out-of-pocket spending on all drugs incurred by specialty drug users (not just spending on specialty drugs) because this reflects the beneficiaries’ true out-of-pocket burden for the year.
Finally, we assessed trends in the relative contribution of different drug classes to overall specialty drug spending among this population of elderly Medicare beneficiaries. For this analysis, we grouped National Drug Codes into drug classes and summed total spending on specialty drugs by therapeutic class and year. We then divided this class-level specialty drug spending by total enrollment in the year. We present results for drug classes that represent a substantial portion of the specialty drug spending, users, or both. All spending is presented in nominal dollars—that is, not adjusted for inflation.
Limitations
We used a nationally representative sample of Medicare beneficiaries. However, we did not adjust trends for potential changes in health status or demographic characteristics of enrollees that could affect specialty drug spending over time.
Furthermore, we defined drugs as specialty or nonspecialty in each year. Thus, our trends could include changes based on which drugs were defined as specialty over time.
Study Results
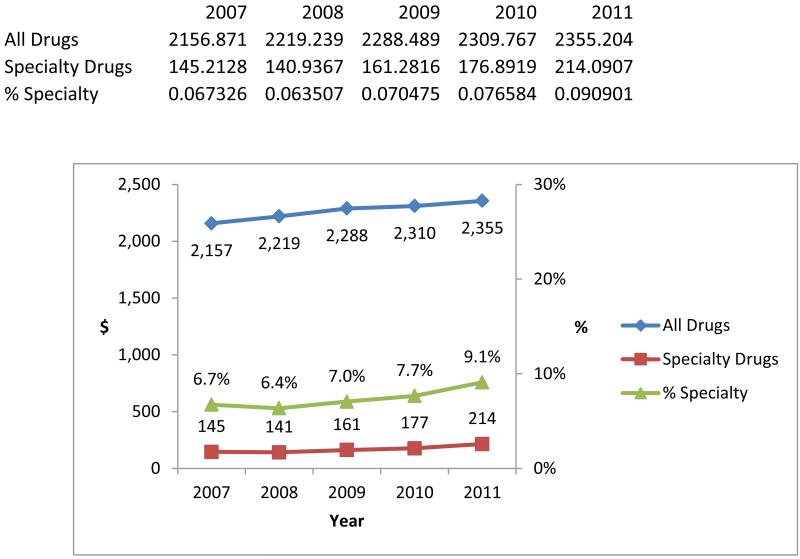
There was a 47 percent increase in per beneficiary spending on specialty drugs during the study period. Nonetheless, specialty drug spending remained a small portion of total drug spending in our sample. From 2007 to 2011, annual specialty drug spending per beneficiary increased from $145 to $214, whereas annual total drug spending per beneficiary increased from $2,157 to $2,355 (Exhibit 1). Thus, spending on specialty drugs increased from 6.7 percent to 9.1 percent of total drug spending.
Exhibit 1 (figure). Annual Per Beneficiary Pharmacy Spending On Specialty And All Drugs Among Elderly Medicare Beneficiaries, 2007–11.
Source/Notes: SOURCE Authors’ analysis of pharmacy claims from a 20 percent sample of Medicare beneficiaries ages sixty-five and older. NOTES Specialty drugs are defined in the text. The sample includes elderly beneficiaries enrolled in stand-alone prescription drug plans or in Medicare Advantage plans that had prescription drug coverage, whether or not they received a low-income subsidy. The red and blue lines depict spending (reported in nominal amounts—that is, not adjusted for inflation) and relate to the left-hand y axis. The green line depicts the percentage of total drug expenditures spent on specialty drugs for each year and relates to the right-hand y axis.
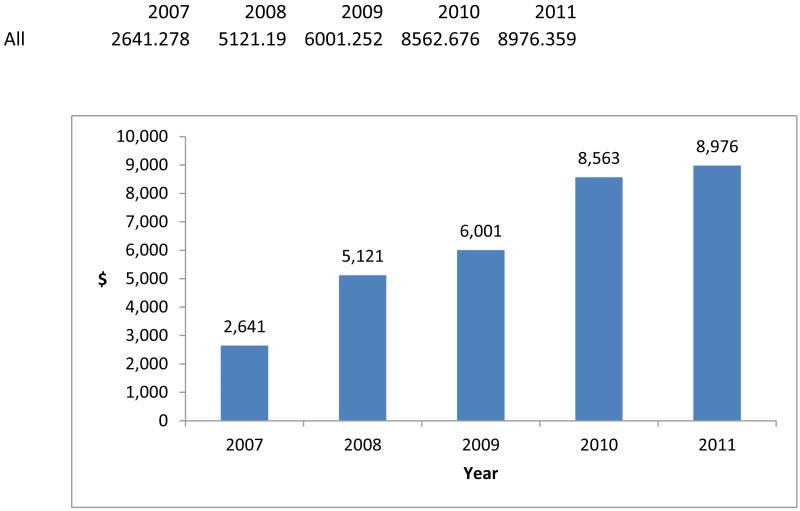
However, the use of specialty drugs was concentrated among a very small portion of beneficiaries, and spending on specialty drugs was considerable for these users. Annual specialty pharmacy spending per specialty drug user increased dramatically during the study period, from $2,641 to $8,976 (Exhibit 2).
Exhibit 2 (figure). Annual Specialty Pharmacy Spending Per Elderly Medicare Specialty Drug User, 2007–11.
Source/Notes: SOURCE Authors’ analysis of pharmacy claims from a 20 percent sample of Medicare beneficiaries ages sixty-five and older. NOTES Specialty drugs are defined in the text. The sample includes elderly beneficiaries who had a specialty drug claim and who were enrolled in stand-alone prescription drug plans or in Medicare Advantage plans that had prescription drug coverage, whether or not they received a low-income subsidy. All amounts are reported in nominal dollars (that is, not adjusted for inflation).
Part of the increase from 2007 to 2008 may be the result of health plans’ dropping comparatively low-price drugs from specialty tiers, since 5.5 percent of the beneficiaries in our sample took specialty drugs in 2007, compared to 2.8 percent in 2008.[18] This reduction in the percentage of users largely reflects the fact that in 2008 Medicare increased the monthly cost threshold at which drugs became eligible for placement on a specialty tier from $500 to $600.
As a sensitivity analysis, we used National Drug Codes to identify drugs that were defined as specialty in 2007 but that did not appear on specialty drug claims during the remainder of our study period. When we excluded beneficiaries taking these drugs from the count of specialty drug users in 2007, the percentage of beneficiaries taking specialty drugs fell to a level quite similar to that of 2008 and the remainder of the study period.[19] This pattern could also reflect changing practices in plans’ use of specialty tiers as they gained familiarity with the Part D program.
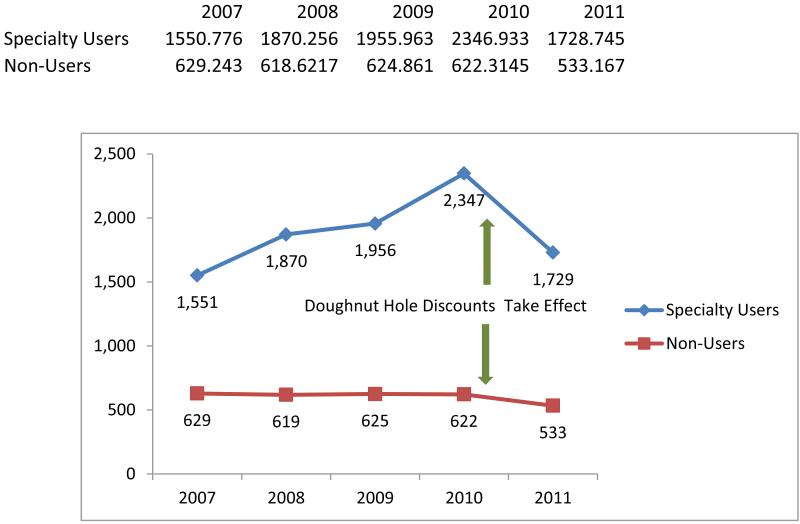
High spending on specialty drugs raises concerns about out-of-pocket burden. Specialty drug users often take many drugs and thus are vulnerable to high out-of-pocket expenses.[20] Exhibit 3 compares trends in annual out-of-pocket pharmacy expenditures per beneficiary for specialty drug users and nonusers. In this analysis we excluded beneficiaries who received a low-income subsidy because they have or no cost sharing.
Exhibit 3 (figure). Annual Per Beneficiary Out-of-Pocket Pharmacy Spending Among Specialty Drug Users And Nonusers, 2007–11.
Source/Notes: SOURCE Authors’ analysis of pharmacy claims from a 20 percent sample of Medicare beneficiaries ages sixty-five and older. NOTES Specialty drugs are defined in the text. The sample includes elderly beneficiaries enrolled in stand-alone prescription drug plans or in Medicare Advantage plans that had prescription drug coverage. Beneficiaries who received a low-income subsidy are excluded. All amounts are reported in nominal dollars (that is, not adjusted for inflation).
We found that the overall annual out-of-pocket burden among nonsubsidized beneficiaries was significantly higher for specialty drug users, compared to nonusers. Moreover, we found that the growth in out-of-pocket burden from 2007 through 2010 was concentrated among specialty drug users, whose annual out-of-pocket spending increased 51 percent—from $1,551 to $2,347 (Exhibit 3). In contrast, nonusers experienced only negligible changes.
However, we also found a 26 percent reduction in out-of-pocket specialty drug users in 2011, with the average annual figure falling $618 from 2010. Average annual out-of-pocket expenditures among nonusers fell by $89 during the same time period, a decline of 14 percent.
We attribute these reductions primarily to the implementation of the ACA provisions for cost-sharing reductions for drugs purchased by beneficiaries while they were in the doughnut hole. The contemporaneous reductions in out-of-pocket expenditures for users and nonusers of specialty drugs is consistent with the fact that the 50 percent manufacturers’ discounts apply to all brand-name drugs, not just specialty drugs. It may also reflect the initial 7 percent doughnut hole cost-sharing reductions for generic drugs that took effect in 2011.
For beneficiaries who received a low-income subsidy, we found no change in out-of-pocket spending during the same time period: It remained very low (for more details, see Appendix Exhibit 3b).[16]
Additionally, we found reductions in the percentage of specialty drug expenditures paid out of pocket over time, as well as a convergence in this percentage for beneficiaries across plan types. Between 2007 and 2011 the share of total specialty drug spending that was paid out of pocket declined from 25 percent to 12 percent for beneficiaries enrolled in prescription drug plans, and from 16 percent to 9 percent for Medicare Advantage enrollees (Appendix Exhibit 4).[16] This reduction occurred during the entire study period, even before the passage of the ACA in 2010. However, there was a sharper reduction from 2010 to 2011 compared to earlier year-over-year changes.
In addition to the doughnut hole cost-sharing subsidies, the ACA includes several other provisions that may have important effects on specialty drug spending. The ACA also likely reduced total and out-of-pocket specialty spending in 2011 through its expansion of the 340B prescription drug discount program. As part of this program, manufacturers give considerable discounts on drugs provided to qualifying patients who are treated by certain providers and at facilities that serve a high volume of Medicare or other high-need, low-income patients. [21]
Beginning in 2011, the ACA expanded program eligibility to include many more providers, including freestanding cancer clinics. We were unable to determine which specialty drugs were provided at 340B facilities or the extent to which the provision of specialty drugs at these facilities increased in 2011. Nonetheless, both total and per beneficiary spending on specialty drugs would likely have been higher in 2011 if this program had not been expanded. However, many beneficiaries who received care from these 340B facilities also received low-income subsidies and were therefore excluded from our calculations of out-of-pocket specialty drug spending shown in Exhibit 3.
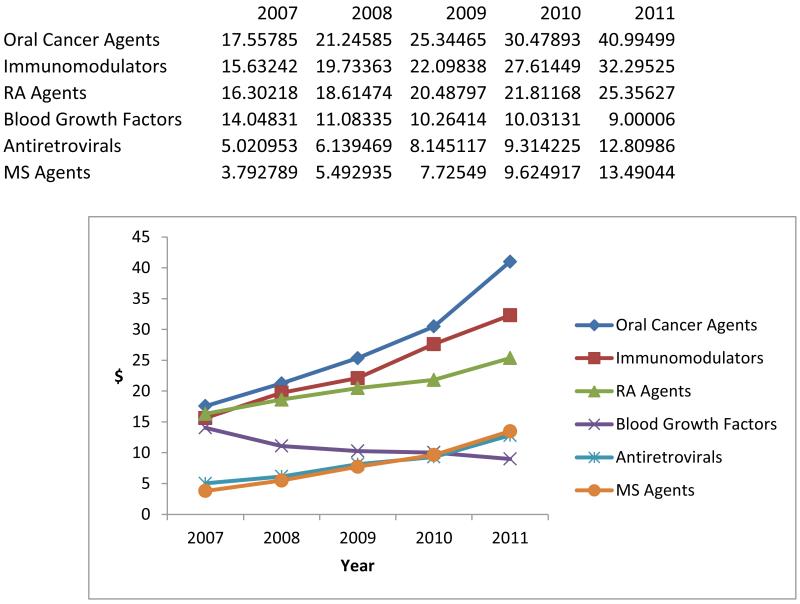
Finally, we assessed the relative contribution of different drug classes to overall growth in specialty pharmacy spending. Exhibit 4 displays trends in average annual expenditures per beneficiary on specialty drugs across six drug classes that represent substantial specialty drug spending, users, or both. We found that specialty drug spending per beneficiary was increasing in all but one of these drug classes. However, expenditures for oral cancer agents and immunomodulators were the primary contributors to overall increases in specialty drug spending in recent years.
Exhibit 4 (figure). Relative Contribution Of Major Specialty Drug Classes To Annual Specialty Pharmacy Spending Per Beneficiary, 2007–11.
Source/Notes: SOURCE Authors’ analysis of pharmacy claims from a 20 percent sample of Medicare beneficiaries ages sixty-five and older. NOTES Specialty drugs ardefined in the text. Drug classes are defined by linking National Drug Codes to drug classes via a crosswalk from the IMS Health drug database. The sample includes elderly beneficiaries enrolled in standalone prescription drug plans or in Medicare Advantage plans that had prescription drug coverage, whether or not they received a low-income subsidy. Immunomodulators are biologic drugs that are used to treat a number of inflammatory conditions, such as inflammatory bowel disease, Crohn’s disease, ulcerative colitis, and other immunebased diseases. RA is rheumatoid arthritis. MS is multiple sclerosis. All amounts are reported in nominal dollars (that is, not adjusted for inflation).
Oral cancer agents include pharmacy-based cancer treatments such as Gleevec and Tarceva. The finding that oral cancer agents contributed significantly to specialty drug spending growth in recent years may suggest that at least part of the observed increase in specialty drug spending is because of a shift in specialty drug coverage from the medical to the pharmacy benefit, resulting from the availability of new pharmacy-based treatment options.
Discussion
In light of the recent attention focused on growth in spending on specialty pharmaceuticals, many payers have contended that covering high-cost specialty drugs will render insurance premiums unaffordable.[22] Moreover, some have noted their inability to effectively negotiate prices for many specialty drugs because of the lack of competing alternative treatments and have declared their intentions to exclude new high-cost specialty drugs from coverage.[23,24]
We found evidence supporting the claim that spending among specialty drug users has rapidly increased in recent years. However, many of these drugs offer considerable therapeutic value to patients and represent significant improvements over alternative treatment options.[25] Moreover, we found that specialty drug spending constituted a small portion of total prescription drug spending (let alone of combined medical and pharmacy spending) at the per beneficiary level.
Thus, we believe that payers should use formularies and other tactics to negotiate the best possible drug prices that they can, but excluding specialty drugs from coverage would harm the small population of beneficiaries who could benefit substantially from their use. In addition, excluding specialty drugs from coverage to be at odds with one of the goals of insurance: to spread the risk of low-probability but high-cost health events over a large pool of people. Nonetheless, the relative contribution of specialty drugs to overall spending per beneficiary should be monitored with the release of new high-price specialty drugs that treat more prevalent diseases, compared to earlier specialty drugs that were less expensive or targeted diseases that affected a very small number of patients.
Another concern related to the high cost of specialty drugs has been the high out-of-pocket burden faced by Medicare beneficiaries who take the drugs. Previous work has shown that the demand for specialty drugs is relatively inelastic.[26-28] However, the use of specialty tiers with high cost sharing has been pervasive among Medicare plans.[29]
We found that out-of-pocket expenditures were considerably higher and increased much more rapidly from 2007 through 2010 for specialty drug users, compared to nonusers. However, the implementation of doughnut hole cost-sharing reductions in 2011 significantly reduced out-of-pocket burden among specialty drug users. These reductions will continue to grow for both brand-name and generic drugs through 2020, at which point all drugs purchased while beneficiaries are in the hole will be discounted by 75 percent.
The reductions should help alleviate the significant out-of-pocket specialty drug users, as well as by nonusers who enter the doughnut hole. The extent which these discounts may affect the growth in prices and the use of specialty and nonspecialty drugs is an important question for future research.
Finally, we found that increasing spending on oral cancer agents accounted for considerable portion of the increase in specialty drug spending in recent years. This may indicate that at least part of the growth in specialty pharmacy spending is a result of a shift in specialty drug coverage from the medical to the pharmacy benefit.[30] This trend likely reflects recent innovations in specialty drugs (such as oral cancer agents) that allow patients to administer the drugs themselves instead of having to visit a provider for an injection or infusion.
We were not able to analyze the extent to which such increases in specialty pharmacy spending were associated with reductions or slower growth in specialty medical spending, but that is an important issue for future work. A shift in coverage of specialty drugs from the medical to the pharmacy benefit could have important implications for Medicare beneficiaries: They may face higher cost sharing under the Part D benefit than under the Part B benefit, especially if they have supplementary coverage for the latter.
In addition, since 2011 the ACA has required that Medicare Advantage plans include out-of-pocket maximum limits, so that enrollees pay no more than $6,700 out of pocket in a given year.[31] However, these limits apply only to services covered under the Parts A and B benefits, not to pharmacy (Part D) spending. Thus, these provisions provide limited financial protection for beneficiaries who take specialty drugs that are covered under the pharmacy benefit.
Other recent Medicare trends may also have important implications for specialty drug spending. Enrollment in Medicare Advantage plans has increased considerably in recent years.[32] Compared to stand-alone prescription drug plans that have no liability for their members’ medical spending, these plans may have different incentives to cover specialty drugs because they are financially responsible for both medical and pharmacy spending. They may also be better situated to integrate specialty drug utilization management across the full spectrum of care. This may affect care patterns for a growing number of beneficiaries who enroll in Medicare Advantage plans.
Other Medicare payment policies may also have important implications related to the shifting coverage of specialty drug spending from Part B to Part D. For example, Part D spending is excluded from the costs for which accountable care organizations (ACOs) must manage spending growth. Therefore, providers that participate in ACOs may face some incentives to shift specialty drug spending from Part B to Part D whenever possible. Additionally, changes affecting physician reimbursement for physician-administered drugs may have effects on provider behavior and on both the types of drugs provided and the settings where provision occurs.[33]
Conclusion
Specialty drug spending per user has increased considerably in recent years. However, this spending represents a small portion of overall drug spending on a per beneficiary basis. Moreover, the implementation of ACA-based doughnut hole cost-sharing reductions has resulted in considerable decreases in out-of-pocket expenditures among specialty drug users. Specialty drugs o1ffer considerable value to a small group of elderly Medicare beneficiaries who have complex conditions. Limiting coverage of these drugs may render treatment options unavailable to these seniors, who stand to gain significant clinical benefit from their use.
Acknowledgments
The research reported in this article was supported by the National Institutes of Health (Grant Nos. P01AG033559 and P30AG024968). Erin Trish also acknowledges support from the Agency for Healthcare Research and Quality (under Grant No. T32HS00046). Dana Goldman is a partner at Precision health Economics, a company providing consulting services to the lifesciences industry. The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health or the Agency for Healthcare Research and Quality.
The authors are grateful to Laura Gascue for expert programming and to the editors and reviewers for helpful comments.
Biographies
Bios for 2014-0538_Trish
Bio 1: Erin Trish is a postdoctoral fellow at the Schaeffer Center for Health Policy and Economics, University of Southern California, and in the Department of Health Policy and Management at the Fielding School of Public Health, University of California, Los Angeles.
Bio 2: Geoffrey Joyce (gjoyce@healthpolicy.usc.edu) is director of health policy at the Schaeffer Center for Health Policy and Economics and an associate professor in the Department of Pharmaceutical Economics and Policy, both at the University of Southern California.
Bio 3: Dana P. Goldman is the Leonard D. Schaeffer Chair and director of the Schaeffer Center for Health Policy and Economics at the University of Southern California.
Contributor Information
Erin Trish, Schaeffer Center for Health Policy and Economics, University of Southern California, and in the Department of Health Policy and Management at the Fielding School of Public Health, University of California, Los Angeles.
Geoffrey Joyce, Schaeffer Center for Health Policy and Economics and an associate professor in the Department of Pharmaceutical Economics and Policy, both at the University of Southern California.
Dana P. Goldman, Schaeffer Center for Health Policy and Economics at the University of Southern California.
Notes
- 1.IMS Institute for Healthcare Informatics . Medicine use and shifting costs of healthcare: a review of the use of medicines in the United States in 2013 [Internet] IMS; Parsippany (NJ): Apr, 2014. [cited 2014 Sep 25]. Available from: http://www.imshealth.com/cds/imshealth/Global/Content/Corporate/IMS%20Health%20Institute/Reports/Secure/IIHI_US_Use_of_Meds_for_2013.pdf. [Google Scholar]
- 2.Thomas K. Prices soaring for specialty drugs, researchers find. New York Times. 2014 Apr 15; [Google Scholar]
- 3.Editorial Board Why $1,000 a pill? Our view. USA Today. 2014 Jul 16; [Google Scholar]
- 4.Wyden R, Grassley CE. Letter to Dr. John C. Martin [Internet] Senate Committee on Finance; Washington (DC): Jul 11, 2014. [cited 2014 Sep 25]. Available from: http://www.finance.senate.gov/imo/media/doc/Wyden-Grassley%20Document%20Request%20to%20Gilead%207-11-141.pdf. [Google Scholar]
- 5.Kliff S. Each of these hepatitis C pills cost $1,000. That’s actually a great deal. Vox [serial on the Internet] [updated 2014 Jul 16; cited 2014 Sep 25]. Available from: http://www.vox.com/2014/7/16/5902271/hepatitis-c-drug-sovaldi-price.
- 6.Saab S. Hepatitis C drug worth the price: opposing view. USA Today. 2014 Jul 16; [Google Scholar]
- 7.Spatz I, McGee N. Health Policy Brief: Specialty pharmaceuticals. Health Affairs [serial on the Internet] 2013 Nov 25; [cited 2014 Sep 21]. Available from: http://www.healthaffairs.org/healthpolicybriefs/brief.php?brief_id=103.
- 8.Express Scripts . The 2013 drug trend report [Internet] Express Scripts Lab; St. Louis (MO): Apr, 2014. [cited 2014 Sep 25]. Available from: http://lab.express-scripts.com/~/media/pdfs/drug%20trend%20report/express%20scripts%202013%20drug%20trend%20report.ashx. [Google Scholar]
- 9.CVS Caremark . Specialty trend management: where to go next [Internet] CVS Health Research Institute; Woonsocket (RI): 2013. [cited 2014 Sep 25]. Available from: http://info.cvscaremark.com/sites/default/files/Insights%202013.pdf. [Google Scholar]
- 10.America’s Health Insurance Plans . Specialty drugs—issues and challenges. AHIP; Washington (DC): Feb, 2014. [cited 2014 Sep 25]. (Issue Brief). Available from: http://www.ahipcoverage.com/wp-content/uploads/2014/02/Specialty-Drugs-Issue-Brief.pdf. [Google Scholar]
- 11.The definition used an amount of $500 in 2007.
- 12.Government Accountability Office . Medicare Part D: spending, beneficiary cost sharing, and cost-containment efforts for high-cost drugs eligible for a specialty tier [Internet] GAO; Washington (DC): Jan, 2010. [cited 2014 Spe 25]. Available from: http://www.gao.gov/assets/310/300528.pdf. [Google Scholar]
- 13.We refer to these as “Medicare Advantage” plans throughout the remainder of this article, although not all Medicare Advantage plans provide prescription drug coverage. We restrict our analyses to those beneficiaries who have prescription drug coverage through their Medicare Advantage plan.
- 14.We excluded nonelderly Medicare beneficiaries, such as people who qualify because of disability.
- 15.This group included all elderly beneficiaries who received a low-income subsidy, whether it was full or partial.
- 16.To access the Appendix, click on the Appendix link in the box to the right of the article online.
- 17.Hargrave E, Hoadley J, Merrell K. Drugs on specialty tiers in Part D [Internet] NORC at the University of Chicago; Bethesda (MD): Feb, 2009. [cited 2014 Sep 29]. Available from: http://permanent.access.gpo.gov/LPS112283/LPS112283_Feb09_DrugsonSpecialtyTiers_CONTRACTOR_RS.pdf. [Google Scholar]
- 18.The percentage of beneficiaries in our sample who took specialty drugs was 2.7 percent in 2009, 2.1 percent in 2010, and 2.4 percent in 2011.
- 19.Additionally, we found that, according to our analysis of National Drug Codes, a considerably higher proportion of drugs changed category from specialty to non-specialty between 2007 and 2008 compared to year-over-year changes during the remainder of the study period. Fifty-one percent of beneficiaries who took specialty drugs in 2007 (a group who accounted for 15 percent of specialty drug spending) were taking drugs that were no longer defined as specialty in 2008. In contrast, during the remainder of the study period only 3.2–4.8 percent of beneficiaries (accounting for 0.5–1.1 percent of specialty drug spending) who took specialty drugs in a given year were taking drugs that were no longer defined as specialty in the following year. Moreover, we found that the average thirty-day price (weighted by use) of the drugs that were classified as specialty in 2007 but not in 2008 was about one-third of the overall weighted average thirty-day price of all drugs defined as specialty in 2007. This indicates that the relatively low-priced drugs were more likely to be dropped from specialty tiers.
- 20.Medicare Payment Advisory Committee . Status Report on Part D, with focus on beneficiaries with high drug spending [Internet] MedPAC; Washington (DC): Mar, 2012. [cited 2014 Sep 26]. Available from: http://www.medpac.gov/documents/reports/mar12_ch13.pdf?sfvrsn=0. [Google Scholar]
- 21.More information on the history of and recent changes to the 340B program is available at: Wynne B. The coming storm over the 340B Rx drug discount program. Health Affairs Blog [blog on the Internet] 2014 May 6; [cited 2014 Sep 26]. Available from: http://healthaffairs.org/blog/2014/05/06/the-coming-storm-over-the-340b-rx-drug-discount-program/
- 22.Express Scripts . State governments may spend $55 billion on hepatitis C medications [Internet] Express Scripts Lab; St. Louis (MO): Jul 17, 2014. [cited 2014 Sep 26]. Available from: http://lab.express-scripts.com/insights/specialty-medications/state-governments-may-spend-%2455-billion-on-hepatitis-c-medications. [Google Scholar]
- 23.Pollack A. Health insurers pressing down on drug prices. New York Times. 2014 Jun 20; [Google Scholar]
- 24.Budnick N. Facing a $168-million price tag for new hepatitis C drugs, Oregon Health Plan balks. Oregonian. 2014 Jun 11; [Google Scholar]
- 25.Fendrick AM, Buxbaum J, Westrich K. Supporting consumer access to specialty medications through value-based insurance design [Internet] University of Michigan Center for Value-Based Insurance Design; Ann Arbor (MI): [cited 2014 Sep 26]. Available from: http://www.sph.umich.edu/vbidcenter/publications/pdfs/2014-vbid-specialty-medications-npc-final-web.pdf. [Google Scholar]
- 26.Goldman DP, Joyce GF, Lawless G, Crown WH, Willey V. Benefit design and specialty drug use. Health Aff (Millwood) 2006;25(5):1319–31. doi: 10.1377/hlthaff.25.5.1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Goldman DP, Jena AB, Lakdawalla DN, Malin JL, Malkin JD, Sun E. The value of specialty oncology drugs. Health Serv Res. 2010;45(1):115–32. doi: 10.1111/j.1475-6773.2009.01059.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Karaca-Mandic P, Joyce GF, Goldman DP, Laouri M. Cost sharing, family health care burden, and the use of specialty drugs for rheumatoid arthritis. Health Serv Res. 2010;45(5 Pt 1):1227–50. doi: 10.1111/j.1475-6773.2010.01117.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Medicare Payment Advisory Committee . Status report on Part D [Internet] MedPAC; Washington (DC): Mar, 2014. [cited 2014 Sep 26]. Available from: http://www.medpac.gov/documents/reports/mar14_ch14.pdf?sfvrsn=0. [Google Scholar]
- 30.Theodorou AA, Palmieri A, Szychowski JA, Sehman ML, Swarna V. Oral oncology: utilization of selected oral antineoplastic enzyme inhibitor agents. Am J Pharm Benefits. 2012;4(4):178–82. [Google Scholar]
- 31.Traditional Medicare has no such limit. However, traditional Medicare beneficiaries may have some sort of annual out-of-pocket maximum if they purchase supplemental coverage such as a Medigap policy.
- 32.Kaiser Family Foundation . Medicare Advantage [Internet] KFF; Menlo Park (CA): May, 2014. [cited 2014 Sep 26]. (Fact Sheet). Available from: http://kaiserfamilyfoundation.files.wordpress.com/2014/05/2052-18-medicare-advantage.pdf. [Google Scholar]
- 33.Jacobson M, Earle CC, Price M, Newhouse JP. How Medicare’s payment cuts for cancer chemotherapy drugs changed patterns of treatment. Health Aff (Millwood) 2010;29(7):1391–9. doi: 10.1377/hlthaff.2009.0563. [DOI] [PubMed] [Google Scholar]