Significance
Much of the available information on NGF-induced neural cell differentiation has been first obtained by the study of a cultured cell line, the pheochromocytoma PC12. Using the pheochromocytoma PC12 cells, we now demonstrate two new aspects of the differentiation process, possibly important also in neurons: (i) the direct binding, activation, and signaling of the NGF receptor TrkA by a neural adhesion protein, L1CAM, when administered as a soluble construct and also when surface-exposed by adjacent cells; and (ii) the expression, in the PC12 exposed for a few hours to NGF treatment, of an exocytic vesicle, the enlargeosome, that cooperates with an endocytic vesicle in the enlargement of the cell surface, which is necessary for neurite outgrowth to take place.
Keywords: NGF receptor, adhesion protein signaling, plasma membrane expansion, d/A surface immunolabeling, VAMP4
Abstract
NGF binding to its protein kinase receptor TrkA is known to induce neurite outgrowth and neural cell differentiation. The plasma membrane expansion, necessary for the process, was shown to be contributed by the VAMP7-dependent exocytosis of endocytic vesicles. Working with wild-type PC12 (wtPC12), a cell model widely used to investigate NGF-induced neurite outgrowth, we found that a few hours of treatment with the neurotrophin (and to a lower extent with basic FGF and EGF) induces the appearance of enlargeosome vesicles competent for VAMP4-dependent exocytosis abundant in high REST-PC12 clones. Both the neurite length assay and the immunocytochemistry of enlargeosomes exocytosis revealed that activation of TrkA is induced not only by NGF, but also by the L1 adhesion protein, L1CAM, whose soluble construct binds the receptor with submicromolar affinity. In the intact wtPC12, the L1CAM construct induced autophosphorylation and internalization of TrkA followed by the activation of the PI3K, MEK, and PKCγ signaling cascades, analogous to the responses induced by NGF. Down-regulation of either VAMP7 or VAMP4 revealed the coparticipation of the two corresponding vesicles to the outgrowth responses induced by NGF and L1CAM. Finally, mixing experiments of wtPC12 cells rich in TrkA with high REST PC12 cells transfected with L1CAM documented the transactivation of the receptor by the adhesion protein surface-exposed in adjacent cells. In view of the known inhomogeneous surface distribution of both L1CAM and TrkA in various neural cells including neurons, their transcellular binding could be restricted to discrete sites, governing local signaling events distinct from those induced by soluble messengers.
The NGF-induced neurite outgrowth of wild-type PC12 (wtPC12) cells (1) is a well-known model of the analogous process occurring in neurons both during development and upon maturation (2–4). A key mechanism of the outgrowth is the traffic of specific membranes sustaining the expansion of the plasma membrane. Work from various laboratories has demonstrated the role in this process of exocytic vesicles of endosomal nature, characterized by markers such as the vSNARE VAMP7 (also known as Ti-VAMP), the Ca2+-sensing protein synaptotagmin VII, and several small GTPases of the Rho family (5–10). Whether these endosomes are the only vesicles sustaining the surface expansion of neurons and wtPC12 cells is, however, unclear. Studies in primary hippocampal neurons from VAMP7 knockout mice have shown neurite outgrowth to be reduced, however, only in part (10), suggesting the involvement of another vSNARE in the endosomal vesicles. Alternatively, the process could be sustained not only by endosome vesicles, but also by other types of vesicles also competent for exocytosis.
Work from our laboratory, carried out in REST PC12 clone defective of neurosecretion, very rich of the transcription repressor REST and transfected with the NGF receptor TrkA (PC12-TrkA clone) (11), had already revealed the participation in neurite outgrowth of another vesicle, the enlargeosome, exocytized by a VAMP4-depenent system (12). Enlargeosomes are lacking in growing wtPC12 cells; however, they have been reported to appear upon NGF treatment. Therefore, the enlargeosomes might be hypothesized to have also a role in neurite outgrowth in wtPC12 cells.
Upon enlargeosome fusion with the plasma membrane, a luminal marker protein of the vesicle, desmoyokin/Ahnak (d/A), is known to appear at the cell surface (11–13). Here we report that the enlargeosome exocytosis, revealed by the surface appearance of d/A, occurs during NGF treatment of wtPC12 and participates in the outgrowth of their neurites. We also report an almost unknown aspect of TrkA function, its activation by L1CAM, a neuronal adhesion protein of the Ig superfamily (14). The latter property of TrkA opened novel perspectives to its signaling and function, important not only in PC12 but also in neurons expressing the neurotrophin receptor.
Results
Enlargeosome Exocytosis in the NGF-Induced Neurite Outgrowth.
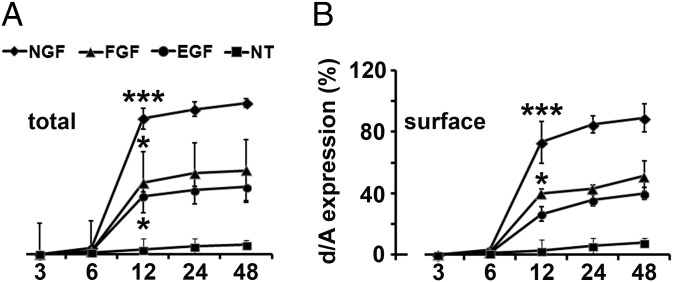
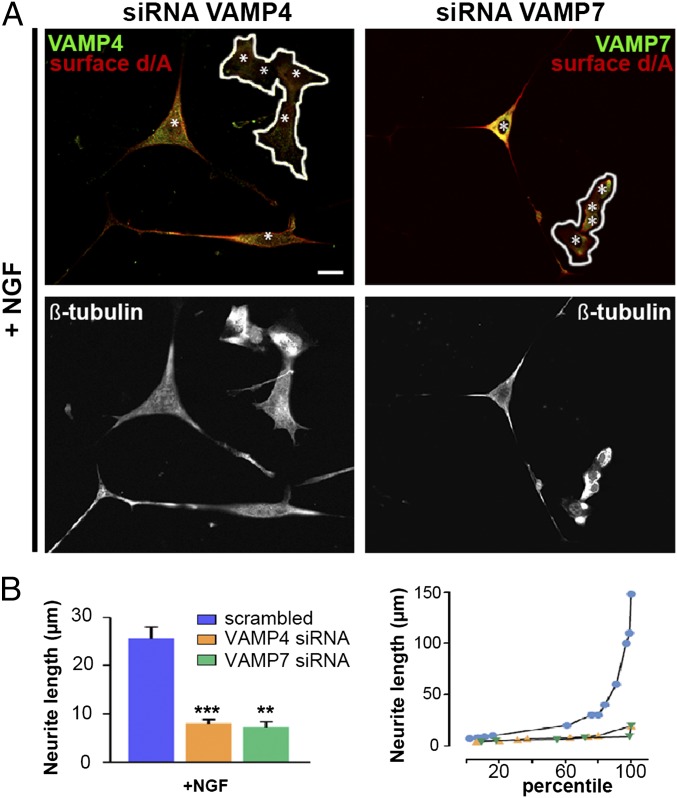
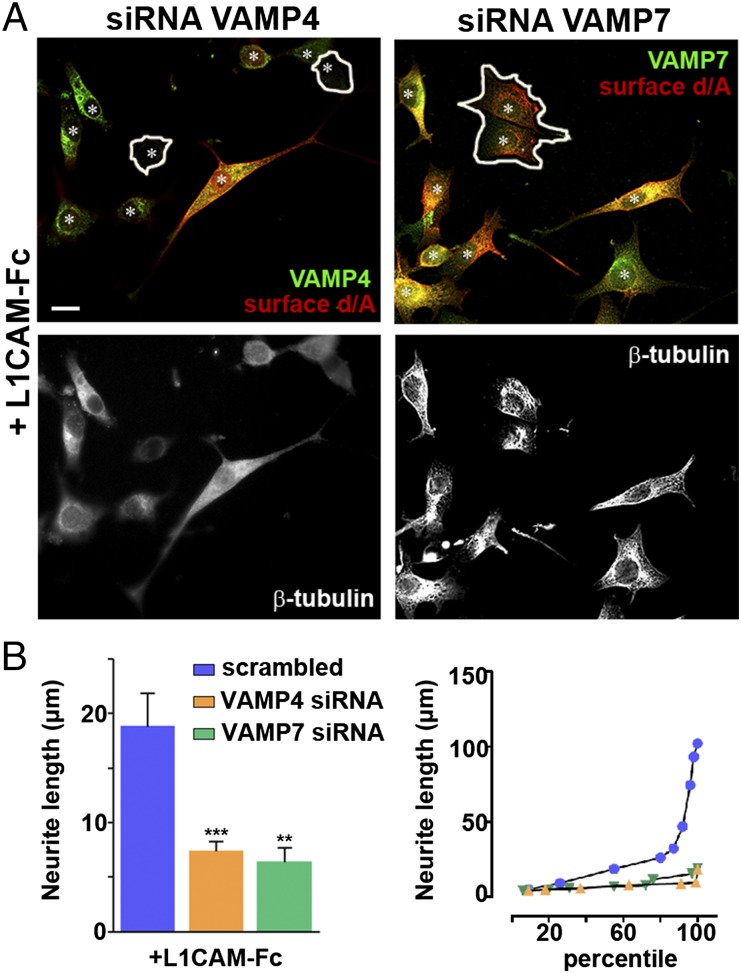
We first investigated whether enlargeosomes are expressed and exocytized by wtPC12 cells during treatment with NGF, basic FGF, or EGF (14–17). The enlargeosome marker d/A appeared in the cells, and at their surface, after 6 h of treatment, stronger with NGF than with the other factors (Fig. 1 A and B and Fig. S1). The d/A surface appearance was most likely due to the exocytosis of enlargeosomes. In fact, (i) in wtPC12 treated with NGF for 12 h, d/A was colocalized not with VAMP2, VAMP7, and VAMP8, the vSNAREs of other membrane fusions (Fig. S2A), but with VAMP4, the vSNARE of enlargeosomes (12); (ii) the surface appearance of d/A was much lower in the cells down-regulated for VAMP4 (Fig. S2B); (iii) the latter reduction was accompanied by a marked decrease (−70%) of the neurite length (Fig. 2A, Left and B), whereas the neurite number remained unchanged. Similar decreases of neurite length were observed in the cells down-regulated for VAMP7, the vSNARE of endosome vesicles (5, 6) (Fig. 2B), however, with no decrease of surface d/A (Fig. 2A, Right). The specificity of the d/A results was confirmed by the marked decrease of the immunolabeling in the cells down-regulated with the d/A shRNA (Fig. S3). The results confirm the role of enlargeosomes in neurite outgrowth, revealed by the d/A surface appearance depending on VAMP4, and not on endosome vesicles. The decrease of long neurite (>25 μm) compared with scramble-transfected wtPC12 cells (Fig. 2B, Right) documented the participation in neurite outgrowth of the exocytosis of both organelles, the endosome vesicles and the enlargeosomes.
Fig. 1.
Expression of d/A in wtPC12 cells treated with growth factors for 3–48 h. Total (Left) and surface (Right) d/A immunolabeling of wtPC12 treated with or without (NT) a growth factor (50 ng/mL) for 3–48 h before fixation. Data from at least 10 fields. Incubation time/growth factor are expressed as percent of the maximum immunolabeling. The significance of differences ± SD with respect to untreated cells is indicated by asterisks (*P < 0.05; **P < 0.01; ***P < 0.001).
Fig. 2.
vSNAREs VAMPs: distribution and function. (A, Top) wtPC12 cells transiently transfected with VAMP4 (Left) and VAMP7 (Right) siRNAs, stimulated with 100 ng/mL NGF for 48 h and immunolabeled for one of the two VAMPs (green) and for surface d/A (red). The same cells, immunolabeled for β-tubulin, are shown at Bottom. In the cells at Top that maintain high levels of either VAMP the surface d/A is strong; in those down-regulated for VAMP4 (Left, encircled), d/A is inappreciable; in those down-regulated for VAMP7 (Right), d/A is unchanged. Nuclei are marked by asterisks. (Scale bar: 10 μm.) (B, Left) Average length of neurites in the wtPC12 transfected with either a scrambled construct (blue), the siRNA of VAMP4 (orange), or VAMP7 (green), and then incubated with 100 ng/mL NGF for 48 h. Significance of the differences of neurite lengths ± SE between the siRNA of VAMPs transfected and nontransfected cells is given as in Fig. 1. **P < 0.01; ***P < 0.001. (B Right) Percentile distribution of neurite length in the three groups illustrated in B Left.
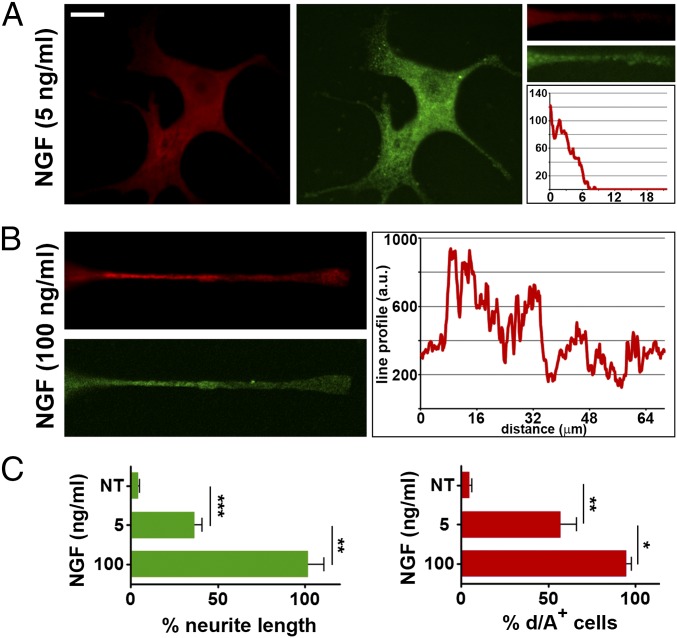
The next question was whether the NGF-induced exocytosis of enlargeosomes sustaining neurite outgrowth shares properties with the exocytosis of other organelles, especially the endosome vesicles. Previous extensive studies demonstrated that, in PC12 cells, neurite outgrowth occurs even when NGF is applied at low concentrations (4). Moreover, in PC12 cells stimulated with staurosporine, the endosome vesicles were shown to spread along the whole outgrowing neurites, preferentially at their top (5, 6). In the case of enlargeosomes, the situation was different (Fig. 3). Upon 72-h treatment with low NGF (2.5–5 ng/mL), the neurites only were slightly positive for surface d/A. Only with higher NGF (50–100 ng/mL) the surface d/A immunolabeling was widely distributed (Fig. 3). The enlargeosomes therefore appear to operate mostly in the reinforcement of the outgrowths induced by strong stimulations by NGF and possibly also by other agents.
Fig. 3.
Neurite outgrowth: Role of enlargeosomes. (A) wtPC12 cells stimulated for 72 h with 5 ng/mL NGF and then immunolabeled for d/A (surface, red) and β-tubulin (green) with focus also on neurites (small images on Right). The trace analysis of d/A immunolabeling in the neurite is shown on Right (cell body to the left). (B) Neurites as in A, images and traces, but from cells treated with 100 ng/mL NGF. (C) Quantized data from at least 10 fields for each value given. (Left) Average neurite length in wtPC12 cells treated with 5 or 100 ng/mL NGF, expressed as percentage of the latter. (Right) Percentage of the same cells treated as on Left, exhibiting surface d/A. Statistical significance of averages ± SD as in Fig. 1. *P < 0.05; **P < 0.01; ***P < 0.001.
TrkA Activation by the Adhesion Protein L1CAM.
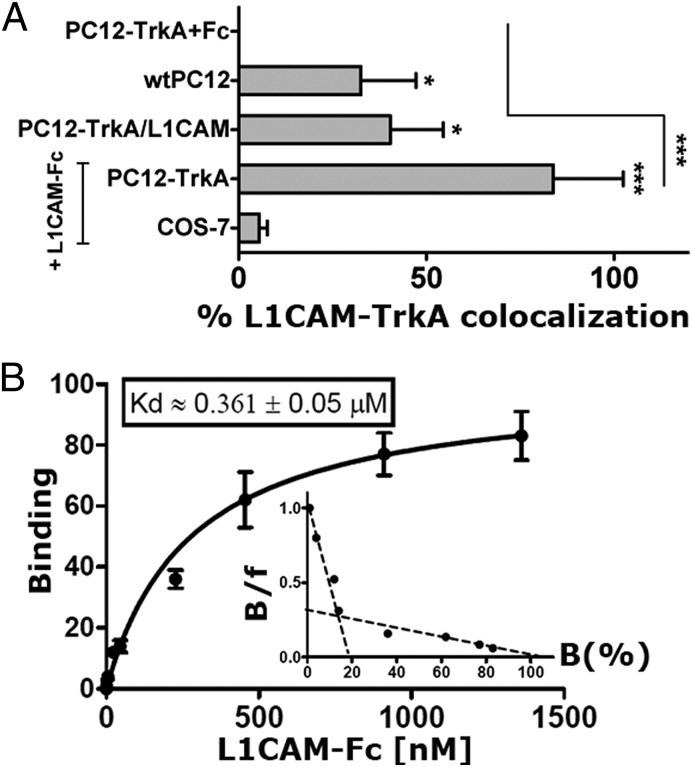
Our previous studies on PC12-TrkA (a high REST-PC12 clone stably transfected with TrkA) had revealed some neurite outgrowth induced by L1CAM activation of TrkA (14). In the new experiments, we used wtPC12 and PC12-TrkA, together with the nonneural COS-7 cells. The Western blots of Fig. S4A illustrate the expression of both L1CAM (similar levels in wtPC12 and PC12-TrkA/L1CAM stably transfected with the adhesion protein) and of TrkA (very high in the PC12-TrkA subclones, with and without L1CAM). Fig. 4A shows quantitative data on the coimmunolocalization of endogenous TrkA and L1CAM in wtPC12 and PC12-TrkA/L1CAM, compared with PC12-TrkA and COS-7 incubated for 2 h with a soluble L1CAM construct (L1CAM-Fc, the ectodomain of human L1CAM combined to an IgG Fc domain) (14, 18). Examples of colocalizations of endogenous TrkA and L1CAM are shown in the Fig. S4 B and C. Upon activation by L1CAM-Fc, TrkA underwent phosphorylation and cointernalization with the adhesion protein and the endosomal marker EEA1 (Fig. S4D). Moreover, in wtPC12 cells predifferentiated with NGF and then stripped mechanically of their neurites, L1CAM-Fc, analogous to reapplication of the neurotrophin, induced the transcription-independent outgrowth of another wave of neurites (Fig. S5). Incubation of the L1CAM-Fc construct with immobilized TrkA-Fc sheets (Fig. 4B) and vice versa revealed, in addition to a low affinity form, also a submicromolar form of binding. The specificity of this binding was confirmed by its decrease by 20 ng/mL of NGF and by the lack of effect of ICAM-1-Fc, a soluble adhesion construct similar in structure to L1CAM-Fc (Fig. S6A). The additive effect of NGF and L1CAM-Fc was shown by the much stronger d/A surface immunolabeling in the cells exposed to the two agents (20 ng/mL) together rather than separate (Fig. S6B). Taken together the results confirm the direct binding of L1CAM to TrkA, likely involving one (or more) surface-exposed Ig domain(s) of the adhesion protein.
Fig. 4.
L1CAM/TrkA interactions. (A) The quantized L1CAM/TrkA immunofluorescence colocalization data (at least 10 fields each) in wtPC12, PC12-TrkA stably transfected with L1CAM, PC12-TrkA, and COS-7 incubated with the water-soluble L1CAM-Fc construct. Examples of immunofluorescent cells corresponding to data shown here are given in Fig. S4 B and C. (B) The dose-dependent curve and the Scatchard analysis of the L1CAM-Fc binding to a plastic-immobilized TrkA water-soluble construct (TrkA-Fc), subtracted of the binding of the Fc construct. The Kd value given refers to the submicromolar binding. Binding of TrkA-Fc to plastic-immobilized L1CAM-Fc yielded analogous results. *P < 0.05; ***P < 0.001.
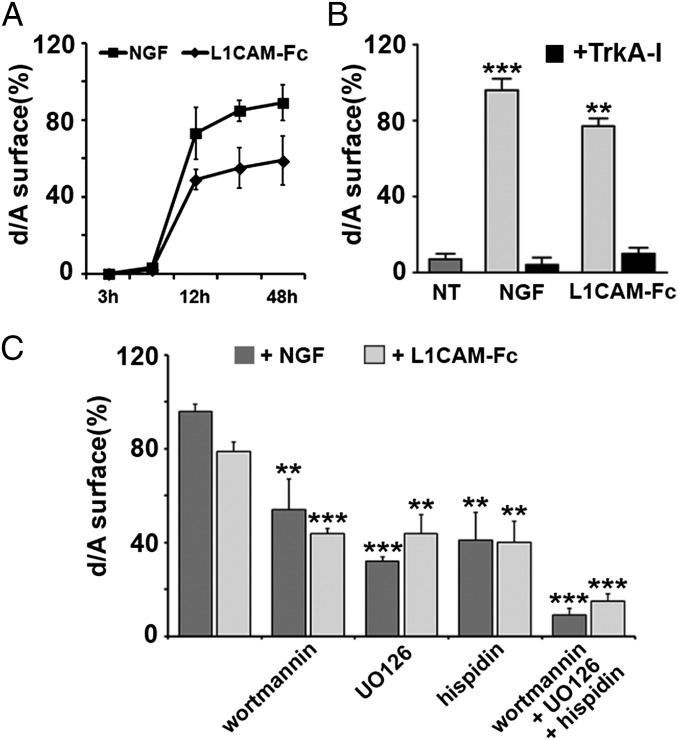
The wtPC12 enlargeosome exocytosis induced by 100 ng/mL L1CAM-Fc was compared with that induced by NGF. Fig. 5 A and B and Fig. S7A show that the d/A surface responses induced by the two agents were (i) quantitatively similar, (ii) additive, and (iii) blocked by a TrkA inhibitory drug, Calbiochem 648450 (14, 19). Moreover, the d/A response was partially inhibited by drugs that affect the various signaling cascades triggered by TrkA activation: wortmannin, a blocker of the PI3K cascade; UO126, of the MEK cascade; and hispidin, of the PKC cascade (20, 21). When the three drugs were administered together, the surface appearance of d/A induced by L1CAM-Fc and NGF was inhibited almost completely (Fig. 5C and Fig. S7B). The neurite outgrowth induced by L1CAM-Fc depended on the exocytosis of both enlargeosomes and endosome vesicles (Fig. 6A). In the cells down-regulated for either VAMP4 or VAMP7, the number of neurites induced by the L1CAM-Fc was unchanged, but their outgrowth was reduced to levels similar to those reported for NGF (compare Fig. 6 A and B, Left to Fig. 2 A and B, Left).
Fig. 5.
The d/A surface immunolabeling of wtPC12 cells, induced by NGF or L1CAM, depends on TrkA and on its three intracellular signaling cascades. (A) The time course of the surface d/A appearance in wtPC12 incubated for 3–48 h with NGF or L1CAM-Fc (50 ng/mL). (B) The surface d/A data (at least 10 fields each) in cells treated with NGF or L1CAM, alone or with the TrkA blocker Calbiochem 648450 (TrkA-I, 15 μM) administered for 2 h before and then during the 12-h stimulation. (C) Analogous data obtained with cascade blocker drugs administered according to the same protocol: the PI3K blocker wortmannin (0.5 μM), the MEK1 and 2 blocker UO126 (10 μM), the PKC blocker hispidin (5 μM), and the three drugs together. Significance of the results ± SD as in Fig. 1. **P < 0.01; ***P < 0.001. Examples of the immunofluorescence data summarized here are shown in the Fig. S7.
Fig. 6.
Role of enlargeosomes and VAMP7-rich vesicles in the neurite outgrowth induced in wtPC12 by 48-h treatment with L1CAM-Fc. (A) The immunolabeling for total VAMP4 or VAMP7 (green) and surface d/A (red) (Top) and for β-tubulin (Bottom) in wtPC12 cells down-regulated (encircled) for VAMP4 or VAMP7 and treated with L1CAM-Fc (100 ng/mL, 48 h). Asterisks mark nuclei. (B, Left) The average length of neurites outgrown from wtPC12 cells transiently transfected with siRNA, scrambled (blue), or specific for VAMP4 (orange) or VAMP7 (green), treated with L1CAM-Fc as in A (analysis of at least 10 fields per group). Statistical significances of the data and SE as in Fig. 1. **P < 0.01; ***P < 0.001. (B, Right) The same data illustrated as percentiles of neurite lengths.
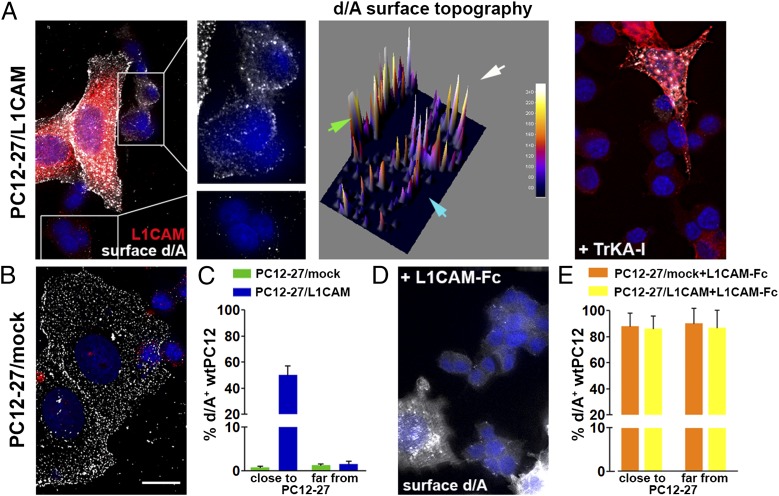
Can the activation of endogenous TrkA by L1CAM occur by direct interaction of two living cells? The cells used were wtPC12 and high REST-PC12 cells, which are very poor of TrkA and stably transfected with L1CAM (PC12-27/L1CAM; ref. 14), identified by their flat shape and high level of d/A (11, 13). Mixed cells seeded on slides coated with polyornithine, incubated for 24 h, were fixed, doubly immunolabeled for d/A and L1CAM, and analyzed at the surface level. When very close to each other, the cells of the two types were expected to interact, with direct binding of the L1CAM of PC12-27/L1CAM cells to the TrkA of wtPC12, and ensuing activation of enlargeosome exocytosis in the latter cells. In contrast, in the cells spread away, the direct L1CAM/TrkA binding and the enlargeosome exocytosis were unlikely. The results showed that ∼50% of the wtPC12 distributed at <10 μm distance from PC12-27/L1CAM cells exhibited clear d/A surface immunolabeling. In contrast, the cells away from the PC12-27/L1CAM were almost all negative (Fig. 7 A and C). The TrkA dependence of the results was confirmed by the blocker drug, Calbiochem 648450 (Fig. 7A, Right). The dependence of the short/long distance results on the L1CAM transstimulation was confirmed by the negative results obtained with PC12-27 cells transfected with a mock construct (Fig. 7 B and C) and by the positive results in almost 100% of the wtPC12 cells incubated with saturating concentrations of L1CAM-Fc (Fig. 7 D and E).
Fig. 7.
Surface d/A appearance triggered in wtPC12 cells by the intercellular interaction of their TrkA with the L1CAM of closely adjacent PC12-27/L1CAM cells. (A, Left) A flat PC12-27 cell, positive for d/A and stably transfected with L1CAM, surrounded by a few semispherical wtPC12 cells, all cultured for 12–18 h. Red, L1CAM; white dots, d/A. Enlargements of the cells in the rectangle, shown as immunolabeling and surface topography (green arrowhead, PC12-27/L1CAM cell; white and blue arrowheads, two wtPC12) appear in the middle. The wtPC12 cells proximal to the PC12-27/L1CAM cell exhibit numerous d/A surface puncta, whereas the cells away from the latter cells are negative. General inhibition of the response by the TrkA inhibitory drug (TrkA-I, 15 μM), even in the cells in close contact, is shown at Right. (B) Cocultures of wtPC12 with mock-transfected PC12-27 cells: no d/A immunolabeling on the wtPC12 cells even when in contact to the flat, intensely d/A dot-labeled PC12-27 cells. (C) Quantitation of the data in A and B Left: The d/A response is in ∼50% of the wtPC12 close-contact cells. D and E illustrate the state of the wtPC12 coseeded with PC12-27/L1CAM cells and then supplemented with soluble L1CAM-Fc (100 ng/mL). The surface positivity for d/A is evident in all wtPC12 cells, no matter whether seeded with the PC12-27/L1CAM or with PC12-27/mock-transfected cells. In A, B, and D, nuclei are stained blue by DAPI.
Discussion
PC12 neurite outgrowth induced by long-term treatment with NGF (1) is widely considered as a useful model of neural cell differentiation (2, 3). In our previous studies, (11–13) interest about this process was focused on the expansion of the plasma membrane necessary for the outgrowth to take place (22). In addition to the endocytic, VAMP7-positive vesicles identified by others (5, 6), we demonstrated the participation of a distinct class of exocytic vesicles, the enlargeosomes, expressed by high REST clones and not by resting wtPC12 cells (11–13). Here, we show that enlargeosomes appear, and are released by, a VAMP4-specific exocytosis, also in wtPC12 treated for more than 6 h with NGF. Interestingly, the exocytosis of enlargeosomes was found to require NGF concentrations higher than those known to stimulate the outgrowth process and the exocytosis of other organelles such as the endosome vesicles (5–8). The enlargeosomes seem therefore to play a role in neurite outgrowth only when the process is strongly stimulated. In conclusion, our results demonstrate and characterize for the first time, to our knowledge, the participation of enlargeosomes in the neurite outgrowth process of a WT neural cell type.
In most previous studies, outgrowth had been assayed by following neurite elongation. The demonstration that enlargeosomes are exocytized by wtPC12 cells long-term treated with NGF provided us with a new assay to investigate the process, i.e., the quantitation of surface immunocytochemistry of the specific marker d/A. The consistency of the results obtained by the new and the classical assays was essential to reveal an unexpected property of the TrkA receptor, its activation not only by NGF but also by L1CAM, an adhesion protein abundant in neural cells. In addition to its homophilic binding, L1CAM was already known to undergo heterophilic bindings to receptors, integrins, and other surface molecules. L1CAM was therefore already known to participate in a spectrum of signaling processes (23–25). The stimulation of neurite outgrowth and the reinforcement of the NGF effects, induced by the L1CAM-Fc construct (14), are shown here to depend on the submicromolar affinity binding of the extracellular domains of L1CAM and TrkA. This binding was shown to trigger the autophosphorylation and internalization of the receptor followed by the activation of its signaling cascades, the PI3K, MEK, and PKC cascades. The ensuing stimulation of neurite outgrowth, analogous to that induced by NGF, was sustained by the exocytosis of both endocytic and enlargeosome vesicles, dependent for their exocytosis of VAMP7 and VAMP4, respectively. Finally, by the study of mixed cell monolayers, we found outgrowth to be induced in wtPC12 cells by closely adjacent cells rich of L1CAM. In these conditions, it is quite likely that the two types of cells interact directly, creating the conditions for the specific, intercellular transactivation of TrkA by the surface L1CAM.
Although obtained in cultured PC12 cells, our results may be of importance also for tissue cells, especially neurons. In the rat brain the enlargeosomes, present in neurons before birth, disappear thereafter (13), most likely due to the extensive decrease of the transcription factor REST. In the mature neurons, however, REST is not stable but increases upon long-term stimulation and during aging (26, 27). Therefore, a participation of enlargeosomes in the traffic of adult neuron membranes cannot be excluded. TrkA and L1CAM are known to be concentrated at discrete sites of neural cells such as growth cones and some synapses (28–30). Activation of TrkA by L1CAM exposed by adjacent neurons could thus play important roles at critical sites, giving rise to long-term signals and contributing to the regulation of local structures and functions.
Materials and Methods
For an extended section, see SI Materials and Methods.
cDNA and siRNA Transfections.
The PcDNA3.1 plasmid of human L1CAM cDNA was a gift of Susan Kenwrick (Institute of Medical Research, Cambridge, UK). siRNAs of mouse VAMP4 (s171259), VAMP7 (s137023), and scrambled sequences were from Ambion. Cells transfected twice (24 h distance) with siRNA and scrambled constructs were treated with NGF or L1CAM-Fc. Transient cDNA transfections were carried out by using Lipofectamine 2000 (Invitrogen). Stably transfected cells were those of ref. 14. shRNA sequences against d/A were from Origene.
Neurite Outgrowth.
PC12 cells on polyornithine coverslips, transfected twice with VAMP4 or VAMP7 siRNA, were serum-starved (6 h) and treated with NGF or L1CAM-Fc (100 ng/mL, 48 h). After fixation and immunolabeling, they were observed under a Leica TCS SP2 confocal microscope. Neurite length were manually outlined in 10 randomly chosen fields (at least three experiments) and measured with the ImageJ software.
Supplementary Material
Acknowledgments
We thank Luigina Tagliavacca for starting the work, and to Susan Kenwrick, Michela Matteoli, and Giovanni Piccoli for the gift of plasmids. This work was supported in part by Telethon Grant GGGP09066.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1406097111/-/DCSupplemental.
References
- 1.Greene LA, Tischler AS. Establishment of a noradrenergic clonal line of rat adrenal pheochromocytoma cells which respond to nerve growth factor. Proc Natl Acad Sci USA. 1976;73(7):2424–2428. doi: 10.1073/pnas.73.7.2424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Tischler AS. Chromaffin cells as models of endocrine cells and neurons. Ann N Y Acad Sci. 2002;971(971):366–370. doi: 10.1111/j.1749-6632.2002.tb04498.x. [DOI] [PubMed] [Google Scholar]
- 3.Vaudry D, Stork PJ, Lazarovici P, Eiden LE. Signaling pathways for PC12 cell differentiation: Making the right connections. Science. 2002;296(5573):1648–1649. doi: 10.1126/science.1071552. [DOI] [PubMed] [Google Scholar]
- 4.Greene LA. Nerve growth factor prevents the death and stimulates the neuronal differentiation of clonal PC12 pheochromocytoma cells in serum-free medium. J Cell Biol. 1978;78(3):747–755. doi: 10.1083/jcb.78.3.747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Martinez-Arca S, Alberts P, Zahraoui A, Louvard D, Galli T. Role of tetanus neurotoxin insensitive vesicle-associated membrane protein (TI-VAMP) in vesicular transport mediating neurite outgrowth. J Cell Biol. 2000;149(4):889–900. doi: 10.1083/jcb.149.4.889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Martinez-Arca S, et al. A common exocytotic mechanism mediates axonal and dendritic outgrowth. J Neurosci. 2001;21(11):3830–3838. doi: 10.1523/JNEUROSCI.21-11-03830.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Arantes RM, Andrews NW. A role for synaptotagmin VII-regulated exocytosis of lysosomes in neurite outgrowth from primary sympathetic neurons. J Neurosci. 2006;26(17):4630–4637. doi: 10.1523/JNEUROSCI.0009-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Alberts P, et al. Cdc42 and actin control polarized expression of TI-VAMP vesicles to neuronal growth cones and their fusion with the plasma membrane. Mol Biol Cell. 2006;17(3):1194–1203. doi: 10.1091/mbc.E05-07-0643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Fujita A, et al. GTP hydrolysis of TC10 promotes neurite outgrowth through exocytic fusion of Rab11- and L1-containing vesicles by releasing exocyst component Exo70. PLoS ONE. 2013;8(11):e79689. doi: 10.1371/journal.pone.0079689. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Sato M, et al. The role of VAMP7/TI-VAMP in cell polarity and lysosomal exocytosis in vivo. Traffic. 2011;12(10):1383–1393. doi: 10.1111/j.1600-0854.2011.01247.x. [DOI] [PubMed] [Google Scholar]
- 11.Schulte C, Racchetti G, D’Alessandro R, Meldolesi J. A new form of neurite outgrowth sustained by the exocytosis of enlargeosomes expressed under the control of REST. Traffic. 2010;11(10):1304–1314. doi: 10.1111/j.1600-0854.2010.01095.x. [DOI] [PubMed] [Google Scholar]
- 12.Cocucci E, Racchetti G, Rupnik M, Meldolesi J. The regulated exocytosis of enlargeosomes is mediated by a SNARE machinery that includes VAMP4. J Cell Sci. 2008;121(Pt 18):2983–2991. doi: 10.1242/jcs.032029. [DOI] [PubMed] [Google Scholar]
- 13.Racchetti G, et al. Rapid neurite outgrowth in neurosecretory cells and neurons is sustained by the exocytosis of a cytoplasmic organelle, the enlargeosome. J Cell Sci. 2010;123(Pt 2):165–170. doi: 10.1242/jcs.059634. [DOI] [PubMed] [Google Scholar]
- 14.Tagliavacca L, Colombo F, Racchetti G, Meldolesi J. L1CAM and its cell-surface mutants: New mechanisms and effects relevant to the physiology and pathology of neural cells. J Neurochem. 2013;124(3):397–409. doi: 10.1111/jnc.12015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rydel RE, Greene LA. Acidic and basic fibroblast growth factors promote stable neurite outgrowth and neuronal differentiation in cultures of PC12 cells. J Neurosci. 1987;7(11):3639–3653. doi: 10.1523/JNEUROSCI.07-11-03639.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kulahin N, Li S, Kiselyov V, Bock E, Berezin V. Identification of neural cell adhesion molecule L1-derived neuritogenic ligands of the fibroblast growth factor receptor. J Neurosci Res. 2009;87(8):1806–1812. doi: 10.1002/jnr.22014. [DOI] [PubMed] [Google Scholar]
- 17.Islam R, Kristiansen LV, Romani S, Garcia-Alonso L, Hortsch M. Activation of EGF receptor kinase by L1-mediated homophilic cell interactions. Mol Biol Cell. 2004;15(4):2003–2012. doi: 10.1091/mbc.E03-05-0333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Roonprapunt C, et al. Soluble cell adhesion molecule L1-Fc promotes locomotor recovery in rats after spinal cord injury. J Neurotrauma. 2003;20(9):871–882. doi: 10.1089/089771503322385809. [DOI] [PubMed] [Google Scholar]
- 19.Lazaridis I, et al. Neurosteroid dehydroepiandrosterone interacts with nerve growth factor (NGF) receptors, preventing neuronal apoptosis. PLoS Biol. 2011;9(4):e1001051. doi: 10.1371/journal.pbio.1001051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Reichardt LF. Neurotrophin-regulated signalling pathways. Philos Trans R Soc Lond B Biol Sci. 2006;361(1473):1545–1564. doi: 10.1098/rstb.2006.1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Skaper SD. The biology of neurotrophins, signalling pathways, and functional peptide mimetics of neurotrophins and their receptors. CNS Neurol Disord Drug Targets. 2008;7(1):46–62. doi: 10.2174/187152708783885174. [DOI] [PubMed] [Google Scholar]
- 22.Chieregatti E, Meldolesi J. Regulated exocytosis: New organelles for non-secretory purposes. Nat Rev Mol Cell Biol. 2005;6(2):181–187. doi: 10.1038/nrm1572. [DOI] [PubMed] [Google Scholar]
- 23.Maness PF, Schachner M. Neural recognition molecules of the immunoglobulin superfamily: Signaling transducers of axon guidance and neuronal migration. Nat Neurosci. 2007;10(1):19–26. doi: 10.1038/nn1827. [DOI] [PubMed] [Google Scholar]
- 24.Schmid RS, Maness PF. L1 and NCAM adhesion molecules as signaling coreceptors in neuronal migration and process outgrowth. Curr Opin Neurobiol. 2008;18(3):245–250. doi: 10.1016/j.conb.2008.07.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Kiefel H, Pfeifer M, Bondong S, Hazin J, Altevogt P. Linking L1CAM-mediated signaling to NF-κB activation. Trends Mol Med. 2011;17(4):178–187. doi: 10.1016/j.molmed.2010.11.005. [DOI] [PubMed] [Google Scholar]
- 26.Pozzi D, et al. REST/NRSF-mediated intrinsic homeostasis protects neuronal networks from hyperexcitability. EMBO J. 2013;32(22):2994–3007. doi: 10.1038/emboj.2013.231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lu T, et al. REST and stress resistance in ageing and Alzheimer’s disease. Nature. 2014;507(7493):448–454. doi: 10.1038/nature13163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Julien J, et al. Trafficking of TrkA-green fluorescent protein chimerae during nerve growth factor-induced differentiation. J Biol Chem. 2003;278(10):8706–8716. doi: 10.1074/jbc.M202401200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Dequidt C, et al. Fast turnover of L1 adhesions in neuronal growth cones involving both surface diffusion and exo/endocytosis of L1 molecules. Mol Biol Cell. 2007;18(8):3131–3143. doi: 10.1091/mbc.E06-12-1101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nishida T, Yoshimura R, Endo Y. Three-dimensional distribution of TrkA neurotrophin receptors in neurite varicosities of differentiated PC12 cells treated with NGF determined by immunoelectron tomography. Cell Tissue Res. 2013;351(1):1–13. doi: 10.1007/s00441-012-1499-0. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.