Abstract
Objective
To understand the prevalence and demographic characteristics of infectious keratitis and infectious corneal blindness.
Methods
A multi-center, population-based cross-sectional study was conducted from January 1 to August 31, 2010. A total of 191,242 individuals of all age groups from 10 geographically representative provinces were sampled using stratified, multi-stage, random and systematic sampling procedures. A majority, 168,673 (88.2%), of those sampled participated in the study. The examination protocol included a structured interview, visual acuity testing, an external eye examination, and an anterior segment examination using a slit lamp. The causes and sequelae of corneal disease were identified using uniform customized protocols. Blindness in one eye caused by infectious keratitis was defined as infectious corneal blindness.
Results
The prevalence of past and active infectious keratitis was 0.192% (95% confidence interval [CI], 0.171–0.213%), and the prevalence of viral, bacterial, and fungal keratitis was 0.11%, 0.075%, and 0.007%, respectively. There were 138 cases of infectious corneal blindness in at least one eye in the study population (prevalence of 0.082% [95%CI, 0.068%–0.095%]). Statistical analysis suggested that ocular trauma, alcoholic consumption, low socioeconomic levels, advanced age, and poor education were risk factors for infectious corneal blindness.
Conclusions
Infectious keratitis is the leading cause of corneal blindness in China. Eye care strategies should focus on the prevention and rehabilitation of infectious corneal blindness.
Introduction
The World Health Organization (WHO) reported in 2001 that corneal disease, as a major cause of blindness, ranks second only to cataracts worldwide [1]. In recent decades, following the implementation of various programs initiated by the WHO, rates of corneal diseases attributable to Chlamydia trachomatis, onchocerciasis, and leprosy have improved. Currently, infectious keratitis is mainly caused by viruses, fungi, bacteria, and Acanthamoeba. In developing countries, most patients with infectious keratitis have limited access to medical care. In addition, the lack of effective drugs, essential operating equipment, and well-trained medical care personnel, together with the lack of legislative guarantee and the shortage of corneal grafts, results in severe outcomes. It is estimated that trauma and corneal ulcers are responsible for 1.5–2.0 million new cases of corneal blindness every year, and this type of blindness has been recognized as a “silent epidemic” [2].
The First China National Sample Survey on Disability showed that cataracts and corneal diseases were the top two causes of blindness and that the prevalence of corneal blindness and low vision was 21/10,000. The survey also suggested that corneal blindness accounted for approximately 1/4 of blindness cases in China, with infectious keratitis being the major cause of corneal blindness [3]. Over the past two decades, following the rapid growth of the Chinese economy, great success in disease control has been achieved. The Chinese government and charity organizations launched many sight-restoring projects that focused on blinding eye diseases, but the prevention of corneal blindness has received less attention. To achieve the goal of the “VISION 2020-The Right to Sight” initiative, which was initiated by the WHO, the prevention of corneal blindness should receive more attention in China.
The prevention of infectious corneal blindness is important for the national control of corneal blindness. It is known that disease control strategies depend on epidemiological data. However, few national epidemiological surveys pertaining to infectious keratitis have been conducted in China. Funded by grants from the Chinese Academy of Engineering, the Shandong Eye Institute began an Epidemiological Study of Infectious Keratitis in China. This was the first national, multi-center, epidemiological survey of infectious keratitis. The aim of the study was to investigate the epidemiological data on infectious keratitis in China for the purpose of providing evidence to encourage the central and provincial governments to develop intervention strategies.
Materials and Methods
Study design
As there are currently no studies that report the prevalence of infectious corneal disease in China, a pre-survey was conducted in urban areas of Beijing and rural areas of Shandong and revealed an estimated prevalence of infectious corneal disease of approximately 0.4%. The sample size was calculated using the formula: n = Z2p(1-p)/B2. For p = 0.004, B = 0.1p, and Z0.05/2 = 1.96, the sample size should be approximately 100000, and the response rate must be greater than 80%. Therefore, considering sample loss, the sample size must be at least 120,000. According to the results from the Fifth National Population Census in 2000, there is a total population of 0.44 billion in the 10 provinces (municipalities or autonomous regions), and the sampling fraction is approximately 3/10000.
Multi-stage stratified cluster random sampling was adopted in the present study. In stage one, ten provinces in mainland China that represented different levels of socioeconomic development within the 31 provinces (municipalities or autonomous regions) were selected based on a list of provinces in each region and on computer-generated random numbers: 2 provinces (Shandong and Guangdong) and 2 municipalities (Beijing and Shanghai) were in the developed east coast region; 2 provinces (Hubei and Heilongjiang) were in the inland middle region; and 4 provinces (Sichuan, Yunnan, Qinghai, and Ningxia) were in the undeveloped west region (Fig. 1). The annual per capita consumption of urban residents in the 10 study areas ranged from ¥5,426 in Ningxia to ¥16,457 in Shanghai and from ¥1,404 in Ningxia to ¥7,516 in Shanghai among rural residents; the sampled study provinces were socioeconomically diverse [4]. In stage two, one district and one county were sampled within each province, and a subdistrict in the district and a township in the county were then selected. In stage three, communities or villages were randomly sampled from the subdistrict or townships. Following the methods in the published literature [5], the sampling frame was formed by geographically defined clusters based on community or village register data. Each cluster had a population of approximately 1,000 individuals (all ages). All clusters were then numbered and sorted, and simple random sampling was adopted to sample the clusters.
Figure 1. Distribution of the 10 provinces (municipalities and autonomous regions) in this study.
Operating procedure
The field survey was carried out during the period from January 1 through August 31, 2010. The survey groups checked and confirmed the demographic data including names, genders, ages, and education levels registered by the communities or villages from house to house. Those who were not registered but who had lived in the clusters for more than 6 months were also included in the current survey.
The examinations were performed in special sites in the communities or villages, and the survey group was composed of pretrained doctors, nurses, and staff. All members of the survey group were trained prior to the investigation, including training on the standard survey procedure, visual examinations, the diagnostic standards for corneal disease, and a questionnaire. Guardians of the participants less than 18 years of age approved the survey on their behalf. Individuals who did not come to the examinations were either revisited and encouraged to participate in the study or they were examined at their homes using portable equipment, including a handheld slit lamp. The response rate was maintained at more than 80% in each cluster.
The field survey was conducted in two phases. In the first phase, presenting visual acuity was measured using the standard logarithmic visual acuity chart. For infants, colored toys were utilized. If there was no obvious abnormality in the eyes and the eyes could rotate with the movement of the toys, the baby was considered to not be blind. Those with visual disturbances, i.e., those whose eyes could not rotate with the movement of the toys, were diagnosed as having visual impairment. A slit lamp was used to examine the anterior segments of the eyes. Cases with corneal ulceration, infiltration, edema, scarring, opacity, vascularization, degeneration, pterygium, corneal transplantation, and foreign objects (arcus senilis was excluded) in the eyes or anophthalmos caused by corneal diseases were forwarded to the second-stage examinations.
In the second stage, the epidemiological questionnaire was completed, the anterior segments of the eyes were photographed with a digital camera or anterior segment camera, and the fundus was also examined. The epidemiological questionnaire contained queries regarding demographics, habits, and customs(smoking and alcoholicbeverage consumption), ocular trauma, and medical history, including ocular or systemic diseases. In the case of minors, the epidemiological questionnaires were completed by their guardians. When the corneal stromal infiltration (regardless of whether there was an epithelial defect) or the corneal ulcer was more than 1 mm2 (regardless of whether there was hypopyon), additional corneal smears and cultures were performed to make a definitive diagnosis [6].
The survey information and diagnostic results of each subject in the second phase were input into predesigned medical records. After the field investigation, each cooperation center checked the survey results to ensure the integrity and accuracy of the data and then logged in to the website created by the Shandong Eye Institute to upload files regarding the survey.
This study was approved by the ethics committees of the Shandong Eye Institute, the Zhongshan Ophthalmic Center of Sun Yat-sen University, the Renmin Hospital of Wuhan University, the First Affiliated Hospital of Kunming Medical College, the West China Eye Center of Sichuan University, and the First Clinical College of Harbin Medical University, and it complied with the Declaration of Helsinki. Informed consent was obtained from all participants following a detailed description of the purpose and potential benefits of the study prior to the examinations. Written consent was preferred, but if the participants were illiterate, verbal consent was recorded by our staff members. The ethics committee approved this consent procedure.
Definition and criteria
Smoking and alcoholic beverage consumption
Smokers were defined as those who had smoked 100 cigarettes and now smoked either every day or some days. Alcoholic beverage consumption was defined as the consumption of more than one drink per day for women and more than two drinks per day for men. One drink was roughly equivalent to 300 ml beer, 150 ml wine, or 50 ml spirits.
Blindness and low vision
The WHO defines visual impairment as acuity less than 20/63, including blindness (visual acuity less than 20/400) and low vision (visual acuity worse than 20/63 but no less than 20/400). Considering that it was difficult to use the available methods and technologies to conduct the visual field tests due to the large number of participants in rural areas, the visual field test was not included in the present study.
Corneal blindness
Visual acuity less than 20/400 in one eye caused by corneal disease is defined as corneal blindness. A presumptive diagnosis was made by the professional doctors in the field based on medical history and examination results. The diagnosis and causes of corneal blindness were validated by specialists in the survey group based on the images and the survey data.
Diagnostic criteria of infectious keratitis
Active infectious keratopathy was diagnosed as follows: (1) bacterial, fungal, and Acanthamoeba keratitis were confirmed by etiological results, and (2) viral keratitis was diagnosed based on recurrent history, characteristic corneal lesions [7], and the clinical criteria for stromal herpetic keratitis [8].
Sequelae of past infectious keratitis
In cases with corneal opacity, scar, or anophthalmos, if the medical history or medical records demonstrated that the sequelae had been caused by bacterial, viral, fungal, Acanthamoeba, or other infectious keratitis, the diagnosis of sequelae of past infectious keratitis was made. The prevalence of corneal diseases (%) was calculated as follows: individuals with a history of corneal diseases plus new emerging cases/the subjects examined ×100%.
Statistical analysis
All data were entered into Excel (Microsoft Corporation; Redmond, WA, USA), and all statistical analyses were performed using the Statistical Package for the Social Sciences Version 17.0 (SPSS 17.0, SPSS Inc.; Chicago, IL, USA). Differences in prevalence were tested for statistical significance with the chi-square test, and those variables which were significant through univariate analysis were included into multivariate analysis. Multivariate analysis was conducted with binary logistic regression analysis. A P-value <0.05 was considered significant.
Results
A total of 191,242 subjects were recruited from 10 provinces (municipalities or autonomous regions), and 168,673 individuals completed the investigation, corresponding to a response rate of 88.2%. The response rates ranged from 80.5% (Ningxia) to 96.8% (Hubei) in rural areas and from 80.0% (Yunnan) to 95.7% (Hubei) in urban areas. There was no significant difference in the characteristics between the individuals who completed and did not complete the survey based on the statistical analysis. Males and females accounted for 48.4% and 51.6% of the subjects examined, respectively; the ages ranged from 0 to 110 years, with a mean age of 40.5±20.5 years. The education levels were as follows: illiterate, 11.6%; primary school, 29.0%; middle school, 43.7%; and university and higher, 15.6%.
Based on the WHO definition of visual impairment, a total of 6,579 individuals were visually impaired; that is, they presented with a visual acuity of <20/63, with a visual impairment prevalence of 3.99% (95% CI: 3.89%–4.08%). Of the total cases with visual impairment, 525 had blindness (PVA <20/400), with a prevalence of 0.32% (95% CI: 0.29%–0.35%), and 6,054 had low vision (PVA ≥20/400, <20/63), with a prevalence of 3.67% (95% CI: 3.58%–3.76%).
There were 4,204 cases of corneal disease sequelae, with a prevalence of 2.49% (95% CI: 2.42%–2.57%). Corneal diseases were found more frequently among females (χ2 = 97.63, p<0.001), the rural population (χ2 = 337.87, p<0.001), and subjects with lower education levels (χ2 = 337.87, p<0.001). Subjects with advanced age were also found to have a higher prevalence of corneal disease (χ2 = 4046.85, p<0.001). The disease prevalence was higher in the eastern and western provinces than in the central regions (χ2 = 626.59, p<0.001) (Table 1). The major corneal diseases were pterygium (3,158 cases, 75.1%), infectious corneal disease (324 cases, 7.7%), and traumatic scarring (147 cases, 3.5%).
Table 1. Univariate analysis of the prevalence of corneal diseases.
| No. of participants | No. with corneal diseases (prevalence %, 95%CI) | χ2 | P | |
| Gender | 168673 | 97.63 | <0.001* | |
| Male | 81564 | 1720 (2.11, 2.01–2.21) | ||
| Female | 87109 | 2484 (2.86, 2.75–2.97) | ||
| Age | 168651 (No data for 22 person) | 4046.99 | <0.001* | |
| 0–14 | 20245 | 20 (0.10, 0.06–0.14) | ||
| 15–59 | 116245 | 1811 (1.56, 1.49–1.63) | ||
| 60 and over | 32161 | 2373 (7.38, 7.09–7.66) | ||
| Education level | 168607 (No data for 66 person) | 2456.85 | <0.001* | |
| Illiterate | 19563 | 1388 (7.09, 6.73–7.45) | ||
| Primary school | 48989 | 1544 (3.16, 3.00–3.31) | ||
| Middle school | 73777 | 1094 (1.49, 1.40–1.57) | ||
| University | 13467 | 115 (0.85, 0.70–1.01) | ||
| Higher | 12811 | 63 (0.49, 0.37–0.61) | ||
| Rural vs. urban | 168673 | 337.87 | <0.001* | |
| Urban | 74902 | 1282 (1.71, 1.62–1.80) | ||
| Rural | 93771 | 2922 (3.12, 3.00–3.23) | ||
| Socioeconomic level | 168673 | 626.59 | <0.001* | |
| Eastern area | 82075 | 2417 (2.95, 2.83–3.06) | ||
| Central area | 39852 | 316 (0.79, 0.71–0.88) | ||
| Western area | 46746 | 1471 (3.15, 2.99–3.31) | ||
* statistically significant difference.
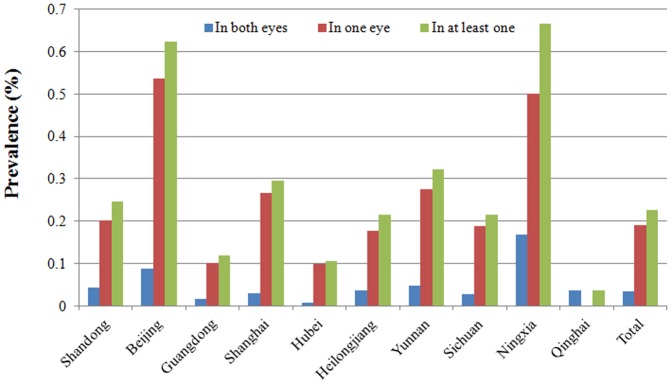
The prevalence of blindness in at least one eye caused by corneal disease was 0.23% (95% CI: 0.20%–0.25%). Infectious corneal disease ranked first among causes of corneal blindness, accounting for 36.4% of cases. Of the 379 cases of corneal blindness, 20 cases (5.3%) had no light perception, or anophthalmos.
The prevalence of corneal blindness increased with age (χ2 = 739.7, p<0.001). Furthermore, corneal blindness had a female preponderance (χ2 = 5.25, p = 0.02) and was more common in the rural populations (χ2 = 60.74, p<0.001). The subjects with lower education levels had a higher prevalence of the condition (χ2 = 416.79, p<0.001) (Table 2). There was a significant difference in the prevalence of blindness among the eastern, central, and western areas (χ2 = 19.93, p<0.001), with the highest prevalence found in the western areas (Fig. 2).
Table 2. Demographic characteristics and the prevalence of corneal blindness.
| Demographic distribution | No. of participants | Blindness in both eyes No. (%) | Blindness in one eye No. (%) | Total No. (%) |
| Gender a | 168673 | |||
| Male | 81564 | 22 (0.027) | 139 (0.170) | 161 (0.197) |
| Female | 87109 | 35 (0.040) | 183 (0.210) | 218 (0.250) |
| Age b | 168651 (No data for 22 persons) | |||
| ≤14 | 20245 | 0 | 0 | 0 |
| 15–59 | 116245 | 9 (0.008) | 70 (0.060) | 79 (0.068) |
| ≥60 | 32161 | 48 (0.149) | 252 (0.784) | 300 (0.933) |
| Education c | 168607 (No data for 66 persons) | |||
| Illiterate | 19563 | 29 (0.148) | 132 (0.675) | 161 (0.823) |
| Primary school | 48989 | 22 (0.045) | 117 (0.239) | 139(0.284) |
| Middle school | 73777 | 6 (0.008) | 62 (0.084) | 68(0.092) |
| University and higher | 26278 | 0 | 11 (0.042) | 11(0.042) |
| Rural vs. urban d | 168673 | |||
| Urban | 74902 | 12 (0.016) | 81 (0.108) | 93(0.124) |
| Rural | 93771 | 45 (0.048) | 241 (0.257) | 286(0.305) |
| Total | 168673 | 57 (0.034) | 322 (0.191) | 379(0.225) |
Comparisons between males and females. Blindness in both eyes, χ2 = 2.18, p = 0.14; blindness in one eye, χ2 = 3.48, p = 0.06; blindness in at least one eye(total), χ2 = 5.25, p = 0.02 (statistically significant difference).
Comparisons between different ages. Blindness in both eyes, χ2 = 131.39, p<0.001 (statistically significant difference); blindness in one eye, χ2 = 608.80, p<0.001 (statistically significant difference); blindness in at least one eye(total), χ2 = 739.70, p <0.001 (statistically significant difference).
Comparisons between different education levels. Blindness in both eyes, χ2 = 100.87, p<0.001 (statistically significant difference); blindness in one eye, χ2 = 321.00, p<0.001 (statistically significant difference); blindness in at least one eye(total), χ2 = 416.79, p≤0.001 (statistically significant difference).
Comparisons between rural and urban areas. Blindness in both eyes, χ2 = 12.60, p<0.001 (statistically significant difference); blindness in one eye, χ2 = 48.43, p<0.001 (statistically significant difference); blindness in at least one eye(total), χ2 = 60.74, p<0.001 (statistically significant difference).
Figure 2. Geographic distribution of the prevalence of corneal blindness.
Prevalence and characteristics of infectious keratitis and infectious corneal blindness
There were 324 subjects with sequelae of infectious keratitis and active ulcers, and the prevalences of infectious keratitis and herpes simplex keratitis were 0.19% (95% CI: 0.17%–0.21%) and 0.11%, respectively. Infectious keratitis was more commonly found in females (χ2 = 10.18, p<0.001), elderly subjects (15 years or older) (χ2 = 495.53, p<0.001), and those who had received less education (χ2 = 112.93, p<0.001). However, there were no significant differences found in the prevalence between urban and rural areas (χ2 = 0.43, p = 0.51) or between various economic development regions (χ2 = 5.50, p = 0.06) (Table 3). The prevalence of blindness in at least one eye caused by infectious keratitis was 0.08% (95% CI: 0.07%–0.10%).
Table 3. Univariate analysis of the prevalence of infectious corneal diseases.
| No. of participants | Number with infectious corneal diseases (prevalence %, 95%CI) | χ2 P | ||
| Gender | 168673 | 10.18<0.001* | ||
| Male | 81564 | 128 (0.16,0.13–0.18) | ||
| Female | 87109 | 196 (0.23,0.19–0.26) | ||
| Age | 168651 (No data for 22 person) | 495.53<0.001* | ||
| 0–14 | 20245 | 1 (0.005,0.00–0.01) | ||
| 15–59 | 116245 | 105 (0.09,0.07–0.11) | ||
| 60 and over | 32161 | 218 (0.68,0.57–0.77) | ||
| Education | 168607 (No data for 66 person) | 112.93<0.001* | ||
| Illiterate | 19563 | 95 (0.49,0.39–0.58) | ||
| Primary school | 48989 | 101 (0.21,0.17–0.25) | ||
| Middle school | 73777 | 104 (0.14,0.11–0.17) | ||
| University | 13467 | 15 (0.11,0.06–0.17) | ||
| Higher | 12811 | 9 (0.07,0.02–0.12) | ||
| Rural vs. urban | 168673 | 0.43 0.51 | ||
| Urban | 74902 | 138 (0.18,0.15–0.21) | ||
| Rural | 93771 | 186(0.20,0.17–0.23) | ||
| Socioeconomic level | 168673 | 5.50 0.06 | ||
| Eastern area | 82075 | 175 (0.21,0.18–0.24) | ||
| Central area | 39852 | 60 (0.15,0.11–0.19) | ||
| Western area | 46746 | 89 (0.19,0.15–0.23) | ||
* statistically significant difference.
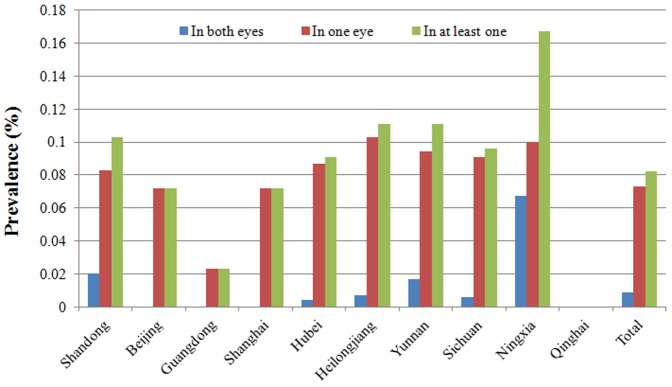
A significant difference was observed in the prevalence of blindness caused by infectious keratitis among the various economic development regions (χ2 = 7.63, p = 0.02), and the lowest prevalence was found in the developed east coast region (Fig. 3).
Figure 3. Geographic distribution of the prevalence of infectious corneal blindness.
There were studies showed that ocular trauma, alcoholic beverage consumption and smoking might be predisposing factors for corneal diseases [9]–[11]. In this study, univariate analysis showed that infectious keratitis caused a higher prevalence of blindness in rural areas (χ2 = 30.61, p<0.001) and among females (χ2 = 4.71, p = 0.03). The prevalence increased with age (χ2 = 260.59, p<0.001). Those who received less education had a higher prevalence (χ2 = 123.23, p<0.001). And higher prevalence was also found to be related to ocular trauma (χ2 = 4.89, p = 0.03), alcoholic beverage consumption (χ2 = 4.20, p = 0.04), and smoking(χ2 = 10.34, p = 0. 001). Furthermore, the logistic regression analysis suggested that ocular trauma, alcoholic beverage consumption, low economic levels, advanced age, and poor education were risk factors for infectious corneal blindness (Table 4). However, gender, hypertension, smoking, and rural vs. urban living were not related.
Table 4. Multivariate analysis of the risk factors for infectious corneal blindness.
| Variables | B | S.E. | Wald | df | Sig. | Exp (B) | 95% CI for Exp (B) | |
| Lower | Upper | |||||||
| Education level | −0.188 | 0.077 | 6.015 | 1 | 0.014 | 0.828 | 0.713 | 0.963 |
| Socioeconomic level | −0.450 | 0.154 | 8.600 | 1 | 0.003 | 0.637 | 0.472 | 0.861 |
| Alcoholic beverage consumption | 0.588 | 0.224 | 6.882 | 1 | 0.009 | 1.801 | 1.160 | 2.796 |
| Ocular trauma | 1.735 | 0.351 | 24.451 | 1 | 0.000 | 5.669 | 2.850 | 11.277 |
| Age | 0.051 | 0.004 | 150.933 | 1 | 0.000 | 1.052 | 1.044 | 1.061 |
| Constant | −10.130 | 0.592 | 292.990 | 1 | 0.000 | 0.000 | ||
Estimates of the prevalence of visual impairment in the population
Based on the population of 1.3 billion revealed by the Fifth National Population Census in 2000 [12], it is estimated that approximately 51.87 million individuals are visually impaired in China, 4.16 million with blindness and 47.71 million with impaired vision. There were 32.37 million cases with sequelae and current corneal diseases, which had caused 2.99 million cases of blindness in at least one eye. Of the 2.47 million individuals with infectious keratitis (including sequelae and active keratopathy), 1.04 million were blind in at least one eye.
Discussion
Corneal disease is a major cause of blindness worldwide. However, the prevalence and causes of corneal blindness vary in different countries, regions, and ethnic groups [1]. Two national sample surveys on disability conducted in China showed that corneal diseases ranked second (11.44%) and third (10.3%) in causes of visual impairment [3], [13]. According to the WHO definition, visual impairment is assessed by testing the eye with better visual acuity. However, corneal disease commonly presents in one eye and impairs vision unilaterally, leading to the underestimation of the actual burden of the condition. The present study is the first nationwide investigation conducted using a population-based, multi-center epidemiological survey in an attempt to understand the burden, causes, and population distribution characteristics of corneal blindness.
Pterygium, infectious keratitis, and traumatic corneal opacity are the most common corneal diseases reported in this study. Consistent with the population distribution of visual impairment reported by the WHO [14], [15], corneal diseases and corneal blindness were more prevalent among females, in rural areas, and among those who had received less education, and the prevalence increased with age. Daily jobs, living environments, and hygienic conditions, together with a lack of disease awareness and effective preventive measures, made these people prone to suffering from corneal lesions. Due to the lack of eye care, limited medical services, and poor economic conditions, the prevalence of corneal blindness is also high in these groups [16], [17]. To reduce the occurrence of corneal diseases and prevent corneal blindness, control strategies should focus on females in rural areas, less educated individuals, and the elderly. The western region had the highest prevalence of corneal blindness because this region is less economically developed.
Pterygium was the most common corneal disease (75.1%) and infectious keratitis was the most common cause of corneal blindness in this study. Up to one-third of the cases of corneal blindness were caused by infectious keratitis. It is estimated that 94.7% of the corneal blindness patients could have their sight restored through corneal transplantation. However, only a small number of hospitals in China have the necessary expertise, technology, and equipment for corneal transplantation. Furthermore, there is a lack of cornea donors in China. As a result, only approximately 5,000 corneal transplantation surgeries are performed annually [18]. Preventive measures against corneal diseases and early treatment for patients are needed to achieve the goal of the “VISION 2020: Right to Sight” initiative.
Infectious keratitis is the leading cause of corneal blindness in China. Traditionally, trachoma ranks first among infectious keratitis. In 1997, the WHO launched the program of “Global Elimination of Blinding Trachoma” (GET 2020) and developed a strategy known by the acronym “SAFE,” which stands for lid surgery (S), antibiotics to treat the infection (A), facial cleanliness (F), and environmental changes (E). Following the implementation of the strategy, the prevalence of trachoma was reduced [19], [20]. Bacterial, viral, and fungal corneal ulcers, as well as Acanthamoeba keratitis, are becoming the major sources of infectious keratitis that impairs vision [21], [22]. In the present study, the prevalence of infectious keratitis was 0.19% and the prevalence of herpes simplex keratitis was 0.11%. Herpes simplex keratitis was the leading infectious corneal disease that led to blindness in developed countries, with an annual incidence of herpes simplex keratitis ranging from 2.07/10000 to 3.15/10000 [7], [23], [24]. From 1950 through 1982, an epidemiological study of ocular herpes simplex virus infection was conducted in Rochester, Minnesota, USA. The survey showed that the prevalence of ocular herpes simplex virus infection in residents was 0.15% in 1980 [7]. A similar result was obtained in the present study, as the prevalence of herpes simplex keratitis was found to be 0.11%.
Infectious keratitis was mainly found in subjects living in poor economic conditions. However, the treatment cost of these conditions is very high. Moreover, there are often no effective drugs available to treat fungal corneal ulcers and Acanthamoeba keratitis in developing countries. In south India, approximately half of the cases of fungal keratitis resulted in blindness despite treatment [25]. With the emergence of resistant isolates and a decrease in effective drugs against bacteria, the rate of blindness associated with bacterial keratitis is also increasing. Because of the poor economic conditions and low health care awareness of females, rural residents, and less educated individuals, together with the lack of specialized doctors for corneal diseases in grassroots hospitals, the diagnosis and treatment of corneal diseases are very difficult. As a result, these residents have a higher prevalence of infectious corneal blindness.
In the present study, infectious keratitis was mainly found in less educated populations who mostly lived in rural areas and had little or no access to health care, and it mainly affected laborers. The disease not only impairs the body and mind but also imposes severe burdens on families and society. Corneal blindness is the final outcome of most infectious keratitis cases. It is of extreme urgency to emphasize the importance of prevention and control of infectious corneal disease and to reduce the number of cases of infectious corneal blindness. Prevention is the most cost-effective approach for reducing the incidence of infected corneal blindness in developing countries [26]. Prevention should focus on strengthening health education, realizing the risk factors and outcomes associated with infectious corneal disease, increasing awareness of eye health, and promoting occupational protection in workplaces. Because most cases of infectious keratitis are the result of corneal trauma, the use of 1% chloromycetin eye ointment for 3 successive days is recommended by the WHO to prevent the development of infectious keratitis [19], [26], [27].
The government should provide economic assistance for patients with infectious keratitis and increase medical insurance reimbursement. It was indicated that infectious keratitis was mainly found in populations with low socioeconomic status [13], [25], [28]. The high cost of corneal transplantation surgery, together with the direct and indirect economic loss caused by the disease, imposes a heavy burden on patients, their families, and society. Increasing the reimbursement percentage and economic assistance to patients would help reduce the incidence of infectious corneal blindness.
Funding Statement
This work was supported by a grant from the Consultation Program of Chinese Academy of Engineering (No. 2009-77). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Whitcher JP, Srinivasan M, Upadhyay MP (2001) Corneal blindness: a global perspective. Bull World Health Organ 79:214–221. [PMC free article] [PubMed] [Google Scholar]
- 2. Whitcher JP, Srinvasan M (1997) Corneal ulceration in developing world: A silent epidemic. Br J Ophthalmol 81:622–623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Zhang SY, Zou LH, Gao YQ, Di Y, Wang XD (1992) National epidemiological survey of blindness and low vision in China. Chin Med J (Engl) 105:603–608. [PubMed] [Google Scholar]
- 4.National Bureau of Statistics of China. China Statistical Yearbook 2003. Table 3–16. Houshold Consumption. Beijing: China Statistics Press; 2003.7. Available at: http://www.stats.gov.cn/english/statisticaldata/yearlydata/yarbook_e.pdf. Accessed Jan. 26, 2011.
- 5. Zhao J, Jia L, Sui R, Ellwein LB (1998) Prevalence of blindness and cataract surgery in Shunyi County, China. Am J Ophthalmol 126:506–514. [DOI] [PubMed] [Google Scholar]
- 6. Lam DSC, Houang E, Fan DSP, Lyon D, Seal D, et al. (2002) Incidence and risk factors for microbial keratitis in Hong Kong: comparison with Europe and North America. Eye 16:608–618. [DOI] [PubMed] [Google Scholar]
- 7. Liesegang T, Melton LJ, Daly PJ, Ilstrup DM (1989) Epidemiology of ocular herpes simplex: incidence in Rochester, Minn.1950 through 1982. Arch Ophthalmol 107:1155–1159. [DOI] [PubMed] [Google Scholar]
- 8. Halberstadt M, Machens M, Gahlenbek KA, Böhnke M, Garweg JG (2002) The outcome of corneal grafting in patients with Stromal keratitis of herpetic and non-herpetic origin. Br J Ophthalmol 86:646–652. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Keay L, Edwards K, Naduvilath T, Taylor HR, Snibson GR, et al. (2006) Microbial keratitis predisposing factors and morbidity. Ophthalmology 113:109–116. [DOI] [PubMed] [Google Scholar]
- 10. Xu L, You QS, Jonas JB (2009) Prevalence of alcohol consumption and risk of ocular diseases in a general population: the Beijing Eye Study. Ophthalmology 116:1872–1879. [DOI] [PubMed] [Google Scholar]
- 11. Solberg Y, Rosner M, Belkin M (1998) The association between cigarette smoking and ocular diseases. Surv Ophthalmol 42:535–547. [DOI] [PubMed] [Google Scholar]
- 12.National Bureau of Statistics of China. Communique on Major Figures of the 2000 Pupulation Census(No. 1). http://www.stats.gov.cn/english/StatisticalCommuniques/. Accessed Jan. 25, 2011.
- 13. Zhao JL (2009) Prevention of blindness is still the great challenge faced by Chinese ophthalmology. Zhonghua Yan ke Za zhi 45:769–771. [PubMed] [Google Scholar]
- 14. World Health Organization. Visual impairment and blindness. Available at: http://www.who.int/mediacentre/factsheets/fs282/en/ Accessed Jan. 25, 2011.. [Google Scholar]
- 15. Resnikoff S (2004) Globle data on visual impairment in the year 2002. Bull WHO 82:844–851. [PMC free article] [PubMed] [Google Scholar]
- 16. Dandona R, Dandona L (2003) Corneal blindness in a southern Indian population: need for health promotion strategies. Br J Ophthalmol 87:133–141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Li Z, Cui H, Zhang L, Liu P, Bai J (2009) Prevalence of and associated factors for corneal blindness in a rural adult population(The Southern Harbin Eye Study. Curr Eye Res 34:646–651. [DOI] [PubMed] [Google Scholar]
- 18. Ament JD, Todani A, Pineda R 2nd, Shen TT, Aldave AJ, et al. (2010) Globle corneal blindness and the Boston keratoprosthesis type. Am J Ophthalmol 149:537–539. [DOI] [PubMed] [Google Scholar]
- 19. Mariotti SP (2004) New steps toward eliminating blinding trachoma. N Engl J Med 351:2004–2007. [DOI] [PubMed] [Google Scholar]
- 20. Ngondi J, Onsarigo A, Matthews F, Reacher M, Brayne C, et al. (2006) Effect of 3 years of SAFE (surgery, antibiotics, facial cleanliness, and environmental change) strategy for trachoma control in southern Sudan: A cross-sectional study. Lancet 368:589–595. [DOI] [PubMed] [Google Scholar]
- 21. Gonzales CA, Srinivasan M, Whitcher JP, Smolin G (1996) Incidence of corneal ulceration in Madurai district, South India. Ophthalmic Epidemiol 3:159–166. [DOI] [PubMed] [Google Scholar]
- 22. Upadhyay MP, Karmacharya PC, Koirala S, Shah DN, Shakya S, et al. (2001) The Bhaktapur Eye Study: Ocular trauma and antibiotic prophylaxis for the prevention of corneal ulceration in Nepal. Br J Ophthalmol 85:388–392. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Liesegang TJ (2001) Herpes simplex virus epidemiology and ocular importance. Cornea 20:1–13. [DOI] [PubMed] [Google Scholar]
- 24. Labetoulle M, Auquier P, Conrad H, Crochard A, Daniloski M, et al. (2005) Incidence of herpes simplex virus keratitis in France. Ophthalmology 112:888–895. [DOI] [PubMed] [Google Scholar]
- 25. Whitcher JP, Srinivasan M, Upadhyay MP (2002) Prevention of corneal ulceration in the developing world. Int Ophthalmol Clin 42:71–77. [DOI] [PubMed] [Google Scholar]
- 26. Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalakshmi R, Whitcher JP (2006) Corneal ulceration in South–East Asia: Prevention of fungal keratitis at the village level in South India using topic antibiotics. Br J Ophthalmol 90:1472–1475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Getshen K, Srinivasan M, Upadhyay MP, Priyadarsini B, Mahalaksmi R, et al. (2006) Corneal ulceration in South East Asia: A model for the prevention of bacterial ulcers at the village level in rural Bhutan. Br J Ophthalmol 90:276–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Naidoo K (2007) Poverty and blindness in Africa. Clin Exp Optom 90:415–421. [DOI] [PubMed] [Google Scholar]