Significance
Rho-associated kinase 2 (ROCK2) is implicated in the regulation of proinflammatory cytokines, such as IL-17 and IL-21, and the development of autoimmunity in mice. However, the role of ROCK2 signaling pathway in regulation of immune responses in humans is still an enigma. Here we show that targeted ROCK2 inhibition down-regulates proinflammatory responses via concurrent regulation of STAT3/STAT5 phosphorylation and shifting Th17/Treg balance in human T cells with a minimal effect on the rest of the immune response. This work provides previously unidentified insights into the molecular mechanism of ROCK2-mediated modulation of the immune response in man and has profound implications for development of a selective ROCK2 inhibitor as a new therapeutic target for autoimmunity treatment.
Keywords: Human T cells, autoimmunity, proinflammatory cytokines, STAT3, STAT5
Abstract
Rho-associated kinase 2 (ROCK2) regulates the secretion of proinflammatory cytokines and the development of autoimmunity in mice. Data from a phase 1 clinical trial demonstrate that oral administration of KD025, a selective ROCK2 inhibitor, to healthy human subjects down-regulates the ability of T cells to secrete IL-21 and IL-17 by 90% and 60%, respectively, but not IFN-γ in response to T-cell receptor stimulation in vitro. Pharmacological inhibition with KD025 or siRNA-mediated inhibition of ROCK2, but not ROCK1, significantly diminished STAT3 phosphorylation and binding to IL-17 and IL-21 promoters and reduced IFN regulatory factor 4 and nuclear hormone RAR-related orphan receptor γt protein levels in T cells derived from healthy subjects or rheumatoid arthritis patients. Simultaneously, treatment with KD025 also promotes the suppressive function of regulatory T cells through up-regulation of STAT5 phosphorylation and positive regulation of forkhead box p3 expression. The administration of KD025 in vivo down-regulates the progression of collagen-induced arthritis in mice via targeting of the Th17-mediated pathway. Thus, ROCK2 signaling appears to be instrumental in regulating the balance between proinflammatory and regulatory T-cell subsets. Targeting of ROCK2 in man may therefore restore disrupted immune homeostasis and have a role in the treatment of autoimmunity.
The immune response is a delicate balancing act, protecting the integrity of the host organism from foreign invaders while not causing autoimmune reactivity (1). IL-21 and IL-17 are proinflammatory cytokines produced by T-helper 17 (Th17) cells that are involved in the pathogenesis of many autoimmune diseases (2–5). The generation of Th17 cells is induced by a combination of several cytokines including transforming growth factor-β (TGF-β1), IL-1β, IL-6, and IL-23, and involves the activation of transcription factors, such as RAR-related orphan receptor (ROR) γt, RORα, IFN regulatory factor (IRF) 4, and signal transducer and activator of transcription 3 (STAT3) (2, 6, 7). However, the signaling pathways that lead to activation of this transcriptional profile are poorly understood and remain unclear.
Rho GTPase-mediated signaling pathways play a central role in the coordination and balancing of T-cell–mediated immune responses, including T-cell receptor (TCR)-mediated signaling, cytoskeletal reorganization, and the acquisition of the appropriate T-cell effector program (8). The Rho kinase family members, consisting of Rho-associated kinase 1 (ROCK1) and ROCK2, are serine–threonine kinases that are activated by Rho GTPases and mediate the phosphorylation of downstream targets in cells (9). Recent studies have demonstrated that ROCK2 regulates the production of both IL-21 and IL-17 and plays an essential role in the development of autoimmunity in mice (10, 11). Indeed, pan ROCK inhibition was reported to effectively down-regulate ongoing autoimmune response in animal models (11, 12). Additionally, ROCK activity was found to be up-regulated in patients with rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) (13, 14), when the production of both IL-21 and IL-17 is deregulated, but to date there is no evidence of selective ROCK2 involvement in the regulation of proinflammatory cytokines in humans.
Results
Regulation of IL-21 and IL-17 Secretion in Human CD4+ T Cells Is ROCK2-Dependent.
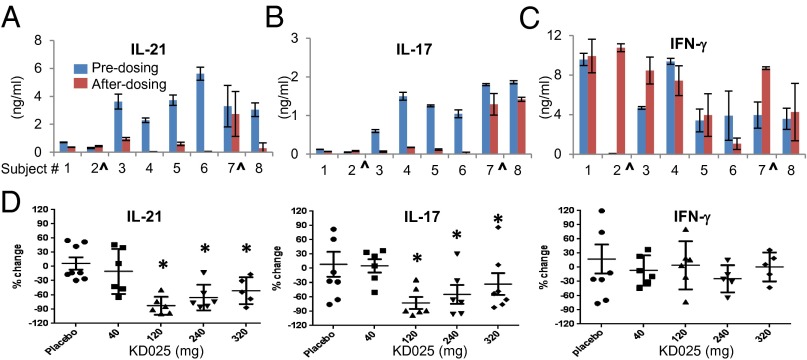
We conducted a placebo-controlled, randomized, phase 1 clinical study in which we show that the selective ROCK2 inhibitor, KD025 (formerly Slx-2119) (15, 16), is orally available, and well tolerated, without significant adverse events related to treatment with the drug (Figs. S1 and S2). KD025 is ATP competitive and 100-fold more selective for the ROCK2 over ROCK1 isoform (16). As part of this study, we purified peripheral blood mononuclear cells (PBMCs) before and after treatment (24 h after the last dosing) and activated them ex vivo by using anti-CD3/CD28 stimulation. Both IL-21 and IL-17 production were reduced by 50–100% in cells from KD025-treated individuals (120 mg dose), but not in placebo-treated human subjects (Fig. 1 A and B). Interestingly, we found that IFN-γ secretion is not affected by KD025 treatment (Fig. 1C). The inhibitory effect of KD025 on IL-21 and IL-17 secretion is observed at doses of 120, 240, and 320 mg, with no effect on IFN-γ (Fig. 1D). The intracellular staining of IL-21, IL-17, and IFN-γ demonstrates that KD025 treatment has no significant effect on frequencies of cytokine-producing cells circulating in peripheral blood (Fig. S3). Thus, oral administration of the selective ROCK2 inhibitor KD025 in normal humans down-regulates the ability of PBMCs to secrete IL-21 and IL-17 in response to stimulation ex vivo.
Fig. 1.
Oral administration of selective ROCK2 inhibitor KD025 down-regulates the IL-17 and IL-21 secretion in human PBMCs upon stimulation ex vivo. Human PBMCs were purified from healthy human subjects before and after (24 h after the last dosing) oral administration of KD025 at doses of 40 mg (D), 120 mg (A–D), 240 mg (D), and 320 mg (D) and stimulated by immobilized mAbs against CD3 and CD28 (anti-CD3/28). The supernatants were analyzed for IL-21(A and D), IL-17 (B and D), and IFN-γ (C and D) after 48 h by ELISA. A–C represent data from individual patients treated with 120 mg of KD025 (^subjects 2 and 7 are placebo-treated). D represents a summary of all patients treated with indicated doses of KD025.
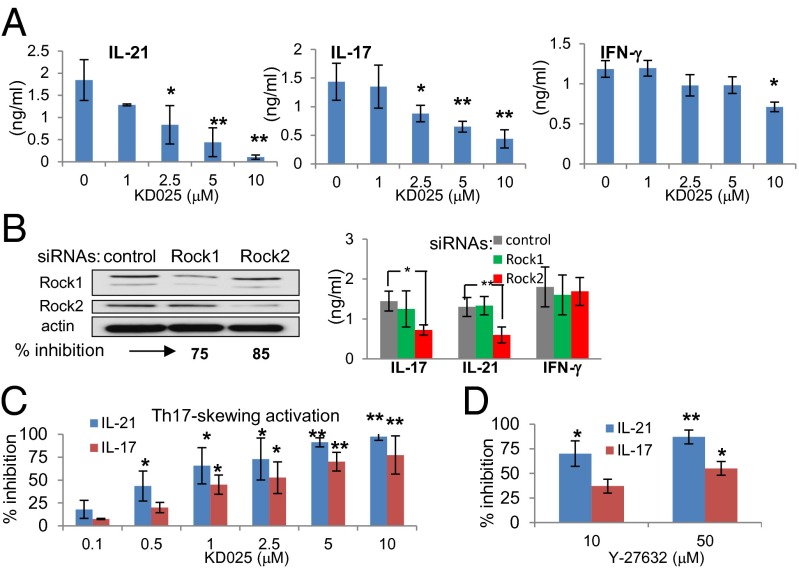
CD4+ T cells are a major source of IL-21 and IL-17 (17, 18). Both ROCK1 and ROCK2 isoforms are equally expressed in purified human CD4+ T cells (Fig. S4 A and B) and are recruited to the immunological synapse (IS), a structured interface between antigen-presenting cells and T cells (19) (Fig. S4C). KD025 inhibits phosphorylation of myosin light chain (MLC), one of the intracellular targets of ROCK (Fig. S5A), without a significant effect on the ability of T cells to form an IS on supported planar bilayers (Fig. S5B) (20). Thus, although ROCK proteins are involved in cytoskeleton organization (9), signaling via other Rho GTPases, such as Cdc42, is sufficient to mediate the cytoskeleton rearrangement that is required for IS formation (21). Consistent with our data from the phase 1 clinical study, treatment of purified CD4+ T cells with KD025 in vitro down-regulates the secretion of IL-21 and IL-17 in a dose-dependent manner, whereas IFN-γ secretion is affected to a lesser extent (Fig. 2A). The same trend of inhibition of inflammatory cytokines is observed when cells are treated with KD025 after TCR stimulation (Fig. S6A). To confirm the involvement of selective ROCK2 inhibition in KD025-mediated effects on cytokine secretion, we used ROCK1- and ROCK2-specific small interfering RNA (siRNA) that reduces expression of ROCK1 and ROCK2 by 75% and 85%, respectively, in human T cells (Fig. 2B). Only ROCK2, but not ROCK1, siRNA significantly down-regulates IL-21 and IL-17 secretion with no effect on IFN-γ (Fig. 2B). Both IL-21 and IL-17 are primarily produced by the subset of CD4+ T cells recently defined as Th17 cells; KD025 was tested on cytokine secretion under Th17-skewing activation, which requires the presence of IL-1β and TGF-β during TCR stimulation (22, 23). We found that under conditions that favor Th17 cells, significant inhibition of IL-21 and IL-17 is achieved even in submicromolar concentrations of KD025 (Fig. 2C), whereas IL-2 secretion and T-cell proliferation are not affected (Fig. S6 B and C). In addition, the secretion of another IL-17 family member, IL-17F, was inhibited by KD025 treatment, whereas IL-17E (IL-25) was undetectable in human T cells (Fig. S6D). A 10-fold higher concentration of the pan ROCK inhibitor Y-27632 is required to achieve a similar level of IL-17 and IL-21 inhibition (Fig. 2D). These data suggest that a ROCK2, but not ROCK1, isoform is specifically involved in regulation of IL-21 and IL-17 secretion in human CD4+ T cells.
Fig. 2.
Regulation of IL-17 and IL-21 secretion in human T cells is ROCK2-dependent. Peripheral blood CD4+ T cells were treated with selective ROCK2 inhibitor KD025 (A and C) or with pan ROCK inhibitor Y-27632 (D) for 1 h and then stimulated by anti-CD3/28 mAbs alone (A and B) or in combination with IL-1β and TGF-β (C and D). IL-21, IL-17, and IFN-γ secretion was analyzed by ELISA after 48 h (A–D). CD4+ T cells were transfected with siRNA targeting ROCK1 and ROCK2 or with control siRNA and plated on anti-CD3/28 mAbs. After 48 h, ROCK1 and ROCK2 expression was defined by Western blot analysis (B, Left), and cytokine secretion was measured by ELISA (B, Right). The average of four different experiments is shown (A–D). *P < 0.05 and **P < 0.01 were calculated by t test.
ROCK2, but Not ROCK1, Inhibition Leads to Down-Regulation of STAT3 Phosphorylation and Transcriptional Activity.
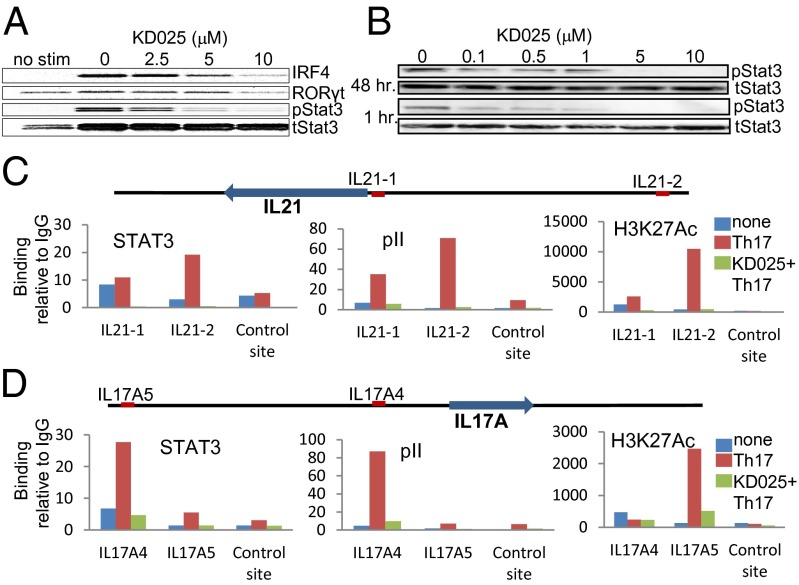
The induction and expression of IL-21 and IL-17 has been shown to be dependent on activation of certain transcription factors including STAT3, IRF4, and RORγt (6, 7, 24). We show that treatment with KD025 under Th17-skewing conditions down-regulates the phosphorylation of STAT3 and leads to reduced levels of IRF4 and RORγt protein in a dose-dependent manner (Fig. 3A). The inhibition of STAT3 phosphorylation occurs within minutes of T-cell exposure to KD025 and at submicromolar concentrations of the inhibitor (Fig. 3B). To test whether ROCK2-mediated regulation of STAT3 phosphorylation acts by controlling STAT3 transcriptional activity, we performed ChIP (chromatin IP) assays in human CD4+ T cells to examine transcription factors binding to the promoters of IL-21 and IL-17. We found that Th17 activation leads to significant enrichment of STAT3, as well as RNA polymerase II (pII), at the site of the promoter of IL-21 (Fig. 3C) and IL-17 (Fig. 3D). Treatment with KD025 significantly reduced the binding of STAT3 and RNA pII to these sites (Fig. 3 C and D). The bindings of STAT3 and pII are consistent with the levels of histone H3K27 acetylation (H3K27Ac), a marker for actively transcribed genes. H3K27AC binding is increased at the IL-21 and IL-17 loci in human CD4+ T cells under Th17-skewing conditions and is significantly reduced in the presence of KD025 (Fig. 3 C and D). These results confirmed that ROCK2 signaling contributes to the regulation of IL-21 and IL-17 in human T cells via a mechanism that involves STAT3 phosphorylation and its consequent transcriptional activity. Consistent with cytokine secretion data, the inhibition of ROCK2 by both KD025 as well a ROCK2-specific siRNA primarily targets STAT3, IRF4, and RORγt (Fig. S7A). There is a minimal effect of ROCK2 inhibition on other molecules, such as STAT1, STAT4, and STAT6 (Fig. S7 B–D). The ROCK1 siRNA efficacy was confirmed by its ability to inhibit phosphorylation of MLC (Fig. S7E). These siRNA studies further separate the ROCK1 and ROCK2 signaling pathways and show that only ROCK2 contributes to the regulation of IL-21 and IL-17 secretion via STAT3, IRF4, and RORγt transcription factors in human CD4+ T cells.
Fig. 3.
ROCK2 but not ROCK1 inhibition leads to down-regulation of STAT3 phosphorylation, IRF4, and RORγt expression. CD4+ T cells were treated with indicated doses of KD025 (A and B) or 10 μM of KD025 (C and D), and then stimulated by anti-CD3/28 mAbs, IL-1β, and TGF-β for 48 h (A, C, and D) or for 1 h (B). Nuclear (A) and cytoplasmic (B) extracts were prepared and analyzed by Western blot. ChIP assays were performed with normal rabbit IgG, anti-RNA pII, STAT3, and histone H3K27 acetylation (H3K27Ac) antibodies (C and D). Binding of these transcription factors to the sites was determined by quantitative PCR and plotted as fold enrichment compared with IgG. One representative of three different experiments is shown.
KD025 Promotes Treg Function via a STAT5-Dependent Mechanism.
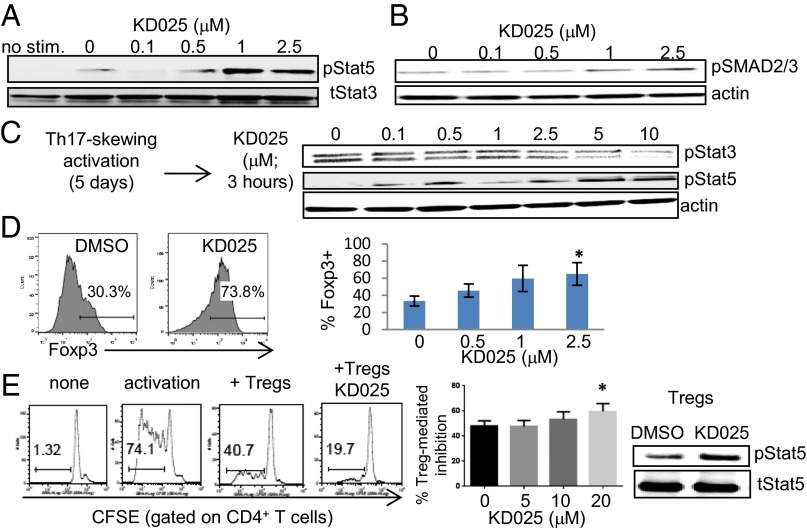
It has been shown that TGF-β is critical for the development and function of both Th17 cells and regulatory forkhead box p3+ (Foxp3+) T cells (Tregs), suggesting an intimate link between the differentiation programs of these two T-cell subsets (25). Indeed, the differential activity of STAT3 and STAT5 in Th17 cells and Tregs may act via their opposing effects on Foxp3 expression (26). ROCK proteins have been implicated in TGF-β signaling (27). It is therefore possible that ROCKs may have a role in TGF-β signaling during Th17/Treg differentiation. TGF-β induces the activation of ROCK2 in human CD4+ T cells in a SMAD2/3-independent manner (Fig. S8A). This is part of a noncanonical TGF-β signaling pathway that has also been reported in other cell types (27). Moreover, we find that the inhibition of ROCK2 with KD025 leads to the up-regulation of STAT5 and SMAD2/3 phosphorylation (Fig. 4 A and B). This occurs at the same doses of KD025 (1–2.5 μM) that were found to inhibit STAT3 phosphorylation. The reciprocal regulation of STAT3 and STAT5 phosphorylation by KD025 was also observed when cells were activated first by Th17-skewing conditions and then exposed to the selective ROCK2 inhibitor (Fig. 4C). This concurrent regulation of STAT3/STAT5 phosphorylation by KD025 is followed by a twofold increase in the percentage of Foxp3+ T cells (Fig. 4D) as well as the up-regulation of IL-10 secretion (Fig. S8B). Although we see the regulation of STAT3/STAT5 phosphorylation in the first hour after treatment with KD025, changes in levels of Foxp3 are observed after 5 d of exposure to the inhibitor. The positive regulation of the Treg subset by KD025 treatment was further confirmed in the suppression assay in vitro. Pretreatment of purified CD4+CD25hiCD127lo regulatory T cells (Tregs are 80% Foxp3+; Fig. S9) with KD025 significantly increases Treg-mediated inhibition of CD4+ T-cell proliferation via a mechanism that involves up-regulation of STAT5 phosphorylation in Tregs (Fig. 4E). This suggests that targeted inhibition of ROCK2 activity modulates immune homeostasis and potentially can shift the balance between Th17 cells and Tregs.
Fig. 4.
KD025 promotes the suppressive function of Tregs via up-regulation of STAT5 phosphorylation and Foxp3 expression. CD4+ T cells were treated with indicated doses of KD025 and then stimulated by anti-CD3/28 mAbs, IL-1β, and TGF-β for 48 h (A and B) or 120 h (D). Whole cellular extracts were prepared and analyzed by Western blot (A–C). Foxp3 expression was tested after 120 h (D). The average of three different experiments is shown. (E) Freshly purified human CD4+CD25hiCD127lo (Tregs) were treated with KD025, mixed with labeled PBMCs (ratio 1:1), and cultured in the presence of soluble anti-CD3 antibodies for 96 h. Cell proliferation was determined by CFSE dilution (E). The average of seven different experiments is shown. STAT5 phosphorylation was evaluated in lysates from purified Tregs treated with KD025 and then activated by anti-CD3/28 mAbs. *P < 0.05.
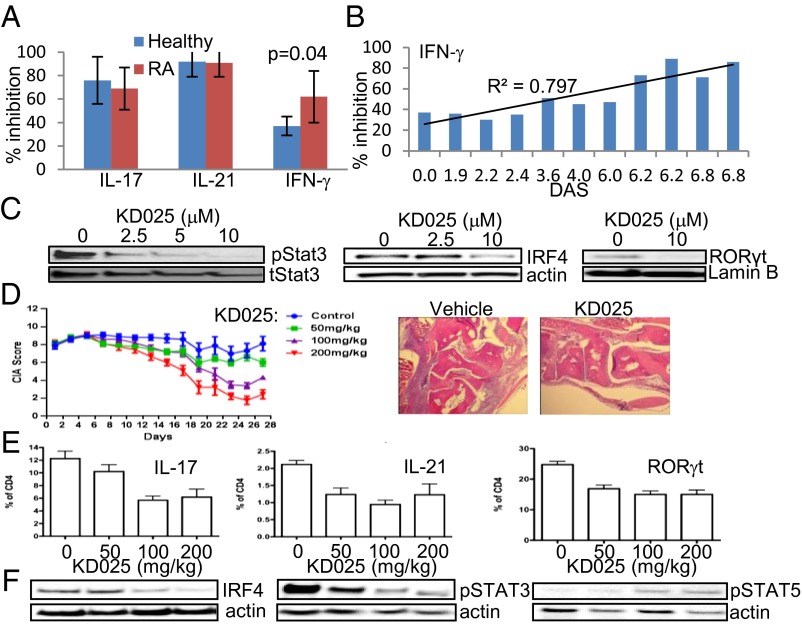
Treatment with KD025 Down-Regulates Proinflammatory Cytokine Secretion in T Cells from RA Patients and Stops the Progression of Collagen-Induced Arthritis in Mice.
RA is a chronic autoimmune disorder characterized by extensive joint inflammation that leads to significant tissue damage (28). Both IL-21 and IL-17 are involved in pathogenesis of RA (5, 29). The IL-17 is produced spontaneously by RA synovial cultures, and IL-17–producing cells were found in T-cell–rich areas (5). CD4+ T cells were purified from the peripheral blood of 11 RA patients with a range of disease activity scores (DASs; Fig. S10A).The inhibition of IL-21 and IL-17 in vitro by KD025 was comparable between RA patients and healthy human volunteers (Fig. 5A). Interestingly, the ability of KD025 to inhibit IFN-γ secretion was significantly higher in CD4+ T cells from RA patients than from healthy controls and was in direct correlation with the DAS (Fig. 5B). Whereas levels of IL-21 and IL-17 are similar between RA patients and healthy controls, IFN-γ secretion is significantly higher in CD4+ T cells purified from RA patients (Fig. S10B). These findings suggest that an increase in disease severity leads to up-regulation of the ROCK2-dependent component of IFN-γ secretion, consistent with previously reported elevated ROCK activity in RA (13). We show that KD025 treatment also down-regulates the phosphorylation of STAT3 (Fig. 5C) and significantly reduces protein levels of IRF4 and RORγt (Fig. 5 D and E) in cells from RA patients. In addition, KD025 treatment reduced protein levels of Tbet, a transcription factor that is known to regulate IFN-γ secretion, in T cells purified from RA patients, but not from healthy subjects (Fig. S10 C and D).
Fig. 5.
Treatment with KD025 down-regulates proinflammatory cytokine secretion in CD4+ T cells purified from RA patients and stops the progression of CIA in mice. Peripheral blood CD4+ T cells from RA patients were treated with 10 μM or indicated doses of KD25 for 1 h and then stimulated by immobilized anti-CD3/28 mAbs alone (A–C) or in combination with IL-1β and TGF-β (D and E). Cytokine secretion was determined after 48 h (A and B), and whole cell lysates were analyzed by Western blot (C). The average of four healthy and 11 different RA patients (A and B) and representative of five (C) different experiments is shown. The P value was calculated by t test. (D) Clinical arthritis scores of CIA mice treated with vehicle or indicated doses of KD025 (n = 10 per group). The treatment began on day 25 after immunization with collagen. Data are presented as the mean clinical score ± SEM. Representative H&E-stained sections in CIA mice killed at day 28 of the treatment. (E) Flow cytometry analysis of spleenocytes from CIA mice (day 28); data are shown as average percentage (n = 10) ± SD of total CD4+ T cells. Levels of IRF4, pSTAT3, and pSTAT5 were determined by Western blot (F). One representative of three different Western blots is shown.
To assess the potential of KD025 to down-regulate autoimmunity in vivo, we used a collagen-induced arthritis (CIA) model in mice that is characterized by a strong T-cell response to collagen, inflammation, and joint destruction. By using a therapeutic approach of treatment (the drug was administered 25 d after initial immunization), we found that KD025 treatment markedly down-regulates the progression of inflammatory arthritis in a dose-dependent manner compared with vehicle-treated animals (Fig. 5D). Histological analysis revealed that KD025 reduces the robust infiltration of immune cells in joints (Fig. 5D). In addition, we found that the anti-inflammatory effect of KD025 in vivo is mediated by targeting and reducing the percentage of IL-21– and IL-17– (Fig. 5E) expressing CD4+ T cells in spleen and lymph nodes compared with the vehicle control group with no effect on the percentage of IFN-γ+ cells (Fig. S11). The frequency of Foxp3+ cells was increased in spleen, but down-regulated in the lymph nodes of mice treated with KD025 (Fig. S11). Additionally, the treatment with KD025 in vivo resulted in dose-dependent down-regulation of RORγt, IRF4 expression, and STAT3 phosphorylation and up-regulation of STAT5 phosphorylation, supporting the molecular mechanism of targeted ROCK2 inhibition (Fig. 5F).
Discussion
A central goal in the study of autoimmune disease is to develop safe and effective therapies that down-regulate autoaggressive immune responses while preserving the ability of the immune system to fight infections and tumors. The approach is to develop therapies that target specific signaling pathways governing immune cell fate and activity rather than general immunosuppression (30). Elevated levels of IL-21 and IL-17 have been implicated in pathology of many autoimmune diseases (2–5, 31) and suggest that the characterization of specific molecular mechanisms that control the inappropriate secretion of these cytokine may provide important therapeutic outcomes. We report here that selective inhibition of ROCK2 by KD025 in purified human CD4+ T cells in vitro down-regulates the secretion of IL-21 and IL-17 via molecular mechanisms involving the inhibition of STAT3 phosphorylation. By using siRNA gene silencing, we further dissected the ROCK1 and ROCK2 signaling pathways and demonstrated that only the ROCK2 isoform contributes to the regulation of the transcription profile that is essential in the control of IL-21 and IL-17 secretion in man. This finding is consistent with previously reported data in mouse models. Furthermore, the secretion of IFN-γ and IL-2 as well as the phosphorylation of STAT1, STAT4, and STAT6 are minimally affected by selective ROCK2 inhibition. Interestingly, inhibition of ROCK2 activity up-regulates STAT5 and SMAD2/3 phosphorylation concomitantly with inhibition of STAT3 phosphorylation and results in increased Treg-suppressive function. This enhancement of Tregs in addition to the attenuation of Th17 cells suggests a potential role for ROCK2 inhibition in modulation of T-cell activity in man.
The down-regulation of STAT3 phosphorylation is the most sensitive readout of KD025 activity in T cells and occurs within minutes of exposure to submicromolar concentrations of this selective ROCK2 inhibitor. These data suggest that there is functional crosstalk between the Rho family GTPases and STAT3 signaling pathways. Indeed, it has been shown that activated Rac1 can directly bind to and activate STAT3 (32) or indirectly activate STAT3 through the induction of IL-6 (33) and that active RhoA, but not Rac1, can stimulate STAT3-responsive gene induction (34). Notably, activation of ROCK pathways can partially activate STAT3, and through the participation of other effectors, such as mDia, ROCK activity is required to fully promote the RhoA-mediated STAT3 activation (35). Because ROCK is a serine/threonine kinase and cannot directly mediate STAT3 phosphorylation on Tyr-705, it is likely that tyrosine kinases such JAK and Src or other intracellular signaling components, which may interface between ROCK and STAT3, are also engaged in this ROCK-dependent activation of STAT3 (36).
The two ROCK isoforms (ROCK1 and ROCK2) appear to be interesting potential targets for the treatment of a wide range of pathological conditions including cancer, neuronal degeneration, and cardiovascular diseases (37). However, because ROCKs play a central role in the organization of the actin cytoskeleton, it might be anticipated that the complete inhibition of these proteins could cause severe adverse events in patients. Our phase 1 clinical trial has shown that the maximum concentration of the selective ROCK2 inhibitor KD025 is linear and dose proportional at doses of 40–500 mg with no significant adverse events during oral administration in healthy human male subjects. Moreover, an analysis of PBMCs from these subjects revealed that KD025 effectively down-regulated the secretion of IL-21 and IL-17, while showing no changes in IFN-γ secretion during stimulation ex vivo. Notably, there was no significant effect of KD025 treatment on frequencies of IL-21–, IL-17–, and IFN-γ–producing cells at a 400-mg dose (Fig. S3), suggesting that selective ROCK2 inhibition modulates the capacity of the immune cells to be activated upon stimulation with no robust effect on the composition of immune cells in healthy subjects with normal immune homeostasis.
The role of ROCK proteins in the modulation of immune cell function may depend on the disease state of the individual. We find that although IFN-γ secretion was minimally affected by KD025 treatment of T cells from healthy donors, ROCK2 inhibition in T cells purified from active RA patients resulted in significant down-regulation of IFN-γ. Because ROCK activity was shown to be increased in SLE and RA patients (13, 14), it is possible that the ROCK2-dependent component of the IFN-γ secretion pathway is elevated with RA progression and this is inhibited directly by KD025. In addition, Treg-suppressive activity has been previously shown to be diminished in RA patients (20, 38) and may contribute to excessive IFN-γ levels observed in these patients (Fig. S10B). Therefore, it is also possible that the KD025-mediated inhibition of IFN-γ secretion that we observed in RA patients occurs indirectly via induction of Treg function that was observed in cells purified from healthy volunteers (Fig. 5). Further studies are required to test these hypotheses.
In conclusion, our data demonstrate the role of ROCK2 in controlling IL-21 and IL-17 secretion in human T cells via a mechanism that involves regulation through STAT3, IRF4, and RORγt. Moreover, targeting ROCK2 with the selective orally available inhibitor KD025 increases the suppressive function of Tregs through up-regulation of STAT5 phosphorylation. This indicates that ROCK2 plays an important role in modulating immune homeostasis in man and the inhibition of ROCK2 shifts the Th17/Treg balance toward the Treg phenotype. This immune modulation capacity suggests that selective ROCK2 inhibition may be an important new therapeutic approach for the treatment of autoimmune diseases.
Materials and Methods
Phase 1 Clinical Study.
We designed this randomized (6:2), double-blind, placebo-controlled, dose-escalating study to evaluate the safety, tolerability, pharmacokinetics (PK), and exploratory pharmacodynamics (PD) of single and seven multiple doses of KD025 (formerly called Slx-2119) (15) in healthy male subjects between the ages of 18 and 55 y. The dose levels studied were 40, 80, 120, 160, 240, 320, 400, and 500 mg. In each cohort, six subjects received KD025, and two subjects received placebo (single dose and then after 7 d, 7-d multiple dosing). The safety and tolerability were assessed and PK measured after single and multiple doses. For the exploratory part of this study, peripheral blood samples were collected before single and after multiple dosing, and PBMCs purified by Ficoll-Paque Plus (Amersham Bioscience) were tested in functional assays in vitro.
Cell Purification and Activation.
CD4+ T cells were purified from the peripheral blood of healthy human donors between the ages of 16 and 75 y (New York Blood Center) or from 11 patients with RA in different stages (accordingly to DAS; Fig. S10A) as previously described (20, 39). The New York University Institutional Review Board has reviewed the use of human specimens for this study. CD4+ T cells were activated with immobilized anti-CD3 mAb (5 μg/mL) and anti-CD28 mAbs (5 μg/mL) (eBioscience) with or without IL-1β (50 ng/mL) and TGF-β (5 ng/mL) (R&D Systems Inc.). Cytokine secretion was determined by ELISA after 48 h by using Human IL-10 and IFN-γ Cytoset (Biosource), IL-17 and IL-2 (R&D Systems Inc.), IL-21 and IL-17F (eBioscience), and IL-17E (Peprotech) or by intracellular staining by using anti–IL-17F-phycoerythrin (eBioscience) and anti–IL-17E-allophycocyanin (R&D Systems Inc.) antibodies. Proliferation was assessed by carboxyfluorescein succinimidyl ester (CFSE) dilution after 72–96 h.
Treg Suppression Assay.
Human CD4+ CD127− CD25+ regulatory T cells (Tregs) were purified using the Stem Cell Technologies human regulatory T-cell kit. Tregs were then incubated with KD025 for 5 h, washed, mixed with total CFSE-labeled PBMCs from the same donor, and then proliferation was analyzed after 4 d with soluble anti-CD3 (1 μg/mL). Percent of inhibition was calculated by comparing activated PBMCs alone to those wells in which PBMCs were cultured in the presence of Tregs.
Inhibitors.
The selective ROCK2 inhibitor KD025 (formerly Slx-2119) (15) was dissolved in DMSO. In addition to ROCK1, KD025 was found to have no significant activity against 300 intracellular kinases and surface receptors (Fig. S12). The pan ROCK inhibitor Y-27632 was purchased from EMD Millipore.
RNA Interference.
A mixture of four ON-TARGETplus SMARTpool siRNAs specific to ROCK1 (l-003536–06, 7, 8, 9) or ROCK2 (l-004610–06, 7, 8, 9) and control siRNA (d-001810-10-20) was purchased from Dharmacon (Thermo Fisher Scientific Inc.). Transfections of freshly purified T cells were performed using the human T-cell Nucleofector kit (Amaxa Biosystems, Lonza). Transfection efficiency was controlled by evaluating ROCK1 and ROCK2 levels using Western blot analysis after 48 h.
ChIP Assay.
Peripheral blood CD4+ T cells were stimulated by anti-CD3/28 mAbs, IL-1β, and TGF-β in the absence or presence of 10 μM of KD025. Cells were harvested at 48 h, and ChIP assays were performed.
Planar Lipid Bilayers.
Planar lipid bilayers containing anti-CD3 antibodies (5 μg/mL) and ICAM-1 (250 molecules/mm2) were prepared in parallel-plate flow cells as described previously (20).
Microscopy.
All total internal reflection fluorescence (TIRF) imaging was performed on the custom automated Nikon inverted fluorescence microscope using the 100×/1.45 N.A. TIRF objective from Nikon. TIRF illumination was set up and aligned according to the manufacturer’s instructions as previously described (40).
CIA in Mice.
Eight-week-old male DBA/1J mice were obtained from Jackson Laboratories, and the study was performed in accordance with institutional guidelines and with approval by the Institutional Animal Care and Use Committee of New York University. KD025 was administered intraperitoneally to mice with established mild arthritis on day 0 (25 d after immunization; clinical score, 6–8). Mice (n = 10 per group) were dosed with 50, 100, and 200 mg/kg of KD025 or vehicle (0.4% methylcellulose) once a day for 28 d. Clinical scores were monitored every other day. On day 28, mice were killed, and spleens and lymph nodes were isolated and analyzed by FACS and Western blot.
Statistics.
Data were analyzed by two-tailed Student t test by using the GraphPad Prism software. Additional detailed materials and methods are presented in SI Materials and Methods.
Supplementary Material
Acknowledgments
We thank Dr. S. Martomo for providing assistance with flow cytometry analysis and Drs. L. Witte, M. Palmer, J. Ryan, and A. Forbes for discussions and critical reading of the manuscript. B.R.B. is supported by NIH Grants R01 HL11879 and P01 CA 065493.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1414189111/-/DCSupplemental.
References
- 1.Medzhitov R, Janeway CA., Jr How does the immune system distinguish self from nonself? Semin Immunol. 2000;12(3):185–188, discussion 257–344. doi: 10.1006/smim.2000.0230. [DOI] [PubMed] [Google Scholar]
- 2.Korn T, Bettelli E, Oukka M, Kuchroo VK. IL-17 and Th17 cells. Annu Rev Immunol. 2009;27:485–517. doi: 10.1146/annurev.immunol.021908.132710. [DOI] [PubMed] [Google Scholar]
- 3.Sallusto F, Lanzavecchia A. Human Th17 cells in infection and autoimmunity. Microbes Infect. 2009;11(5):620–624. doi: 10.1016/j.micinf.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 4.Zhou L, et al. IL-6 programs T(H)-17 cell differentiation by promoting sequential engagement of the IL-21 and IL-23 pathways. Nat Immunol. 2007;8(9):967–974. doi: 10.1038/ni1488. [DOI] [PubMed] [Google Scholar]
- 5.Chabaud M, et al. Human interleukin-17: A T cell-derived proinflammatory cytokine produced by the rheumatoid synovium. Arthritis Rheum. 1999;42(5):963–970. doi: 10.1002/1529-0131(199905)42:5<963::AID-ANR15>3.0.CO;2-E. [DOI] [PubMed] [Google Scholar]
- 6.Chen Z, Laurence A, O’Shea JJ. Signal transduction pathways and transcriptional regulation in the control of Th17 differentiation. Semin Immunol. 2007;19(6):400–408. doi: 10.1016/j.smim.2007.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ivanov II, Zhou L, Littman DR. Transcriptional regulation of Th17 cell differentiation. Semin Immunol. 2007;19(6):409–417. doi: 10.1016/j.smim.2007.10.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Tybulewicz VL, Henderson RB. Rho family GTPases and their regulators in lymphocytes. Nat Rev Immunol. 2009;9(9):630–644. doi: 10.1038/nri2606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Riento K, Ridley AJ. Rocks: Multifunctional kinases in cell behaviour. Nat Rev Mol Cell Biol. 2003;4(6):446–456. doi: 10.1038/nrm1128. [DOI] [PubMed] [Google Scholar]
- 10.Biswas PS, Bhagat G, Pernis AB. IRF4 and its regulators: Evolving insights into the pathogenesis of inflammatory arthritis? Immunol Rev. 2010;233(1):79–96. doi: 10.1111/j.0105-2896.2009.00864.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Biswas PS, et al. Phosphorylation of IRF4 by ROCK2 regulates IL-17 and IL-21 production and the development of autoimmunity in mice. J Clin Invest. 2010;120(9):3280–3295. doi: 10.1172/JCI42856. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sun X, et al. The selective Rho-kinase inhibitor Fasudil is protective and therapeutic in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2006;180(1-2):126–134. doi: 10.1016/j.jneuroim.2006.06.027. [DOI] [PubMed] [Google Scholar]
- 13.He Y, et al. Antiinflammatory effect of Rho kinase blockade via inhibition of NF-kappaB activation in rheumatoid arthritis. Arthritis Rheum. 2008;58(11):3366–3376. doi: 10.1002/art.23986. [DOI] [PubMed] [Google Scholar]
- 14.Isgro J, et al. Enhanced rho-associated protein kinase activation in patients with systemic lupus erythematosus. Arthritis Rheum. 2013;65(6):1592–1602. doi: 10.1002/art.37934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Boerma M, et al. Comparative gene expression profiling in three primary human cell lines after treatment with a novel inhibitor of Rho kinase or atorvastatin. Blood Coagul Fibrinolysis. 2008;19(7):709–718. doi: 10.1097/MBC.0b013e32830b2891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lee JH, et al. Selective ROCK2 inhibition in focal cerebral ischemia. Annals Clin Transl Neurol. 2014;1(1):2–14. doi: 10.1002/acn3.19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Dong C. TH17 cells in development: An updated view of their molecular identity and genetic programming. Nat Rev Immunol. 2008;8(5):337–348. doi: 10.1038/nri2295. [DOI] [PubMed] [Google Scholar]
- 18.Spolski R, Leonard WJ. Interleukin-21: Basic biology and implications for cancer and autoimmunity. Annu Rev Immunol. 2008;26:57–79. doi: 10.1146/annurev.immunol.26.021607.090316. [DOI] [PubMed] [Google Scholar]
- 19.Fooksman DR, et al. Functional anatomy of T cell activation and synapse formation. Annu Rev Immunol. 2010;28:79–105. doi: 10.1146/annurev-immunol-030409-101308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Zanin-Zhorov A, et al. Protein kinase C-theta mediates negative feedback on regulatory T cell function. Science. 2010;328(5976):372–376. doi: 10.1126/science.1186068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Chemin K, et al. Cytokine secretion by CD4+ T cells at the immunological synapse requires Cdc42-dependent local actin remodeling but not microtubule organizing center polarity. J Immunol. 2012;189(5):2159–2168. doi: 10.4049/jimmunol.1200156. [DOI] [PubMed] [Google Scholar]
- 22.Chung Y, et al. Critical regulation of early Th17 cell differentiation by interleukin-1 signaling. Immunity. 2009;30(4):576–587. doi: 10.1016/j.immuni.2009.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Manel N, Unutmaz D, Littman DR. The differentiation of human T(H)-17 cells requires transforming growth factor-beta and induction of the nuclear receptor RORgammat. Nat Immunol. 2008;9(6):641–649. doi: 10.1038/ni.1610. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Brüstle A, et al. The development of inflammatory T(H)-17 cells requires interferon-regulatory factor 4. Nat Immunol. 2007;8(9):958–966. doi: 10.1038/ni1500. [DOI] [PubMed] [Google Scholar]
- 25.Zhou L, Chong MM, Littman DR. Plasticity of CD4+ T cell lineage differentiation. Immunity. 2009;30(5):646–655. doi: 10.1016/j.immuni.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 26.Bettelli E, et al. Reciprocal developmental pathways for the generation of pathogenic effector TH17 and regulatory T cells. Nature. 2006;441(7090):235–238. doi: 10.1038/nature04753. [DOI] [PubMed] [Google Scholar]
- 27.Edlund S, Landström M, Heldin CH, Aspenström P. Transforming growth factor-beta-induced mobilization of actin cytoskeleton requires signaling by small GTPases Cdc42 and RhoA. Mol Biol Cell. 2002;13(3):902–914. doi: 10.1091/mbc.01-08-0398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2(5):364–371. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 29.Lubberts E. Th17 cytokines and arthritis. Semin Immunopathol. 2010;32(1):43–53. doi: 10.1007/s00281-009-0189-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Steinman L, Merrill JT, McInnes IB, Peakman M. Optimization of current and future therapy for autoimmune diseases. Nat Med. 2012;18(1):59–65. doi: 10.1038/nm.2625. [DOI] [PubMed] [Google Scholar]
- 31.Sarra M, Franzè E, Pallone F, Monteleone G. Targeting interleukin-21 in inflammatory diseases. Expert Opin Ther Targets. 2011;15(6):695–702. doi: 10.1517/14728222.2011.561319. [DOI] [PubMed] [Google Scholar]
- 32.Simon AR, et al. Regulation of STAT3 by direct binding to the Rac1 GTPase. Science. 2000;290(5489):144–147. doi: 10.1126/science.290.5489.144. [DOI] [PubMed] [Google Scholar]
- 33.Faruqi TR, Gomez D, Bustelo XR, Bar-Sagi D, Reich NC. Rac1 mediates STAT3 activation by autocrine IL-6. Proc Natl Acad Sci USA. 2001;98(16):9014–9019. doi: 10.1073/pnas.161281298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Aznar S, et al. Simultaneous tyrosine and serine phosphorylation of STAT3 transcription factor is involved in Rho A GTPase oncogenic transformation. Mol Biol Cell. 2001;12(10):3282–3294. doi: 10.1091/mbc.12.10.3282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Debidda M, Wang L, Zang H, Poli V, Zheng Y. A role of STAT3 in Rho GTPase-regulated cell migration and proliferation. J Biol Chem. 2005;280(17):17275–17285. doi: 10.1074/jbc.M413187200. [DOI] [PubMed] [Google Scholar]
- 36.Sanz-Moreno V, et al. ROCK and JAK1 signaling cooperate to control actomyosin contractility in tumor cells and stroma. Cancer Cell. 2011;20(2):229–245. doi: 10.1016/j.ccr.2011.06.018. [DOI] [PubMed] [Google Scholar]
- 37.Olson MF. Applications for ROCK kinase inhibition. Curr Opin Cell Biol. 2008;20(2):242–248. doi: 10.1016/j.ceb.2008.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Nie H, et al. Phosphorylation of FOXP3 controls regulatory T cell function and is inhibited by TNF-α in rheumatoid arthritis. Nat Med. 2013;19(3):322–328. doi: 10.1038/nm.3085. [DOI] [PubMed] [Google Scholar]
- 39.Prevoo ML, et al. Modified disease activity scores that include twenty-eight-joint counts. Development and validation in a prospective longitudinal study of patients with rheumatoid arthritis. Arthritis Rheum. 1995;38(1):44–48. doi: 10.1002/art.1780380107. [DOI] [PubMed] [Google Scholar]
- 40.Varma R, Campi G, Yokosuka T, Saito T, Dustin ML. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity. 2006;25(1):117–127. doi: 10.1016/j.immuni.2006.04.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.