Significance
There are no treatments for congenital intellectual disabilities. Here we show that newly discovered timing rules for maximizing hippocampal long-term potentiation predict training regimens that offset defects in synaptic chemistry and memory in the fragile X mental retardation 1 (Fmr1) KO model of fragile X syndrome. Wild-type mice required far less training to form stable memories when given three training trials separated by 1 hour as opposed to one extended session; shorter or longer intervals were ineffective. The same spaced training protocol rescued memory in Fmr1 KO mice and restored activation of synaptic ERK1/2, a kinase critical for both LTP and learning. These results suggest a readily implementable, neurobiologically based therapeutic strategy for a prevalent form of intellectual disability.
Keywords: Fmr1 KO, hippocampus, object location memory, massed training, novel object recognition
Abstract
Recent studies have shown that short, spaced trains of afferent stimulation produce much greater long-term potentiation (LTP) than that obtained with a single, prolonged stimulation episode. The present studies demonstrate that spaced training regimens, based on these LTP timing rules, facilitate learning in wild-type (WT) mice and can offset learning and synaptic signaling impairments in the fragile X mental retardation 1 (Fmr1) knockout (KO) model of fragile X syndrome. We determined that 5 min of continuous training supports object location memory (OLM) in WT but not Fmr1 KO mice. However, the same amount of training distributed across three short trials, spaced by one hour, produced robust long-term memory in the KOs. At least three training trials were needed to realize the benefit of spacing, and intertrial intervals shorter or longer than 60 min were ineffective. Multiple short training trials also rescued novel object recognition in Fmr1 KOs. The spacing effect was surprisingly potent: just 1 min of OLM training, distributed across three trials, supported robust memory in both genotypes. Spacing also rescued training-induced activation of synaptic ERK1/2 in dorsal hippocampus of Fmr1 KO mice. These results show that a spaced training regimen designed to maximize synaptic potentiation facilitates recognition memory in WT mice and can offset synaptic signaling and memory impairments in a model of congenital intellectual disability.
Fragile X syndrome (FXS) is the most common cause of inherited intellectual disability (ID) (1). Currently no treatments exist for cognitive deficits associated with FXS or other neurodevelopmental disorders with ID. Research on the fragile X mental retardation 1 (Fmr1) KO mouse model of FXS has identified impairments in synaptic signaling required to produce lasting synaptic modifications (2–6) with corresponding disturbances in the activation threshold and stabilization of hippocampal long-term potentiation (LTP) (7, 8). These findings suggest specific synaptic disturbances underlie learning problems in FXS as well as targets for therapeutic interventions to improve cognitive function.
The present experiments tested predictions from LTP studies as to how modified training paradigms might rescue synaptic signaling and learning in Fmr1 KO mice. There is a deep literature demonstrating that individuals learn better when trained in short trials spaced in time than in a single, extended training episode (9). We recently found that LTP exhibits a synaptic analog for this “spaced trials effect” (10). Specifically, in hippocampal field CA1 short trains of theta burst afferent stimulation spaced by 60 min elicit far greater synaptic potentiation than can be achieved with long theta trains or by repeated trains applied at shorter intervals. As LTP is considered a mechanism of memory encoding, we propose that spaced training regimens that use the same 60-min periodicity should facilitate hippocampus-dependent learning and, thereby, may offset deficits associated with congenital ID. This hypothesis was tested for Fmr1 KO mice using the object location memory (OLM) task that both is highly sensitive to the duration of training and depends on dorsal hippocampal field CA1 (11) which exhibits LTP impairments in the KOs (7).
Our results show that given short training trials spaced by 1 h, wild-type (WT) mice learn object location in a fraction of the time needed with continuous training, and Fmr1 KOs perform at WT levels. In KOs, this robust behavioral rescue is accompanied by restoration of training-induced synaptic activation of ERK1/2, a kinase required for hippocampal LTP and learning (12, 13).
Results
Fmr1 KOs Have an Elevated Threshold for Enduring OLM.
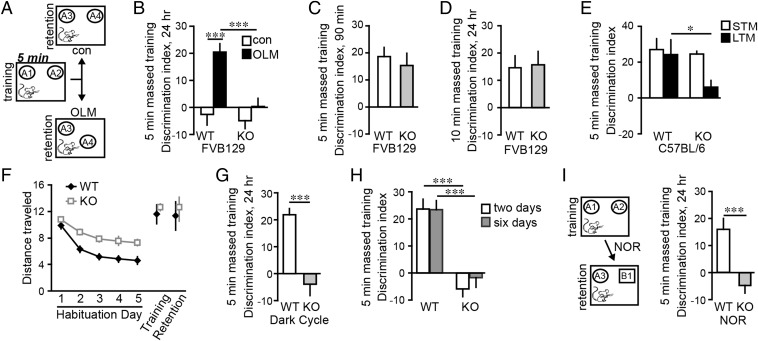
Mice were exposed to two identical objects in an open arena during training, and later returned to the arena in which one object had been moved to a novel location (Fig. 1A); preferential exploration of the novel location object indicates learning of the original location. In WT mice, a single 5- or 10-min training session elicits both short-term (90-min latency) and long-term (24-h latency) OLM whereas 3 min of training is not sufficient (14). Accordingly we used near-threshold, 5-min training to test the prediction from LTP studies that Fmr1 KO mice have an elevated threshold for this form of memory. WTs exhibited robust long-term OLM, whereas KOs did not (Fig. 1B). The deficit in KO mice was not due to initial encoding impairments; retention tested after 90 min was comparable between genotypes (Fig. 1C). As expected from the threshold argument, training for 10 min produced similar retention scores in KOs and WTs (Fig. 1D), suggesting that the KOs defect involves the amount of training needed to transfer information into long-term storage.
Fig. 1.
Long-term object location memory (OLM) and novel object recognition (NOR) are impaired in Fmr1 KOs. (A) For OLM, mice were given 5 min of continuous (massed) training with two identical objects (A1 and A2). For retention testing 24 h later, one object was in the familiar location (A3) and one was placed in a novel location (A4); for control (“con”) mice, objects were in the familiar location. (B) With 5-min training, OLM was robust in WT mice but absent in KOs (***P < 0.001; n ≥ 8 per group). (C) Short-term memory (STM) was comparable between genotypes (P = 0.57; n ≥ 10 per group). (D) KOs and WTs expressed long-term OLM when trained for 10 min (P = 0.88; n ≥ 8 per group). (E) Fmr1 KOs on the C57BL/6 background had deficient long-term OLM (*P < 0.05; n ≥ 7 per group) but control level short-term OLM (P = 0.68; n ≥ 7 per group) with 5-min massed training. (F) KOs traveled greater distance (meters) than WTs on habituation days 2–5 (P < 0.01 for each day), but not during training or retention trials (P > 0.50 both; n ≥ 8 per group). (G) KOs trained and tested in their dark cycle did not express OLM (***P < 0.001; n ≥ 10 per group). (H) KO mice given 5-min massed training on two or six successive days failed to express long-term OLM although memory was robust in WTs (***P < 0.001 vs. WTs; n ≥ 8 per group). (I) With 5-min training, WTs showed robust long-term NOR whereas KOs did not (***P < 0.001; n ≥ 12 per group).
We tested whether the above results, obtained using FVB129-background mice, also hold for KOs on the C57BL/6 background: Short-term OLM was again comparable for KO and WT mice but long-term OLM was absent in KOs given 5-min massed training (Fig. 1E).
Potential Contributors to the OLM Defect in KO Mice.
Like FXS patients, Fmr1 KOs are excessively anxious (15), which could reduce object exploration during training. However, object interaction times during training and retention trials were comparable in WTs and KOs (Fig. S1). Fmr1 KOs are also hyperactive in open-field tests (16). In accord with this, distance traveled for KOs decayed more slowly than for WTs across habituation sessions, resulting in greater locomotion on pretraining days 2–5. However, with objects present, the genotypes traveled the same distance (Fig. 1F).
To assess the robustness of the OLM impairment, we tested mice during the dark phase of their day/night cycle. Long-term OLM was still absent in KOs (Fig. 1G) although total object exploration times were not different between genotypes during training or retention testing.
Next we tested whether 5 min of continuous (i.e., massed) training produces a memory trace that is too weak to elicit quantifiable retention. If so, then additional training should increase trace strength, producing measurable discrimination indices. Mice were trained for 5 min and 24 h later for 5 min more. Tested 1 d later, WT mice showed strong OLM but KOs did not (Fig. 1H). We gave additional mice 5-min training daily for 6 d: OLM was still absent in the KOs indicating that they do not form a partial memory trace after 5 min of training.
Fmr1 KOs Have Impaired, Long-Term Novel Object Recognition (NOR).
We focused on OLM because its retrieval depends on hippocampal field CA1 (11, 14), which exhibits an elevated LTP threshold in Fmr1 KOs (7, 8) and enhanced potentiation with spaced stimulation in WTs (10, 17). However, Fmr1 KOs have more robust LTP impairments in other regions including cortex (6, 18–21). Therefore, we tested if the KOs have deficits in enduring NOR memory which depends, at least in part, on perirhinal and insular cortices for retrieval (14, 22, 23). Short-term NOR impairments have been described for Fmr1 KOs (6, 24). Mice were trained as for OLM for 5 min. For retention testing 24 h later, one of the familiar objects was replaced with a novel object but there was no change in arena-context or object location. WT mice spent more time sampling the novel object but KOs did not (Fig. 1I). The total time spent exploring objects was comparable for WTs and KOs during training but somewhat longer for KOs during retention testing (Fig. S2). NOR deficits in the KOs persisted after 10 min of training (Fig. S3). We conclude that in Fmr1 KO mice impairments in OLM and NOR reflect defects in long-term object recognition memory and, as with LTP impairments, the effects of genotype are more robust for measures with greater dependence upon cortical function.
Spaced Training Facilitates WT Learning and Rescues Memory in Fmr1 KOs.
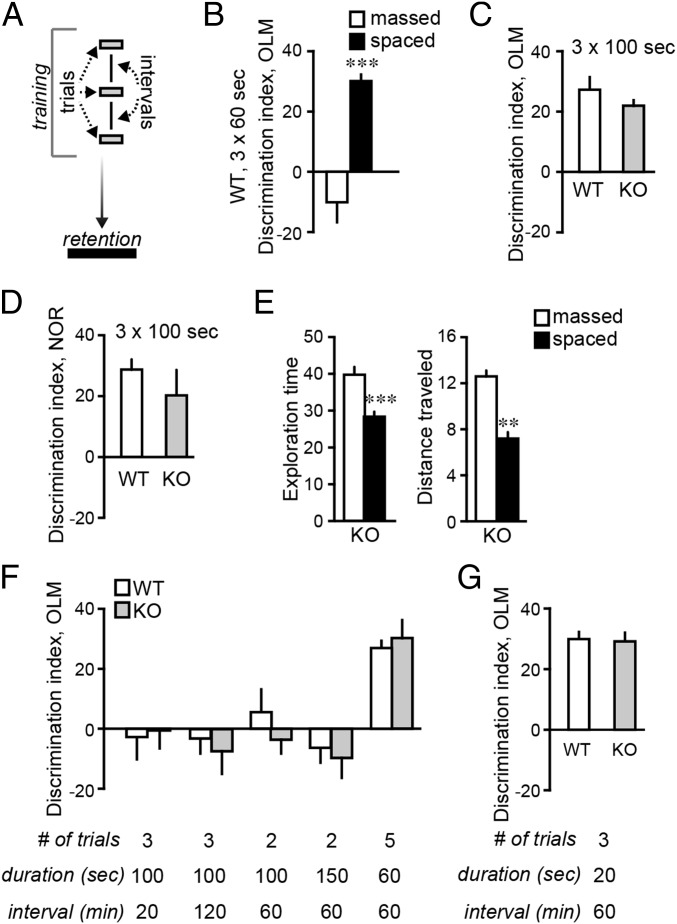
As WT mice do not form OLM with 3 min of continuous training (14), we first tested whether dividing training into three 60 s long trials produces enduring memory (Fig. 2A). A 60-min intertrial interval was selected because in LTP studies successive stimulation trains separated by this interval enhance potentiation in normal rodents (10). As shown (Fig. 2B), in WT mice, three 60-s trials supported robust OLM, whereas 3 min of continuous training did not.
Fig. 2.
Spaced training facilitates long-term memory in WTs and normalizes learning in Fmr1 KOs. (A) Training was distributed across 3 trials so that total training time was equivalent to massed trial durations that did not elicit long-term memory in WTs (3 min) or Fmr1 KOs (5 min). Training trials were spaced by 60 min; mice were tested 24 h after training. (B) WTs did not learn object location given one, 3 min long (“massed”) training trial, but did exhibit robust OLM after three 60-s trials “spaced” by 60 min (***P < 0.001; n ≥ 7 per group). (C) WT and KO mice trained in three 100-s trials, spaced by 60 min, exhibited long-term OLM (n ≥ 8 per group p); KOs given the same total training en masse did not (Fig. 1B). (D) Spaced training rescued long-term novel object recognition (NOR) in KOs (P = 0.33; n ≥ 12 per group). (E) With spaced training, KOs spent less time (seconds) exploring objects and traveled less distance (meters) than in a 5 min massed trial (**P < 0.01, ***P < 0.001; n ≥ 9 per group). (F) In both Fmr1 KO and WT mice, OLM was not supported by three 100-s trials spaced by 20 or 120 min, or by two 100-s– or 150-s–long trials spaced by 60 min. Five 60-s trials separated by 60 min supported long-term OLM in both genotypes. (G) WT and KO mice trained in three 20-s trials, spaced by 60 min, for 1 min total training, exhibited robust and comparable long-term OLM (P = 0.83 WT vs. KO; n ≥ 10 per group).
To test whether spaced training also decreases the learning threshold in Fmr1 KOs, mice were given 5 min of training divided into three 100-s trials spaced by 60 min. This regimen produced robust OLM in KOs that was comparable to measures in WTs (Fig. 2C); the same amount of training in a single 300-s trial did not support OLM in KO mice (Fig. 1B). A potent spacing effect was also observed for NOR: KOs that received three 100 s training trials spaced by 60 min performed as well as WTs when tested 24 h later (Fig. 2D). These results constitute, to our knowledge, the first evidence that spaced training modeled on LTP timing rules can rescue long-term memory in a mouse model of ID and further show the approach is effective in tasks that depend on dorsal hippocampus (OLM) or on both hippocampal and cortical fields (NOR) for retrieval.
One hypothesis for the advantages of spacing posits that the animal’s interest is greatest at the beginning of training, so that learning declines during a trial (25). If so, overall exploration would be greater for mice given spaced as opposed to continuous training. This was not the case. Total distance traveled over three spaced trials was less than in a single massed trial as was the time spent exploring objects (Fig. 2E).
Alternatively, spacing may more readily engage neurobiological processes regulating synaptic plasticity. The LTP spaced trials effect is not obtained if stimuli are separated by <50 min (10). Thus, we tested whether spacing effects on OLM are still present with shorter intertrial intervals. KO and WT mice trained with three 100-s trials spaced by 20 min did not express long-term OLM (Fig. 2F). Moreover, three 100-s trials separated by 120 min were not effective in either genotype. These results describe a surprisingly narrow between-trials window for facilitating memory by spaced training.
We next sought to define minimum conditions for lowering the training threshold for OLM with spaced training. For both genotypes, two 100-s trials, or two 150-s trials (5 min total), spaced by 1 h, did not support OLM, whereas at least three trials spaced by 1 h did (Fig. 2F). Thus, at least three trials are needed to facilitate encoding. To assess the minimum duration of training required, three trials separated by 60 min were used but each trial was decreased from 100 to just 20 s. Remarkably, when appropriately spaced, a total of 1-min training was sufficient for both WT and KO mice to encode robust, long-term memory (Fig. 2G). These results point to an unprecedented potency for spaced training, and indicate that spacing can normalize this form of learning in Fmr1 KOs.
Spaced Training Rescues Synaptic ERK1/2 Activation in Fmr1 KOs.
The above results suggest that in KOs spaced training overcomes defects in synaptic mechanisms that promote encoding. Several points implicate disturbances in ERK1/2 signaling. First, the kinase helps stabilize LTP and memory (13) and is critical for recognition memory (12). Second, although effects of the FXS mutation on ERK1/2 activation are variably described (2, 4, 26, 27), we found that synaptic, but not overall, levels of the activated kinase are elevated, and activity-driven increases in synaptic ERK1/2 phosphorylation are stunted in KO relative to WT mice (5). Here, we tested whether (i) object location learning activates synaptic ERK1/2 in WTs, (ii) the effect is impaired in Fmr1 KOs, and (iii) spaced training offsets the signaling defect in the KOs.
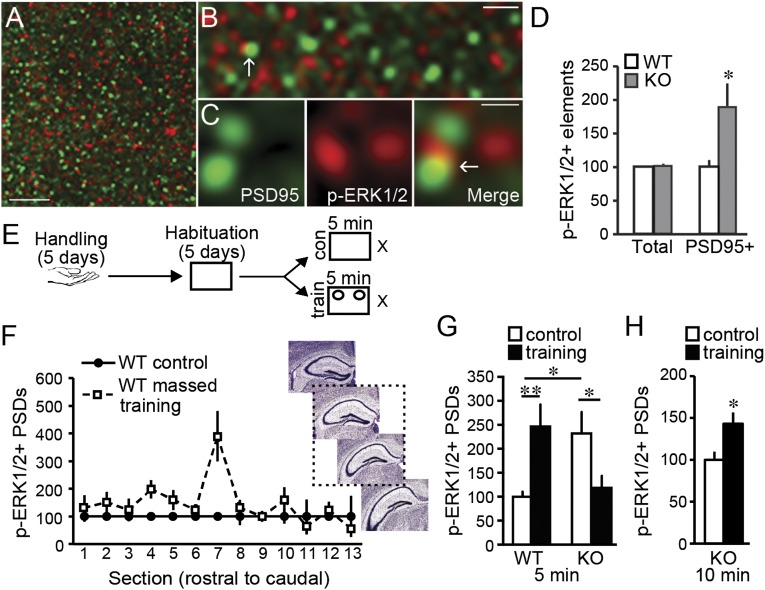
Synaptic levels of activated (Thr202/Tyr204 phosphorylated, p-) ERK1/2 were measured in CA1 stratum radiatum (SR) using quantitative fluorescence deconvolution tomography (5, 28). Sections were dual-immunolabeled for the excitatory synapse scaffold protein PSD95 (29) and p-ERK1/2 (Fig. 3 A–C). The KOs had elevated numbers of densely p-ERK1/2 immunopositive (+) synapses in CA1 SR (Fig. 3D), although total numbers of ERK1/2+ synapses were not different between genotypes.
Fig. 3.
Increases in synaptic ERK1/2 activation are associated with object location memory (OLM) in WT mice and absent in KOs given massed training. (A–C) Deconvolved images of immunolabeling for PSD95 (green) and p-ERK1/2 (red) in CA1; overlap appears yellow (arrow). (Scale bars: 5 µm, 1.25 µm, and 0.5 µm in A, B, and C, respectively.) (D) In CA1 stratum radiatum (SR), “Total” numbers of densely p-ERK1/2+ elements did not differ between genotypes (P = 0.73; normalized to WT means) but those colocalized with PSD95 (PSD95+) were more numerous in KOs than WTs (*P < 0.05; N ≥ 10 per group). (E) Mice were handled and habituated and then given 5-min massed training with (“train”) and without (“con”) objects present; p-ERK1/2+ PSDs was quantified for CA1 SR sample fields (Fig. S6). (F) Numbers of densely p-ERK1/2+ PSDs were greater in section 7, ∼2.16 mm posterior to Bregma (2 way ANOVA P = 0.002, interaction between section and group; P < 0.001, section 7 vs. others) of WT mice given spaced training. The dashed box around images shows the approximate region of further analysis (images from Allen Institute for Brain Science). (G) After 5-min massed training, numbers of densely p-ERK1/2+ PSDs were increased in WTs (**P < 0.01) but decreased in Fmr1 KOs (*P < 0.05, **P < 0.01; n ≥ 10 per group; normalized to WT control mean). Note: KOs had constitutively greater numbers of p-ERK1/2 enriched contacts. (H) Ten minutes of massed training increased numbers of densely p-ERK1/2+ PSDs in KOs (*P < 0.05; n ≥ 10 per group: normalized to KO controls).
As synaptic ERK1/2 is activated 2 min after LTP induction in WTs (5), we used this latency to test whether massed training, which supports OLM, produces similar activation in WT mice (Fig. 3E). Synaptic p-ERK1/2 levels were evaluated in every 10th section through hippocampus to determine where OLM-driven effects map onto the septotemporal arc of field CA1 (Fig. 3F). There was a significant elevation in p-ERK1/2+ PSDs ∼2.16 mm posterior to Bregma. Therefore, as a conservative measure of training-induced synaptic changes, we quantified densely p-ERK1/2+ contacts from this plane and the two adjacent sections: numbers of densely p-ERK1/2+ synapses were 2.5-fold greater in WTs given 5-min training compared with controls (Fig. 3F).
As expected, densely p-ERK1/2+ PSDs were twice as numerous in control KOs than in control WTs (Fig. 3G). For the KOs, a single 5-min training session with objects present, which does not elicit long-term OLM, failed to further increase numbers of densely p-ERK1/2+ synapses. Indeed, a significant decrease, relative to KO controls, was observed (Fig. 3G). However, 10 min of continuous training, which support OLM in KOs, increased numbers of p-ERK1/2+ synapses in CA1 SR (Fig. 3H). Thus, in both WT and KO mice, effective training activated synaptic ERK1/2 in CA1 of midrostral hippocampus.
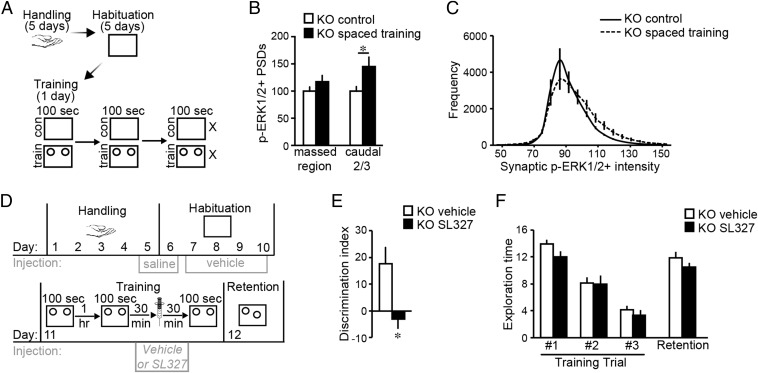
We next tested if three 100-s training trials spaced by 1 h increase synaptic p-ERK1/2 in Fmr1 KOs (Fig. 4A). In contrast to effects of 5-min massed training, spaced trials significantly increased numbers of densely p-ERK1/2+ PSDs. The increase was located in the caudal two-thirds of the segment in which increases were found after massed training in WTs (Fig. 4B) and involved a rightward shift in the p-ERK1/2 immunolabeling intensity frequency distribution (Fig. 4C) suggesting an increase in levels of activated ERK1/2 as opposed to an increase in numbers of contacts that are p-ERK1/2+. Finally, we tested if ERK1/2 activation was required for the rescue of OLM with spaced training in KOs using the brain-permeable ERK1/2 activation inhibitor SL327 (13). Mice received three 100-s–long training trials spaced by 1 h, and were injected with vehicle or SL327 (at a dose verified to depress synaptic p-ERK1/2 levels, Fig. S4) 30 min before the last trial (Fig. 4D). When tested 24 h later, vehicle-treated KOs exhibited robust OLM whereas those receiving SL327 did not (Fig. 4E); exploration times and distance traveled were comparable between groups (Fig. 4F and Fig. S5).
Fig. 4.
Synaptic ERK1/2 is activated with spaced training, and required for OLM in Fmr1 KOs. (A) Experimental Fmr1 KOs were given three 100 s OLM training trials separated by 1 h; control (“con”) mice were placed in the apparatus without objects present. Brains were harvested after the third arena session. (B) When quantified for the three planes (“massed region”) evaluated in WTs, numbers of p-ERK1/2+ PSDs in KO spaced training mice were not different from those in KO control mice (P = 0.22). However, numbers of p-ERK1/2+ PSDs were elevated in the caudal two-thirds of this span (*P < 0.05; n ≥ 9 per group; normalized to KO control mean). (C) Intensity frequency distribution for p-ERK1/2+ PSDs in CA1 planes activated by spaced training shows a rightward-shift in immunolabeling intensities of p-ERK1/2+ contacts in trained KOs. (D) Paradigm for testing effects of ERK1/2 activation inhibitor SL327 (50 mg/kg) on OLM with spaced training in KOs: injections were given 30 min before arena sessions on days 5–10 and 30 min before the last trial on the training day. (E) In KOs, OLM was blocked by SL327 but was robust with vehicle-treatment (*P < 0.05; n = 10 per group). (F) Exploration times (seconds) were comparable between vehicle- and SL327-treated KOs during spaced training and retention testing (P > 0.05 all vehicle vs. SL327 comparisons; n = 10 per group).
Discussion
The present results demonstrate that a spaced training regimen modeled on timing rules that optimize hippocampal LTP dramatically facilitates two forms of memory in WT and Fmr1 KO mice. Indeed, short training sessions spaced by one hour enabled learning with a fraction of the total training time normally required for WTs and seemingly normalized learning threshold in the KOs. Moreover, in Fmr1 KO mice, spaced training rescued otherwise deficient synaptic signaling (i.e., ERK1/2 activation) thought to be critical for memory encoding.
Fmr1 KO mice model the most common cause of inherited ID (1), but their cognitive impairments are subtle and inconsistently observed (19, 21, 30). As increased anxiety likely disrupts performance in many tasks (31), we sought a low-stress paradigm in which the KOs have a robust memory impairment. OLM satisfied these criteria. Extensive handling, several days of habituation, and an absence of strong stimuli or salient rewards during training likely minimized anxiety-inducing features. Despite the expected hyperactivity over pretraining days, measures of object interaction and exploration were comparable in KOs and WTs during training and retention trials, as was short-term memory. Nevertheless long-term memory in both OLM and NOR tasks was completely and consistently absent with conventional massed training in Fmr1 KO mice.
In the KOs, enduring LTP is impaired in the same hippocampal field required for OLM retrieval (11, 14) although this potentiation defect can be overcome by increasing the duration of inducing stimulation above that needed in WTs (7, 8). A similar phenomenon was found for the memory deficit in KO mice: Increasing massed OLM training from 5 to 10 min yielded WT retention levels. These findings suggest that the FXS mutation impairs plasticity by raising the threshold of a normally used signaling cascade or relies upon a “redundant” pathway with a high threshold. Our results support the former hypothesis: (i) KOs have more densely p-ERK1/2+ synapses than WTs, (ii) their memory impairment following a single massed trial was associated with an absence of training-induced synaptic ERK1/2 activation, (iii) spaced training, which fully restores OLM in the KOs, induced robust synaptic ERK1/2 activation, and (iv) realization of the spacing effect on learning in KO mice required ERK1/2 activation. These findings build on a rapidly expanding literature implicating ERK1/2 signaling in neurodevelopmental disabilities, including FXS (5, 32). How might abnormal regulation of synaptic ERK1/2 signaling affect long-term encoding events? One possibility is suggested by studies showing that in association with LTP the stabilization of changes to the actin cytoskeleton is impaired in Fmr1 KOs (3); ERK1/2 normally contributes to this process via signaling to actin cross linking proteins including cortactin (5, 33). Thus, disturbances in synaptic ERK1/2 regulation and filamentous (F-) actin stabilization with massed training may underlie, or importantly contribute to, the Fmr1 KO's elevated threshold for memory encoding.
Although, in WT rodents, hippocampus-dependent forms of memory rely on activity-induced ERK1/2 phosphorylation (13), we provide, to our knowledge, the first demonstration of synaptic ERK1/2 activation with learning in WT mice and its impairment in a model of intellectual disability. Moreover, evidence that training-induced ERK1/2 activation is localized to a particular septotemporal segment of hippocampus is unprecedented but accords with evidence that spatial learning activates LTP-related signaling in a limited span in the septal third of hippocampal field CA1 in rat (34). Although our results do not shed light on the basis of this topography, they raise intriguing possibilities regarding the distribution of synapses encoding different forms of memory.
Whatever its origins, the memory impairment in Fmr1 KO mice was corrected by spaced training, a well-described process in which brief, temporally separated training episodes are used to enhance memory (9, 25, 35, 36). The studies also demonstrate that the facilitating effects of spacing, for both genotypes, align with the periodicity of afferent stimulation required to enhance hippocampal LTP (10, 17). This results in a narrow time window for augmenting learning: Spaced trials were effective with a minimum of three trials spaced by 60 min while 20- or 120-min intervals did not improve retention scores. The theory that spaced training focuses learning on constant, memory-relevant cues while excluding transient stimuli (25) is not consistent with an a priori optimal spacing interval. A second hypothesis posits that the decline in attention that accompanies prolonged learning can be avoided by using several short trials. There again is no evident reason why spacing by 60 min would be better than shorter or longer intervals for reengaging attention. Moreover, we found that interaction with objects during training was not greater with spaced versus continuous training. A third widely considered benefit of spacing—that increasing the strength of newly encoded memory with additional training can occur only after the first memory trace stabilizes—does align with our finding of a minimal effective interval. We have identified a delayed consolidation phase for both LTP and OLM that emerges 50 min after stimulation or training, respectively (10, 37). The secondary LTP consolidation phase coincides with the delayed capacity for inducing augmented potentiation. The spacing effect and consolidation are thus correlated mechanistically and temporally for LTP and OLM.
The present results point to a readily implemented, noninvasive strategy for offsetting cognitive disabilities that characterize FXS. Such a behavioral approach might complement pharmacological treatments shown to facilitate learning in Fmr1 KOs or other models of congenital intellectual disability (6, 31, 32, 38). The likelihood for successful outcome is increased by the dramatic potency of spaced training. For OLM, three 20-s trials produced robust memory whereas three times (WTs) or five times (KOs) this amount of training was ineffective when given in a single session. The FXS mutation appears to have more severe consequences in humans than in mice and many of these symptoms are extrahippocampal in nature (1). It is thus noteworthy that in the KOs spaced training also rescued enduring NOR, a task that relies upon perirhinal and insular cortices for retrieval (14, 22). Beyond this, the extremely simple conditions used for the present OLM and NOR tasks may not be predictive of results for everyday circumstances in which distracting stimuli and alternative choices are present. Studies incorporating these elements constitute a next step in evaluating the translational potential of spaced training.
Materials and Methods
For greater detail, refer to SI Materials and Methods.
Animals.
Adult (3–5 mo old) male Fmr1 KO and WT mice on FVB129 and C57BL/6 backgrounds were used. Unless otherwise specified, experiments were performed on the light cycle in the FVB129 line. Studies were conducted in accordance with NIH guidelines for the care and use of laboratory animals and protocols approved by the Institutional Animal Care and Use Committee.
Behavioral Analysis.
Animal handling, training, and testing for OLM and NOR tasks were performed as described (14) with 5 min retention testing 90 min or 24 h after the last training. Arena sessions were video recorded with an overhead camera; movements were tracked, and total distance traveled was assessed, using ANY-Maze software (Stoelting). Mice were scored as interacting with an object when sniffing with nose touching or within 0.5 cm from the object. To assess preferential attention to an object, a discrimination index was calculated as 100 × (tnovel − tfamiliar)/(tnovel + tfamiliar): A positive discrimination index represents a preference for the novel object (NOR) or novel object location (OLM). In studies involving inhibition of ERK1/2 activation, the MEK inhibitor SL327 (50 mg/kg at 2.5 mg/mL; Tocris Bioscience) (13), or vehicle (DMSO) was given 30 min before the third training trial in spacing studies or 30 min before sacrifice for immunofluorescence.
Immunofluorescence/Fluorescence Deconvolution Tomography.
Mice were euthanized 2 min after the last training session and brains were cryostat sectioned (20 µm, coronal) through hippocampus. Every 10th section was processed for dual immunofluorescence localization of PSD95 and either p-ERK1/2 Thr202/Tyr204 or total ERK1/2 as described (5, 28, 39). To quantify synaptic immunolabeling, 2–3 nonoverlapping image z-stacks per section were collected at 63× (NA1.4) from CA1 SR (Fig. S6). Stacks were individually processed for FDT (28) which entailed restorative deconvolution, normalization of background fluorescence and construction of a 3D montage of each z-stack (which constituted each 42,840 µm3 sample field). Automated systems then counted and measured in 3D the size and fluorescence intensities of all synapse-sized, single- and double-labeled elements per sample field. Contacts were designated double-labeled if there was any overlap in the boundaries of labeling with the different fluorophores as assessed in 3D. Double-labeled PSD counts were normalized to the total number of PSD95+ elements in a given sample field; normalized values for each field were averaged to obtain a mean value for each brain. Intensity frequency distributions were constructed for p-ERK1/2 immunolabeling: Elements considered to be “densely” p-ERK1/2 immunoreactive had a labeling intensity of 100 or above.
Statistical Analysis.
Two-way repeated-measures ANOVAs and two-tailed Student t tests were used to assess statistical significance (considered as P ≤ 0.05). A single N was one animal for behavioral and immunofluorescence analyses. Data points that were more than four SDs removed from the group mean were not included in analyses. Values in text and figures show group means ± SEM.
Supplementary Material
Acknowledgments
We thank M. Weinberg and Drs. J. Haettig, A. Vogel-Cierna, M. A. Wood, and L. C. Palmer for assistance with behavioral studies, and Dr. J. C. Lauterborn for comments on the manuscript. This work was supported by National Science Foundation Grant 1146708, National Institutes of Health Grants MH082042 and NS04260, and the UC Irvine Center for Autism Research and Treatment. R.R.S. was supported by T32-GM0862 and National Institute of Mental Health Fellowship FMH095432A.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1413335111/-/DCSupplemental.
References
- 1.Hagerman RJ, Hagerman PJ. Fragile X Syndrome: Diagnosis, Treatment, and Research. Johns Hopkins University Press; Baltimore: 2002. [Google Scholar]
- 2.Hu H, et al. Ras signaling mechanisms underlying impaired GluR1-dependent plasticity associated with fragile X syndrome. J Neurosci. 2008;28(31):7847–7862. doi: 10.1523/JNEUROSCI.1496-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Chen LY, et al. Physiological activation of synaptic Rac>PAK (p-21 activated kinase) signaling is defective in a mouse model of fragile X syndrome. J Neurosci. 2010;30(33):10977–10984. doi: 10.1523/JNEUROSCI.1077-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gross C, et al. Excess phosphoinositide 3-kinase subunit synthesis and activity as a novel therapeutic target in fragile X syndrome. J Neurosci. 2010;30(32):10624–10638. doi: 10.1523/JNEUROSCI.0402-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Seese RR, et al. LTP induction translocates cortactin at distant synapses in wild-type but not Fmr1 knock-out mice. J Neurosci. 2012;32(21):7403–7413. doi: 10.1523/JNEUROSCI.0968-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Franklin AV, et al. Glycogen synthase kinase-3 inhibitors reverse deficits in long-term potentiation and cognition in fragile X mice. Biol Psychiatry. 2014;75(3):198–206. doi: 10.1016/j.biopsych.2013.08.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lauterborn JC, et al. Brain-derived neurotrophic factor rescues synaptic plasticity in a mouse model of fragile X syndrome. J Neurosci. 2007;27(40):10685–10694. doi: 10.1523/JNEUROSCI.2624-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lee HY, et al. Bidirectional regulation of dendritic voltage-gated potassium channels by the fragile X mental retardation protein. Neuron. 2011;72(4):630–642. doi: 10.1016/j.neuron.2011.09.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Crowder R. Principles of Learning and Memory. Erlbaum; Hillsdale, NJ: 1976. [Google Scholar]
- 10.Kramár EA, et al. Synaptic evidence for the efficacy of spaced learning. Proc Natl Acad Sci USA. 2012;109(13):5121–5126. doi: 10.1073/pnas.1120700109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barrett RM, et al. Hippocampal focal knockout of CBP affects specific histone modifications, long-term potentiation, and long-term memory. Neuropsychopharmacology. 2011;36(8):1545–1556. doi: 10.1038/npp.2011.61. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kelly A, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. J Neurosci. 2003;23(12):5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Thomas GM, Huganir RL. MAPK cascade signalling and synaptic plasticity. Nat Rev Neurosci. 2004;5(3):173–183. doi: 10.1038/nrn1346. [DOI] [PubMed] [Google Scholar]
- 14.Haettig J, et al. HDAC inhibition modulates hippocampus-dependent long-term memory for object location in a CBP-dependent manner. Learn Mem. 2011;18(2):71–79. doi: 10.1101/lm.1986911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Spencer CM, Alekseyenko O, Serysheva E, Yuva-Paylor LA, Paylor R. Altered anxiety-related and social behaviors in the Fmr1 knockout mouse model of fragile X syndrome. Genes Brain Behav. 2005;4(7):420–430. doi: 10.1111/j.1601-183X.2005.00123.x. [DOI] [PubMed] [Google Scholar]
- 16.Mineur YS, Sluyter F, de Wit S, Oostra BA, Crusio WE. Behavioral and neuroanatomical characterization of the Fmr1 knockout mouse. Hippocampus. 2002;12(1):39–46. doi: 10.1002/hipo.10005. [DOI] [PubMed] [Google Scholar]
- 17.Cao G, Harris KM. Augmenting saturated LTP by broadly spaced episodes of theta-burst stimulation in hippocampal area CA1 of adult rats and mice. J Neurophysiol. 2014 doi: 10.1152/jn.00297.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Larson J, Jessen RE, Kim D, Fine AK, du Hoffmann J. Age-dependent and selective impairment of long-term potentiation in the anterior piriform cortex of mice lacking the fragile X mental retardation protein. J Neurosci. 2005;25(41):9460–9469. doi: 10.1523/JNEUROSCI.2638-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Zhao MG, et al. Deficits in trace fear memory and long-term potentiation in a mouse model for fragile X syndrome. J Neurosci. 2005;25(32):7385–7392. doi: 10.1523/JNEUROSCI.1520-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Meredith RM, Holmgren CD, Weidum M, Burnashev N, Mansvelder HD. Increased threshold for spike-timing-dependent plasticity is caused by unreliable calcium signaling in mice lacking fragile X gene FMR1. Neuron. 2007;54(4):627–638. doi: 10.1016/j.neuron.2007.04.028. [DOI] [PubMed] [Google Scholar]
- 21.Eadie BD, Cushman J, Kannangara TS, Fanselow MS, Christie BR. NMDA receptor hypofunction in the dentate gyrus and impaired context discrimination in adult Fmr1 knockout mice. Hippocampus. 2012;22(2):241–254. doi: 10.1002/hipo.20890. [DOI] [PubMed] [Google Scholar]
- 22.Winters BD, Saksida LM, Bussey TJ. Object recognition memory: Neurobiological mechanisms of encoding, consolidation and retrieval. Neurosci Biobehav Rev. 2008;32(5):1055–1070. doi: 10.1016/j.neubiorev.2008.04.004. [DOI] [PubMed] [Google Scholar]
- 23.Broadbent NJ, Gaskin S, Squire LR, Clark RE. Object recognition memory and the rodent hippocampus. Learn Mem. 2010;17(1):5–11. doi: 10.1101/lm.1650110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ventura R, Pascucci T, Catania MV, Musumeci SA, Puglisi-Allegra S. Object recognition impairment in Fmr1 knockout mice is reversed by amphetamine: Involvement of dopamine in the medial prefrontal cortex. Behav Pharmacol. 2004;15(5-6):433–442. doi: 10.1097/00008877-200409000-00018. [DOI] [PubMed] [Google Scholar]
- 25.Hintzman D. Theoretical implications of the spacing effect. In: Solso R, editor. Theories in Cognitive Psychology: The Loyola Symposium. Wiley; Potomac, MD: 1974. pp. 77–97. [Google Scholar]
- 26.Hou L, et al. Dynamic translational and proteasomal regulation of fragile X mental retardation protein controls mGluR-dependent long-term depression. Neuron. 2006;51(4):441–454. doi: 10.1016/j.neuron.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 27.Osterweil EK, et al. Lovastatin corrects excess protein synthesis and prevents epileptogenesis in a mouse model of fragile X syndrome. Neuron. 2013;77(2):243–250. doi: 10.1016/j.neuron.2012.01.034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Seese RR, et al. Synaptic abnormalities in the infralimbic cortex of a model of congenital depression. J Neurosci. 2013;33(33):13441–13448. doi: 10.1523/JNEUROSCI.2434-13.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aoki C, et al. Electron microscopic immunocytochemical detection of PSD-95, PSD-93, SAP-102, and SAP-97 at postsynaptic, presynaptic, and nonsynaptic sites of adult and neonatal rat visual cortex. Synapse. 2001;40(4):239–257. doi: 10.1002/syn.1047. [DOI] [PubMed] [Google Scholar]
- 30.Paradee W, et al. Fragile X mouse: Strain effects of knockout phenotype and evidence suggesting deficient amygdala function. Neuroscience. 1999;94(1):185–192. doi: 10.1016/s0306-4522(99)00285-7. [DOI] [PubMed] [Google Scholar]
- 31.Bilousova TV, et al. Minocycline promotes dendritic spine maturation and improves behavioural performance in the fragile X mouse model. J Med Genet. 2009;46(2):94–102. doi: 10.1136/jmg.2008.061796. [DOI] [PubMed] [Google Scholar]
- 32.Seese RR, Maske AR, Lynch G, Gall CM. Long-term memory deficits are associated with elevated synaptic ERK1/2 activation and reversed by mGluR5 antagonism in an animal model of autism. Neuropsychopharmacology. 2014;39(7):1664–1673. doi: 10.1038/npp.2014.13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Kruchten AE, Krueger EW, Wang Y, McNiven MA. Distinct phospho-forms of cortactin differentially regulate actin polymerization and focal adhesions. Am J Physiol Cell Physiol. 2008;295(5):C1113–C1122. doi: 10.1152/ajpcell.00238.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Chen LY, Rex CS, Pham DT, Lynch G, Gall CM. BDNF signaling during learning is regionally differentiated within hippocampus. J Neurosci. 2010;30(45):15097–15101. doi: 10.1523/JNEUROSCI.3549-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Anderson MJ, Jablonski SA, Klimas DB. Spaced initial stimulus familiarization enhances novelty preference in Long-Evans rats. Behav Processes. 2008;78(3):481–486. doi: 10.1016/j.beproc.2008.02.005. [DOI] [PubMed] [Google Scholar]
- 36.da Silva BM, Bast T, Morris RG. Spatial memory: Behavioral determinants of persistence in the watermaze delayed matching-to-place task. Learn Mem. 2014;21(1):28–36. doi: 10.1101/lm.032169.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Babayan AH, et al. Integrin dynamics produce a delayed stage of long-term potentiation and memory consolidation. J Neurosci. 2012;32(37):12854–12861. doi: 10.1523/JNEUROSCI.2024-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Michalon A, et al. Chronic metabotropic glutamate receptor 5 inhibition corrects local alterations of brain activity and improves cognitive performance in fragile X mice. Biol Psychiatry. 2014;75(3):189–197. doi: 10.1016/j.biopsych.2013.05.038. [DOI] [PubMed] [Google Scholar]
- 39.Rex CS, et al. Different Rho GTPase-dependent signaling pathways initiate sequential steps in the consolidation of long-term potentiation. J Cell Biol. 2009;186(1):85–97. doi: 10.1083/jcb.200901084. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.