Significance
Humans, like chimpanzees, engage in coalitionary violence: Members of both species coordinate lethal activity against conspecifics. The origin and adaptive functions of this behavior are poorly understood, and data from tribal populations are rare. We examine the composition of lethal coalitions from the Yanomamö, a tribal society in Amazonia. In contrast to chimpanzees, Yanomamö coalitions are composed of individuals from different lineages and natal communities. Many coalition partners are ideal marriage exchange partners. Men who kill together more often are more likely to live together in the same village later in life and to engage in marriage exchange. Our results highlight connections between coalitionary aggression and alliance formation and illuminate differences in social structure distinguishing humans from other primates.
Keywords: internal warfare, male coalitions, fraternal interest groups, strategic alliances, Yanomamö
Abstract
Some cross-cultural evidence suggests lethal coalitionary aggression in humans is the product of residence and descent rules that promote fraternal interest groups, i.e., power groups of coresident males bonded by kinship. As such, human lethal coalitions are hypothesized to be homologous to chimpanzee (Pan troglodytes) border patrols. However, humans demonstrate a unique metagroup social structure in which strategic alliances allow individuals to form coalitions transcending local community boundaries. We test predictions derived from the fraternal interest group and strategic alliance models using lethal coalition data from a lowland South American population, the Yanomamö. Yanomamö men who kill an enemy acquire a special status, termed unokai. We examine the social characteristics of co-unokais or men who jointly kill others. Analyses indicate co-unokais generally are (i) from the same population but from different villages and patrilines, (ii) close age mates, and (iii) maternal half-first cousins. Furthermore, the incident rate for co-unokai killings increases if men are similar in age, from the same population, and from different natal communities. Co-unokais who have killed more times in the past and who are more genetically related to each other have a higher probability of coresidence in adulthood. Last, a relationship exists between lethal coalition formation and marriage exchange. In this population, internal warfare unites multiple communities, and co-unokais strategically form new residential groups and marriage alliances. These results support the strategic alliance model of coalitionary aggression, demonstrate the complexities of human alliance formation, and illuminate key differences in social structure distinguishing humans from other primates.
The evolution of lethal coalitionary aggression remains a pivotal topic across the biological and social sciences (1–12). Revealing the ultimate and proximate factors responsible for the emergence and dynamics of warfare is of practical and theoretical importance across a wide range of contexts, including the evolution of human ultra-sociality, coalitionary psychology, ethnic identity, leadership, and political behavior. Surprisingly few detailed analyses exist concerning the social composition of lethal coalitions in small-scale societies. This lack is problematic, because the particular form that lethal coalitions take sheds light on the adaptive function of this behavior and the phylogenetic roots of coalitionary aggression with chimpanzees (Pan troglodytes).
Humans, like chimpanzees, demonstrate a capacity to coordinate behavior with others to kill conspecifics (11–15). Although the scope of lethal coalitionary aggression is far greater in humans, a number of similarities exist between the two species, namely coordinated groups of adult males defending home territories and aggressing against individuals from other communities with low-cost but lethal intergroup killings (12–17). These similarities have led some researchers to hypothesize that lethal coalitionary aggression represents a homology, with the last common ancestor between humans and chimpanzees having a similar capacity for coordinated violence (11–15). In chimpanzees, the primary proximate mechanism driving lethal coalitionary aggression appears to be local imbalances of power which lead to group-level benefits such as larger territories, more food, and greater reproductive opportunities to the aggressors (11, 12, 15). Anthropologists, on the other hand, have postulated a number of mechanisms responsible for lethal intergroup conflict in small-scale societies that span a variety of causal levels and empirical support, such as population regulation (18, 19), within-culture individual rewards (status and reproductive opportunities) (20, 21), between-group competition (22, 23), and novel response to contact with the Western world (24). One mechanism that conforms to the homology hypothesis—the fraternal interest group model—highlights how particular social structures modulate the emergence and intensity of lethal coalitionary aggression in tribal-level human societies (25–29). Specifically, the fraternal interest group model suggests that coalitions for intergroup conflict emerge in tribal societies when individuals experience overlapping group ties, i.e., in situations in which interest group allegiances reinforce one another and few ties of loyalty, such as marriage links, exist between groups. Cross-cultural analyses (26, 29, 30) demonstrate overlapping group ties are attained most readily under conditions of patriliny and patrilocality. Patrifocal descent and residence rules promote segmentary, factional polities, coalitions of males with common interests that stem from genetic and social kinship and that are cemented psychologically through common residence and a history of repeated interactions (1, 8, 31). Male-centered, descent-based power groups cause affiliated males to coreside with one another throughout life and organize individuals of varying degrees of consanguinity and age classes into cooperative units that compete violently against other similarly organized factional polities in cycles of retaliatory violence and internal warfare (1, 26, 28, 31).
In contrast to the effects of overlapping group ties for organizing coordinated violence, coalitionary aggression purportedly is dampened by cross-cutting ties (32–36) through which individuals owe allegiance to groups with conflicting special interests. Cross-cutting ties produce a landscape of conflicting loyalties that prevent actors from expressing any single position, thus halting cleavages between groups and reducing coordinated conflict (28, 37). Taken together, the fraternal interest group model allows us to make several predictions concerning the nature of coalitionary aggression in patrilineal–patrilocal tribal societies: (i) coalitions will be composed of males from the same patriline and place of birth who occupy a variety of age and genetic relatedness categories; and (ii) lethal aggression will be suppressed between males linked by cross-cutting ties (i.e., those from different places of birth and patrilines) relative to those who are linked by overlapping ties (i.e., those from a common birth place and patriline). Furthermore, adult male coresidence patterns should reflect fraternal group interests; thus, males will live with members of their natal community and patriline throughout life.
The fraternal interest group model of coalitionary aggression is consistent with genetic group selection models for the evolution of human ultra-sociality (2, 5) and research across the social sciences that suggests humans have a unique coalitional psychology that facilitates within-group cooperation for between-group competition (38–41). Furthermore, it converges with primatological evidence concerning chimpanzee social structure, in which female dispersal causes males of varying degrees of consanguinity to coreside throughout life and form coalitions with members of their natal group (1, 12, 13, 42). However, qualitative differences exist between the two species (8, 31, 43). Chimpanzees form no broader coalitions beyond the local community, but humans demonstrate a unique form of multitiered social structure in which marriage, social kinship, alliances, trade, and communication bond multiple descent groups, residential communities, and even ethnolinguistic units (32, 44–47). Human metagroup social structure involves a concomitant increase in cooperation and competition in wider networks that extend beyond the local community (45, 46). A wide net of social ties enables the formation of coalitions pitted against other groups in cycles of ongoing violence (1, 20, 48, 49), motivated by a variety of factors (21), and generating multicommunity conflicts at scales unparalleled by other species (50).
Given the uniquely flexible and multitiered structure of human society, Rodseth and Wrangham (8) and Rodseth (43) have amended the fraternal interest group model to explain the composition of human coalitions, which we term the “strategic alliance model of coalitionary aggression.” This model posits that, although males in tribal societies may prefer to form lethal coalitions with same-sex adult kin, coalitions can be composed of additional classes of males as long as they reside within practical visiting distance of one another and social institutions allow them to maintain amicable relationships through mutual monitoring and the exchange of strategic resources (8). Although a variety of social institutions could link human communities, one in particular—descent-group exogamy—is thought to play a primary role in between-group alliance and coalition formation (32, 45, 51). Descent-group exogamy causes individuals to seek marriage partners outside their socially defined lineage. In patrilineal societies, marriageable females represent a strategic resource that males use to negotiate alliances with individuals from different descent groups. These marriage alliances are thought to form the structural foundation for organizing lethal coalitions (8). As a result, neither common residence nor lineal membership may structure lethal coalitions.
In these contexts, males and the descent groups in which they are embedded exist within a social marketplace for alliance partners (sensu 52–57); marriage alliances and coalitions are strategically formed and terminated as economic, social, and political opportunities ebb and flow (28, 31). Under these conditions, coalitionary aggression acts as a signal of partnership intent (sensu 58, 59) and can be used for organizing cooperation in other domains in life. Therefore males who signal greater partnership intent with one another through more acts of coordinated lethal aggression should be more likely to cooperate with one another postviolence. Two avenues for cooperation postviolence are coresidence and the exchange of marriage partners. The strategic alliance model is consistent with social science research demonstrating the malleability of in-group membership (60–62) and with anthropological research that shows lethal coalitions in tribal societies can number in the thousands and draw men from multiple settlements (6, 22, 63–65).
The strategic alliance model of coalitionary aggression makes the following propositions about lethal human coalitions: raiding groups will be composed of males who (i) live in practical visiting distance of one another but may not necessarily emanate from the same residential group; (ii) are a mix of close, distant, and nongenetic kin; (iii) are from multiple descent groups; and (iv) are ideal marriage-exchange partners. Furthermore, it predicts that adult male coresidence patterns should reflect the micropolitics of coalition formation: Males who form lethal coalitions with one another more often should show a higher probability of coresidence, because coresidence allows them to capture the benefits of cooperation in linked contexts over relatively long periods of time. Last, it predicts a relationship between coalition formation and marriage alliances.
Here, we investigate whether the fraternal interest group model or the strategic alliance model of coalitionary aggression better characterizes the dynamics of lethal coalitions among the Yanomamö. We begin by examining Yanomamö social organization. Next, we examine the composition of lethal coalitions at the raiding group and dyadic level in terms of genetic relatedness, patrilineal affiliation, village coresidence at birth, age, and practical visiting distance (indexed by the variable “population block”; see Methods). Third, conditional on individuals having participated together in lethal raids, we examine the factors that contribute to some dyads forming lethal coalitions more often than others. Fourth, we examine the history of coresidence patterns of Yanomamö warriors following lethal raids. Last, we examine the relationship between lethal coalition formation and marriage exchange. The goals are to understand better the social relationships among men who have participated in coalitionary killings and to probe the broader relevance of these relationships in social spheres of kinship networks, residential choice, and marriage exchange.
The Yanomamö
The Yanomamö are an autonomous, indigenous, tribal population who inhabit the northern portion of the Amazon basin between the border region of southern Venezuela and the Brazilian state of Roraima (66–69). Currently, this population is experiencing political and economic distress related to gold mining and missionization. A number of primary-source ethnographic texts exist concerning Yanomamö economic, social, and political life (66–70). Until the early 1950s, no European outsider had any sustained contact with the Yanomamö; however, a few explorers reported fleeting contacts during the 19th and early 20th centuries (71–73). During the 1950s, the first reliable reports on the Venezuelan Yanomamö began to appear in the anthropological literature based on the sustained contact of members of the New Tribes Mission (74, 75). During the period of N.A.C.’s research (1964–1993), Yanomamö economic life rested largely on swidden agriculture with a heavy reliance on plantains, bananas, and manioc (76), but the Yanomamö supplemented their diet with a variety of foraged game animals (77). Although the exact population size was difficult to reconstruct, because a number of tribes were isolated, it is estimated that the Yanomamö numbered ∼25,000 people across 250 villages (68). Typical village size was ∼100 individuals and ranged between 25 and 400 people, depending on elevation, soil drainage, and their immediate warfare circumstances (78). In the area where N.A.C. spent the majority of his research career, intervillage warfare was a chronic problem, and villages were larger than in other portions of the Yanomamö tribal distribution. The Yanomamö complained frequently that when villages became too large, fighting over women became chronic and eventually, intolerable (66, 78). Although most individuals expressed a preference for living in smaller villages, these communities were vulnerable to attack by larger villages and could not fall below the critical threshold of 40 individuals, the size of a village needed to field a raiding party of 10 adult men. As a result, many Yanomamö were forced to live with the discomforts of a larger village because of its greater political security. Elsewhere, where warfare was less intense, villages were much smaller and could include as few as 25 people.
Yanomamö tribal warfare is typical for lowland South America where groups of men generally attack an enemy village at dawn to kill several enemies and then quickly retreat (49, 66, 79–84). Yanomamö raiding parties involve around 10–20 adult males and may take several days to complete (20). When raiding parties reach a village to attack, they divide into two or more groups and attack the first person they encounter. Yanomamö men who have killed or participated in a killing by shooting an enemy with an arrow during a raid later go through a ritual purification (unokaimou) and acquire a special status known as “unokai” (20). We refer to men who kill together in this context as co-unokai.
Normatively, the Yanomamö were a patrilineal, patrilocal population, and most villages comprised at least two lineages. These subgroups had a large number of kinsmen in nearby villages who would welcome them and would be willing to forget past grievances if they rejoined that village. This process would be more likely to occur if the subgroup included men who were willing to fight for the new village’s security. In a sense, Yanomamö warriors with kinsmen in a different village would be attractive as immigrants and would be more likely to be welcomed than a group that did not include warriors, because only the former demonstrated a willingness to defend their kin and community.
During the period of N.A.C.’s research, the Yanomamö maintained a preference for descent-group exogamy and cross-cousin marriage (66). Within villages, elder men, especially those of larger lineages, arranged most marriages and influenced the marriage arrangements of people who were not members of their patriline. The acquisition of material wealth was not a prerequisite to acquiring wives among the Yanomamö. Instead, a demonstrated prowess in fighting, the relative size of one’s lineage, and the number of adult male kinsmen and allies were important determinants of marriage success. Females leveraged little choice over marriage partners (66, 68).
Yanomamö marriage should be viewed as a life-historical process (85). From the vantage of a male in a situation where spouses were scarce, the ideal situation would be to have at least one wife; however, most men expressed the culturally desired goal of having several wives. Early in life a male might have to share the wife of an older brother and be part of a polyandrous household. Later, this brother might “give” this woman to him, so that the man becomes monogamous. If he acquires more status through fighting or because his lineage is large, he might acquire a second or third wife and become the head of a polygynous household.
Results
Composition of Lethal Coalitions.
If the fraternal interest group model better captures the dynamics of Yanomamö coalitionary aggression, then coalitions will be composed of males from the same lineage and place of birth who occupy a variety of age and genetic relatedness categories. If the strategic alliance model fits Yanomamö lethal coalitionary aggression, then coalitions should be composed of individuals who are from multiple places of birth and from multiple patrilines and who are genetically related as maternal kin at the level of first and second cousins; such persons in Yanomamö kinship terms are ideal “wife-givers.” We evaluated the fraternal interest group and alliance models by analyzing coalitions at the level of the group associated with each victim and at the level of the co-unokai dyad.
Our sample includes 47 Yanomamö individuals who each died at the hands of a raiding party, involving a total of 118 unokais. The number of co-unokais per lethal raid ranges from 2 to 15 (median, 3 men). The typical Yanomamö unokai coalition involves individuals from multiple villages of birth (median, two villages) (Fig. 1, Left) and from multiple patrilines (median, two patrilines) (Fig. 1, Right) and who live within practical visiting distance (i.e., they come from the same population block) (median, one population block).
Fig. 1.
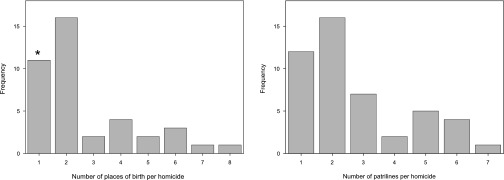
The social composition of Yanomamö lethal coalitions. (Left) Histogram of the number of places of birth associated with unokais per homicide (n = 40). Note that for seven cases information related to place of birth is missing (in the column denoted by *); thus these cases provide a lower bound estimate on the total number of places of birth per homicide. (Right) Histogram of the number of patrilines associated with unokais per homicide (n = 47).
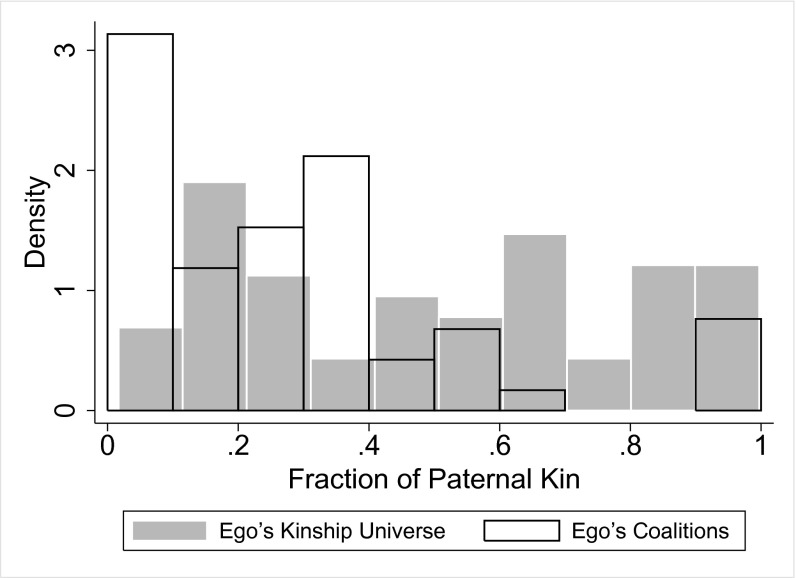
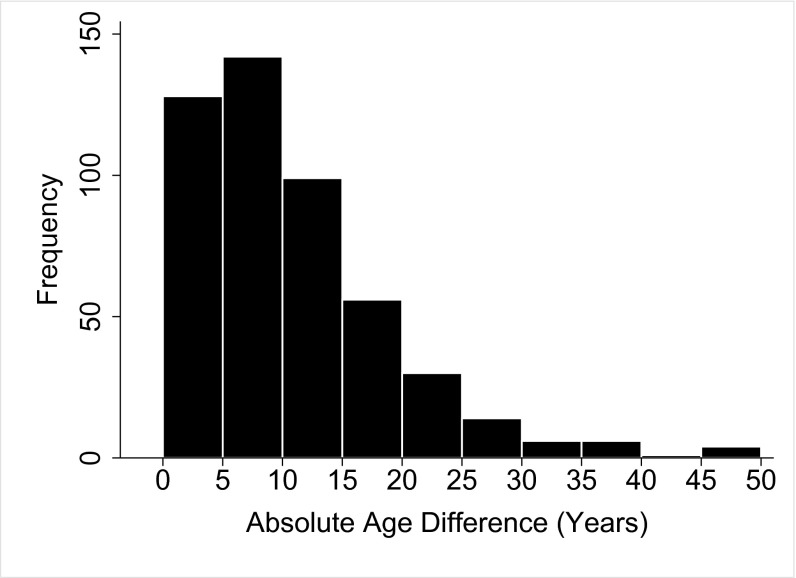
The 118 unokais generate 509 co-unokai dyads [for example, a group composed of four unokais results in six unique unokai dyads ; however, in our case, not every possible unokai dyad is achieved for each of the 118 men, because groups are bounded by 47 discrete events spread over time and space]. Of 509 co-unokai dyads for which there was sufficient information, 89% were from the same population block, 34% were from the same place of birth, and only 22% were from the same patriline (Table 1). Average genetic relatedness (r) among co-unokais is 0.08 (median, 0.0625; n = 509) (Fig. 2A). By plotting genetic relatedness on patrilineal membership (Fig. 2 B and C), we can determine the fraction of dyads composed of genetically related maternal and paternal kin. Of the dyads related to one another at a fraction greater than zero, 73% involve maternal kin, and 27% involve paternal kin. The low percentage of paternal-kin coalition partners is not a simple artifact of egos having more maternal kin relative to paternal kin. A two-sample Kolmogorov–Smirnov test shows that the distribution of paternal kin in egos’ lethal coalition is not drawn from the same distribution as egos’ kinship universe (D = 0.43, P < 0.001, n = 118) (Fig. 3). Last, males demonstrate a preference for forming coalitions with similarly aged individuals (median age difference, 8 y; n = 486), despite the substantial range in age (0–50 y) (Fig. 4). In no case did a father and son form a lethal coalition with one another.
Table 1.
Descriptive statistics for co-unokai dyads
| Independent variable | Yes | No | n | Mean (SD) | Minimum | Maximum |
| Same population | 443 | 53 | 496 | |||
| Same place of birth | 129 | 246 | 375 | |||
| Same patriline | 113 | 396 | 509 | |||
| Coresident as adults | 149 | 269 | 418 | |||
| Coefficient of relatedness | 509 | 0.08 (0.09) | 0 | 0.56 | ||
| Absolute age difference | 486 | 10.1 (8.4) | 0 | 50 | ||
| Times dyad was co-unokai | 509 | 1.8 (2.1) | 1 | 13 |
Fig. 2.
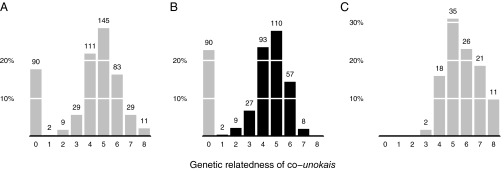
Genetic relatedness of co-unokai dyads. (A) Histogram of coefficients of relatedness among co-unokais grouped into nine relatedness categories (n = 509): 0 = no relatedness; 1 = 0.0039–0.0077; 2 = 0.0078–0.014; 3 = 0.015–0.03; 4 = 0.031–0.062; 5 = 0.063–0.124; 6 = 0.125–0.249; 7 = 0.25–0.49; 8 ≥ 0.5. (B) Histogram of coefficients of relatedness among co-unokais who are from different patrilines, grouped into nine relatedness categories (n = 396). Dark bars represent maternal kin. (C) Histogram of coefficients of relatedness among co-unokais who are from the same patriline, grouped into nine relatedness categories (n = 113). The numbers above the bars are counts of co-unokai dyads.
Fig. 3.
Histogram showing the fraction of paternal kin in egos’ kinship universe (gray bars) and lethal coalitions (open bars) (n = 118).
Fig. 4.
Histogram of the absolute age difference in years between co-unokais (n = 486).
In sum, the typical co-unokai relationship involves men who stem from the same population but who do not share a common place of birth or patriline. Most co-unokais are similar in age and are maternal kin who are related to each other at the level of half-first cousins. The modal co-unokai therefore is an ideal marriage-exchange partner.
Number of Times Men Act as Co-Unokai.
We examine why some unokai dyads commit more acts of lethal violence than others. If Yanomamö coalitionary aggression fits the fraternal interest group model, then men with overlapping ties—those from the same place of birth and patriline—should commit more acts of violence together than those with cross-cutting ties or those from different places of birth and patrilines. For this analysis, the outcome variable is the number of times two men have committed lethal acts of violence together while controlling for data structural autocorrelation around dyads. The incidence rate for co-unokai violence increases if men are similar in age, stem from the same population, and are from different places of birth (Wald χ2 = 132.1; P < 0.001; n = 728) contra the fraternal interest group model (Table 2). Neither patrilineal membership nor genetic relatedness predicted an increase in the number of times dyads killed together.
Table 2.
Model coefficients associated with the number of times men were co-unokais together
| Independent variable | IRR (semirobust SE) | Z | P |
| Dyad from same population block | 1.28 (0.04) | 8.7 | <0.001 |
| Absolute age difference | 0.99 (0.002) | −4.2 | <0.001 |
| Dyad from same place of birth | 0.86 (0.04) | −3.6 | <0.001 |
| Dyad from same patriline | 0.93 (0.05) | −1.3 | 0.21 |
| Coefficient of relatedness | 1.22 (0.25) | 0.9 | 0.35 |
| Constant | 0.56 (0.02) | −21.3 | <0.001 |
IRR, incidence rate ratio. Data coding: 0 = no; 1 = yes.
Residential Patterns of Co-Unokais.
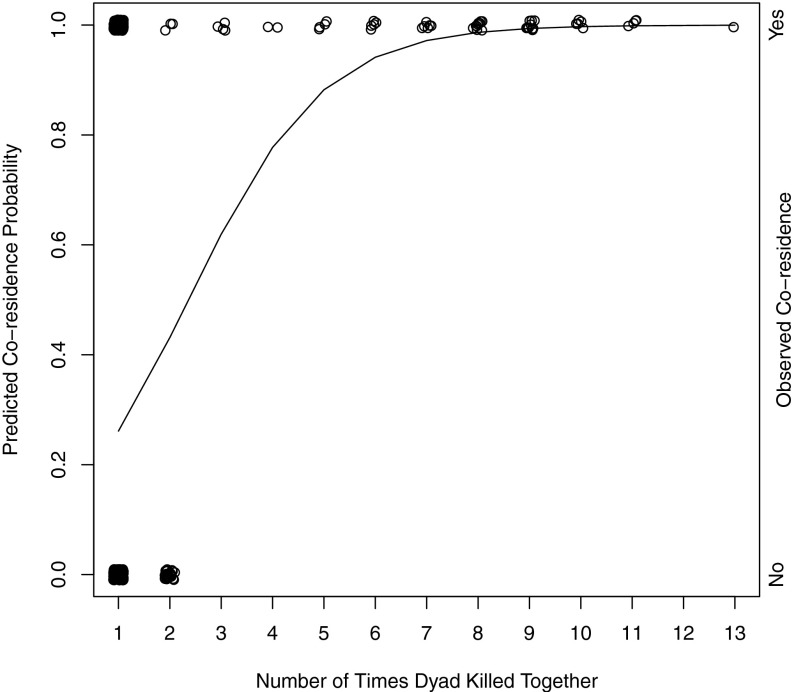
The fraternal interest group model predicts that adult male coresidence patterns should reflect fraternal group interests; thus males commonly will live with members of their natal community and patriline throughout much of life to maintain descent group power. The strategic alliance model predicts that the micropolitics of coalition formation will affect adult male coresidence patterns, so that males who more often form lethal coalitions with one another will demonstrate a higher probability of coresidence in adulthood. We use logistic regression to model the factors shaping whether two men coreside after they have killed together. We find that dyads are statistically more likely to coreside with one another as the number of times they have killed together increases and the more genetically related they are to one another (Wald χ2 = 57.6; P < 0.001; n = 598) (Fig. 5 and Table 3). In fact, for each additional time men kill together, the odds of coresiding later in life more than double. Neither place of birth nor patrilineal affiliation was significantly related to coresidence in adulthood.
Fig. 5.
Scatterplot of the relationship between the number of times two men killed together and whether the dyad was coresident after the successful raid(s) (n = 598). (Data points have been “jittered” to show data mass.)
Table 3.
Model coefficients associated with whether co-unokais shared a residence after a successful raid
| Odds ratio (semirobust SE) | Z | P | |
| No. of times co-unokais | 2.6 (0.4) | 7.0 | <0.001 |
| Coefficient of relatedness | 36.7 (59.0) | 2.2 | 0.03 |
| Dyad from same population block | 3.1 (2.5) | 1.4 | 0.16 |
| Absolute age difference | 1.03 (0.2) | 1.3 | 0.19 |
| Dyad from same place of birth | 0.69 (0.2) | −1.2 | 0.25 |
| Dyad from same patriline | 1.1 (0.4) | 0.1 | 0.90 |
| Constant | 0.02 (0.02) | −4.6 | <0.001 |
Data coding: 0 = no; 1 = yes.
Lethal Coalitions and Marriage Exchange.
The strategic alliance model posits a relationship between coalition formation and marriage alliances. Because the Yanomamö lack calendric records detailing when lethal coalitions were formed, we cannot determine whether marriages between lineages occur before or after the formation of lethal coalitions. Therefore we examine simple patterns of marriage exchange in light of lethal coalition formation using three different reference points: the individual unokai (n = 118), the individual marriage (n = 223), and the co-unokai dyad (n = 509). Of the 118 unokai, 102 married at least one female [mean number of wives (SD) = 1.9 (1.4); range, 0–7] involving a total of 206 women and 223 marriages (16 women were married to more than one unokai, and one of these women was married to three unokais). All but one of the 102 married unokai had sufficient information for reconstructing whether he married a female from the same patriline as a co-unokai. Seventy-one unokai (70% of all married unokais and 60% of all unokai) married at least one female who was from the same patriline as a co-unokai. Of the 223 total marriages, 215 had sufficient information on unokais’ spouses’ patrilineal membership. One hundred twenty-five marriages (58%) occurred between co-unokai patrilines. Of the 509 co-unokai dyads, 56 (11%) exchanged at least two females between their respective patrilines, resulting in each unokai acquiring at least one marriage partner from his co-unokai’s patriline. One hundred forty-nine of the 509 co-unokai dyads (29%) engaged in a one-way transfer of at least one female, resulting in only one unokai of the co-unokai pair receiving a marriage partner. The remaining 304 co-unokai dyads (60%) did not engage in marriage exchange; however, 113 of these dyads were composed of males from the same patriline and therefore were not ideal marriage-exchange partners. Finally, a significant positive relationship exists between the number of times two men killed together and whether they married a female from one another’s respective patrilines (Spearman’s ρ = 0.14; P < 0.001; n = 509). In sum, more than half of all unokais marry a female who is descended from at least one co-unokai’s patriline, more than half of all unokai marriages occur between co-unokai patrilines, and nearly half of all unokai dyads engaged in some form of marriage exchange.
Discussion
Our analyses suggest the strategic alliance model captures the dynamics of Yanomamö lethal coalitionary aggression better than the fraternal interest group model. First, co-unokais commonly are from different villages and patrilines, and the modal coalitionary partner is a maternal half-first cousin, who in Yanomamö terminology is an ideal marriage-exchange partner. Second, co-unokais commit more acts of lethal violence when they are similar in age, reside within practical visiting distance of one another, and come from different places of birth. Third, co-unokais who have killed more times in the past are much more likely to coreside in the same village later in life despite coming from different natal communities. Last, a relationship exists between the formation of a lethal coalition and the exchange of marriage partners between co-unokai lineages. These results differ markedly from those predicted by the fraternal interest group model and stand in stark contrast to the patterning of chimpanzee border patrol coalitions and related intra- and intercommunity social dynamics (13, 15). Instead, we find support for the strategic alliance model in documenting longitudinal patterns of alliance formation based around coalitionary aggression.
Yanomamö men appear to be embedded in a social market; individuals seek to establish cooperative partnerships lying outside the domain circumscribed by genetic kinship, lineage membership, and the natal community. In this context, lethal coalitionary violence serves as a venue to attain prestige and partnerships, in addition to satisfying culturally prescribed rules governing revenge (20). These partnerships (i) bind individuals, lineages, and villages together; (ii) provide the foundations for new communities; and (iii) form the structural basis for a variety of resources to flow between them, the most important of which appears to be reproductive opportunities. Cooperation during successful raids likely represents a psychologically meaningful act that binds men together through mutual commitments and trust. Once a partnership is formed, some unokai move to a co-unokai’s village, whereas others form new villages with their co-unokai despite their coming from different natal communities. Alliances made between co-unokais who also are maternal kin form the structural basis for a social group with dual organization. Many Yanomamö villages have two major lineages, each with its own leader, who almost always is the brother-in-law of the other headman because they have married females from each other’s patriline (66, 68). There are strong personal bonds between these men who exchange females in marriage. A large social anthropological literature suggests that the social institution of descent group exogamy forms the structural basis upon which multiple human communities organize to form segmental groups for coalitionary violence (8, 32, 45). The Yanomamö appear to fit this model, because many males form coalitions with individuals who are ideal marriage exchange partners, and a sizable portion of unokai marriages occur with females from their respective co-unokai’s lineage.
Unokai are accorded a great deal of respect, can obtain wives more successfully from men in other patrilineages, and have more than twice as many wives and children than non-unokai (20). Our analyses provide a mechanism explaining why Yanomamö warriors have higher reproductive success than nonwarriors: Males who participate in and are successful at warfare have greater access to marriage partnerships than non-unokai. In this respect, our analyses dovetail with arguments that the motivation for warfare in small-scale societies lies in the within-culture individual rewards that one can obtain by participating (21).
Neither social nor genetic kinship was found to be an important mechanism for organizing lethal raiding; however, genetic kinship was found to play a role in unokai residential decision-making. The latter finding is consistent with research demonstrating that Yanomamö men have a preference for coresiding with close genetic kin, especially brothers (86). Although social and genetic kinship play a pivotal role in within-community alliance formation (87, 88), unokai alliances seem to be more salient for uniting multiple lineages and villages. That genetic kinship organizes cooperation at some levels of social organization but not others is consistent with research demonstrating that the modular structure of multilevel societies, in conjunction with the presence of female exogamy and conspecific male threats, can promote cooperation between distantly or unrelated males in some primate species (89, 90).
Although the fraternal interest group model does not explain the patterning of lethal coalitionary aggression and alliance formation within Yanomamö society, it may explain the cross-cultural patterning of internal warfare in societies where land and resources other than marriage opportunities are the primary economic motivation for coalitionary aggression. Furthermore, it is still plausible that human and chimpanzee lethal coalitionary aggression share a common evolutionary origin. One possibility is that humans and chimpanzees inherited a common ancestry and psychology for coalitionary violence that initially was restricted to genetically related males from the same natal community; however, after pair bonding and the metagroup level of social organization evolved in humans, that psychology was co-opted to motivate alliances with genetic and social kin residing in other communities. If this notion is correct, a potential scenario for the evolution of human social structure involves an initial phase in which pair-bonding, bilateral kinship, and descent-group exogamy set favorable conditions for the recognition of cross-cousins and affines living in different communities (45, 46). Once these social institutions were established, males would be in a position to recognize these individuals as potential coalitionary partners for aggressive or lethal purposes and could use warfare as a vehicle to vet potential social partners for marriage exchange. In this context, the formation of lethal coalitions among cross-cousins, affines, and other classes of males helped forge strong bonds between them, which could lead to coresidence later in life.
Although some popular and academic accounts idealize the social composition of lethal coalitions in small-scale societies as simply a “band of brothers” (e.g., 14), our analyses suggest a more apt description might be a “band of brothers-in-law.” We demonstrate some of the long-term complexities of lethal coalitionary violence and alliance formation in Yanomamö warfare. Our results illuminate several key differences in multicommunity coalitions that distinguish humans from other primates and support the strategic alliance model of human coalitionary aggression.
Methods
Yanomamö Unokai Dataset.
N.A.C. made 30 field trips to the Yanomamö between 1964 and 1993, visiting ∼60 different villages, mostly in two major population blocks (clusters of villages that share a common history, are of recent origin in the last 100 y or so, and are named for a specific mountain or river basin). By 1990, N.A.C. collected demographic information for ∼2,000 Yanomamö individuals with estimated year of birth, village of birth, village of residence, parental identification, and all marriages (67).
N.A.C. obtained the names of men who participated in the killing of particular victims over three field seasons between 1985 and 1987. Informants who knew the raiding history of unokais provided this information, but occasionally an unokai himself would volunteer personal accounts of how victims were dispatched. At the time N.A.C. obtained this information, the Yanomamö lacked accurate calendric records for these lethal events; as a result, we do not know when they occurred. Because of the sporadic and chaotic nature of raiding, not all perpetrators of acts of violence were known for each victim (and vice versa). Data were filtered to include only cases in which both the victim and all offenders associated with the act of violence were known and there was information for reconstructing genealogical relatedness. This process resulted in 100 victims and 138 perpetrators. However, because our interest centers on the composition of coalitional aggression, we examine only cases in which more than one individual is associated with a victim, resulting in 47 victims, 118 perpetrators (seven of whom also died violent deaths), and 509 dyadic relationships between co-perpetrators. The number of victims per offender ranges from 1 to 11 with a median of 1 (n = 118). Of the 118 individuals who committed an act of group violence, sufficient information existed for reconstructing all 118 individuals’ patrilineage membership, 110 individuals’ population block, 87 individuals’ place of birth, and 112 individuals' age. Complete information about the unokai’s place of birth was recorded for 24 victims; for 16 victims place-of-birth information was available for a subset of unokais; and seven victims were associated with unokais for whom there was no information regarding place of birth. We include victims with partial information to reconstruct the composition of lethal coalitions. This method provides conservative estimates of the diversity of these groups. Hagen’s (91) Descent program was used to calculate coefficients of genetic relatedness and to establish patrilineal membership.
Statistical Analyses.
The unit of analysis is the co-offending dyad, and no natural ordering exists to determine which ego is entered first (each dyad is represented twice in all analyses), thus necessitating the use of a generalized estimating equation (GEE) to account for data structural autocorrelation in STATA (92). Multivariate GEEs were used to model the factors affecting the number of times unokai formed lethal coalitions together (multivariate GEE negative binomial regression) and to model the probability that co-unokai coresided after successful raids (multivariate GEE logistic regression). The outcome variable of the times men unokai together shows evidence of overdispersion (the variance is ∼2.5 times larger than the mean), necessitating the use of a negative binomial regression.
Acknowledgments
We thank our colleagues Karthik Panchanathan, Martin Daly, Ray Hames, Luke Glowacki, Kristen Hawkes, and Robert Lynch for help and advice and the Yanomamö of the 60 villages that N.A.C. visited, especially the older men and women who tried to make certain that what he wrote was the truth.
Footnotes
See Profile on page 16636.
The authors declare no conflict of interest.
References
- 1.Boehm C. Retaliatory violence in human prehistory. Br J Criminol. 2011;51(3):518–534. [Google Scholar]
- 2.Bowles S. Group competition, reproductive leveling, and the evolution of human altruism. Science. 2006;314(5805):1569–1572. doi: 10.1126/science.1134829. [DOI] [PubMed] [Google Scholar]
- 3.Bowles S. Being human: Conflict: Altruism’s midwife. Nature. 2008;456(7220):326–327. doi: 10.1038/456326a. [DOI] [PubMed] [Google Scholar]
- 4.Bowles S. Did warfare among ancestral hunter-gatherers affect the evolution of human social behaviors? Science. 2009;324(5932):1293–1298. doi: 10.1126/science.1168112. [DOI] [PubMed] [Google Scholar]
- 5.Choi JK, Bowles S. The coevolution of parochial altruism and war. Science. 2007;318(5850):636–640. doi: 10.1126/science.1144237. [DOI] [PubMed] [Google Scholar]
- 6.Mathew S, Boyd R. Punishment sustains large-scale cooperation in prestate warfare. Proc Natl Acad Sci USA. 2011;108(28):11375–11380. doi: 10.1073/pnas.1105604108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Pinker S. The Better Angels of Our Nature. Viking; New York: 2011. [Google Scholar]
- 8.Rodseth L, Wrangham RW. In: Human kinship: A Continuation of Politics by Other Means? Kinship and Behavior in Primates. Chapais B, Berman CM, editors. Oxford Univ Press; Oxford, UK: 2004. pp. 389–419. [Google Scholar]
- 9.Turchin P. Warfare and the evolution of social complexity: A multilevel-selection approach. Structure and Dynamics. 2011;4(3):1–37. [Google Scholar]
- 10.Walker RS, Bailey DH. Body counts in lowland South American violence. Evol Hum Behav. 2013;34(1):29–34. [Google Scholar]
- 11.Wilson ML, et al. Lethal aggression in Pan is better explained by adaptive strategies than human impacts. Nature. 2014;513(7518):414–417. doi: 10.1038/nature13727. [DOI] [PubMed] [Google Scholar]
- 12.Wrangham RW, Glowacki L. Intergroup aggression in chimpanzees and war in nomadic hunter-gatherers: Evaluating the chimpanzee model. Hum Nat. 2012;23(1):5–29. doi: 10.1007/s12110-012-9132-1. [DOI] [PubMed] [Google Scholar]
- 13.Manson JH, Wrangham RW. Intergroup aggression in chimpanzees and humans. Curr Anthropol. 1991;32(4):369–390. [Google Scholar]
- 14.Wrangham RW, Peterson D. Demonic Males: Apes and the Origins of Human Violence. Houghton Mifflin Company; New York: 1996. [Google Scholar]
- 15.Wrangham RW. Evolution of coalitionary killing. Am J Phys Anthropol. 1999;110(Suppl 29):1–30. doi: 10.1002/(sici)1096-8644(1999)110:29+<1::aid-ajpa2>3.3.co;2-5. [DOI] [PubMed] [Google Scholar]
- 16.Goodall J. The Chimpanzees of Gombe: Patterns of Behavior. Harvard Univ Press; Cambridge, MA: 1986. [Google Scholar]
- 17.Wilson ML, Wallauer WR, Pusey AE. New cases of intergroup violence among chimpanzees in Gombe National Park, Tanzania. Int J Primatol. 2004;25(3):523–549. [Google Scholar]
- 18.Rappaport RA. Pigs for the Ancestors. Yale Univ Press; New Haven, CT: 1968. [Google Scholar]
- 19.Harris M. Cows, Pigs, Wars, and Witches: The Riddles of Culture. Random House; New York: 1974. [Google Scholar]
- 20.Chagnon NA. Life histories, blood revenge, and warfare in a tribal population. Science. 1988;239(4843):985–992. doi: 10.1126/science.239.4843.985. [DOI] [PubMed] [Google Scholar]
- 21.Glowacki L, Wrangham RW. The role of rewards in motivating participation in simple warfare. Hum Nat. 2013;24(4):444–460. doi: 10.1007/s12110-013-9178-8. [DOI] [PubMed] [Google Scholar]
- 22.Meggitt M. Blood Is Their Argument: Warfare Among the Mae Enga Tribesmen of the New Guinea Highland. Mayfield Publishing Co; Palo Alto, CA: 1977. [Google Scholar]
- 23.Ember C, Ember M. Warfare, aggression, and resource problems: Cross-cultural codes. Field Methods. 1992;26(1–4):169–226. [Google Scholar]
- 24.Ferguson B. Yanomami Warfare: A Political History. School of American Research; Santa Fe, NM: 1995. [Google Scholar]
- 25.Murphy RF. Intergroup hostility and social cohesion. Am Anthropol. 1957;59(6):1018–1035. [Google Scholar]
- 26.Otterbein KF, Otterbein CS. An eye for an eye, a tooth for a tooth: A cross-cultural study of feuding. Am Anthropol. 1965;67(6):1470–1482. [Google Scholar]
- 27.Divale WT. Migration, external warfare, and matrilocal residence. Cross-Cultural Res. 1974;9(2):75–133. [Google Scholar]
- 28.Paige J. Kinship and polity in stateless societies. J Sociol (Melb) 1974;80(2):301–320. [Google Scholar]
- 29.Göhlen R. Fraternal interest groups and violent conflict management: A social-structural hypothesis. Z Ethnol. 1990;115:45–55. [Google Scholar]
- 30.van Velzen HUET, van Wetering W. Residence, power groups, and intra-societal aggression. Arch Int Ethnogr. 1960;49:169–200. [Google Scholar]
- 31.Boehm C. Segmentary 'warfare' and the management of conflict: Comparison of East-African chimpanzees and patrilineal-patrilocal humans. In: Harcourt AH, De Waal FBM, editors. Coalitions and Alliances in Humans and Other Animals. Oxford Univ Press; Oxford, UK: 1992. pp. 137–172. [Google Scholar]
- 32.Levi-Strauss C. Les Structures Élémentaires de la Parenté. Presses Universitaires de France; Paris: 1949. [Google Scholar]
- 33.Service ER. Primitive Social Organization: An Evolutionary Perspective. Random House; New York: 1962. [Google Scholar]
- 34.Tylor EB. Anthropology: An Introduction to the Study of Man and Civilization. Appleton; New York: 1888. [Google Scholar]
- 35.White LA. The Science of Culture: A Study of Man and Civilization. Farrar, Straus & Cudahy; New York: 1949. [Google Scholar]
- 36.White LA. The Evolution of Culture: The Development of Civilization to the Fall of Rome. McGraw Hill; New York: 1959. [Google Scholar]
- 37.van der Dennen JMG. The Origin of War: The Evolution of a Male Coalitional Reproductive Strategy. Origin; Groningen, The Netherlands: 1995. [Google Scholar]
- 38.Alexander RD. Evolution of the human psyche. In: Mellars P, Stringer C, editors. The Human Revolution: Behavioral and Biological Perspective on the Origins of Modern Humans. Princeton Univ Press; Princeton, NJ: 1989. pp. 455–513. [Google Scholar]
- 39.Flinn MV, Geary DC, Ward CV. Ecological dominance, social competition, and coalitionary arms races: Why humans evolved extraordinary intelligence. Evol Hum Behav. 2005;26(1):10–46. [Google Scholar]
- 40.Flinn MV, Ponzi D, Muehlenbein MP. Hormonal mechanisms for regulation of aggression in human coalitions. Hum Nat. 2012;23(1):68–88. doi: 10.1007/s12110-012-9135-y. [DOI] [PubMed] [Google Scholar]
- 41.van Vugt M. The male warrior hypothesis: The evolutionary psychology of intergroup conflict, tribal aggression, and warfare. In: Shackelford TK, Weekes-Shackelford VA, editors. The Oxford Handbook of Evolutionary Perspective on Violence, Homicide, and War. Oxford Univ Press; Oxford, UK: 2012. pp. 291–300. [Google Scholar]
- 42.Langergraber KE, Mitani JC, Vigilant L. The limited impact of kinship on cooperation in wild chimpanzees. Proc Natl Acad Sci USA. 2007;104(19):7786–7790. doi: 10.1073/pnas.0611449104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Rodseth L. From bachelor threat to fraternal security: Male associations and modular organization in human societies. Int J Primatol. 2012;33(5):1194–1214. [Google Scholar]
- 44.Rodseth L, Wrangham RW, Harrigan AM, Smuts BB. The human community as a primate society. Curr Anthropol. 1991;32(3):221–254. [Google Scholar]
- 45.Chapais B. Primeval Kinship: How Pair-Bonding Gave Birth to Human Society. Harvard Univ Press; Cambridge, MA: 2008. [Google Scholar]
- 46.Chapais B. Monogamy, strongly bonded groups, and the evolution of human social structure. Evol Anthropol. 2013;22(2):52–65. doi: 10.1002/evan.21345. [DOI] [PubMed] [Google Scholar]
- 47.Hill KR, et al. Co-residence patterns in hunter-gatherer societies show unique human social structure. Science. 2011;331(6022):1286–1289. doi: 10.1126/science.1199071. [DOI] [PubMed] [Google Scholar]
- 48.Knauft B. Reconsdering violence in simple human societies: Homicide among the Gebusi of New Guinea. Curr Anthropol. 1987;28(4):457–500. [Google Scholar]
- 49.Beckerman S, Valentine P. Revenge in the Cultures of Lowland South America. Univ Press of Florida; Gainesville, FL: 2008. [Google Scholar]
- 50.Alexander RD. 1990. How did humans evolve? Reflections on the uniquely unique species. University of Michigan Museum of Zoology Special Publication 1:1–38.
- 51.Fox R. Kinship and Marriage: An Anthropological Perspective. Cambridge Univ Press; Cambridge, UK: 1967. [Google Scholar]
- 52.Noë R, Hammerstein P. Biological markets: Supply and demand determine the effect of partner choice in cooperation, mutualism, and mating. Behav Ecol Sociobiol. 1994;35(1):1–11. [Google Scholar]
- 53.Noë R, Hammerstein P. Biological markets. Trends Ecol Evol. 1995;10(8):336–339. doi: 10.1016/s0169-5347(00)89123-5. [DOI] [PubMed] [Google Scholar]
- 54.Nesse R. Runaway social selection for displays of partner value and altruism. Biol Theory. 2007;2(2):143–155. [Google Scholar]
- 55.Macfarlan SJ, Remiker M, Quinlan RJ. Competitive altruism explains labor exchange variation in a Dominican village. Curr Anthropol. 2012;35(1):118–124. [Google Scholar]
- 56.Macfarlan SJ, Quinlan R, Remiker M. 2013. Cooperative behaviour and prosocial reputation dynamics in a Dominican village. Proc Biol Soc 280(1761):20130557. [DOI] [PMC free article] [PubMed]
- 57.Barclay P. Strategies for cooperation in biological markets, especially for humans. Evol Hum Behav. 2013;34(3):164–175. [Google Scholar]
- 58.Hawkes K, Bliege Bird R. Showing off, handicap signaling, and the evolution of men's work. Evol Anthropol. 2002;11(2):58–67. [Google Scholar]
- 59.Bliege Bird R, Smith EA. Signaling theory, strategic interaction, and symbolic capital. Curr Anthropol. 2005;46(2):221–248. [Google Scholar]
- 60.Tajfel H. Social psychology of intergroup relations. Annu Rev Psychol. 1982;33:1–39. [Google Scholar]
- 61.Waller J. Becoming Evil: How Ordinary People Commit Genocide and Mass Killing. Oxford Univ Press; Oxford, UK: 2002. [Google Scholar]
- 62.Eibl-Eibesfeldt I. Human Ethology. Aldine Transaction; New Brunswick, NJ: 2007. [Google Scholar]
- 63.Boehm C. Montenegrin Social Organization and Values. AMS; New York: 1983. [Google Scholar]
- 64.Evans-Pritchard EE. The Nuer: A Description of the Modes of Livelihood and Political Institutions of a Nilotic People. Clarendon; Oxford, UK: 1940. [Google Scholar]
- 65.Wiessner P, Pupu N. Toward peace: Foreign arms and indigenous institutions in a Papua New Guinea society. Science. 2012;337(6102):1651–1654. doi: 10.1126/science.1221685. [DOI] [PubMed] [Google Scholar]
- 66.Chagnon NA. Yanomamö: The Fierce People. Holt, Rinehart, & Winston; New York: 1968. [Google Scholar]
- 67.Chagnon NA. Studying the Yanomamö. Holt, Rinehart & Winston; New York: 1974. [Google Scholar]
- 68.Chagnon NA. Noble Savages: My Life Among Two Dangerous Tribes – The Yanomamö and the Anthropologists. Simon & Schuster; New York: 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Lizot J. The Yanomami in the Face of Ethnocide. International Work Group for Indigenous Affairs; Copenhagen: 1976. [Google Scholar]
- 70.Lizot J. Tales of the Yanomami: Daily Life in the Venezuelan Forest. Cambridge Univ Press; Cambridge, UK: 1991. [Google Scholar]
- 71.Humboldt A, Bonpland A. 1821. Personal Narrative of Travels to the Equinoctial regions of the New Continent, During the years 1799–1804, Vol 5 (Longman, Hurst, Rees, Orme, and Brown, London) trans Williams HM [originally published as Voyage aux régions du Nouveau Continent équinoxiales: fait en 1799, 1800, 1801, 1803, et 1804 (1820), N. Maze, Paris]
- 72.Schomburgk RH. Journey from Esmeralda, on the Orinoco, to San Carlos and Moura on the Rio Negro, and thence by Fort San Joaquim to Demerara, in the Spring of 1839. Journal of the Royal Geographic Society of London. 1840;10:248–267. [Google Scholar]
- 73.Koch-Grünberg T. Vom Roraima Zum Orinoco. Vol III Ethnographie; Stuttgart: 1923. [Google Scholar]
- 74.Barker JP. Memoria sobre la cultura de los Guaika. Boletín Indigenista Venezolano. 1953;1:433–499. [Google Scholar]
- 75.Barker JP. Las incursions entre los Guaika. Boletín Indigenista Venezolano. 1959;7:151–168. [Google Scholar]
- 76.Hames R. Monoculture, polyculture, and polyvariety in tropical forest swidden cultivation. Hum Ecol Interdiscip J. 1983;11(1):13–34. [Google Scholar]
- 77.Hames R. A comparison of the efficiencies of the shotgun and the bow in neotropical forest hunting. Hum Ecol Interdiscip J. 1979;7(3):219–252. [Google Scholar]
- 78.Chagnon NA. Genealogy, solidarity, and relatedness: Limits to local group size and patterns of fissioning in an expanding population. Yearb Phys Anthropol. 1975;19:95–110. [Google Scholar]
- 79.Larrick JW, Yost JA, Kaplan J, King G, Mayhall J. Part I: Patterns of health and disease among the Waorani Indians of eastern Ecuador. Med Anthropol. 1979;3(2):147–189. [Google Scholar]
- 80.Ross JB. A Balance of Deaths: Revenge Feuding Among the Achuarä Jívaro of the Northwest Peruvian Amazon. Columbia Univ Press; New York: 1988. [Google Scholar]
- 81.Conklin BA. 1989. Images of Health, Illness and Death among the Wari' (Pakaas Novos) of Rondonia, Brazil. PhD dissertation (Univ of California, San Francisco)
- 82.Verswijver G. The Club-Fighters of the Amazon: Warfare Among the Kaiapo Indians of Central Brazil. University of Ghent; Ghent, Belgium: 1992. [Google Scholar]
- 83.Fausto C. Inimigos Fiéis: Historía, Guerra e Xamanismo na Amazônia. Editora da Universidade de São Paulo; São Paulo, Brazil: 2001. [Google Scholar]
- 84.Beckerman S, Yost J. 2007. Upper Amazonian warfare. Latin American Indigenous Warfare and Ritual Violence, eds Chacon RJ, Mendoza RG (Univ of Arizona Press, Tucson, AZ), pp 142–179.
- 85.Hames R. Costs and benefits of monogamy and polygyny for Yanomamö women. Ethol Sociobiol. 1996;17(3):181–199. [Google Scholar]
- 86.Walker RS, et al. Living with kin in lowland horticultural societies. Curr Anthropol. 2013;54(1):96–103. [Google Scholar]
- 87.Chagnon NA, Bugos P. 1979. Kin selection and conflict: An analysis of a Yanomamö ax fight. Evolutionary Biology and Human Social Behavior: An Anthropological Perspective, eds Chagnon NA, Irons W (Duxbury, North Scituate, MA), pp 213–238.
- 88.Alvard M. Kinship and cooperation: The axe fight revisited. Hum Nat. 2009;20(4):394–416. [Google Scholar]
- 89.Grueter CC, Chapais B, Zinner D. Evolution of multilevel social systems in nonhuman primates and humans. Int J Primatol. 2012;33(5):1002–1037. doi: 10.1007/s10764-012-9618-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Patzelt A, et al. Male tolerance and male-male bonds in a multilevel primate society. Proc Natl Acad Sci USA. 2014;111(41):14740–14745. doi: 10.1073/pnas.1405811111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 91.Hagen E. (nd) Descent. Available at http://code.google.com/p/descent/. Accessed May 15, 2014.
- 92.Rabe-Hesketh S, Skrondal A. Multilevel and Longitudinal Modeling Using Stata. STATA; College Station, TX: 2008. [Google Scholar]