Significance
Long noncoding RNAs (lncRNAs) provide new layers of complexity to gene expression control. We report on the functional consequences of the interaction between the ssRNA-binding protein K homology-type splicing regulatory protein (KSRP) with H19 lncRNA (H19) in multipotent C2C12 cells able to differentiate in culture toward myotubes in response to activation of cell signaling pathways, including AKT. KSRP and H19 interact exclusively in undifferentiated C2C12 cells, and this favors KSRP’s ability to interact with the promyogenic transcript myogenin and to favor its degradation. AKT activation induces KSRP dissociation from H19 and, as a consequence, from myogenin mRNA that is stabilized. H19 likely acts as a scaffold that favors KSRP-mediated degradation of myogenin to contribute to the maintenance of the undifferentiated state of C2C12 cells.
Keywords: long noncoding RNA, mRNA decay, RNA-binding proteins
Abstract
Long noncoding RNAs (lncRNAs) interact with protein factors to regulate different layers of gene expression transcriptionally or posttranscriptionally. Here we report on the functional consequences of the unanticipated interaction of the RNA binding protein K homology-type splicing regulatory protein (KSRP) with the H19 lncRNA (H19). KSRP directly binds to H19 in the cytoplasm of undifferentiated multipotent mesenchymal C2C12 cells, and this interaction favors KSRP-mediated destabilization of labile transcripts such as myogenin. AKT activation induces KSRP dismissal from H19 and, as a consequence, myogenin mRNA is stabilized while KSRP is repurposed to promote maturation of myogenic microRNAs, thus favoring myogenic differentiation. Our data indicate that H19 operates as a molecular scaffold that facilitates effective association of KSRP with myogenin and other labile transcripts, and we propose that H19 works with KSRP to optimize an AKT-regulated posttranscriptional switch that controls myogenic differentiation.
Regulatory noncoding RNAs and RNA-binding proteins (RBPs) act side-by-side to convert extracellular signals into changes of gene expression regulated at posttranscriptional level.
Among small noncoding RNAs, microRNAs (miRNAs) repress gene expression by inhibiting translation and/or promoting decay of target mRNAs (1), with RBPs being able to control miRNA maturation from precursors or influence miRNA function (1, 2). A new class of transcripts referred to as long noncoding RNAs (lncRNAs, arbitrarily defined as longer than 200 nt) has recently moved to the forefront of regulatory RNA research (3). lncRNAs had been originally considered epigenetic regulators of gene expression, but the emphasis placed on the ways they regulate chromatin state likely obscures the full repertoire of their functions (4–6). Other roles of lncRNAs include posttranscriptional regulation, posttranslational control of protein activity, organization of protein complexes, as well as cell–cell signaling (4, 6). lncRNAs have been implicated in cellular events as different as cell cycle regulation, pluripotency, apoptosis, and DNA damage response, to name just a few (5, 6). Not surprisingly, lncRNA expression is altered in cancer, and it is becoming clear that some lncRNAs can control cell transformation by regulating vital cellular functions (7). Nevertheless, the composition and function of ribonucleoprotein complexes, including lncRNAs, is generally uncharacterized.
Studies performed in primary and cultured cells as well as in mice proved that the K homology (KH)-type splicing regulatory protein (KSRP), a single-stranded RBP that interacts with nucleic acids through four hnRNP KH domains, is able to integrate different levels of gene expression and is required for proper immune response, lipid metabolism, cell fate decisions, and tissue regeneration (see ref. 8 for a recent review). We and others have found that KSRP negatively regulates gene expression via at least two distinct and integrated posttranscriptional mechanisms: (i) by promoting decay of unstable mRNAs [mainly targeting AU-rich elements (AREs) in their 3′UTRs] (8, 9) and (ii) by favoring maturation of select miRNAs from precursors (8, 10). Briefly, KSRP recruits the RNA exosome and other enzymes to labile transcripts and promotes decay of inherently unstable mRNAs that encode factors critical for disparate cell functions (8, 9). On the contrary, KSRP associates with Drosha and Dicer complexes and promotes maturation of precursors to generate miRNAs that exert important functions in cell proliferation, differentiation, and metabolism in response to extracellular stimuli (8, 10–12). The critical role exerted by KSRP in the myogenic differentiation of multipotent mesenchymal C2C12 cells and the profound transcriptome reshaping we recently described in these cells upon KSRP silencing (13, 14) emphasizes the possibility that KSRP modulates a wide range of RNA targets.
To systematically detect regulatory RNA species interacting with KSRP, we have performed a transcriptome-wide analysis of KSRP-interacting RNAs in C2C12 cells and identified, among other RNAs, the H19 lncRNA (hereafter indicated as H19). We demonstrate that KSRP directly interacts with H19 in the cytoplasm of proliferating undifferentiated C2C12 cells, and that this interaction favors the decay-promoting function of KSRP on labile transcripts such as myogenin. AKT activation induces KSRP dissociation from H19 and, as a consequence, myogenin mRNA is stabilized whereas KSRP is able to promote maturation of myogenic miRNAs, thus favoring myogenic differentiation.
Results
KSRP Directly Interacts with the H19 lncRNA.
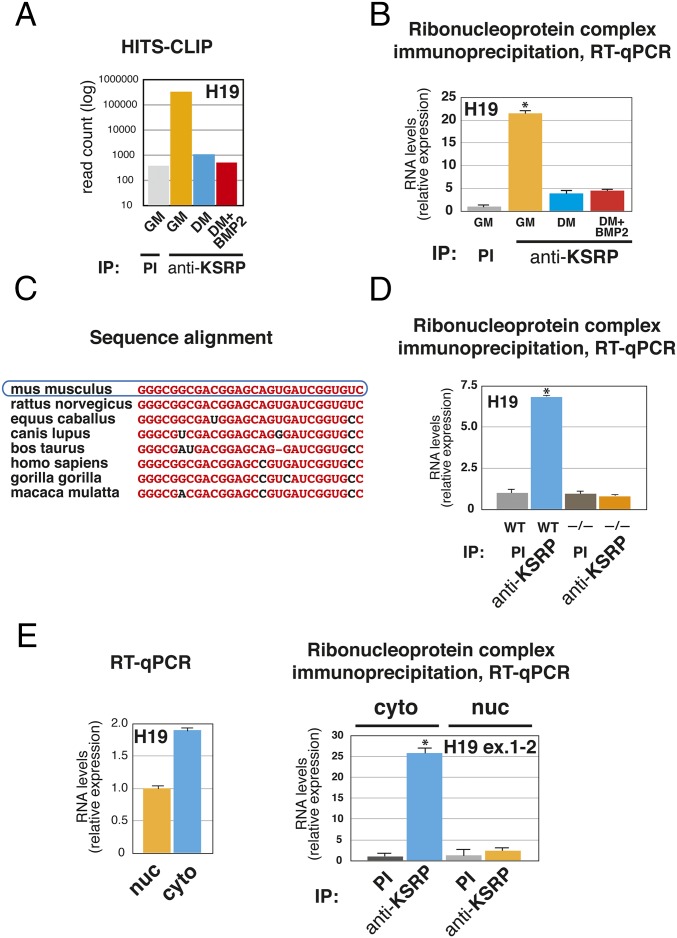
An unbiased search for potential KSRP interactions with target RNAs was performed by high-throughput sequencing of RNA isolated by crosslinking immunoprecipitation (HITS-CLIP), a method that has been used to map the in vivo interaction of proteins with their RNA targets (15, 16). Experiments were performed in undifferentiated multipotent mesenchymal C2C12 cells [growth medium (GM)] as well as in C2C12 cells induced to differentiate toward myotubes upon serum withdrawal [differentiation medium (DM)] or toward osteoblasts upon addition of BMP2 to DM (14). Extracts from UV-crosslinked cells were immunoprecipitated by using a polyclonal anti-KSRP antibody or the corresponding preimmune serum (9). As shown in Fig. S1A, bioinformatic analysis of HITS-CLIP results revealed KSRP interaction with exonic sequences (including 3′ and 5′ UTRs), intronic sequences, as well as intergenic regions of the transcriptome. In this study, we focused on the unanticipated interaction of KSRP with H19, an intergenic lncRNA (Fig. 1A and Fig. S1 B and C).
Fig. 1.
KSRP directly interacts with H19 lncRNA in the cytoplasm of proliferating C2C12 cells. (A) C2C12 cells were cultured in GM, DM, or DM supplemented with 300 ng/mL BMP2 for 24 h, UV-crosslinked, lysated and subjected to HITS-CLIP analysis. Immunoprecipitations were performed by using anti-KSRP polyclonal antibody or the respective preimmune serum (PI). The results of a representative experiment (of three distinct performed) are expressed as read count mapped to mouse H19 lncRNA (on a logarithmic scale). (B) C2C12 cells were cultured in GM, DM, or DM plus 300 ng/mL BMP2 for 24 h. Total cell extracts were immunoprecipitated as indicated. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect H19. (C) Alignment of the murine H19 sequence corresponding to the KSRP binding site (as revealed by HITS-CLIP analysis) with sequences belonging to different mammals. (D) Mouse embryonic fibroblasts (MEFs) were prepared from embryonic day 14.5 WT or Ksrp−/− embryos, total extracts were prepared and immunoprecipitated as indicated. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect H19. (E) RNA was prepared from nuclear or cytoplasmic fractions of C2C12 cells cultured in GM, and H19 levels were quantified by RT-qPCR (Left). Cytoplasmic or nuclear extracts from C2C12 cells cultured in GM were immunoprecipitated as indicated. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect lncRNA H19 using primers spanning the exon 1–exon 2 junction (Right). The values of RT-qPCR experiments shown are averages (±SEM) of three independent experiments performed in triplicate (*P < 0.001, Student t test).
H19 is the developmentally regulated product of a paternally imprinted gene located on chromosome 7 in mouse, and its first exon encodes the precursor of two conserved miRNAs: miR-675–5p and miR-675–3p (17, 18) (Fig. S1 B and C). HITS-CLIP and ribonucleoprotein complex immunoprecipitation (RIP) analyses revealed that KSRP/H19 interaction takes place in proliferating undifferentiated cells but is abrogated by myogenic or osteoblastic differentiation (Fig. 1 A and B; a negative control is shown in Fig. S1D). Notably, KSRP levels were unaffected by the differentiation state of C2C12 cells, whereas H19 expression increased in C2C12 cells cultured in DM and remained unchanged in cells cultured in DM plus BMP2 (Fig. S1E). The KSRP target sequence is located at the 3′ end of the first exon of H19 (Fig. S1B) and is well conserved among mammalian species (Fig. 1C). This sequence does not display any AU-rich motif, whereas it presents short G-rich stretches that we previously demonstrated to efficiently interact with KSRP (8, 10). The specificity of the anti-KSRP immunoprecipitations was validated by RIP analysis in MEFs derived from WT or Ksrp−/− mice (Fig. 1D). Further, we ruled out the possibility that KSRP interacts with H19 only in cells in which the lncRNA is very abundant (such as C2C12 cells and MEFs) by showing KSRP–H19 association also in tissues that express limited amounts of H19 such as adipose tissue (Fig. S1F). H19 is present in nuclear and cytoplasmic fractions of C2C12 cells cultured in GM even though it is enriched in the cytoplasm (Fig. 1E, Left), where its interaction with KSRP takes place (Fig. 1E, Right). Altogether, these data revealed that KSRP interacts with the H19 lncRNA in the cytoplasm of proliferating C2C12 cells.
KSRP–H19 Interaction Is Abrogated by AKT Signaling Activation.
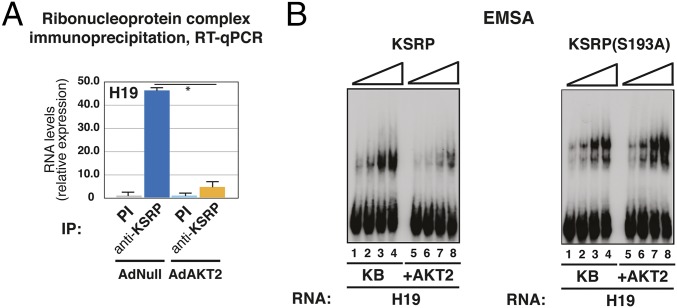
AKT signaling is rapidly activated in C2C12 cells cultured in DM, and we have previously shown that AKT phosphorylates KSRP on Ser-193, affecting its function during myogenic differentiation (13, 19). Based on the evidence that KSRP dissociates from H19 upon the induction of differentiation (Fig. 1 A and B), we investigated whether AKT activation impairs KSRP–H19 interaction. Expression of constitutively active AKT2 in C2C12 cells cultured in GM strongly reduced the interaction of KSRP with H19 (Fig. 2A). To explore whether this effect was caused by direct KSRP phosphorylation by AKT, purified recombinant KSRP was preincubated with active recombinant AKT2 or kinase buffer alone. As shown in Fig. 2B (Left), in vitro phosphorylation impaired KSRP-H19 interaction. Conversely, phosphorylation by AKT2 did not affect the interaction of recombinant purified KSRP mutated in the AKT phosphorylation site (S193A) (19) with H19 (Fig. 2B, Right). Accordingly, an aspartic acid mutant (S193D) that we have reported to destabilize the structure of the first KH domain (KH1), where Ser-193 is located (20), impaired the interaction of KSRP with murine H19 transiently expressed in HEK-293 cells (Fig. S2A).
Fig. 2.
KSRP–H19 interaction is abrogated by AKT signaling activation. (A) C2C12 cells cultured in GM were treated with a negative control adenoviral vector (AdNull) or with a constitutively active myristoylated AKT2-expressing adenoviral vector (AdAKT2) for 48 h. Total cell extracts were immunoprecipitated as indicated, and RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect H19. The values of RT-qPCR experiments shown are averages (±SEM) of three independent experiments performed in triplicate (*P < 0.001, Student t test). (B) WT or S193A mutant recombinant purified KSRP (increasing amounts from 30 to 300 nM) was preincubated in AKT2 kinase assay buffer (KB) alone or supplemented with active recombinant AKT2 (30 min at 30 °C, +AKT2). The interaction between 32P-labeled RNAs (as indicated) and KSRP was evaluated by EMSA. Representative autoradiograms are shown.
We have previously shown that KSRP also undergoes phosphorylation by MAPK p38 (henceforth “p38”) during myogenic differentiation (21). However, as shown in Fig. S2B, p38 activation by a constitutively active form of the upstream kinase MKK6 (MKK6EE) failed to affect KSRP–H19 interaction in transfected HEK-293 cells whereas, as predicted, constitutively active AKT2 impaired the interaction in the same cellular context (Fig. S2C). Altogether, these data suggest that AKT activation impairs the interaction of KSRP with H19 in C2C12 cells.
H19 Silencing in Undifferentiated C2C12 Cells Promotes Myogenin mRNA Stabilization and Maturation of Myogenic miRNAs from Precursors.
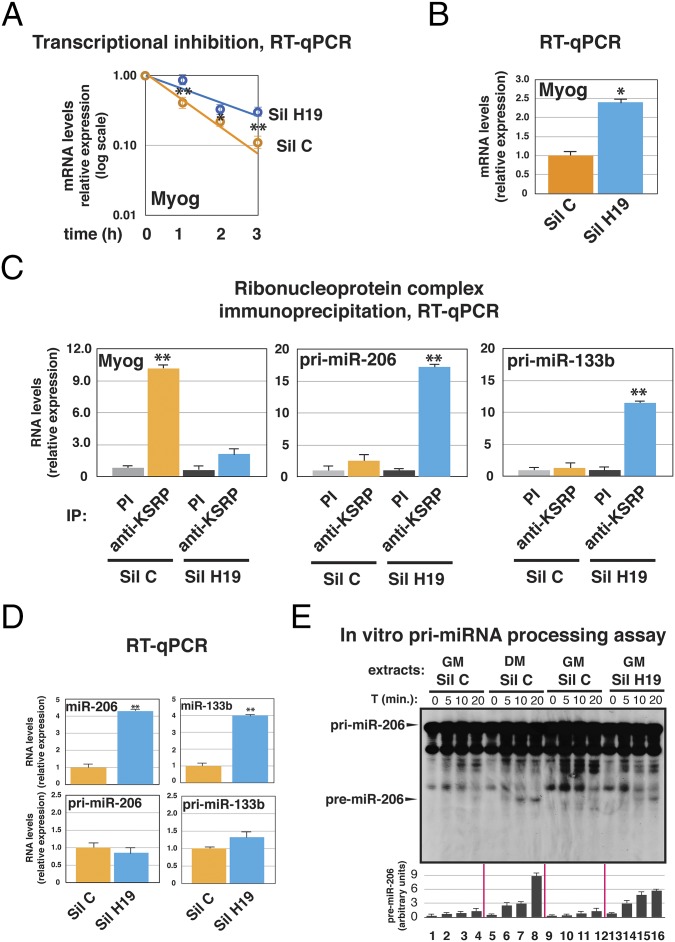
To gain functional insights into KSRP–H19 interaction, we efficiently silenced H19 in proliferating C2C12 cells (Fig. S3A). The expression of myogenin is transcriptionally and posttranscriptionally regulated during myogenic differentiation of C2C12 cells (22–24), and we have previously reported that KSRP silencing impairs its rapid decay (21). As shown in Fig. 3A, H19 silencing induced stabilization of myogenin mRNA and significantly enhanced its expression (Fig. 3B) in proliferating C2C12 cells. In vitro decay assays supported that this stabilization is mediated through the ARE present in the myogenin 3′ UTR (Fig. S3B). Further, H19-depleted C2C12 cells, although cultured in GM, showed increased expression levels of promyogenic factors including muscle creatine kinase, myoglobin, and Igf2 (Fig. S3C).
Fig. 3.
H19 silencing in undifferentiated C2C12 cells promotes myogenin mRNA stabilization and maturation of myomiRs from precursors. H19 was transiently silenced in C2C12 cells cultured in GM by using a combination of a sequence-specific siRNA and a GAPmeR (collectively indicated as Sil H19). Parallel cultures were mock-silenced by using a combination of a control siRNA and a control GAPmeR (collectively indicated as Sil C). Cells were used for experiments 48 h after transfection. (A) Cells were treated with 100 μM 5,6-Dichlorobenzimidazole 1-β-D-ribofuranoside (DRB), and total RNA was isolated at different times (as indicated) after the addition of DRB and analyzed by RT-qPCR to detect myogenin mRNA expression. (B) RNA was prepared from transfected C2C12 cells, and myogenin levels were quantified by RT-qPCR. (C) Total cell extracts were immunoprecipitated as indicated. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect myogenin or primary miRNAs. (D) RNA was prepared from transfected C2C12 cells, and the levels of the indicated miRNAs and primary miRNAs were quantified by RT-qPCR. (E) In vitro pri-miR-206 processing assays performed by using total extracts from C2C12 cells mock- (Sil C) or H19- (Sil H19) silenced and cultured in GM or DM (for 36 h). Internally 32P-labeled pri-miR-206 RNA substrate was added, and its processing was monitored as described under Materials and Methods. A representative autoradiogram is shown. The intensity of background bands is ascribable to the long exposure of gels as a result of the low processing efficiency of pri-miR-206. The arrow points to premiR-206 band that is visible only in experiments performed by using extracts from cells treated with DM/Sil C or GM/Sil H19. The bar graph below the autoradiogram is a quantification of the premiR-206 levels measured in two distinct processing assays. The intensity of the bands corresponding to pri–miR-206 and premiR-206, quantified with ImageJ software (http://rsb.info.nih.gov/ij/index.html), was expressed as percentage (±SEM calculated on two experiments) of premiR-206 generated from pri-miR-206 at each time point. To avoid signal saturation, the quantification was performed on underexposed autoradiograms. The values of RT-qPCR experiments shown are averages (±SEM) of three independent experiments performed in triplicate (*P < 0.01 and **P < 0.001, Student t test).
Based on our previous observations that AKT activation impairs KSRP association with myogenin mRNA whereas it enhances its association with myogenic miRNA primary transcripts (13), we investigated the consequences of H19 silencing on the formation of ribonucleoprotein complexes including KSRP. Our RIP experiments showed that H19 silencing impairs KSRP association with myogenin mRNA (Fig. 3C) and favors KSRP association with pri-miR-206 and pri-miR-133b (Fig. 3C), with accumulation of mature miR-206 and miR-133b (myogenic miRNAs, hereafter indicated as myomiRs), without affecting the levels of their primary transcripts (Fig. 3D). Further, in vitro processing assays confirmed that extracts from H19-depleted C2C12 cells cultured in GM were able to promote the formation of premiR-206 from the primary transcript, similarly to extracts from cells induced to myogenic differentiation (i.e., DM; Fig. 3E).
The first exon of H19 encodes two miRNAs, miR-675–5p and miR-675–3p (18) (schematic in Fig. S1B), that are expressed, although at low levels, in C2C12 cells cultured in GM and increase over the course of myogenic differentiation (25). To investigate whether the observed effects of H19 silencing can be ascribed, at least in part, to the abrogation of miR-675–5p and miR-675–3p function, we silenced each miRNA in proliferating C2C12 cells. As presented in Fig. S3D, we did not observe any difference in myogenin or myomiR expression upon transfection of miR-675–5p or miR-675–3p antagomirs. Similarly, overexpression of miR-675–5p and miR-675–3p in proliferating C2C12 cells did not affect myogenin or myomiR expression (Fig. S3E).
Altogether, our silencing experiments suggest that H19 plays a critical role in favoring KSRP-mediated myogenin mRNA decay and in preventing KSRP-dependent maturation of myomiRs.
The Interaction with H19 Favors the Decay-Promoting Function of KSRP.
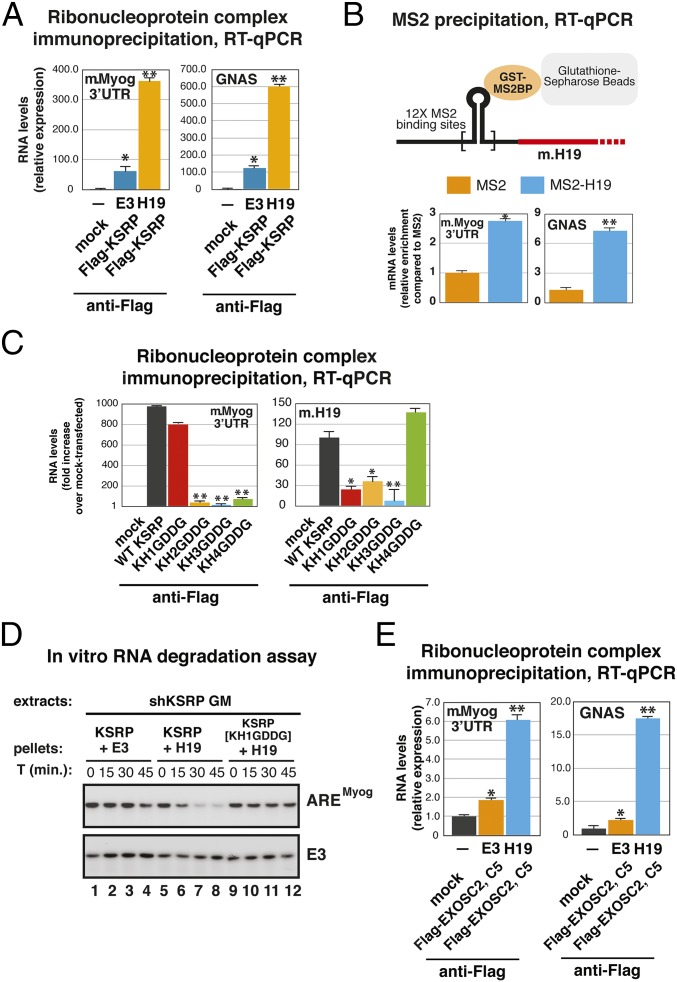
To gain molecular insight into the way H19 regulates the decay-promoting function of KSRP, we transiently transfected HEK-293 cells with a vector expressing murine H19 or the control sequence E3 [a region derived from the mouse Pitx2 3′UTR that does not interact with KSRP (26)] together with Flag-tagged KSRP. Murine H19 ectopically expressed in HEK-293 cells associated with KSRP (Fig. S4A) and favored its interaction with coexpressed myogenin 3′UTR or endogenous GNAS [guanine nucleotide binding protein (G protein), alpha stimulating activity polypeptide], a labile mRNA that we previously characterized as a target of KSRP (27) (Fig. 4A). Notably, Flag-tagged KSRP was similarly immunoprecipitated in the presence of E3 or H19 (Fig. S4B). This observation, together with the evidence that KSRP binds to myogenin mRNA and H19 in undifferentiated C2C12 cells whereas it fails to interact with both transcripts in C2C12 cells differentiating toward myoblasts (13) (Fig. 1B), prompted us to investigate whether H19 is present in a complex including myogenin mRNA and, possibly, other KSRP target mRNAs. We expressed murine H19 tagged with MS2 RNA hairpins (MS2-H19) in HEK-293 cells together with myogenin 3′UTR as well as GST-fused MS2 binding protein (GST-MS2BP). Total cell extracts were precipitated by using glutathione–Sepharose beads, and GST-MS2BP–associated RNA was extracted and analyzed by quantitative RT-PCR (RT-qPCR). Results presented in Fig. 4B indicate that MS2-H19 expression enhanced the association of GST-MS2BP with transfected myogenin 3′UTR. Importantly, also the association of the endogenous labile mRNA GNAS was similarly regulated.
Fig. 4.
The interaction with H19 favors the decay-promoting function of KSRP. (A) HEK-293 cells were transiently cotransfected with pTAG2B-myog 3′UTR together with empty vector (mock control cells), or pTAG2B-E3 (E3) plus pCDNA3-Flag-KSRP (flag-KSRP), or pTAG2B-H19 (H19) plus pCDNA3-Flag-KSRP (flag-KSRP). Total cell extracts were prepared 48 h after transfection and immunoprecipitated with anti-Flag antibody. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect transfected murine myogenin 3′UTR (m.Myog 3′UTR) or endogenous GNAS. (B) HEK-293 cells were transiently transfected with GST-fused MS2 binding protein (GST-MS2BP) together with pTAG2B-myog 3′UTR and pTAG2B-MS2-12XH19 (in which murine H19 was tagged with MS2 RNA hairpins; MS2-H19) or pTAG2B-MS2-12X (empty vector; MS2). Total cell extracts were prepared 48 h after transfection and precipitated by glutathione–Sepharose beads. RNA was purified and analyzed by RT-qPCR to detect transfected murine myogenin 3′UTR (m.Myog 3′UTR) or endogenous GNAS. (C) HEK-293 cells were transiently cotransfected with pCMV-TAG2B-KSRP (expressing Flag-tagged WT KSRP) or pCMV-TAG2B expressing the indicated Flag-tagged KSRP mutants (KH1GDDG, KH2GDDG, KH3GDDG, KH4GDDG) together with pTAG2B-myog 3′UTR and pTAG2B-H19. Total cell extracts were prepared 48 h after transfection and immunoprecipitated by anti-Flag antibody. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect myogenin 3′UTR (m.Myog 3′UTR) or transfected murine H19 (m.H19). (D) In vitro RNA degradation assays using S100 extracts from shKSRP C2C12 cells preincubated (for 90 min at 4 °C) with anti-Flag immunoprecipitates from HEK-293 cells transiently transfected with Flag-tagged WT KSRP (KSRP) or Flag-tagged KH1GDDG mutant (KSRP[KH1GDDG]) together with the E3 sequence or murine H19 cloned in expression vectors. Internally 32P-labeled and capped RNA substrates were incubated with the aforementioned reaction mixtures, and their decay was monitored for the indicated times. RNA was analyzed by denaturing polyacrylamide gel electrophoresis followed by autoradiography. E3 is a stable transcript used to detect background decay. Representative autoradiograms are displayed. (E) HEK-293 cells were transiently cotransfected with pTAG2B-myog 3′UTR together with empty vector (mock control cells), or pTAG2B-E3 (E3) plus pCDNA3-Flag-EXOSC2 and pCDNA3-Flag-EXOSC5, or pTAG2B-H19 (H19) plus pCDNA3-Flag-EXOSC2 and pCDNA3-Flag-EXOSC5. Total cell extracts were prepared 48 h after transfection and immunoprecipitated with anti-Flag antibody. RNA was purified from immunocomplexes and analyzed by RT-qPCR to detect transfected murine myogenin 3′UTR (m.Myog 3′UTR) or endogenous GNAS. The values of RT-qPCR experiments shown are averages (±SEM) of three independent experiments performed in triplicate (*P < 0.01 and **P < 0.001, Student t test.)
Based on these observations, we wanted to explore the mode of KSRP interaction with H19 or labile mRNAs. We have recently generated a series of KSRP mutants in which the hallmark GxxG RNA binding loop present in each KH domain is substituted by the GDDG sequence (28). Thus, we obtained four KSRP mutants in which the RNA binding ability of each single KH domain is abrogated whereas the structure and stability of the protein is unaffected (KH1GDDG, KH2GDDG, KH3GDDG, KH4GDDG) (28). We transiently expressed each KSRP mutant (or WT KSRP) in HEK-293 cells (Fig. S4C) together with murine H19 and myogenin 3′UTR. As shown in Fig. 4C and Fig. S4D, the interaction of KSRP mutants with transfected myogenin 3′UTR or endogenous KSRP mRNA targets (GNAS and CTNNB1, also known as β-catenin) occurred according to the previously described mode with KH2, KH3, and KH4 playing the major role in the RNA recognition and KH1 resulting dispensable (28, 29). The interaction of KSRP with H19 was unaffected by mutations in KH4 but was impaired not only by mutations in KH2 and 3 but also in KH1 (Fig. 4C). The fact that KH1 is required for optimal KSRP binding to H19 but not to ARE-containing mRNAs (Fig. 4C and Fig. S4D) (29), together with results shown in Figs. 3C and 4B, made it reasonable to hypothesize that H19 operates as a molecular scaffold favoring KSRP binding to mRNA targets and its decay-promoting activity.
To verify this hypothesis, Flag-tagged KSRP was immunoprecipitated from extracts of HEK-293 cells transiently transfected with H19 or E3 negative control sequence. Immunocomplexes were preincubated with S100 extracts prepared from KSRP-silenced C2C12 cells cultured in GM that are unable to promote decay of labile mRNAs (14). As shown in Fig. 4D, KSRP immunopurified from H19 transfected cells promoted myogenin 3′UTR decay more efficiently than KSRP immunopurified from cells transfected with the negative control E3 sequence. On the contrary, KH1GDDG mutant immunoprecipitated from H19 cotransfected HEK-293 cells was not able to induce myogenin 3′UTR rapid decay (Fig. 4D).
Next, we explored whether H19 expression was able to favor the interaction of the RNA exosome with ARE-containing RNAs. RIP analysis was performed by using extracts from HEK-293 transiently transfected with two distinct Flag-tagged exosome components (EXOSC2 and EXOSC5) together with H19 or the E3 sequence. As shown in Fig. 4E, the coexpression of H19 significantly enhanced the interaction of the RNA exosome with cotransfected Myog 3′UTR or endogenous GNAS mRNA. Predictably, H19 was able to favor the interaction of the RNA exosome with KSRP as revealed by coimmunoprecipitation experiments performed in transiently transfected HEK-293 cells (Fig. S4E). Notably, the expression of myogenin 3′UTR and GNAS mRNA was reduced in H19-transfected cells, emphasizing H19 relevance in the control of the steady-state levels of KSRP-regulated unstable mRNAs (Fig. S4F).
On the whole, our data indicate that H19, interacting with KSRP, favors its decay-promoting function and recruitment of the RNA exosome to labile mRNAs.
Discussion
We have identified H19 as an lncRNA that directly interacts with the multifunctional RNA binding-protein KSRP and defined its role as a regulator of rapid KSRP-dependent mRNA decay in undifferentiated multipotent mesenchymal C2C12 cells.
H19 is expressed in all embryonic and neonatal tissues, but, after birth, it is generally down-regulated, with the exception of skeletal muscle, in which it remains abundant (reviewed in ref. 17). Although the role of H19 in tumorigenesis is still debated, it is considered as an oncogenic lncRNA with protumorigenic properties in a variety of cell types and has also been reported to play an active role in promoting tumor metastasis (30–32). However, the molecular mechanisms underlying its function(s) are poorly understood.
lncRNAs, like other regulatory RNAs, are endowed with the ability to interact with protein factors and nucleic acids, thus displaying the potential to direct ribonucleoprotein complexes to specific RNA or DNA target sites (4, 6, 33). Thus, it is not surprising that different roles have been described for lncRNAs in regulating various stages of gene expression (4, 6, 33). Besides the originally reported ability to interact with transcriptional regulators modulating chromatin accessibility, a few lncRNAs recently proved capable of associating with RBPs implicated in various RNA metabolism checkpoints (5, 6). Interestingly, recent reports indicated that some lncRNAs can function as competing endogenous RNAs (ceRNAs) by base-paring to and sequestering specific miRNAs (34), whereas others can modulate mRNA stability by interacting with RBPs (35–37).
In this report, we have identified an unanticipated mechanism by which cytoplasmic H19 posttranscriptionally modulates gene expression in proliferating C2C12 cells. We propose that H19 acts as a scaffold to favor the interaction of KSRP and the RNA exosome, with target mRNAs enhancing the mRNA decay-promoting function of KSRP on myogenin mRNA (and, possibly, other labile transcripts). The modulation of KSRP function operated by H19 contributes to the maintenance of the undifferentiated state in these cells.
We previously showed that AKT signaling activation determines a series of changes in KSRP functions (including interaction with the adaptor protein 14–3-3 and nuclear translocation) that enable the protein to switch from the cytoplasmic mRNA decay-promoting activity to the nuclear primary myomiR processing activity (13, 19, 20). We propose that H19 silencing, similarly to AKT activation, impairs the function of a cytoplasmic “mRNA decay-promoting domain” in which KSRP accumulates to exert its role in undifferentiated C2C12 cells. A model summarizing our idea on the mechanism by which H19 can affect myogenin mRNA decay through KSRP regulation is presented in Fig. S5.
Induction of myogenic differentiation involves the activation of at least two distinct cell signaling pathways—AKT and MAPK p38 (22)—and we previously reported that KSRP phosphorylation by these kinases (in distinct protein domains) is instrumental to achieve some of the gene expression changes crucial to myogenic differentiation (13, 21). The AKT phosphorylation site is located in KH1, a domain that does not actively participate in KSRP interaction with labile mRNAs (19, 20). Our experiments have now revealed that KH1 is required for optimal KSRP–H19 binding. Thus, it is plausible that KH1 phosphorylation by AKT, abrogating the interaction with H19, limits the decay-promoting function of KSRP. Conversely, although we have demonstrated in the past that phosphorylation by p38 is able to limit KSRP interaction with myogenin and other labile myogenic transcripts (21), it does not impair KSRP interaction with H19. Based on our present and previous experimental evidence, we can speculate that dual phosphorylation of KSRP is required to achieve the complete inhibition of the KSRP-dependent mRNA decay-promoting function.
Interestingly, HuR, an RBP that controls decay of labile mRNAs and also interacts with lncRNA similarly to KSRP (36, 38, 39), associates with KSRP to destabilize the mRNA encoding the cell cycle regulator Nucleophosmin in C2C12 cells (40). Considering that HuR also has been reported to associate with H19 (41), it will be interesting to investigate in the future whether H19, KSRP, and HuR collaborate during C2C12 differentiation.
While our studies were in progress, Huang and coworkers reported that H19 functions as a ceRNA for let-7 miRNA to control muscle differentiation and that H19 silencing induces myogenic differentiation in a let-7–dependent way (42). Conflicting results were published by Dutta and coworkers that showed a promyogenic function of H19 attributable to miR-675–5p and -3p (processed from H19 exon 1) whose expression is induced by culturing C2C12 cells in DM (25). Our data are in agreement with the antimyogenic function of H19 described by Huang and coworkers (42) but point to a different molecular mechanism independent of let-7 modulation and dependent on H19 ability to favor the mRNA decay-promoting function of KSRP in undifferentiated C2C12 cells. Further, our experiments cannot exclude that, in the course of myogenic differentiation, miR-675–5p and miR-675–3p might exert a promyogenic function as proposed by Dutta and coworkers (25).
The analysis of the noncoding transcriptome has suggested that the expression of lncRNAs is more cell type-restricted than the expression of protein-coding genes (43). This, together with our evidence that KSRP associates with distinct lncRNAs in other cell types (Fig. S4G), suggests the possibility that the role played by H19 in C2C12 cells to modulate KSRP function might be operated by different lncRNAs in other cell types.
Our work suggests a cell-specific role for select lncRNAs in amplifying and consolidating the function of RBPs ultimately controlling cell fate and differentiation programs.
Materials and Methods
HITS-CLIP Experiments.
To perform HITS-CLIP experiments, we adopted the procedure used by Yeo and coworkers (44) with the following modifications. Subconfluent C2C12 cells (16 × 100 mm dishes per experimental point) were UV-irradiated (120 mJ/cm2, 254 nm) at 4 °C. Cross-linked lysates were treated with RNase I (Ambion). Immunoprecipitations were carried out by using the anti-KSRP polyclonal antibody (9) or the correspondent preimmune serum. A detailed protocol and a list of linkers and primers used will be provided upon request.
Cell Cultures and Transfections.
Murine mesenchymal C2C12 cells [CRL-1772, lot no. 58236521; American Type Culture Collection (ATCC)] were cultured in DMEM plus 20% (vol/vol) FBS (i.e., GM). Myogenic differentiation of C2C12 cells was induced by incubation in DMEM plus 2% (vol/vol) horse serum (i.e., DM). Osteoblastic differentiation of C2C12 cells was induced by the addition of 300 ng/mL of recombinant BMP2 (R&D Systems). HEK-293 cells (ATCC) were cultured in DMEM plus 10% (vol/vol) FBS.
C2C12 cells were transfected by using the Nucleofector II (Amaxa) according to the manufacturer’s instructions, and, to generate stable transfectants, Puromycin (Invivogen) was used at 1.5 μg/mL for selection. Pools of transfected cells were used for experiments. HEK-293 cells were transfected using Attractene transfection Reagent (Qiagen). Mouse embryonic fibroblasts (MEFs) were prepared from WT and Ksrp−/− mice as described previously (45).
Plasmids, Recombinant Proteins, and Antibodies.
To generate pTAG2B-H19 expression vector, a fragment of 2,166 nt from murine H19 obtained by RT-qPCR was cloned in the EcoRI/SalI sites of pCMV-TAG2B plasmid (Agilent). To generate pTAG2B-myog 3′UTR expression vector, a fragment of 595 nt from murine Myogenin 3′ UTR obtained by RT-qPCR was cloned in the SalI/XhoI sites of pCMV-TAG2B plasmid. To generate pTAG2B-E3 expression vector, a fragment of 122 nt of the murine E3 region from Pitx2 locus (22) obtained by RT-qPCR was cloned in the SalI/XhoI sites of pCMV-TAG2B plasmid. To generate the pTAG2B-MS2-12X vector, a fragment encompassing 12 MS2 binding sites was excised from the pSLMS2-12X (Addgene) and cloned in the EcoRI/EcoRV sites of pCMV-TAG2B. To generate the pTAG2B-MS2-12XH19 expression plasmid, a fragment of 1,695 nt from murine H19 was obtained by RT-qPCR and cloned in the EcoRV/XhoI sites of the pTAG2B-MS2-12X plasmid. All plasmids were sequenced before their use. pcDNA3-Flag-KSRP, pCMV-TAG2B-KSRP, pCMV-TAG2B-KSRP(S193D), pCMV-TAG2B-KH1GDDG, pCMV-TAG2B-KH2GDDG, pCMV-TAG2B-KH3GDDG, pCMV-TAG2B-KH4GDDG, pcDNA-3-myrAKT2, pcDNA3-MKK6EE, pcDNA3-EXOSC2, and pcDNA3-EXOSC5 plasmids were described elsewhere (13, 14, 21, 28, 46). Adenoviral vector expressing myristoylated AKT2 and the respective negative control were from Vector Biolabs.
GST-KSRP and GST-KSRP(S193A) were previously described (14). Affinity-purified rabbit polyclonal anti-KSRP antibody and the corresponding preimmune serum were previously described (9); anti–phospho-AKT and anti–phospho-p38 were from Cell Signaling Technology, M2 anti-Flag antibody was from Sigma.
Gene Silencing.
siRNAs used to knock down murine H19 were from Ambion (38). GAPmeRs used to knock down murine H19 expression were from Exiqon (Table S1 shows sequences). KSRP silencing was obtained as previously described (13). Anti-miR-675–5p, anti-miR-675–3p, mature miR-675–5p, and mature miR-675–3p were from Qiagen.
While a list of primers used in RT-qPCR analysis is provided in Table S2, bioinformatic analysis, RT-qPCR, pri-miR in vitro processing assays, RNA in vitro degradation, RIP assays, nuclear and cytoplasmic extracts preparation, MS2 precipitation, and EMSA are described in detail in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. Gene Yeo (University of California at San Diego) for sharing his detailed HITS-CLIP protocol and for discussions and Dr. Myriam Gorospe (National Institute on Aging) for discussion and reagents. This project has been supported in part by Associazione Italiana per la Ricerca sul Cancro Investigator Grant 10090, Association for International Cancer Research Grant 10-0527, Ministero della Salute Grant 129/RF-2010-2306205 (to R.G.), and Medical Research Council Grant MC_PC_13051 (to A.R.).
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415098111/-/DCSupplemental.
References
- 1.Fabian MR, Sonenberg N, Filipowicz W. Regulation of mRNA translation and stability by microRNAs. Annu Rev Biochem. 2010;79:351–379. doi: 10.1146/annurev-biochem-060308-103103. [DOI] [PubMed] [Google Scholar]
- 2.Winter J, Diederichs S. MicroRNA biogenesis and cancer. Methods Mol Biol. 2011;676:3–22. doi: 10.1007/978-1-60761-863-8_1. [DOI] [PubMed] [Google Scholar]
- 3.Cech TR, Steitz JA. The noncoding RNA revolution-trashing old rules to forge new ones. Cell. 2014;157(1):77–94. doi: 10.1016/j.cell.2014.03.008. [DOI] [PubMed] [Google Scholar]
- 4.Wang KC, Chang HY. Molecular mechanisms of long noncoding RNAs. Mol Cell. 2011;43(6):904–914. doi: 10.1016/j.molcel.2011.08.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Yoon JH, Abdelmohsen K, Gorospe M. Posttranscriptional gene regulation by long noncoding RNA. J Mol Biol. 2013;425(19):3723–3730. doi: 10.1016/j.jmb.2012.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Fatica A, Bozzoni I. Long non-coding RNAs: New players in cell differentiation and development. Nat Rev Genet. 2014;15(1):7–21. doi: 10.1038/nrg3606. [DOI] [PubMed] [Google Scholar]
- 7.Prensner JR, Chinnaiyan AM. The emergence of lncRNAs in cancer biology. Cancer Discov. 2011;1(5):391–407. doi: 10.1158/2159-8290.CD-11-0209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Gherzi R, Chen C-Y, Ramos A, Briata P. KSRP controls pleiotropic cellular functions. Semin Cell Dev Biol. 2014;34C:2–8. doi: 10.1016/j.semcdb.2014.05.004. [DOI] [PubMed] [Google Scholar]
- 9.Gherzi R, et al. A KH domain RNA binding protein, KSRP, promotes ARE-directed mRNA turnover by recruiting the degradation machinery. Mol Cell. 2004;14(5):571–583. doi: 10.1016/j.molcel.2004.05.002. [DOI] [PubMed] [Google Scholar]
- 10.Trabucchi M, et al. The RNA-binding protein KSRP promotes the biogenesis of a subset of microRNAs. Nature. 2009;459(7249):1010–1014. doi: 10.1038/nature08025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Lin YY, et al. KSRP and MicroRNA 145 are negative regulators of lipolysis in white adipose tissue. Mol Cell Biol. 2014;34(12):2339–2349. doi: 10.1128/MCB.00042-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Chou CF, et al. KSRP ablation enhances brown fat gene program in white adipose tissue through reduced miR-150 expression. Diabetes. 2014;63(9):2949–2961. doi: 10.2337/db13-1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Briata P, et al. PI3K/AKT signaling determines a dynamic switch between distinct KSRP functions favoring skeletal myogenesis. Cell Death Differ. 2012;19(3):478–487. doi: 10.1038/cdd.2011.117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Pasero M, Giovarelli M, Bucci G, Gherzi R, Briata P. Bone morphogenetic protein/SMAD signaling orients cell fate decision by impairing KSRP-dependent microRNA maturation. Cell Reports. 2012;2(5):1159–1168. doi: 10.1016/j.celrep.2012.10.020. [DOI] [PubMed] [Google Scholar]
- 15.Ule J, Jensen K, Mele A, Darnell RB. CLIP: a method for identifying protein-RNA interaction sites in living cells. Methods. 2005;37(4):376–386. doi: 10.1016/j.ymeth.2005.07.018. [DOI] [PubMed] [Google Scholar]
- 16.Jensen KB, Darnell RB. CLIP: Crosslinking and immunoprecipitation of in vivo RNA targets of RNA-binding proteins. Methods Mol Biol. 2008;488:85–98. doi: 10.1007/978-1-60327-475-3_6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Gabory A, Ripoche MA, Yoshimizu T, Dandolo L. The H19 gene: Regulation and function of a non-coding RNA. Cytogenet Genome Res. 2006;113(1-4):188–193. doi: 10.1159/000090831. [DOI] [PubMed] [Google Scholar]
- 18.Cai X, Cullen BR. The imprinted H19 noncoding RNA is a primary microRNA precursor. RNA. 2007;13(3):313–316. doi: 10.1261/rna.351707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gherzi R, et al. The RNA-binding protein KSRP promotes decay of beta-catenin mRNA and is inactivated by PI3K-AKT signaling. PLoS Biol. 2006;5(1):e5. doi: 10.1371/journal.pbio.0050005. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 20.Díaz-Moreno I, et al. Phosphorylation-mediated unfolding of a KH domain regulates KSRP localization via 14-3-3 binding. Nat Struct Mol Biol. 2009;16(3):238–246. doi: 10.1038/nsmb.1558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Briata P, et al. p38-dependent phosphorylation of the mRNA decay-promoting factor KSRP controls the stability of select myogenic transcripts. Mol Cell. 2005;20(6):891–903. doi: 10.1016/j.molcel.2005.10.021. [DOI] [PubMed] [Google Scholar]
- 22.Puri PL, Sartorelli V. Regulation of muscle regulatory factors by DNA-binding, interacting proteins, and post-transcriptional modifications. J Cell Physiol. 2000;185(2):155–173. doi: 10.1002/1097-4652(200011)185:2<155::AID-JCP1>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 23.Figueroa A, et al. Role of HuR in skeletal myogenesis through coordinate regulation of muscle differentiation genes. Mol Cell Biol. 2003;23(14):4991–5004. doi: 10.1128/MCB.23.14.4991-5004.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.van der Giessen K, Di-Marco S, Clair E, Gallouzi IE. RNAi-mediated HuR depletion leads to the inhibition of muscle cell differentiation. J Biol Chem. 2003;278(47):47119–47128. doi: 10.1074/jbc.M308889200. [DOI] [PubMed] [Google Scholar]
- 25.Dey BK, Pfeifer K, Dutta A. The H19 long noncoding RNA gives rise to microRNAs miR-675-3p and miR-675-5p to promote skeletal muscle differentiation and regeneration. Genes Dev. 2014;28(5):491–501.35. doi: 10.1101/gad.234419.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Briata P, et al. The Wnt/beta-catenin—>Pitx2 pathway controls the turnover of Pitx2 and other unstable mRNAs. Mol Cell. 2003;12(5):1201–1211. doi: 10.1016/s1097-2765(03)00407-6. [DOI] [PubMed] [Google Scholar]
- 27.Ruggiero T, et al. Identification of a set of KSRP target transcripts upregulated by PI3K-AKT signaling. BMC Mol Biol. 2007;8:28. doi: 10.1186/1471-2199-8-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hollingworth D, et al. KH domains with impaired nucleic acid binding as a tool for functional analysis. Nucleic Acids Res. 2012;40(14):6873–6886. doi: 10.1093/nar/gks368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.García-Mayoral MF, et al. The structure of the C-terminal KH domains of KSRP reveals a noncanonical motif important for mRNA degradation. Structure. 2007;15(4):485–498. doi: 10.1016/j.str.2007.03.006. [DOI] [PubMed] [Google Scholar]
- 30.Zhu M, et al. lncRNA H19/miR-675 axis represses prostate cancer metastasis by targeting TGFBI. FEBS J. 2014;281(16):3766–3775. doi: 10.1111/febs.12902. [DOI] [PubMed] [Google Scholar]
- 31.Zhang EB, et al. c-Myc-induced, long, noncoding H19 affects cell proliferation and predicts a poor prognosis in patients with gastric cancer. Med Oncol. 2014;31(5):914. doi: 10.1007/s12032-014-0914-7. [DOI] [PubMed] [Google Scholar]
- 32.Matouk IJ, et al. Oncofetal H19 RNA promotes tumor metastasis. Biochim Biophys Acta. 2014;1843(7):1414–1426. doi: 10.1016/j.bbamcr.2014.03.023. [DOI] [PubMed] [Google Scholar]
- 33.Rinn JL, Chang HY. Genome regulation by long noncoding RNAs. Annu Rev Biochem. 2012;81:145–166. doi: 10.1146/annurev-biochem-051410-092902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Cesana M, et al. A long noncoding RNA controls muscle differentiation by functioning as a competing endogenous RNA. Cell. 2011;147(2):358–369. doi: 10.1016/j.cell.2011.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Faghihi MA, et al. Expression of a noncoding RNA is elevated in Alzheimer’s disease and drives rapid feed-forward regulation of beta-secretase. Nat Med. 2008;14(7):723–730. doi: 10.1038/nm1784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Yoon JH, et al. LincRNA-p21 suppresses target mRNA translation. Mol Cell. 2012;47(4):648–655. doi: 10.1016/j.molcel.2012.06.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Gong C, Maquat LE. lncRNAs transactivate STAU1-mediated mRNA decay by duplexing with 3′ UTRs via Alu elements. Nature. 2011;470(7333):284–288. doi: 10.1038/nature09701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Simone LE, Keene JD. Mechanisms coordinating ELAV/Hu mRNA regulons. Curr Opin Genet Dev. 2013;23(1):35–43. doi: 10.1016/j.gde.2012.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Legnini I, Morlando M, Mangiavacchi A, Fatica A, Bozzoni I. A feedforward regulatory loop between HuR and the long noncoding RNA linc-MD1 controls early phases of myogenesis. Mol Cell. 2014;53(3):506–514. doi: 10.1016/j.molcel.2013.12.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Cammas A, et al. Destabilization of nucleophosmin mRNA by the HuR/KSRP complex is required for muscle fibre formation. Nat Commun. 2014;5:4190. doi: 10.1038/ncomms5190. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Keniry A, et al. The H19 lincRNA is a developmental reservoir of miR-675 that suppresses growth and Igf1r. Nat Cell Biol. 2012;14(7):659–665. doi: 10.1038/ncb2521. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kallen AN, et al. The imprinted H19 lncRNA antagonizes let-7 microRNAs. Mol Cell. 2013;52(1):101–112. doi: 10.1016/j.molcel.2013.08.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Derrien T, et al. The GENCODE v7 catalog of human long noncoding RNAs: Analysis of their gene structure, evolution, and expression. Genome Res. 2012;22(9):1775–1789. doi: 10.1101/gr.132159.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Zisoulis DG, et al. Comprehensive discovery of endogenous Argonaute binding sites in Caenorhabditis elegans. Nat Struct Mol Biol. 2010;17(2):173–179. doi: 10.1038/nsmb.1745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin WJ, et al. Posttranscriptional control of type I interferon genes by KSRP in the innate immune response against viral infection. Mol Cell Biol. 2011;31(16):3196–3207. doi: 10.1128/MCB.05073-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Lin WJ, Duffy A, Chen CY. Localization of AU-rich element-containing mRNA in cytoplasmic granules containing exosome subunits. J Biol Chem. 2007;282(27):19958–19968. doi: 10.1074/jbc.M702281200. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.