Significance
Within the cell nucleus, the genetic material is organized into loop domains that transiently bring together genes separated by various distances along linear DNA. We asked whether the insulin (INS) gene in human pancreatic beta cells, which secrete the insulin protein, makes contact with other genes that play some role in insulin function. We show that the INS promoter contacts the anoctamin 1 (ANO1) gene, located far away on the same chromosome, stimulating its expression, and that ANO1, a chloride ion channel protein, plays a role in insulin secretion. Measurements of long-range interactions detect regulatory pathways not evident from other kinds of studies. In this case, such experiments reveal previously unrecognized mechanisms that could affect susceptibility to human diabetes.
Keywords: chloride channel, diabetes, insulin secretion
Abstract
We used circular chromatin conformation capture (4C) to identify a physical contact in human pancreatic islets between the region near the insulin (INS) promoter and the ANO1 gene, lying 68 Mb away on human chromosome 11, which encodes a Ca2+-dependent chloride ion channel. In response to glucose, this contact was strengthened and ANO1 expression increased, whereas inhibition of INS gene transcription by INS promoter targeting siRNA decreased ANO1 expression, revealing a regulatory effect of INS promoter on ANO1 expression. Knockdown of ANO1 expression caused decreased insulin secretion in human islets, establishing a physical proximity-dependent feedback loop involving INS transcription, ANO1 expression, and insulin secretion. To explore a possible role of ANO1 in insulin metabolism, we carried out experiments in Ano1+/− mice. We observed reduced serum insulin levels and insulin-to-glucose ratios in high-fat diet–fed Ano1+/− mice relative to Ano1+/+ mice fed the same diet. Our results show that determination of long-range contacts within the nucleus can be used to detect novel and physiologically relevant mechanisms. They also show that networks of long-range physical contacts are important to the regulation of insulin metabolism.
Mammalian genomes are organized in the nucleus into megabase- and submegabase-sized topological domains (1–4). Genes and regulatory elements are engaged through chromatin interactions within and between these physical domains to form gene regulatory networks (5–8). This genome organization seems to play a role in gene regulation (9), given that expression of the genes within a domain appears to be positively correlated (2), and disruption of domain formation leads to deregulation of expression of genes within the domain (7). Physical interactions between gene promoters are widespread as well, and interacting promoters are capable of regulating one another’s activities in human cells (10).
Genetic linkage and association studies have revealed thousands of disease-associated loci in the human genome; however, the molecular mechanisms through which these loci contribute to disease susceptibility are largely unknown. Because most of these loci are either large in size or located far from coding genes, the target genes or regulatory elements ultimately contributing to disease susceptibility are not immediately obvious. For that reason, techniques for detecting contacts between distant genomic sites within the nucleus are valuable tools for identifying those targets. As we and others have shown previously (5, 11–15), chromatin conformation capture-based methods (3C, 4C, 5C, Hi-C, and Capture-C) can be used effectively to reveal physiologically significant contacts between distant regulatory elements and, in principle, to uncover gene regulatory pathways that could contribute to genetic susceptibility to human diseases.
In previous work, we used 4C-Seq analysis to detect the large-scale interaction landscape of the insulin (INS) gene locus in human pancreatic islets (14). That study uncovered a role for the INS promoter in the long-range regulation of SYT8, a gene that we identified as important for insulin secretion in human islets. In the case of SYT8, physical contact was influenced by glucose level. Our results suggested that the INS promoter can make physical contact with distant genes, stimulating their transcription and creating an INS promoter-associated regulatory network important for β cell function. We reasoned that, in addition to SYT8, separated by only ∼300 kb from the INS promoter, there should be other genes within these newly identified INS-associated loci that could play a role in the regulation of β cell function.
Here we report the identification of such a gene, coding for a calcium-activated chloride channel protein, anoctamin 1 (ANO1), located more than 68 Mb away from the INS gene on human chromosome 11. In the nuclei of pancreatic islet cells, the INS promoter contacts the ANO1 locus. We found that both the strength of the contact and the level of ANO1 expression were increased on the addition of glucose. Inhibiting ANO1 expression by siRNA in islets decreased insulin secretion, consistent with a role for ANO1 in insulin metabolism. Chemical inhibition and activation of ANO1 function had corresponding effects on insulin secretion. Similar observations of the effect of an ANO1 inhibitor have been reported recently (16).
Results
INS–ANO1 Contacts in Human Islets.
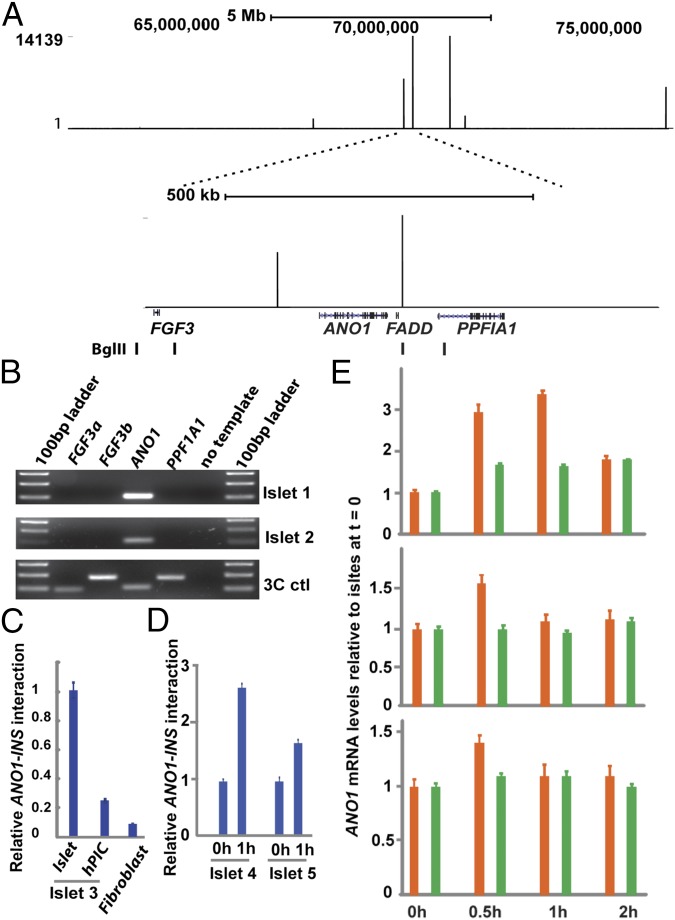
Examination of our previously reported 4C-Seq data in human islets for INS contacts with distant sites revealed that the INS promoter interacts strongly with a region on chromosome 11q13, at a site ∼68 Mb away from the INS gene, that was previously reported to be within a type 1 diabetes susceptibility locus, IDDM4 (Fig. 1A). Our 4C analysis revealed within this region two strong INS-interacting sites in the ANO1 (also called TMEM16A) gene locus, a 250-kb region flanked by FGF3 and FADD genes on each side (Fig. 1A). We found that in human islets, FGF3 mRNA is not detectable, whereas ANO1 and FADD genes are expressed at significant levels (SI Appendix, Fig. S1). ANO1 is a calcium-activated chloride channel protein (17–19); chloride ion levels in β cells are known to influence insulin secretion (20–23), and it has been proposed that one or more chloride channels contribute to the regulation of plasma membrane electrical activity and insulin secretion in β cells (24, 25). ANO1 protein and its closest homolog ANO2 are expressed mainly in the plasma membrane and act as membrane calcium-activated chloride channels (17–19), but whether other members of the ANO family are also plasma membrane chloride channels in vivo remains unclear (26, 27). In human islets, ANO1 is coexpressed with insulin, and its mRNA levels are twofold to threefold higher in isolated human islets than in the whole pancreas (28). In addition, the mouse ortholog Ano1 is expressed at high levels in the pancreas as well as in other electrolyte- transporting tissues, whereas Ano2 is not (29). Thus, we focused the remainder of the present study on the ANO1 gene.
Fig. 1.
4C-Seq analysis reveals the physical association of the ANO1 gene locus with the INS promoter in human pancreatic islets. (A) 4C-Seq analysis of INS-associated loci in the IDDM4 locus in chromosome 11 (NCBI36/hg18) (Upper) and the ANO1 gene locus within the IDDM4 (Lower). The four Bgl II sites used in 3C-PCR for confirmation of 4C results are shown. (B) 3C-PCR analysis of the interactions of the INS promoter with the ANO1/FADD genes and the nearby FGF3 and PPF1A1 genes in human islets cultured in the basal islet media containing 5.5 mM glucose. (C) TaqMan quantitative 3C analysis of the INS–ANO1 interactions in islets, islet-derived hIPCs, and primary human fibroblasts. Data are mean ± SEM (n = 8). (D) TaqMan quantitative 3C analysis of the INS–ANO1 interactions in islets from two donors before and after treatment with 25 mM glucose for 1 h. Data are mean ± SEM (n = 8). (E) qRT-PCR analysis of ANO1 (orange bar) and FADD (green bar) gene expression in islets from three donors before and after treatment with 25 mM glucose for the indicated times. The RNA levels are normalized to those of HPRT1. Plotted are mRNA levels relative to those at t = 0. Data are mean ± SEM (n = 8).
To confirm the 4C results, we carried out a 3C PCR analysis in human islets with specific primers designed to detect INS–ANO1 interactions. We examined interactions of INS with four Bgl II restriction sites within this region, including one INS-interacting site detected by 4C-Seq analysis (Fig. 1A). The results show that the INS promoter physically interacts with this Bgl II site in the ANO1 gene locus (Fig. 1B). In contrast, we detected no INS interactions with the neighboring FGF3 and PPF1A1 genes (Fig. 1B). DNA sequencing of the 3C PCR products and TaqMan quantitative 3C PCR analysis further confirmed the interactions between INS and ANO1 (SI Appendix, Fig. S2). These interactions were nearly fivefold weaker in human islet-derived precursor cells (hIPCs), fibroblast-like cells that largely have lost the ability to express insulin (30), and weaker still in human primary fibroblasts, compared with those detected in human islets (Fig. 1C). This finding suggests that the INS–ANO1 interactions are cell type-specific.
Our earlier studies of the interaction between INS and SYT8, which are separated by ∼300 kb, showed that the strength of the contact and the level of SYT8 expression increased when glucose was added to the islet culture medium. We asked whether the INS–ANO1 interaction, extending over more than 68 Mb, behaved similarly. Glucose treatment indeed increased the INS–ANO1 interactions in human islets from two donors (Fig. 1D). Time-course experiments also revealed a 1.4- to 3-fold increase in ANO1 gene expression at 30 min after the addition of glucose to human islets from three donors (Fig. 1E). In contrast, FADD gene expression was little affected in human islets from two of three donors, but enhanced but to a lesser extent in human islets from the third donor (Fig. 1E).
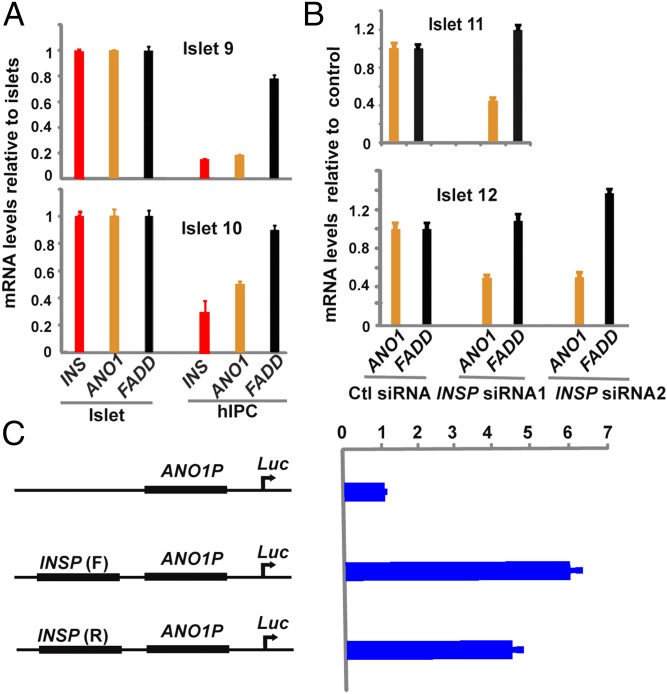
We previously reported that the INS promoter positively regulates expression of both SYT8 and TNNI2, two genes that physically associate with it (14). More recently, Li et al. (10) similarly found that in human cell lines, promoter–promoter interactions are widespread and that interacting promoters influence each other’s transcriptional activity. To determine whether the INS promoter could similarly regulate expression of genes in the ANO1 gene locus, we first compared the mRNA levels of INS, ANO1, and FADD genes in human islets before and after dedifferentiation into hIPCs. As reported earlier, INS gene expression was dramatically reduced in hIPCs compared with that observed in corresponding primary human islets from two different donors (Fig. 2A). ANO1 gene expression also was decreased by twofold to fivefold in hIPCs, whereas FADD gene expression was little affected. These results indicate that expression of ANO1, but not of FADD, is correlated with that of the INS gene in hIPCs.
Fig. 2.
The INS promoter positively regulates ANO1 gene expression in human islets. (A) qRT-PCR analysis of INS (red bar), ANO1 (orange bar), and FADD (black bar) gene expression in islets and islet-derived hIPCs from two donors. Plotted are mRNA levels in hIPCs relative to those in the corresponding islets. Data are mean ± SEM (n = 8). (B) qRT-PCR analysis of ANO1 (orange bar) and FADD (black bar) gene expression in islets from two donors treated with nontargeting control (Ctl) siRNA or one of the two siRNAs targeting to the INS promoter (INSP). The mRNA levels are plotted relative to the nontargeting control. (C) Promoter luciferase reporter assays in transfected MIN6 mouse β cells. The human ANO1 promoter luciferase constructs without (Top) or with insertion of human INS promoter in the forward (Middle) or reverse (Bottom) orientation was transfected into MIN6 cells. Plotted are the reporter luciferase activities relative to the ANO1 promoter luciferase construct without the INS promoter. Data are mean ± SEM (n = 12).
To directly investigate the role of the INS promoter in ANO1 expression, we treated human islets with siRNAs targeted to the INS promoter and measured ANO1 and FADD gene expression. We had reported earlier that INS gene transcription was reduced by 40–60% by this siRNA treatment (14). Quantitative RT-PCR (qRT-PCR) analysis using the same islet specimen described above showed that ANO1 gene expression was reduced by approximately twofold after the reduction in INS gene transcription (Fig. 2B), whereas FADD expression was slightly increased. Thus, similar to the response of SYT8 and TNNI2 genes reported earlier, ANO1 is also subject to transcriptional activation by the INS promoter in human islets.
Although the mechanism by which the INS promoter activates ANO1 gene expression remains to be determined, we asked whether the INS promoter, when coupled to the ANO1 promoter, could stimulate ANO1 promoter activity. As shown in Fig. 2C, the INS promoter, acting in cis, did increase ANO1 promoter activity by fourfold to sixfold in transfected mouse β cells in an orientation-independent manner. This in vitro assay suggests that the INS promoter might be capable of functioning similarly if brought physically close together with the ANO1 promoter via long-range contact, as seen in human islets (Fig. 1).
ANO1 and Insulin Secretion in Human Islets.
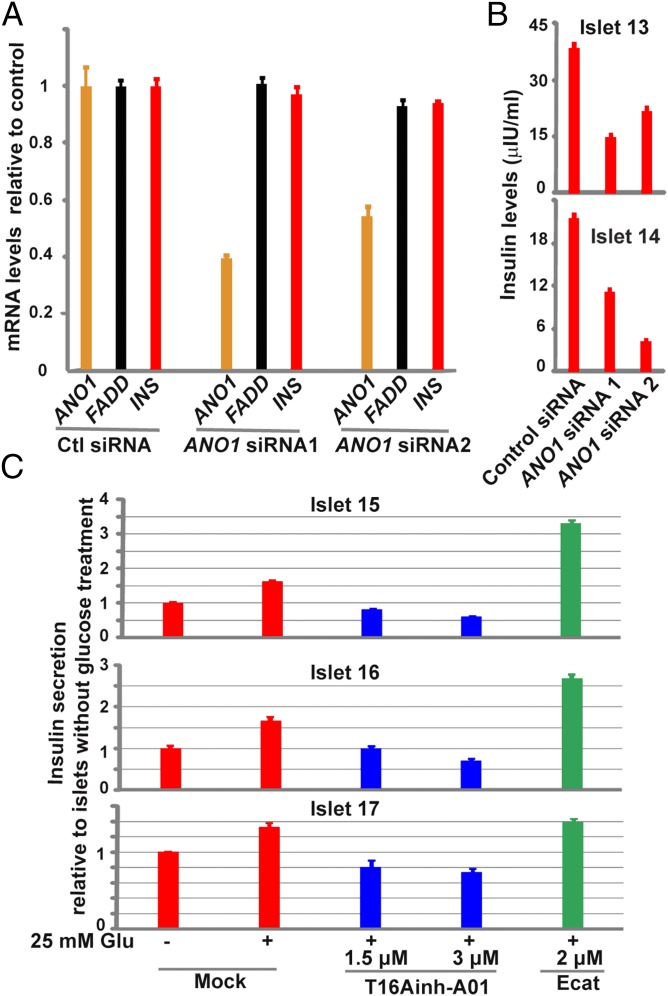
Chloride ions are transported by an active mechanism in pancreatic β cells (21). Glucose increases chloride efflux in β cells, which could contribute to membrane depolarization (20–23). Although the identity of the chloride channel(s) in β cells remains unclear, it has been proposed that certain unidentified chloride channels expressed in the plasma membrane might be involved in the regulation of electrical activity and insulin secretion in β cells (24, 25). Because glucose quickly increases ANO1 gene expression, we investigated whether ANO1, the membrane calcium-activated chloride channel gene known to be expressed at a significant level in human islets (Fig. 1E and SI Appendix, Fig. S1), could be involved in glucose-induced insulin secretion. We tested this by determining the effects on insulin secretion of siRNA-mediated knockdown of ANO1 gene expression. Treatment with siRNA has been shown to knock down ANO1 RNA and protein expression and to reduce cell membrane chloride currents in various human cell lines (19, 31). We treated human islets with ANO1 gene-specific siRNAs for 4.5 d. Incubation of human islets with one of two ANO1-specific siRNAs caused a 50–60% reduction in ANO1 gene expression (Fig. 3A), but had no effect on expression of INS or FADD.
Fig. 3.
ANO1 is a positive regulator of insulin secretion in human islets. (A) qRT-PCR analysis of ANO1 (orange bar), FADD (black bar), and INS (red bar) gene expression in islets treated with nontargeting control siRNA or with one of the two ANO1-gene specific siRNAs. The mRNA levels are plotted relative to nontargeting control. (B) ELISA analysis of insulin levels in the medium for islets from two donors treated separately as in A. The insulin levels were normalized to the number of islets needed to produce 1 µg of total RNA. Data are mean ± SEM (n = 4). (C) ELISA analysis of insulin levels in the medium for islets from three donors treated with mock (DMSO, red bars), 1.5 or 3 μM ANO1 channel inhibitor T16Ainh–A01 (blue bars), or 2 μM ANO1 channel activator Ecat (green bar). Insulin levels were normalized to the amount of genomic DNA made from the corresponding islets. Plotted are insulin levels relative to those in islets without 25 mM glucose treatment (column 1). Data are mean ± SEM (n = 4).
We then asked about the effects of this depletion of ANO1 gene expression on insulin secretion. Two independent experiments showed that, compared with islets treated with nontargeting control siRNA, glucose-induced insulin secretion from ANO1-depleted human islets was decreased by ∼45–80% (Fig. 3B), indicating that ANO1 is a positive regulator of insulin secretion in intact human pancreatic islets. A similar conclusion using an ANO1 chemical inhibitor has been reported by Edlund et al. (16) since the completion of our work.
ANO1 protein has been shown to physically interact with SNARE proteins, including Syntaxin 4 and Syntaxin 7 (32), which are important components of the insulin exocytosis machinery. Thus, reduced insulin secretion by ANO1 gene knockdown could be attributed to either its physical interaction with SNARE proteins or its chloride channel activity. To distinguish between these two possibilities, we treated human islets with a chemical ANO1 channel inhibitor (T16Ainh-A01) or an activator (Ecat). These small molecules have been shown to repress or stimulate, respectively, the chloride channel activity of ANO1 and ANO2, but not that of the CFTR chloride channel (33, 34). Three independent experiments showed that although glucose increased insulin secretion in mock (DMSO)-treated human islets, treatment of human islets with T16Ainh-A01 caused a dose-related reduction in insulin secretion in islets from all three donors (Fig. 3C). In the presence of 3 μM T16Ainh-A01, glucose failed to stimulate insulin secretion in human islets (compare columns 1 and 4, Fig. 3C). In contrast to the effects of treatment with T16Ainh-A01, addition of the ANO1 channel activator Ecat markedly increased glucose-induced insulin secretion in islets from two of three donors (Fig. 3C). These results suggest that the channel activity of ANO1 contributes to glucose-induced insulin secretion in human islets. They confirm a very recent study, published after the present work was completed, showing that T16Ainh-A01 inhibits glucose-induced insulin secretion in human and mouse islets (16).
Effects of Ano1 Knockout on Glucose Metabolism in Mice.
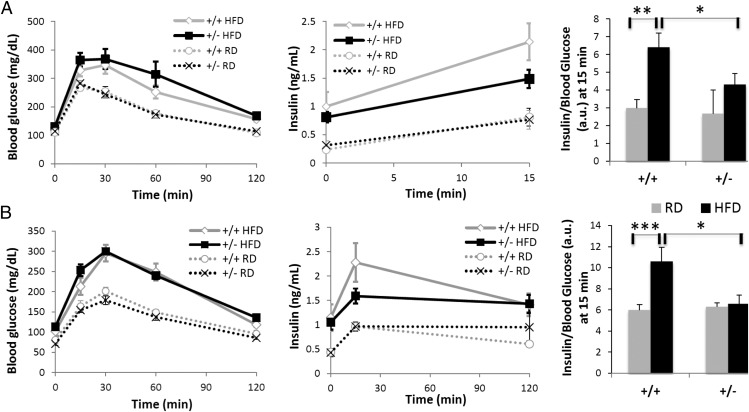
To further evaluate the physiological significance of the ANO1 gene in animals, we asked whether Ano1 deficiency affects insulin secretion and glucose metabolism in mice. Mice deficient for Ano1 (Ano1−/−) have a reduced life span owing to abnormal tracheal development, with fewer than 10% of mice surviving past postnatal day 10. This low survival rate made it difficult to determine whether the fluctuations in serum insulin and glucose levels observed in some Ano1−/− KO mice compared with their control littermates were a consequence of the pups’ poor nutrition or a direct cause of the absence of Ano1 in the pancreas. To get around this issue, we examined the phenotype of adult heterozygous (Ano1+/−) male mice fed regular chow or a high-fat diet (HFD) for 3 mo. The HFD induced obesity and glucose intolerance in both Ano1+/+ and Ano1+/− mice; however, there was no difference in body weight, fat mass, lean mass, randomly fed glucose and lipid levels, or insulin tolerance between the two genotypes (Fig. 4 and SI Appendix, Fig. S3 and Table S2). Interestingly, after 4 wk of HFD feeding, Ano1+/− mice showed significantly reduced serum insulin levels and insulin-to-glucose ratios compared with WT controls (SI Appendix, Table S2), suggesting a defect in insulin secretion. A similar trend was observed after 3 mo of HFD feeding.
Fig. 4.
Impaired insulin response to a glucose load in HFD-fed Ano1+/− mice. Ano1+/+ and Ano1+/− mice fed either a regular diet (RD; n = 8–9) or an HFD (n = 9) were subjected to a GTT after 5 wk (A) or 13 wk (B) of challenge. After an overnight fast, mice were injected i.p. with 2 mg/g glucose. Blood glucose (Left) was measured before the injection (t0) and at 15, 30, 60, 120 min after the injection. Plasma insulin (Center) was measured at t0 and at 15 and 120 min after the injection. Values are presented with respect to time (Left and Center) and as a ratio (a.u., arbitrary unit) for the 15-min time point (Right). *P ≤ 0.05; **P ≤ 0.01; ***P ≤ 0.005, t test.
Glucose tolerance tests (GTTs) revealed that HFD-fed Ano1+/− mice had an impaired early response to glucose compared with Ano1+/+ mice fed the same diet (Fig. 4A). At 15 min after glucose administration, blood glucose levels were higher and insulin levels were lower in HFD-fed Ano1+/− mice than in HFD-fed Ano1+/+ mice, resulting in a statistically lower insulin-to-glucose ratio in the former (Fig. 4A, Right). This difference persisted at that time point over the 13 wk of challenge (Fig. 4B). A three-way ANOVA analysis of the GTT results revealed a significant interaction effect of genotype and diet on blood glucose [F(1,62) = 6.28; P < 0.05]. Although supportive of a functional link between Ano1 and insulin secretion, the interaction effects of genotype and diet on insulin [F(1,62) = 2.82; P < 0.1], as well as the insulin-to-glucose ratio [F(1,62) = 2.92; P < 0.1] did not reach statistical significance (SI Appendix, Table S3). A post hoc analysis of the ANOVA results further confirmed the cumulative effect of genotype and diet on insulin and glucose levels, separately or as a ratio, at 5 and 13 wk of challenge (starred cells in SI Appendix, Table S3). Taken together, these results thus indicate that, although subtle and restricted to certain challenging conditions, changes in the insulin response to glucose in Ano1+/− mice reveal the existence of a conserved, functional link between calcium-activated chloride channel Ano1 and insulin secretion.
Discussion
Here we report that the INS promoter interacts with and affects the expression of the calcium-activated chloride channel gene ANO1 (or TMEM16A) in human islets, and that ANO1 in turn is directly involved in control of insulin secretion. Our results show that long-range interactions, in this case over distances of more than half a chromosome, can have significant regulatory consequences. They also show that 3C and 4C methods can serve as tools to detect such interactions, as we showed earlier for INS and SYT8 (14). There is considerable evidence in other systems that such interactions may have important regulatory consequences (5, 10, 12, 13, 15). We find that INS–ANO1 contacts are strengthened by the addition of glucose to islets, and that this is accompanied by an increase in ANO1 gene expression. In contrast, inhibition of INS gene transcription by targeting its promoter leads to a decrease in ANO1 expression. Thus, our results suggest that, in addition to SYT8, ANO1 is a downstream target regulated from a distance by the INS promoter in human islets.
ATP-sensitive potassium channels (KATP channels) are essential for the regulation of electrical activity and oscillatory Ca2+ signaling that subsequently induces insulin secretion in pancreatic β cells. KATP channels are not the sole mechanism for the regulation of these complex processes, however. Glucose still induces electrical activity and insulin secretion even when KATP channels are inactivated (24, 25, 35, 36). Chloride levels in β cells are known to be important for insulin secretion, and chloride channel(s) have been proposed as part of the background channels contributing to the regulation of electrical activity and insulin secretion in β cells, although the identity of such a channel remains elusive (24, 25). It has been reported that ANO1 knockdown reduces chloride ion transport in diverse cell types (37), although islets have not been studied. Quite recently, human islet studies using an ANO1 chemical inhibitor have suggested a role for ANO1 in insulin secretion (16). Our similar results obtained by siRNA-mediated ANO1 gene knockdown in human islets, a more direct approach, lends further support to this conclusion.
ANO1 is located within a previously reported type 1 diabetes susceptibility locus more than 68 Mb away from the INS gene (38–42), although recent genome-wide association studies (GWAS) have not confirmed this association (43). Nonetheless, we note that ANO1 (TMEM16A) overexpression has been shown to suppress proinflammatory cytokine expression in human cystic fibrosis bronchial epithelia (44). Another study identified an activated immune response specific to the pancreas in newborn pigs with cystic fibrosis (45). It seems possible that defects in the ANO1 gene or in its expression in pancreatic islets could affect cytokine expression and evoke an immune response resulting in β cell death.
Our results demonstrate that ANO1 has a positive role in insulin secretion, suggesting that it contributes to β cell function. Furthermore, the interaction between the INS promoter and the ANO1 gene provides an example of a network system, based on long-range physical contacts, for the regulation of β cell function. Because the physical interactions are affected by glucose levels, this regulatory network might act in response to glucose to integrate various environmental and cellular signals in human β cells. It seems reasonable to expect that other such networks involving physical contact and regulatory interaction with INS exist, and that their identification may shed light on both normal and abnormal function in human pancreatic β cells.
Materials and Methods
Culture of Human Islets and hIPCs.
Human pancreatic islets from independent cadaver donors were obtained through the Integrated Islet Distribution Program. Human islets, hIPCs, and normal human primary fibroblasts were cultured essentially as described previously (14). Human islets were maintained in the basal islet medium containing 5.5 mM d-glucose (99-663-cv; Cellgro) except in the glucose treatment experiments, in which additional 25 mM d-glucose was added to make the stimulating islet medium mimic a supraphysiological condition, allowing examination of the transient effects of glucose on insulin secretion and gene regulation.
4C-Seq and 3C Analyses.
4C-Seq and 3C analyses of INS-associated loci in human islets have been reported previously (14).
ANO1 Gene Knockdown in Intact Human Islets.
Human islets were mixed with 1 μM nontargeting control siRNA duplexes (D-001910–10; Dharmacon) or either one of the two ANO1 gene-specific siRNA duplexes (5′-CCAUUAUGCAGGGAAUAUU-3′ and 5′-GGCUGAUCUUCAAGGCUUU-3′), and cultured at 1,000 islet equivalents (IEQ)/mL of the basal islet medium for 4 d, 20 h. siRNA-treated islets on cell strainers were washed twice with fresh islet medium and cultured in the fresh medium for 6 h before total cellular RNAs were prepared.
qRT-PCR Analysis.
SYBR Green qRT-PCR analyses were performed as described previously (14). The steady-state levels of ANO1, FADD, and INS mRNAs were determined by qRT-PCR and normalized to those of HPRT1, whose expression is stable in human islets before and after glucose treatment (SI Appendix, Fig. S4).
Analysis of Insulin Secretion in Human Islets.
siRNA-treated islets were cultured in stimulating islet medium for 1 h. The supernatants were collected for measurement of the insulin levels using a human insulin ELISA (IS130D; Calbiotech), and the pellets of human islets were used to prepare total cellular RNA. Human islets were also treated for 3 h or overnight with mock (DMSO), 1.5 or 3 μM ANO1 channel inhibitor T16Ainh-A01, or 2 μM ANO1 channel activator Ecat.
Supplementary Material
Acknowledgments
We thank Tatyana Chanturiya and Ruifeng Teng [National Institute of Diabetes and Digestive and Kidney Diseases (NIDDK) Mouse Metabolic Core] and Huiyan Lu and Jennifer Portas (NIDDK Laboratory of Animal Sciences) for their technical assistance. We thank Dr. A. S. Verkman for providing ANO1 inhibitors and activators. We also thank Dr. Jason Rock for kindly providing Ano1+/− mice. This work was supported by the NIDDK’s Intramural Research Program.
Footnotes
The authors declare no conflict of interest.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1419240111/-/DCSupplemental.
References
- 1.Lieberman-Aiden E, et al. Comprehensive mapping of long-range interactions reveals folding principles of the human genome. Science. 2009;326(5950):289–293. doi: 10.1126/science.1181369. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Hou C, Li L, Qin ZS, Corces VG. Gene density, transcription, and insulators contribute to the partition of the Drosophila genome into physical domains. Mol Cell. 2012;48(3):471–484. doi: 10.1016/j.molcel.2012.08.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Sexton T, et al. Three-dimensional folding and functional organization principles of the Drosophila genome. Cell. 2012;148(3):458–472. doi: 10.1016/j.cell.2012.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Dixon JR, et al. Topological domains in mammalian genomes identified by analysis of chromatin interactions. Nature. 2012;485(7398):376–380. doi: 10.1038/nature11082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dunham I, et al. ENCODE Project Consortium An integrated encyclopedia of DNA elements in the human genome. Nature. 2012;489(7414):57–74. doi: 10.1038/nature11247. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Sanyal A, Lajoie BR, Jain G, Dekker J. The long-range interaction landscape of gene promoters. Nature. 2012;489(7414):109–113. doi: 10.1038/nature11279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Nora EP, et al. Spatial partitioning of the regulatory landscape of the X-inactivation centre. Nature. 2012;485(7398):381–385. doi: 10.1038/nature11049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Cheutin T, Bantignies F, Leblanc B, Cavalli G. Chromatin folding: From linear chromosomes to the 4D nucleus. Cold Spring Harb Symp Quant Biol. 2010;75:461–473. doi: 10.1101/sqb.2010.75.029. [DOI] [PubMed] [Google Scholar]
- 9.Göndör A, Ohlsson R. Chromosome crosstalk in three dimensions. Nature. 2009;461(7261):212–217. doi: 10.1038/nature08453. [DOI] [PubMed] [Google Scholar]
- 10.Li G, et al. Extensive promoter-centered chromatin interactions provide a topological basis for transcription regulation. Cell. 2012;148(1-2):84–98. doi: 10.1016/j.cell.2011.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Hughes JR, et al. Analysis of hundreds of cis-regulatory landscapes at high resolution in a single, high-throughput experiment. Nat Genet. 2014;46(2):205–212. doi: 10.1038/ng.2871. [DOI] [PubMed] [Google Scholar]
- 12.Xu Z, Lefevre GM, Felsenfeld G. Chromatin structure, epigenetic mechanisms and long-range interactions in the human insulin locus. Diabetes Obes Metab. 2012;14(Suppl 3):1–11. doi: 10.1111/j.1463-1326.2012.01645.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.French JD, et al. GENICA Network kConFab Investigators Functional variants at the 11q13 risk locus for breast cancer regulate cyclin D1 expression through long-range enhancers. Am J Hum Genet. 2013;92(4):489–503. doi: 10.1016/j.ajhg.2013.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Xu Z, Wei G, Chepelev I, Zhao K, Felsenfeld G. Mapping of INS promoter interactions reveals its role in long-range regulation of SYT8 transcription. Nat Struct Mol Biol. 2011;18(3):372–378. doi: 10.1038/nsmb.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Harismendy O, et al. 9p21 DNA variants associated with coronary artery disease impair interferon-γ signalling response. Nature. 2011;470(7333):264–268. doi: 10.1038/nature09753. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Edlund A, Esguerra JL, Wendt A, Flodström-Tullberg M, Eliasson L. CFTR and Anoctamin 1 (ANO1) contribute to camp-amplified exocytosis and insulin secretion in human and murine pancreatic beta-cells. BMC Med. 2014;12(1):87. doi: 10.1186/1741-7015-12-87. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Schroeder BC, Cheng T, Jan YN, Jan LY. Expression cloning of TMEM16A as a calcium-activated chloride channel subunit. Cell. 2008;134(6):1019–1029. doi: 10.1016/j.cell.2008.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Yang YD, et al. TMEM16A confers receptor-activated calcium-dependent chloride conductance. Nature. 2008;455(7217):1210–1215. doi: 10.1038/nature07313. [DOI] [PubMed] [Google Scholar]
- 19.Caputo A, et al. TMEM16A, a membrane protein associated with calcium-dependent chloride channel activity. Science. 2008;322(5901):590–594. doi: 10.1126/science.1163518. [DOI] [PubMed] [Google Scholar]
- 20.Best L. Glucose-induced electrical activity in rat pancreatic beta-cells: Dependence on intracellular chloride concentration. J Physiol. 2005;568(Pt 1):137–144. doi: 10.1113/jphysiol.2005.093740. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Sehlin J. Interrelationship between chloride fluxes in pancreatic islets and insulin release. Am J Physiol. 1978;235(5):E501–E508. doi: 10.1152/ajpendo.1978.235.5.E501. [DOI] [PubMed] [Google Scholar]
- 22.Eberhardson M, Patterson S, Grapengiesser E. Microfluorometric analysis of Cl− permeability and its relation to oscillatory Ca2+ signalling in glucose-stimulated pancreatic beta-cells. Cell Signal. 2000;12(11-12):781–786. doi: 10.1016/s0898-6568(00)00122-4. [DOI] [PubMed] [Google Scholar]
- 23.Malaisse WJ, Zhang Y, Louchami K, Jijakli H. Stimulation by d-glucose of 36Cl− efflux from prelabeled rat pancreatic islets. Endocrine. 2004;25(1):23–25. doi: 10.1385/ENDO:25:1:23. [DOI] [PubMed] [Google Scholar]
- 24.Best L, Brown PD, Sener A, Malaisse WJ. Electrical activity in pancreatic islet cells: The VRAC hypothesis. Islets. 2010;2(2):59–64. doi: 10.4161/isl.2.2.11171. [DOI] [PubMed] [Google Scholar]
- 25.Henquin JC, Nenquin M, Ravier MA, Szollosi A. Shortcomings of current models of glucose-induced insulin secretion. Diabetes Obes Metab. 2009;11(Suppl 4):168–179. doi: 10.1111/j.1463-1326.2009.01109.x. [DOI] [PubMed] [Google Scholar]
- 26.Almaça J, et al. TMEM16 proteins produce volume-regulated chloride currents that are reduced in mice lacking TMEM16A. J Biol Chem. 2009;284(42):28571–28578. doi: 10.1074/jbc.M109.010074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Duran C, Hartzell HC. Physiological roles and diseases of Tmem16/Anoctamin proteins: Are they all chloride channels? Acta Pharmacol Sin. 2011;32(6):685–692. doi: 10.1038/aps.2011.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Hanzu FA, et al. Expression of TMEM16A and SLC4A4 in human pancreatic islets. Cell Physiol Biochem. 2012;29(1-2):61–64. doi: 10.1159/000337587. [DOI] [PubMed] [Google Scholar]
- 29.Schreiber R, et al. Expression and function of epithelial anoctamins. J Biol Chem. 2010;285(10):7838–7845. doi: 10.1074/jbc.M109.065367. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Gershengorn MC, et al. Epithelial-to-mesenchymal transition generates proliferative human islet precursor cells. Science. 2004;306(5705):2261–2264. doi: 10.1126/science.1101968. [DOI] [PubMed] [Google Scholar]
- 31.Thomas-Gatewood C, et al. TMEM16A channels generate Ca²+-activated Cl− currents in cerebral artery smooth muscle cells. Am J Physiol Heart Circ Physiol. 2011;301(5):H1819–H1827. doi: 10.1152/ajpheart.00404.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Perez-Cornejo P, et al. Anoctamin 1 (Tmem16A) Ca2+-activated chloride channel stoichiometrically interacts with an ezrin-radixin-moesin network. Proc Natl Acad Sci USA. 2012;109(26):10376–10381. doi: 10.1073/pnas.1200174109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Namkung W, Yao Z, Finkbeiner WE, Verkman AS. Small-molecule activators of TMEM16A, a calcium-activated chloride channel, stimulate epithelial chloride secretion and intestinal contraction. FASEB J. 2011;25(11):4048–4062. doi: 10.1096/fj.11-191627. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Namkung W, Phuan PW, Verkman AS. TMEM16A inhibitors reveal TMEM16A as a minor component of calcium-activated chloride channel conductance in airway and intestinal epithelial cells. J Biol Chem. 2011;286(3):2365–2374. doi: 10.1074/jbc.M110.175109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Szollosi A, Nenquin M, Aguilar-Bryan L, Bryan J, Henquin JC. Glucose stimulates Ca2+ influx and insulin secretion in 2-week-old beta-cells lacking ATP-sensitive K+ channels. J Biol Chem. 2007;282(3):1747–1756. doi: 10.1074/jbc.M609875200. [DOI] [PubMed] [Google Scholar]
- 36.Henquin JC, et al. In vitro insulin secretion by pancreatic tissue from infants with diazoxide-resistant congenital hyperinsulinism deviates from model predictions. J Clin Invest. 2011;121(10):3932–3942. doi: 10.1172/JCI58400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Huang F, Wong X, Jan LY. International Union of Basic and Clinical Pharmacology. LXXXV: Calcium-activated chloride channels. Pharmacol Rev. 2012;64(1):1–15. doi: 10.1124/pr.111.005009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nakagawa Y, et al. Bart’s-Oxford Family Study Group Fine mapping of the diabetes-susceptibility locus, IDDM4, on chromosome 11q13. Am J Hum Genet. 1998;63(2):547–556. doi: 10.1086/301974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Eckenrode S, et al. Fine-mapping of the type 1 diabetes locus (IDDM4) on chromosome 11q and evaluation of two candidate genes (FADD and GALN) by affected sibpair and linkage-disequilibrium analyses. Hum Genet. 2000;106(1):14–18. doi: 10.1007/s004399900186. [DOI] [PubMed] [Google Scholar]
- 40.Hashimoto L, et al. Genetic mapping of a susceptibility locus for insulin-dependent diabetes mellitus on chromosome 11q. Nature. 1994;371(6493):161–164. doi: 10.1038/371161a0. [DOI] [PubMed] [Google Scholar]
- 41.Davies JL, et al. A genome-wide search for human type 1 diabetes susceptibility genes. Nature. 1994;371(6493):130–136. doi: 10.1038/371130a0. [DOI] [PubMed] [Google Scholar]
- 42.Concannon P, et al. A second-generation screen of the human genome for susceptibility to insulin-dependent diabetes mellitus. Nat Genet. 1998;19(3):292–296. doi: 10.1038/985. [DOI] [PubMed] [Google Scholar]
- 43.Noble JA, Erlich HA. Genetics of type 1 diabetes. Cold Spring Harb Perspect Med. 2012;2(1):a007732. doi: 10.1101/cshperspect.a007732. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Veit G, et al. Proinflammatory cytokine secretion is suppressed by TMEM16A or CFTR channel activity in human cystic fibrosis bronchial epithelia. Mol Biol Cell. 2012;23(21):4188–4202. doi: 10.1091/mbc.E12-06-0424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Abu-El-Haija M, et al. An activated immune and inflammatory response targets the pancreas of newborn pigs with cystic fibrosis. Pancreatology. 2011;11(5):506–515. doi: 10.1159/000332582. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.