Abstract
Although major research efforts have focused on how specific components of foodstuffs affect health, relatively little is known about a more fundamental aspect of diet, the frequency and circadian timing of meals, and potential benefits of intermittent periods with no or very low energy intakes. The most common eating pattern in modern societies, three meals plus snacks every day, is abnormal from an evolutionary perspective. Emerging findings from studies of animal models and human subjects suggest that intermittent energy restriction periods of as little as 16 h can improve health indicators and counteract disease processes. The mechanisms involve a metabolic shift to fat metabolism and ketone production, and stimulation of adaptive cellular stress responses that prevent and repair molecular damage. As data on the optimal frequency and timing of meals crystalizes, it will be critical to develop strategies to incorporate those eating patterns into health care policy and practice, and the lifestyles of the population.
Keywords: metabolism, circadian rhythm, time-restricted feeding, feeding behavior, obesity
Obesity and associated diseases of modern societies (diabetes, cardiovascular/cerebrovascular disease, cancers, and Alzheimer’s disease) are overwhelming health care systems. Unfortunately, the common knowledge that reducing overall calorie intake and regular exercise can help optimize body weight and reduce disease risk has, in many cases, not been implemented successfully. Some of the advice provided by physicians and dieticians to their patients is consistent with the current scientific evidence, including the benefits of vegetables, fruits, fiber, nuts, and fish, and the value of reducing or eliminating snacks. However, there are many myths and presumptions concerning diet and health, including that it is important to eat three or more meals per day on a regular basis (1, 2). Although many aspects of diet and lifestyle influence metabolic status and disease trajectory during the life course, emerging findings suggest that the influences of the frequency and timing of meals on health may be large, but are difficult to characterize with any generality. Here we describe the physiological responses of laboratory animals and human subjects to controlled variations in meal size, frequency, and circadian timing, and their impact on health and disease in modern societies. Three experimental dietary regimens are considered: (i) caloric restriction (CR), in which daily calorie intake is reduced by 20–40%, and meal frequency is unchanged; (ii) intermittent energy restriction (IER), which involves eliminating (fasting) or greatly reducing (e.g., 500 calories per day) daily intake food/caloric beverage intake intermittently, for example 2 d/wk; and (iii) time-restricted feeding (TRF), which involves limiting daily intake of food and caloric beverages to a 4- to 6-h time window. We also consider the cultural and industrial barriers to implementing evidence-based healthy eating patterns, and strategies for removing or circumventing those barriers.
Evolutionary and Cultural Considerations
Unlike modern humans and domesticated animals, the eating patterns of many mammals are characterized by intermittent energy intake. Carnivores may kill and eat prey only a few times each week or even less frequently (3, 4), and hunter-gatherer anthropoids, including those living today, often eat intermittently depending upon food availability (5, 6). The ability to function at a high level, both physically and mentally, during extended periods without food may have been of fundamental importance in our evolutionary history. Many adaptations for an intermittent food supply are conserved among mammals, including organs for the uptake and storage of rapidly mobilizable glucose (liver glycogen stores) and longer-lasting energy substrates, such as fatty acids in adipose tissue. Behavioral adaptations that enable food acquisition and storage permeate the behavioral repertoire of all species, including humans. Indeed, the higher cognitive capabilities of humans compared with other species likely evolved for the purpose of acquiring food resources; evidence suggests that the earliest tools (7) and languages (8) were invented to aid in food acquisition.
The agricultural revolution, which began ∼10,000 y ago, resulted in the constant year-round availability of food typical of modern societies. Our agrarian ancestors adopted a three meals per day eating pattern, presumably because it provided both social and practical benefits for the daily work and school schedules. More recently, within the past 50 y, high calorie density foodstuffs (refined grains, sugar, cooking oils, corn syrup, and so on) have permeated these three daily meals (9). When superimposed on increasingly sedentary lifestyles, the consumption of high-energy meals multiple times each day plausibly contributed to the emergence of obesity and related diseases as major causes of morbidity and mortality (Fig. 1). Obesity has also become a major health problem in dogs and cats, which are often fed ad libitum (10), and even laboratory rodents can often be considered overfed and sedentary (11, 12). Indeed, animals in the wild and hunter-gatherer humans rarely, if ever, suffer from obesity, diabetes, and cardiovascular disease (5).
Fig. 1.
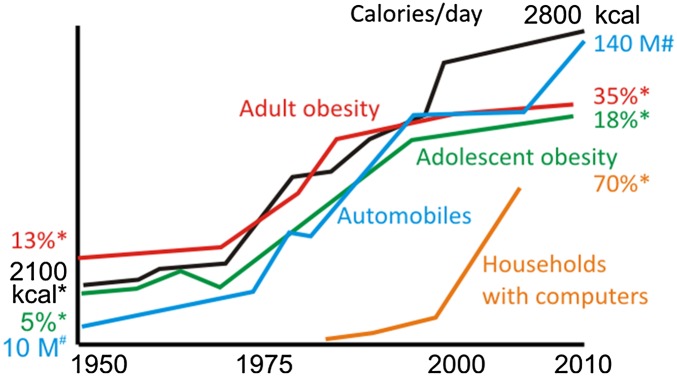
The rising tide of obesity is strongly associated with daily calorie intake and sedentary lifestyle-promoting transportation (refs. 84–86; www.earth-policy.org/data_center/C23). *US, approximate value. #Worldwide auto production.
Circadian Rhythms, Meal Timing, and Health
Circadian rhythms are self-sustained ∼24-h oscillations in behavior, physiology, and metabolism. These rhythms have evolved and permit organisms to effectively respond to the predictable daily change in the light:dark cycle and the resultant rhythms in food availability in nature. Gene-expression studies have revealed more than 10% of expressed genes in any given organ exhibit circadian oscillation (13). These rhythmic transcripts encode key rate-determining steps in neuroendocrine, signaling, and metabolic pathways. Such regulation temporally separates incompatible cellular processes and optimizes cellular and organismal fitness. Although the circadian clock is cell-autonomous and is present in the majority of tissue types, the circadian system is organized in a hierarchical manner in which the hypothalamic suprachiasmatic nucleus (SCN) functions as the master circadian clock that uses both diffusible and synaptic mechanisms to orchestrate circadian rhythms in the peripheral organs at appropriate phase. Photoreceptive retinal ganglion cells send ambient light information to the SCN through monosynaptic connection to ensure that the circadian system is entrained to the daily light:dark cycle (14).
Whereas light is the dominant timing cue for the SCN oscillator, time of food intake affects the phase of the clocks in peripheral tissues (15), including liver, muscle, and adipose tissues. For millions of years in the absence of artificial light, the circadian clock—in conjunction with the retinal light input—imposed diurnal rhythms in physiology and behaviors, including the activity/rest and feeding/fasting cycle. For many of our ancestors, food was probably scarce and primarily consumed during daylight hours, leaving long hours of overnight fasting. With the advent of affordable artificial lighting and industrialization, modern humans began to experience prolonged hours of illumination every day and resultant extended consumption of food. The modern lifestyle perturbed the human circadian system in three primary ways: shift work, exposure to prolonged hours of artificial light, and erratic eating patterns. Although it is difficult to separate the consequence of each of these perturbations on metabolism and physiology, animal models and recent experimental human studies have begun to elucidate the mechanisms and consequence of these circadian disruptions. In industrial societies nearly 10% of the workforce performs night-shift work: either permanent night work, rotating shifts, or irregular schedules, in which the individuals typically switch their wakeful hours back to the daytime during days off to maintain a typical social life on those days. During night-shift work the individuals are subject to both prolonged hours of artificial lighting and an abnormal eating schedule. Furthermore, during the weekend the tendency to maintain a day-active social life imposes a jet-lag–type paradigm in which both central and peripheral clocks attempt to adjust to a weekend lifestyle. Although such internal desynchrony has never been demonstrated directly in humans, based on animal experimental work this is presumed to result in chronic disruption of circadian rhythms, which may help explain the known association between night work and several diseases, including cardiovascular disease, diabetes, obesity, certain types of cancer, and neurodegenerative diseases (16, 17).
In addition to shift work, modern human societies experience prolonged illumination (18) and erratic eating patterns, both of which are known to perturb the circadian system. In nocturnal rodent models, extended illumination has been shown to increase predisposition to metabolic diseases. Conversely, in diurnal flies a shift to nighttime feeding compromises fat metabolism and fecundity (19). In humans, a 12-h shift of the sleep/wake and fasting/feeding cycle compared with the central circadian system, while maintaining an isocaloric diet, reduced glucose tolerance, increased blood pressure, and decreased the satiety hormone leptin (20). These studies highlight the importance of temporal organization of sleep and feeding relative to the circadian system. Both nutrient quality and genetic factors appear to affect meal timing in rodents. Mutation in the circadian clock gene Per1 affecting a conserved phosphorylation site causes mice to consume more food during the daytime and predisposes them to metabolic diseases (21). The widely used diet-induced obesity model in mice also perturbs feeding; mice fed a high-fat diet ad libitum consume small meals throughout day and night (22). Both diet-induced obesity and obesity in Per1 mutant mice can be prevented by restricting access to high-fat diet only during the nighttime (23). The surprising effectiveness of TRF without altering caloric intake or source of calories suggests a potentially effective meal-timing intervention for humans. Indeed, recent human studies suggest that earlier meal timing associates with improved effectiveness of weight-loss therapy in overweight and obese patients (24, 25).
The mechanism underlying the beneficial effect of TRF is likely complex and acts on multiple pathways. The daily fasting and feeding episodes trigger alternative activation of fasting-responsive cAMP response element binding protein (CREB) and AMP kinase, and feeding responsive insulin-dependent mammalian target of rapamycin (mTOR) pathways implicated in metabolic homeostasis. In addition, these pathways also impinge on the circadian clock and improve robustness of oscillation of clock components and downstream targets (23). Accordingly, gene-expression studies indicate that TRF supports circadian rhythmicity of thousands of hepatic transcripts (26).
The confluence of genomics and genetics in mice is unraveling the pathways from the core clock components to specific nutrient metabolism. The nuclear hormone receptors REV-ERBs are integral to the circadian clock and directly regulate transcription of several key rate-determining enzymes for fatty acid and cholesterol metabolism (27). Although cryptochrome proteins are strong transcriptional suppressors, they also inhibit cAMP signaling and thereby tune CREB-mediated gluconeogenesis (28). Circadian clock downstream transcription factors DBP/TEF/HLF regulate xenobiotic metabolism (29), and KLF15 mediates nitrogen metabolism (30). These and other modes of regulation (31) provide a mechanistic framework by which meal-timing affects the circadian clock and, in turn, affects metabolic homeostasis in mammals.
Not only does circadian phase influence the metabolic response to food intake, food intake itself has recently been demonstrated to be under control by the endogenous circadian system, independent of the sleep/wake and fasting/feeding cycle (32), possibly helping explain why breakfast is often the smallest meal of the day or even skipped all together.
Cellular and Molecular Mechanisms: Insight from Intermittent Energy Restriction and Fasting
Compared with those fed ad libitum, the lifespans of organisms from yeast and worms, to mice and monkeys can be extended by dietary energy restriction (33–35). Data collected from individuals practicing severe dietary restriction indicate that humans undergo many of the same molecular, metabolic, and physiologic adaptations typical of long-lived CR rodents (36). IER/fasting can forestall and even reverse disease processes in animal models of various cancers, cardiovascular disease, diabetes, and neurodegenerative disorders (2). Here we briefly highlight four general mechanisms by which IER protects cells against injury and disease.
Adaptive Stress Responses.
Compared with their usual ad libitum feeding conditions, laboratory animals maintained on IER exhibit numerous changes, suggesting heightened adaptive stress responses at the molecular, cellular, and organ system levels. Alternate-day fasting prevents age-related decrements in the antioxidant enzymes superoxide dismutase 1 and catalase in the liver cells of rats (37). IER increases levels of the antioxidant enzymes NADH-cytochrome b5 reductase and NAD(P)H-quinone oxidoreductase 1 in muscle cells of mice, and these effects are accentuated by exercise (38). Numerous studies have shown that IER can protect neurons against oxidative, metabolic, and proteotoxic stress in animal models of neurodegenerative disorders, including Alzheimer’s and Parkinson’s diseases (39). IER can also protect the heart against ischemic damage in an animal model of myocardial infarction (40). Alternate-day fasting stimulates the production of several different neuroprotective proteins, including the antioxidant enzyme heme oxygenase 1, proteins involved in mitochondrial function, and the protein chaperones HSP-70 and GRP-78 (41, 42). Moreover, IER increases the production of trophic factors that promote neuronal survival, neurogenesis, and the formation and strengthening of synapses in the brain (43). Taken together, these data suggest that beneficial effects of IER involve the general biological phenomenon of “hormesis” or “preconditioning,” in which exposure of cells and organisms to a mild stress results in adaptive responses that protect against more severe stress.
Bioenergetics.
When humans switch from eating three full meals per day to an IER diet, such as one moderate size meal every other day or only 500–600 calories 2 d/wk, they exhibit robust changes in energy metabolism characterized by increased insulin sensitivity, reduced levels of insulin and leptin, mobilization of fatty acids, and elevation of ketone levels (44–47). Ketones, such as β-hydroxybutyrate, are known to have beneficial effects on cells with a high energy demand, such as neurons in the brain (Fig. 2) (48, 49). In mice, alternate-day fasting can greatly increase insulin sensitivity even without a major reduction in body weight (50), and in humans IER can increase insulin sensitivity more than daily calorie restriction that achieves similar weight loss (45, 51). Dietary energy restriction can prevent age-related decline in mitochondrial oxidative capacity in skeletal muscle, and can induce mitochondrial biogenesis (52). Brain bioenergetics may also be bolstered by IER. For example, brain-derived neurotrophic factor (BDNF), which is up-regulated in hippocampal neurons in response to IER and exercise, activates the transcription factor CREB, which then induces peroxisome proliferator-activated receptor gamma coactivator 1-α (PGC-1α) expression and mitochondrial biogenesis (53). The latter study showed that PGC-1α and mitochondrial biogenesis are critical for the formation of synapses in developing hippocampal neurons and the maintenance of synapses in the hippocampus of adult mice. Because impaired mitochondrial biogenesis and function occur during aging and chronic disease states, such as sarcopenia and neurodegenerative disorders, it is important to consider the impact of the frequency and circadian timing of meals on the development and progression of such disorders.
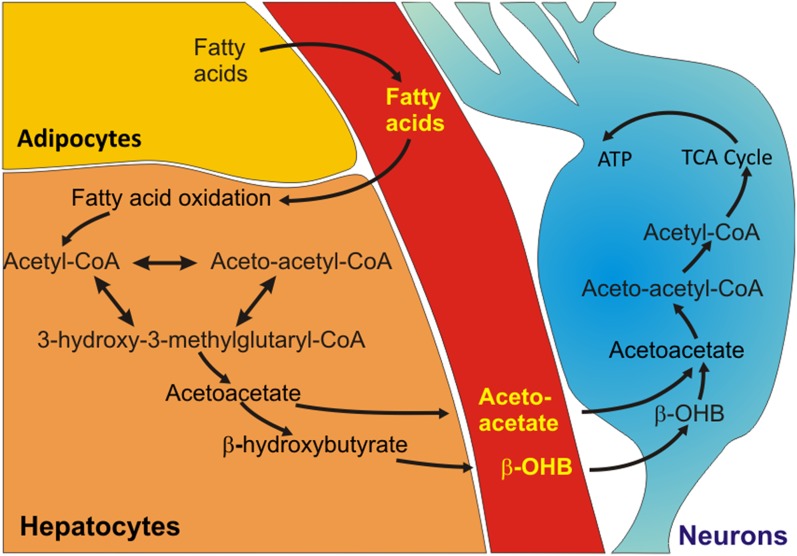
Fig. 2.
A metabolic shift to ketogenesis that occurs with fasting bolsters neuronal bioenergetics. Liver glycogen stores are typically depleted within 10–12 h of fasting, which is followed by liberation of fatty acids from adipose tissue cells into the blood. The fatty acids are then transported into liver cells where they are oxidized to generate Acetyl-CoA. Acetyl-CoA is then converted to 3-hydroxy-3-methylgluaryl-CoA, which is in turn used to generate the ketones acetoacetate and β-hydroxybutyrate (β-OHB). The ketones are released into the blood and are transported into various tissues, including the brain, where they are taken up by neurons and used to produce acetyl-CoA. Acetyl-CoA enters the tricarboxylic acid (TCA) cycle to generate ATP.
Whereas IER/fasting is beneficial and overeating detrimental for many types of normal cells, the converse is true for tumor cells. Cells in tumors exhibit major mitochondrial abnormalities and generate their ATP primarily from glycolysis rather than oxidative phosphorylation (54). Moreover, tumors are highly vascularized and so their cells have access to large amounts of circulating glucose. Animal models have consistently shown that IER inhibits and even reverses the growth of a range of tumors, including neuroblastoma, breast, and ovarian cancers (55). The shift to ketogenesis may play an important role in suppression of tumor growth by IER/fasting because many tumor cells are largely unable to use ketones as an energy source; accordingly, ketogenic diets may potentiate the antitumor effects of IER (54). Although preliminary, recent case studies in human patients suggest potential applications of IER in the treatment of a range of cancers, including breast, ovarian, prostate, and glioblastoma (56, 57). Indeed, evolutionary theory predicts that collected random mutations will prevent tumor cells from making the necessary metabolic adaptations to IER (58).
Inflammation.
All major diseases, including cardiovascular disease, diabetes, neurodegenerative disorders, arthritis, and cancers involve chronic inflammation in the affected tissues and, in many cases, systemically (59). Local tissue inflammation involves hyperactivation of macrophages (microglia in the brain) which produce proinflammatory cytokines (TNF, IL-1β, IL-6) and reactive oxygen species. Overweight and obesity promote inflammation, and IER suppresses inflammation in human subjects and animal models of diseases. Obese women who changed their diet from multiple daily meals to alternate-day energy restriction exhibited significant reductions in levels of circulating TNF and IL-6 (60). In asthma patients, 2 mo of alternate-day energy restriction reduced circulating TNF and markers of oxidative stress, and improved asthma symptoms and airway resistance (44). However, because weight loss may reduce inflammation regardless of the dietary change inducing the weight loss, it will be important to determine if and how eating patterns modify inflammation independently of weight loss. Multiple studies have shown that fasting can lessen symptoms in patients with rheumatoid arthritis (61), and data from animal studies suggest that the pathogenesis of other autoimmune disorders may also be counteracted by IER, including multiple sclerosis (62), lupus erythematosus (63), and type I diabetes (64). In a mouse model of stroke, IER suppressed elevations of TNF and IL-1β in the ischemic cerebral cortex and striatum, which was associated with improved functional outcome (41). Inflammation is increasingly recognized as a contributing factor for cancer cell growth (65) and, because excessive energy intake promotes inflammation, it is likely that suppression of inflammation plays a role in the inhibition of tumor growth by IER. Whereas inhibiting immune responses to autoantigens and sterile tissue injuries can be beneficial, suppression of immune responses to infectious agents is detrimental. It will therefore be important to determine whether eating regimens such as TRF and IER affect immune responses to pathogens, an as yet unexplored area of investigation.
Improved Repair and Removal of Damaged Molecules and Organelles.
Cells possess dedicated mechanisms for the removal of damaged molecules and organelles. One mechanism involves the molecular “tagging” of damaged proteins with ubiquitin, which targets them for degradation in the proteasome (66). In a second and more elaborate mechanism called autophagy, damaged and dysfunctional proteins, membranes, and organelles are directed to and degraded in lysosomes (67). Energy and nutrient (particularly amino acids) intake have been shown to have consistent effects on autophagy. When organisms ingest regular meals, their cells receive a relatively steady supply of nutrients and so remain in a “growth mode” in which protein synthesis is robust and autophagy is suppressed (68). The nutrient-responsive mTOR pathway negatively regulates autophagy. Accordingly, fasting inhibits the mTOR pathway and stimulates autophagy in cells of many tissues, including liver, kidney, and skeletal muscle (69–71). In this way, fasting “cleanses” cells of damaged molecules and organelles.
Rats maintained on energy-restricted diets exhibit reduced accumulation of polyubiquitinated proteins and evidence of increased autophagy in peripheral nerves compared with rats fed ad libitum (72). In a mouse model of Charcot-Marie-Tooth type 1A, an inherited disorder characterized by demyelination of peripheral nerves, IER improved motor performance and reduced demyelination by a mechanism involving enhanced autophagy and reduced accumulation of myelin protein PMP22 aggregates (73). A common feature of many major chronic diseases is the abnormal/excessive accumulation of protein aggregates within and outside of cells; examples include intracellular α-synuclein in Parkinson’s disease and extracellular amyloid β-peptide and intracellular Tau protein in Alzheimer’s disease (74, 75). In addition to the frequency of meals, the circadian timing of meals is likely to affect the responses of the cellular machineries for clearance of damaged proteins and organelles (76). Autophagy is regulated in a diurnal rhythm in many cell types, and this rhythm can be altered by changing the timing of food intake. It is therefore reasonable to consider that meal timing has an impact on diseases that involve impaired or insufficient autophagy.
Society-Wide Implications
The high rates of childhood and adult obesity and the diseases they foster is a major burden to our society. As findings from basic research studies and controlled interventional trials accrue, consensus recommendations for healthy patterns of meal frequency and diurnal timing may eventually emerge. If sufficient evidence does emerge to support public health and clinical recommendations to alter meal patterning, there will be numerous forces at play in the acceptance or resistance to such recommendations. First and perhaps foremost is cultural tradition. Three meals plus snacks daily has become the norm during the past half-century, such that a majority of American children are accustomed to this eating pattern. Second, the agriculture, food processing, food retail, and restaurant industries and all of the affiliated industries that serve or promote food—from airlines to concert stadiums to television cooking shows to advertising and others—still all have established practices and financial interests and these interests may affect receptivity to proposed shifts in eating patterns and potential decreases in total food purchased. Third, the willingness and ability of the American health care system, including medical training and practice, to emphasize prevention and lifestyles will be a key factor in success or lack thereof.
We believe that it is important to consider how “prescriptions” for meal frequency and timing can be developed, validated, and implemented in light of the current industrial, cultural, and institutional pressures to maintain the status quo of daily overconsumption of food. In doing so, it will be important to ensure that we provide the public with accurate information on eating patterns and health. For example, despite equivocal and even contradictory scientific evidence, breakfast is often touted as a weight-control aid (77), but recent evidence has suggested that it may not be (78). Primary education and media outlets should dispense up-to-date information on healthy eating, including the frequency and circadian timing of meals. Although regulatory agencies must play an important role in developing recommendations and facilitating their implementation, it may also be helpful for parents to lead by example and establish healthy eating patterns in their children. Additionally, the inclusion of science-based information on eating patterns and health in primary and secondary education may help stem the rising tide of overeating and related poor health in our children. The medical community could play a central role in developing and implementing prescriptions for long-term daily energy restriction or IER that can be incorporated into most daily home and workplace environments. Examples of such prescriptions include fasting or caloric restriction (e.g., 500 calories) on alternate days or 2 d each week, or forgoing breakfast and lunch several days each week (Fig. 3). The available evidence suggests that patients may be able to comply with such diets when there is rigorous follow-up (44, 45, 47), and it will be important to determine if compliance would increase further if patients were able to choose an eating pattern-based prescription that best fits their weekly routines. Recent findings suggest that it may be possible for many people to adopt a long-term change in their lifestyle from eating three meals plus snacks every day to an IER diet if they are able to keep on the new eating pattern during a transition period of approximately 1 mo (45). Moreover, for many people who are overweight IER may facilitate their maintenance of an overall reduction in energy intake compared with prescriptions for daily caloric restriction.
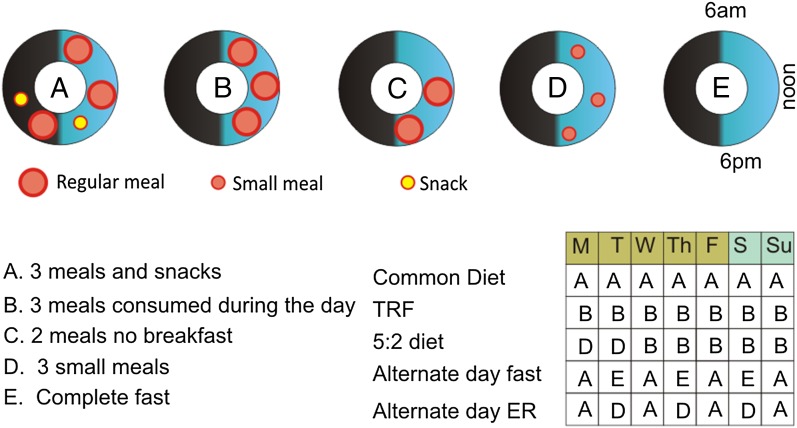
Fig. 3.
Patterns of daily and weekly food consumption. The upper illustration shows five different patterns of food consumption during a 24-h period. A: Eating three large meals plus snacks spread throughout a 16-h period of wakefulness; this is the common eating pattern of food consumption upon which the epidemic of obesity, diabetes, and associated chronic diseases has emerged. B–D: Examples if time-restricted eating patterns in which food is consumed as three (B) or two (C) regular size meals, or three small meals (D). E: Complete fast. Examples of weekly eating schedules are shown in the lower right. ER, energy restriction; IER, intermittent energy restriction; TRF, time-restricted feeding.
Future Directions
Further animal studies are required to better elucidate the cellular and molecular mechanisms by which meal frequency, IER, and TRF affect health and disease susceptibility, as well as the impact of eating patterns on extant disease processes in various experimental models. For example, it will be of great interest to know the effects of IER and TRF on gene expression, epigenetic markers (methylation and acetylation), and disease-relevant pathways in multiple tissues throughout the body and nervous system. The overlapping and complementary effects of exercise and healthy eating patterns on functionality and disease resistance should be elucidated. Intervention studies of IER and TRF, particularly randomized controlled trials (RCTs), should be performed in various groups of human subjects, including those who are healthy and those with diseases, such as obesity, diabetes, cancer, cardiovascular disease, and neurodegenerative disorders. RCTs should include functional outcomes as well as biomarkers relevant to disease risk and pathogenesis. Thus, far, relatively few RCTs of IER and TRF have been performed in human subjects, with the results of several studies of alternate-day and twice weekly energy restriction demonstrating weight loss and abdominal fat reduction and suggesting improvements in indicators of energy and lipid metabolism and inflammation (44–46, 51, 61). On the other hand, a study of TRF in which healthy normal weight subjects consumed a balanced daily food intake within a 4-h or 12-h time period each day revealed no improvement (79, 80), which is similar to the lack of any short-term benefit of TRF in mice when the animals are fed a balanced diet (23). This finding suggests that the short-term benefits of TRF might depend on the diet and body composition. It will also be critical to evaluate long-term adherence of different subject populations to IER and TRF protocols to evaluate their feasibility for broad applications for sustained weight reduction and disease risk reduction.
Genetic factors can determine whether the lifespan of a particular strain of mouse or rat is increased, unaffected, or even decreased, by lifelong CR or TRF, with inbred animals generally responding less well to CR (81). Understanding the mechanism of TRF will help to predict whether a certain eating pattern is beneficial or whether individuals with specific genotype are predisposed to erratic eating patterns. Missense mutation in circadian clock component Per1 has been shown to affect eating patterns in mice (21). However, the presence of intact food anticipatory activity in SCN ablated rodents or those lacking functional circadian oscillator genes points to yet-unidentified genes and circuits in eating-pattern determination (82, 83). Humans are highly heterogeneous with regard to their genetic composition, epigenetic landscape, and the environmental factors to which they are exposed throughout life. It is therefore likely that there will be considerable variability among human subjects in the responses of their cells and organ systems (and overall health) to different eating patterns. Although there is sufficient evidence to suggest that CR and IER can improve health indicators in most or all obese human subjects (2), data are lacking with regard to normal weight subjects. Insight into genetic and epigenetic factors that affect responses to specific eating patterns could be obtained from RCTs of TRF and IER regimens in normal weight subjects in which biomarkers of health and disease risk are measured (blood pressure, heart rate variability, insulin resistance, lipid profiles, adipokines, ketones, and so forth).
It would be particularly valuable to design RCT in human subjects with head-to-head comparisons of multiple eating patterns, such as those shown in Fig. 3. Once the eating patterns that promote optimal health are established, what can be done to encourage, enable and empower individuals to modify their food choices and eating patterns? Implementing any such changes will be challenging, as a half-century of research on behavioral approaches to weight control suggests. That said, the field of behavioral science is continually evolving, as is the growth and quality of mobile information technology, which may serve to buttress efforts. We are hopeful that in the future, we may be better able to help individuals achieve the healthy behavior changes they desire.
Acknowledgments
This article incorporates information from a workshop on “Eating Patterns and Disease,” which can be viewed at videocast.nih.gov/summary.asp?Live=13746&bhcp=1, and was supported by the National Institute on Aging Intramural Research Program and the Glenn Foundation for Medical Research. Relevant research in the authors' laboratories are supported by NIH intramural support (to M.P.M.); NIH Grants P30DK056336 (to D.B.A.), P01AG034906 (to V.D.L.), R01NS041012 (to L.N.), P30DK072476 (to E.R.), R01DK099512 (to F.A.J.L.S.), R01NS055195 (to T.N.S.), R01HL106228 (to K.A.V.), and R01DK091618 (to S.P.); the European Union's Seventh Framework Programme MOPACT [mobilising the potential of active ageing in Europe; FP7-SSH-2012-1 Grant 320333 (to L.F.)]; a grant from Genesis Breast Cancer Prevention, UK (to M.H.); and Belgian Foundation for Scientific Medical Research Grant 3.4520.07 (to W.J.M.).
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission.
References
- 1.Casazza K, et al. Myths, presumptions, and facts about obesity. N Engl J Med. 2013;368(5):446–454. doi: 10.1056/NEJMsa1208051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Longo VD, Mattson MP. Fasting: Molecular mechanisms and clinical applications. Cell Metab. 2014;19(2):181–192. doi: 10.1016/j.cmet.2013.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gervasi V, et al. Predicting the potential demographic impact of predators on their prey: A comparative analysis of two carnivore-ungulate systems in Scandinavia. J Anim Ecol. 2012;81(2):443–454. doi: 10.1111/j.1365-2656.2011.01928.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Metz MC, Smith DW, Vucetich JA, Stahler DR, Peterson RO. Seasonal patterns of predation for gray wolves in the multi-prey system of Yellowstone National Park. J Anim Ecol. 2012;81(3):553–563. doi: 10.1111/j.1365-2656.2011.01945.x. [DOI] [PubMed] [Google Scholar]
- 5.Cordain L, Eaton SB, Miller JB, Mann N, Hill K. The paradoxical nature of hunter-gatherer diets: Meat-based, yet non-atherogenic. Eur J Clin Nutr. 2002;56(Suppl 1):S42–S52. doi: 10.1038/sj.ejcn.1601353. [DOI] [PubMed] [Google Scholar]
- 6.Ströhle A, Hahn A, Sebastian A. Estimation of the diet-dependent net acid load in 229 worldwide historically studied hunter-gatherer societies. Am J Clin Nutr. 2010;91(2):406–412. doi: 10.3945/ajcn.2009.28637. [DOI] [PubMed] [Google Scholar]
- 7.van Schaik CP, Deaner RO, Merrill MY. The conditions for tool use in primates: Implications for the evolution of material culture. J Hum Evol. 1999;36(6):719–741. doi: 10.1006/jhev.1999.0304. [DOI] [PubMed] [Google Scholar]
- 8.Allen JS. “Theory of food” as a neurocognitive adaptation. Am J Hum Biol. 2012;24(2):123–129. doi: 10.1002/ajhb.22209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tillotson JE. America’s obesity: Conflicting public policies, industrial economic development, and unintended human consequences. Annu Rev Nutr. 2004;24:617–643. doi: 10.1146/annurev.nutr.24.012003.132434. [DOI] [PubMed] [Google Scholar]
- 10.Zoran DL. Obesity in dogs and cats: A metabolic and endocrine disorder. Vet Clin North Am Small Anim Pract. 2010;40(2):221–239. doi: 10.1016/j.cvsm.2009.10.009. [DOI] [PubMed] [Google Scholar]
- 11.Martin B, Ji S, Maudsley S, Mattson MP. “Control” laboratory rodents are metabolically morbid: Why it matters. Proc Natl Acad Sci USA. 2010;107(14):6127–6133. doi: 10.1073/pnas.0912955107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Klimentidis YC, et al. Canaries in the coal mine: A cross-species analysis of the plurality of obesity epidemics. Proc Biol Sci. 2011;278(1712):1626–1632. doi: 10.1098/rspb.2010.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Panda S, Hogenesch JB, Kay SA. Circadian rhythms from flies to human. Nature. 2002;417(6886):329–335. doi: 10.1038/417329a. [DOI] [PubMed] [Google Scholar]
- 14.Hatori M, Panda S. The emerging roles of melanopsin in behavioral adaptation to light. Trends Mol Med. 2010;16(10):435–446. doi: 10.1016/j.molmed.2010.07.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stokkan KA, Yamazaki S, Tei H, Sakaki Y, Menaker M. Entrainment of the circadian clock in the liver by feeding. Science. 2001;291(5503):490–493. doi: 10.1126/science.291.5503.490. [DOI] [PubMed] [Google Scholar]
- 16.Stevens RG, Brainard GC, Blask DE, Lockley SW, Motta ME. Breast cancer and circadian disruption from electric lighting in the modern world. CA Cancer J Clin. 2014;64(3):207–218. doi: 10.3322/caac.21218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Morris CJ, Yang JN, Scheer FA. The impact of the circadian timing system on cardiovascular and metabolic function. Prog Brain Res. 2012;199:337–358. doi: 10.1016/B978-0-444-59427-3.00019-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Wright KP, Jr, et al. Entrainment of the human circadian clock to the natural light-dark cycle. Curr Biol. 2013;23(16):1554–1558. doi: 10.1016/j.cub.2013.06.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gill S, Panda S. Feeding mistiming decreases reproductive fitness in flies. Cell Metab. 2011;13(6):613–614. doi: 10.1016/j.cmet.2011.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Scheer FA, Hilton MF, Mantzoros CS, Shea SA. Adverse metabolic and cardiovascular consequences of circadian misalignment. Proc Natl Acad Sci USA. 2009;106(11):4453–4458. doi: 10.1073/pnas.0808180106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Liu Z, et al. PER1 phosphorylation specifies feeding rhythm in mice. Cell Rep. 2014;7(5):1509–1520. doi: 10.1016/j.celrep.2014.04.032. [DOI] [PubMed] [Google Scholar]
- 22.Kohsaka A, et al. High-fat diet disrupts behavioral and molecular circadian rhythms in mice. Cell Metab. 2007;6(5):414–421. doi: 10.1016/j.cmet.2007.09.006. [DOI] [PubMed] [Google Scholar]
- 23.Hatori M, et al. Time-restricted feeding without reducing caloric intake prevents metabolic diseases in mice fed a high-fat diet. Cell Metab. 2012;15(6):848–860. doi: 10.1016/j.cmet.2012.04.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Garaulet M, et al. Timing of food intake predicts weight loss effectiveness. Int J Obes (Lond) 2013;37(4):604–611. doi: 10.1038/ijo.2012.229. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Jakubowicz D, Barnea M, Wainstein J, Froy O. High caloric intake at breakfast vs. dinner differentially influences weight loss of overweight and obese women. Obesity (Silver Spring) 2013;21(12):2504–2512. doi: 10.1002/oby.20460. [DOI] [PubMed] [Google Scholar]
- 26.Vollmers C, et al. Circadian oscillations of protein-coding and regulatory RNAs in a highly dynamic mammalian liver epigenome. Cell Metab. 2012;16(6):833–845. doi: 10.1016/j.cmet.2012.11.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Cho H, et al. Regulation of circadian behaviour and metabolism by REV-ERB-α and REV-ERB-β. Nature. 2010;485(7396):123–127. doi: 10.1038/nature11048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhang EE, et al. Cryptochrome mediates circadian regulation of cAMP signaling and hepatic gluconeogenesis. Nat Med. 2010;16(10):1152–1156. doi: 10.1038/nm.2214. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gachon F, Olela FF, Schaad O, Descombes P, Schibler U. The circadian PAR-domain basic leucine zipper transcription factors DBP, TEF, and HLF modulate basal and inducible xenobiotic detoxification. Cell Metab. 2006;4(1):25–36. doi: 10.1016/j.cmet.2006.04.015. [DOI] [PubMed] [Google Scholar]
- 30.Jeyaraj D, et al. Klf15 orchestrates circadian nitrogen homeostasis. Cell Metab. 2006;15(3):311–323. doi: 10.1016/j.cmet.2012.01.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Bass J, Takahashi JS. Circadian integration of metabolism and energetics. Science. 2010;330(6009):1349–1354. doi: 10.1126/science.1195027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Scheer FA, Morris CJ, Shea SA. The internal circadian clock increases hunger and appetite in the evening independent of food intake and other behaviors. Obesity (Silver Spring) 2013;21(3):421–423. doi: 10.1002/oby.20351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Goodrick CL, Ingram DK, Reynolds MA, Freeman JR, Cider NL. Effects of intermittent feeding upon growth, activity, and lifespan in rats allowed voluntary exercise. Exp Aging Res. 1983;9(3):203–209. doi: 10.1080/03610738308258453. [DOI] [PubMed] [Google Scholar]
- 34.Honjoh S, Yamamoto T, Uno M, Nishida E. Signalling through RHEB-1 mediates intermittent fasting-induced longevity in C. elegans. Nature. 2009;457(7230):726–730. doi: 10.1038/nature07583. [DOI] [PubMed] [Google Scholar]
- 35.Colman RJ, et al. Caloric restriction reduces age-related and all-cause mortality in rhesus monkeys. Nat Commun. 2014;5:3557. doi: 10.1038/ncomms4557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Rizza W, Veronese N, Fontana L. What are the roles of calorie restriction and diet quality in promoting healthy longevity? Ageing Res Rev. 2014;13:38–45. doi: 10.1016/j.arr.2013.11.002. [DOI] [PubMed] [Google Scholar]
- 37.Pieri C, et al. Food restriction in female Wistar rats: V. Lipid peroxidation and antioxidant enzymes in the liver. Arch Gerontol Geriatr. 1992;14(1):93–99. doi: 10.1016/0167-4943(92)90010-2. [DOI] [PubMed] [Google Scholar]
- 38.Rodríguez-Bies E, Navas P, López-Lluch G. Age-dependent effect of every-other-day feeding and aerobic exercise in ubiquinone levels and related antioxidant activities in mice muscle. J Gerontol A Biol Sci Med Sci. 2014 doi: 10.1093/gerona/glu002. [DOI] [PubMed] [Google Scholar]
- 39.Mattson MP. Energy intake and exercise as determinants of brain health and vulnerability to injury and disease. Cell Metab. 2012;16(6):706–722. doi: 10.1016/j.cmet.2012.08.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Ahmet I, Wan R, Mattson MP, Lakatta EG, Talan M. Cardioprotection by intermittent fasting in rats. Circulation. 2005;112(20):3115–3121. doi: 10.1161/CIRCULATIONAHA.105.563817. [DOI] [PubMed] [Google Scholar]
- 41.Arumugam TV, et al. Age and energy intake interact to modify cell stress pathways and stroke outcome. Ann Neurol. 2010;67(1):41–52. doi: 10.1002/ana.21798. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Poon HF, et al. Proteomics analysis provides insight into caloric restriction mediated oxidation and expression of brain proteins associated with age-related impaired cellular processes: Mitochondrial dysfunction, glutamate dysregulation and impaired protein synthes-is. Neurobiol Aging. 2006;27(7):1020–1034. doi: 10.1016/j.neurobiolaging.2005.05.014. [DOI] [PubMed] [Google Scholar]
- 43.Marosi K, Mattson MP. BDNF mediates adaptive brain and body responses to energetic challenges. Trends Endocrinol Metab. 2014;25(2):89–98. doi: 10.1016/j.tem.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Johnson JB, et al. Alternate day calorie restriction improves clinical findings and reduces markers of oxidative stress and inflammation in overweight adults with moderate asthma. Free Radic Biol Med. 2007;42(5):665–674. doi: 10.1016/j.freeradbiomed.2006.12.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Harvie MN, et al. The effects of intermittent or continuous energy restriction on weight loss and metabolic disease risk markers: A randomized trial in young overweight women. Int J Obes (Lond) 2011;35(5):714–727. doi: 10.1038/ijo.2010.171. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Klempel MC, Kroeger CM, Varady KA. Alternate day fasting (ADF) with a high-fat diet produces similar weight loss and cardio-protection as ADF with a low-fat diet. Metabolism. 2013;62(1):137–143. doi: 10.1016/j.metabol.2012.07.002. [DOI] [PubMed] [Google Scholar]
- 47.Varady KA, et al. Alternate day fasting for weight loss in normal weight and overweight subjects: A randomized controlled trial. Nutr J. 2013;12(1):146. doi: 10.1186/1475-2891-12-146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kashiwaya Y, et al. A ketone ester diet exhibits anxiolytic and cognition-sparing properties, and lessens amyloid and tau pathologies in a mouse model of Alzheimer’s disease. Neurobiol Aging. 2013;34(6):1530–1539. doi: 10.1016/j.neurobiolaging.2012.11.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Newman JC, Verdin E. Ketone bodies as signaling metabolites. Trends Endocrinol Metab. 2014;25(1):42–52. doi: 10.1016/j.tem.2013.09.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Anson RM, et al. Intermittent fasting dissociates beneficial effects of dietary restriction on glucose metabolism and neuronal resistance to injury from calorie intake. Proc Natl Acad Sci USA. 2003;100(10):6216–6220. doi: 10.1073/pnas.1035720100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Harvie M, et al. The effect of intermittent energy and carbohydrate restriction v. daily energy restriction on weight loss and metabolic disease risk markers in overweight women. Br J Nutr. 2013;110(8):1534–1547. doi: 10.1017/S0007114513000792. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Martin-Montalvo A, de Cabo R. Mitochondrial metabolic reprogramming induced by calorie restriction. Antioxid Redox Signal. 2013;19(3):310–320. doi: 10.1089/ars.2012.4866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Cheng A, et al. Involvement of PGC-1α in the formation and maintenance of neuronal dendritic spines. Nat Commun. 2012;3:1250. doi: 10.1038/ncomms2238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Seyfried TN, Flores RE, Poff AM, D’Agostino DP. Cancer as a metabolic disease: Implications for novel therapeutics. Carcinogenesis. 2014;35(3):515–527. doi: 10.1093/carcin/bgt480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lee C, et al. Fasting cycles retard growth of tumors and sensitize a range of cancer cell types to chemotherapy. Sci Transl Med. 2012;4(124):124ra27. doi: 10.1126/scitranslmed.3003293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Safdie FM, et al. Fasting and cancer treatment in humans: A case series report. Aging (Albany, NY Online) 2009;1(12):988–1007. doi: 10.18632/aging.100114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Zuccoli G, et al. Metabolic management of glioblastoma multiforme using standard therapy together with a restricted ketogenic diet: Case report. Nutr Metab (Lond) 2010;7:33. doi: 10.1186/1743-7075-7-33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Seyfried TN. Cancer as a Metabolic Disease: On the Origin, Management and Prevention of Cancer. John Wiley & Sons; Hoboken, NJ: 2012. pp. 261–275. [Google Scholar]
- 59.McDade TW. Early environments and the ecology of inflammation. Proc Natl Acad Sci USA. 2012;109(Suppl 2):17281–17288. doi: 10.1073/pnas.1202244109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kroeger CM, et al. Improvement in coronary heart disease risk factors during an intermittent fasting/calorie restriction regimen: Relationship to adipokine modulations. Nutr Metab (Lond) 2012;9(1):98. doi: 10.1186/1743-7075-9-98. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Müller H, de Toledo FW, Resch KL. Fasting followed by vegetarian diet in patients with rheumatoid arthritis: A systematic review. Scand J Rheumatol. 2001;30(1):1–10. doi: 10.1080/030097401750065256. [DOI] [PubMed] [Google Scholar]
- 62.Esquifino AI, Cano P, Jimenez-Ortega V, Fernández-Mateos MP, Cardinali DP. Immune response after experimental allergic encephalomyelitis in rats subjected to calorie restriction. J Neuroinflammation. 2007;4:6. doi: 10.1186/1742-2094-4-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Muthukumar A, Zaman K, Lawrence R, Barnes JL, Fernandes G. Food restriction and fish oil suppress atherogenic risk factors in lupus-prone (NZB x NZW) F1 mice. J Clin Immunol. 2003;23(1):23–33. doi: 10.1023/a:1021996130672. [DOI] [PubMed] [Google Scholar]
- 64.Belkacemi L, et al. Intermittent fasting modulation of the diabetic syndrome in streptozotocin-injected rats. Int J Endocrinol. 2012;2012:962012. doi: 10.1155/2012/962012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Elinav E, et al. Inflammation-induced cancer: Crosstalk between tumours, immune cells and microorganisms. Nat Rev Cancer. 2013;13(11):759–771. doi: 10.1038/nrc3611. [DOI] [PubMed] [Google Scholar]
- 66.Maupin-Furlow J. Proteasomes and protein conjugation across domains of life. Nat Rev Microbiol. 2012;10(2):100–111. doi: 10.1038/nrmicro2696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Rubinsztein DC, Mariño G, Kroemer G. Autophagy and aging. Cell. 2011;146(5):682–695. doi: 10.1016/j.cell.2011.07.030. [DOI] [PubMed] [Google Scholar]
- 68.Speakman JR, Mitchell SE. Caloric restriction. Mol Aspects Med. 2011;32(3):159–221. doi: 10.1016/j.mam.2011.07.001. [DOI] [PubMed] [Google Scholar]
- 69.Donati A, Recchia G, Cavallini G, Bergamini E. Effect of aging and anti-aging caloric restriction on the endocrine regulation of rat liver autophagy. J Gerontol A Biol Sci Med Sci. 2008;63(6):550–555. doi: 10.1093/gerona/63.6.550. [DOI] [PubMed] [Google Scholar]
- 70.Kume S, et al. Calorie restriction enhances cell adaptation to hypoxia through Sirt1-dependent mitochondrial autophagy in mouse aged kidney. J Clin Invest. 2010;120(4):1043–1055. doi: 10.1172/JCI41376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Wohlgemuth SE, Seo AY, Marzetti E, Lees HA, Leeuwenburgh C. Skeletal muscle autophagy and apoptosis during aging: Effects of calorie restriction and life-long exercise. Exp Gerontol. 2010;45(2):138–148. doi: 10.1016/j.exger.2009.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lee S, Notterpek L. Dietary restriction supports peripheral nerve health by enhancing endogenous protein quality control mechanisms. Exp Gerontol. 2013;48(10):1085–1090. doi: 10.1016/j.exger.2012.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Madorsky I, et al. Intermittent fasting alleviates the neuropathic phenotype in a mouse model of Charcot-Marie-Tooth disease. Neurobiol Dis. 2009;34(1):146–154. doi: 10.1016/j.nbd.2009.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Moreno-Gonzalez I, Soto C. Misfolded protein aggregates: Mechanisms, structures and potential for disease transmission. Semin Cell Dev Biol. 2011;22(5):482–487. doi: 10.1016/j.semcdb.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Jucker M, Walker LC. Pathogenic protein seeding in Alzheimer disease and other neurodegenerative disorders. Ann Neurol. 2011;70(4):532–540. doi: 10.1002/ana.22615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Ma D, Li S, Molusky MM, Lin JD. Circadian autophagy rhythm: A link between clock and metabolism? Trends Endocrinol Metab. 2012;23(7):319–325. doi: 10.1016/j.tem.2012.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Brown AW, Bohan Brown MM, Allison DB. Belief beyond the evidence: Using the proposed effect of breakfast on obesity to show 2 practices that distort scientific evidence. Am J Clin Nutr. 2013;98(5):1298–1308. doi: 10.3945/ajcn.113.064410. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Dhurandhar EJ, et al. The effectiveness of breakfast recommendations on weight loss: A randomized controlled trial. Am J Clin Nutr. 2014;100(2):507–513. doi: 10.3945/ajcn.114.089573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Stote KS, et al. A controlled trial of reduced meal frequency without caloric restriction in healthy, normal-weight, middle-aged adults. Am J Clin Nutr. 2007;85(4):981–988. doi: 10.1093/ajcn/85.4.981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Carlson O, et al. Impact of reduced meal frequency without caloric restriction on glucose regulation in healthy, normal-weight middle-aged men and women. Metabolism. 2007;56(12):1729–1734. doi: 10.1016/j.metabol.2007.07.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Swindell WR. Dietary restriction in rats and mice: A meta-analysis and review of the evidence for genotype-dependent effects on lifespan. Ageing Res Rev. 2012;11(2):254–270. doi: 10.1016/j.arr.2011.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Pendergast JS, Oda GA, Niswender KD, Yamazaki S. Period determination in the food-entrainable and methamphetamine-sensitive circadian oscillator(s) Proc Natl Acad Sci USA. 2012;109(35):14218–14223. doi: 10.1073/pnas.1206213109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mulder CK, Papantoniou C, Gerkema MP, Van Der Zee EA. Neither the SCN nor the adrenals are required for circadian time-place learning in mice. Chronobiol Int. 2014;31(9):1075–1092. doi: 10.3109/07420528.2014.944975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Fryar CD, Carroll MD, Ogden CL. 2012 Prevalence of Overweight, Obesity, and Extreme Obesity Among Adults: United States, Trends 1960–1962 Through 2009–2010 (National Center for Health Statistics, Hyattsville, MD). Available at www.cdc.gov/nchs/data/hestat/obesity_adult_09_10/obesity_adult_09_10.htm. Accessed November 3, 2014.
- 85.United States Department of Agriculture . Agriculture Fact Book. US Department of Agriculture; Washington, DC: 2003. Profiling food consumption in America; pp. 13–21. [Google Scholar]
- 86.Day JC, Janus A, Davis J. 2005 Computer and Internet Use in the United States: 2003 (US Census Bureau, Washington, DC). Available at www.census.gov/prod/2005pubs/p23-208.pdf. Accessed November 3, 2014.