Significance
The auditory sensory epithelium contains two major cell types: hair cells and supporting cells. Mammalian auditory hair cells do not regenerate after damage or loss, resulting in permanent hearing impairment. How supporting cell loss affects auditory function remains to be determined. Here, we demonstrate that inner border and inner phalangeal cells, the two types of supporting cells surrounding inner hair cells, can be replenished completely after selective ablation in the neonatal cochlea, allowing hearing to be preserved. Our findings challenge the view that mammalian auditory sensory epithelium has limited intrinsic regenerative capacity and provide previously unindetified opportunities for replacement of damaged auditory cells and restoration of hearing.
Keywords: deafness, cell ablation, hair cell, organ of Corti, glia
Abstract
Supporting cells in the cochlea play critical roles in the development, maintenance, and function of sensory hair cells and auditory neurons. Although the loss of hair cells or auditory neurons results in sensorineural hearing loss, the consequence of supporting cell loss on auditory function is largely unknown. In this study, we specifically ablated inner border cells (IBCs) and inner phalangeal cells (IPhCs), the two types of supporting cells surrounding inner hair cells (IHCs) in mice in vivo. We demonstrate that the organ of Corti has the intrinsic capacity to replenish IBCs/IPhCs effectively during early postnatal development. Repopulation depends on the presence of hair cells and cells within the greater epithelial ridge and is independent of cell proliferation. This plastic response in the neonatal cochlea preserves neuronal survival, afferent innervation, and hearing sensitivity in adult mice. In contrast, the capacity for IBC/IPhC regeneration is lost in the mature organ of Corti, and consequently IHC survival and hearing sensitivity are impaired significantly, demonstrating that there is a critical period for the regeneration of cochlear supporting cells. Our findings indicate that the quiescent neonatal organ of Corti can replenish specific supporting cells completely after loss in vivo to guarantee mature hearing function.
Inner hair cells (IHCs), the sensory cells of the mammalian auditory sensory epithelium, are surrounded by specialized supporting cells (SCs) called “inner border cells” (IBCs) and “inner phalangeal cells” (IPhCs) (Fig. S1A). IBCs and IPhCs, together with other SCs, are known to play critical roles during the development and maturation of the organ of Corti, in processes such as patterning of the epithelium, synaptogenesis, and initiation of electrical activity in auditory nerves before the onset of hearing and formation of extracellular matrices (1–7). SCs also are essential for the function of the mature organ of Corti, where they contribute to the maintenance of the reticular lamina at the apical surface of the epithelium (8), control the extracellular concentration of ions (e.g., K+) (9, 10) and neurotransmitters (e.g., glutamate) (11), and support hair cell (HC) and auditory sensory neuron survival (5, 12–15). SCs also have been proposed to regulate the effects of insults on HCs by releasing molecules that either promote (e.g., ERK1 and 2) (16) or reduce (e.g., heat shock protein 70) (17) HC death. Additionally, SCs impact the extent of damage in the auditory epithelium through scar formation and clearance of HC debris (18). Furthermore, SCs are considered a potential source of cells for HC replacement in mammals, because SCs are a documented source of new HCs in cultured neonatal cochlea (19) and in adult utricles (20). Additionally, nonmammalian vertebrates regenerate HCs and SCs after damage and recover hearing, with the SCs being the source of the regenerative response (21–23). Indeed, if SCs are damaged by insults, the regenerative response is severely compromised (1, 24). Thus, it is assumed that the presence of these cells in the postnatal cochlea is essential for hearing, but specific roles of IBCs and IPhCs in HC maintenance and cochlear function have not been established.
To determine the consequences of neonatal IBC and IPhC loss on the mature organ of Corti, we ablated these cells in vivo using an inducible diphtheria toxin fragment A (DTA) transgenic approach (25). Unexpectedly, we found that when these IHC supporting cells are eliminated immediately after birth, they are replaced efficiently within days. Moreover, this regeneration preserves the structure and function of the organ of Corti, so that mice with transient IBC/IPhC loss retain normal hearing as adults. In contrast, IBCs and IPhCs do not regenerate if ablation occurs after the onset of hearing, resulting in IHC loss and severe hearing impairment. Our studies also indicate that IBC and IPhC replacement in the neonatal cochlea results from transdifferentiation of less-specified SCs within the neighboring greater epithelial ridge (GER or Kölliker’s organ), which does not require cell proliferation. The unexpected regenerative capacity of SCs in the early postnatal organ of Corti in vivo may provide new strategies to regenerate its nonsensory and sensory cells after damage.
Results
Inducible Diphtheria Toxin-Mediated Ablation of IBCs/IPhCs in Neonatal Mice.
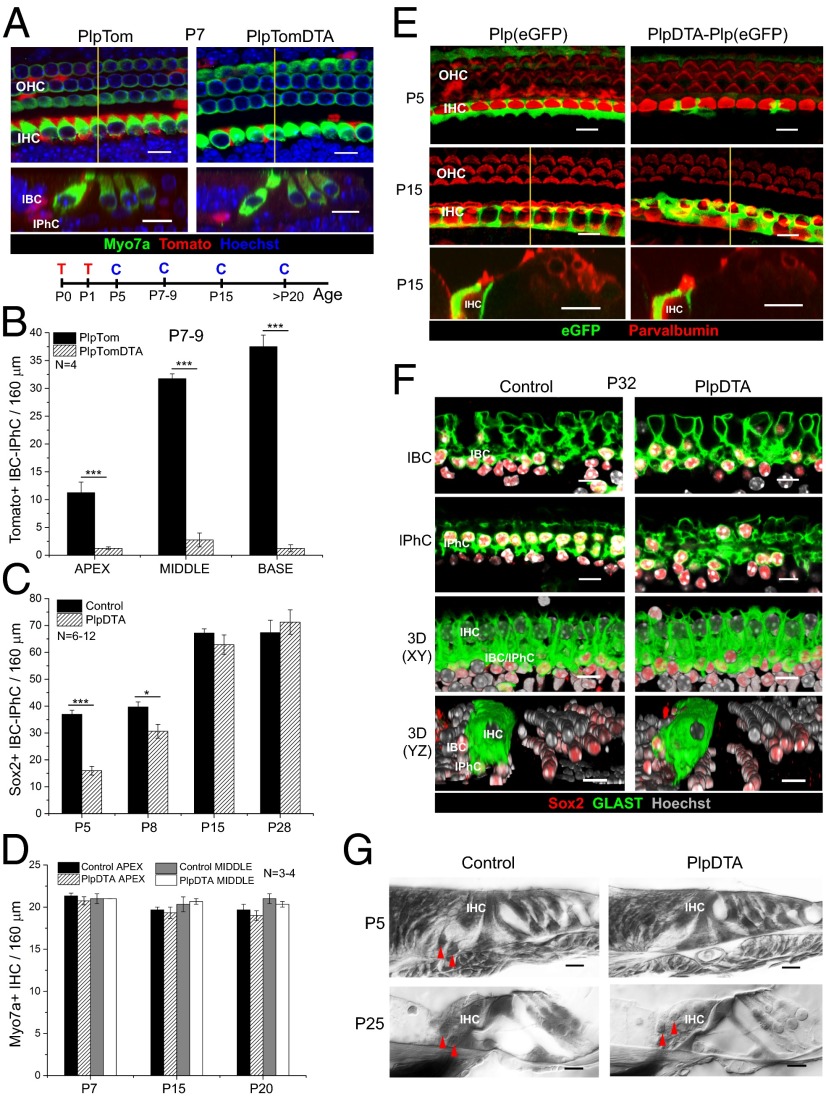
To determine if IBCs and IPhCs are necessary for long-term survival and function of IHCs and/or spiral ganglion neurons, we explored the consequences of neonatal IBC/IPhC diphtheria toxin-mediated ablation. To do so, we created mice expressing the tamoxifen-dependent Cre recombinase (CreERT) under the control of the proteolipid protein 1 (Plp1) promoter (PlpCreERT) (26, 27) and carrying an inducible flox-stop DTA transgene (Rosa26DTA) (25) together with an inducible Tomato reporter (Ai14:Rosa26tdTom) (28). Tamoxifen treatment of neonatal [postnatal day (P)0–1] PlpCreERT;Ai14:Rosa26tdTom (PlpTom) mice resulted in specific and effective gene recombination in IBCs/IPhCs in all regions of the cochlea (apex, 25.9 ± 4.1%; middle, 71.9 ± 2.3%; base, 86.1 ± 4.2%; mean ± SEM; statistical differences exist only between the apex and the other cochlear turns) (Fig. 1 A and B and Fig. S1 A and B; note that cell numbers, not percentages, are plotted in Fig. 1B). This same cell specificity was described previously using the Rosa26LacZ reporter (27). Activation of the DTA transgene leads to cell-autonomous death via apoptosis (29–31). Triple-transgenic mice [PlpCreERT;Ai14:Rosa26tdTom;Rosa26DTA (PlpTomDTA)] and their PlpTom littermates were injected with tamoxifen at P0 and P1, and their cochleae were analyzed at P7. As anticipated, mice with DTA expression showed significant loss of Tomato+ IBCs and IPhCs in all turns of the cochlea, leaving a very small number of Tomato+ cells in each region (apex, 1.3 ± 0.3; middle, 2.8 ± 1.3; base, 1.2 ± 0.6; mean ± SEM, no significant difference between turns) (Fig. 1 A and B). At this age a few Tomato+ pillar cells (PCs) and Deiters cells (DCs) are present but do not change significantly with DTA expression (Fig. S2A). Notably, loss of IBCs/IPhCs did not alter the number of myosin 7a+ IHCs at P7 (Fig. 1 A and D). Thus, this model produces rapid and effective IBC/IPhC ablation without compromising neighboring CreER−/DTA− cells.
Fig. 1.
IBCs/IPhCs are specifically ablated in the neonatal organ of Corti of PlpDTA mice and are completely repopulated between P8 and P15. (A) Representative optical sections of whole mounts (Upper) and optical cross-sections extracted from z-stacks (Lower) from the middle turn of the cochleae of P7 PlpTom and PlpTomDTA mice showing Tomato+ IBCs/IPhCs in red, Myo7a+ HCs in green, and nuclei labeled with Hoechst 33342 in blue. The location of the section is indicated by the yellow line in whole mounts. The timeline shows the protocol for tamoxifen injection (T) and cochleae collection (C). (B) Quantification of the total number of Tomato+ IBCs/IPhCs in 160-μm regions of each turn of the cochleae of P7–9 PlpTom and PlpTomDTA mice (mean ± SEM, n = 4). Statistical differences in the number of Tomato+ cells were determined by two-way ANOVA followed by Student t test with Bonferroni correction (C) Quantification of Sox2+ IBC/IPhC nuclei surrounding IHCs in 160-μm regions of the middle turn of the cochleae in PlpDTA and control mice at P5, P8, P15, and P21 (mean ± SEM; n = 6–12). Statistical differences between groups were determined using Student’s t test. (D) Quantification of IHC numbers in 160-μm regions of the apical and middle turns of the cochleae of PlpDTA and control mice at P7, P15, and P20 (mean ± SEM, n = 3–4). (E, Top and Middle) Representative optical projections of whole mounts from the apical turn of the cochleae of Plp(eGFP) and PlpDTA-Plp(eGFP) mice at P5 (Top) and P15 (Middle). (Bottom) Optical cross-sections extracted from z-stacks at P15. eGFP (green) reports Plp gene expression in cells around the IHCs (labeled with parvalbumin in red). The location of the section is indicated by the yellow line in P15 whole mounts. (F) Representative optical sections and 3D reconstructions in two planes (x–y and y–z) of whole mounts from the apical turns of the cochleae of PlpDTA and control mice at P32. The two sets of upper panels show optical planes at IBC and IPhC levels, respectively, showing GLAST expression in green, Sox2+ nuclei in red, and Hoechst in gray scale. The two lower sets of panels show 3D reconstruction of z-stacks of the x–y and y–z axes, respectively. (G) Plastic cross-sections of the P5 and P25 organ of Corti of PlpDTA and control mice showing the lack of IBC/IPhC nuclei at P5 but not at P25 in PlpDTA mice. Red arrowheads indicate the positions of IBC/IPhC nuclei in PlpDTA and control mice. (Scale bars: 10 μm.) *P < 0.05, ***P < 0.001.
Repopulation of IBCs/IPhCs After Neonatal Ablation in the Mouse Organ of Corti in Vivo.
To define the time course of IBC/IPhC loss, we quantified the number of Sox2+ nuclei adjacent to IHCs at different ages in PlpCreERT;Rosa26DTA (PlpDTA) and control mice after tamoxifen injections at P0 and P1 (Fig. 1C and Fig. S2B). Sox2 (sex-determining region Y box 2) is a transcription factor expressed by SCs in the early postnatal organ of Corti (32, 33). At early postnatal ages Sox2 is expressed in IBCs and IPhCs, as well as in cells of the GER, PCs, DCs, and some cells of the lesser epithelial ridge lateral to the third row of DCs (34). However, Sox2+ IBCs and IPhCs can be recognized at these ages by their location relative to the IHCs (Figs. S1A and S2B). As expected, the number of Sox2+ cells surrounding IHCs was significantly reduced in PlpDTA mice at P5 (∼43% of control). Remarkably, by P8 the total number of Sox2+ cells in the IHC area was increased significantly (∼75% of control; P = 0.0002 compared with PlpDTA mice at P5; Fig. 1C and Fig. S2B). By P15, cell numbers in PlpDTA mice were indistinguishable from those in control littermates, a recovery that was maintained at later ages (Fig. 1C and Fig. S2B). The numbers of IBCs/IPhCs, PCs, and DCs were indistinguishable in PlpDTA and control mice in all turns of the cochlea at P20 (Fig. S2C). IHCs appeared unaffected by this wave of Sox2+ cell ablation and regeneration, and their numbers remained normal along the cochlea at P7, P15, and P20 (Fig. 1D). These results show that SCs surrounding IHCs regenerate rapidly in all turns of the cochlea after neonatal ablation and that their temporary loss does not affect IHC survival.
To determine if the new Sox2+ cells surrounding IHCs exhibited characteristics of IBCs/IPhCs, we tested if they expressed known molecular markers of these cell types. IBCs/IPhCs express Plp1 in the neonatal organ of Corti (35) and express glutamate-aspartate transporter (GLAST) in the juvenile and adult organ of Corti (11, 36). Experiments with mice carrying a Plp(eGFP) allele (37) in combination with PlpCreERT and Rosa26DTA alleles [PlpDTA-Plp(eGFP)] showed that the eGFP+ IBCs/IPhCs surrounding IHCs are lost at P5 but reappear by P15 (Fig. 1E). Similarly, GLAST expression by IBCs and IPhCs around IHCs was similar in PlpDTA and control mice at P32 (Fig. 1F). Thus, the new cells appear to be bona fide IBCs/IPhCs. The absence of IBCs/IPhCs around the IHCs P5 was evident in plastic sections, as it was their recovery by P25 (Fig. 1G). However, these new IBCs/IPhCs showed subtle morphological alterations, i.e., their nuclei were closer to the apical region of the epithelium than in controls at P25 (Fig. 1G). Morphological changes in IBCs/IPhCs also can be observed in the PlpDTA-Plp(eGFP) model at P15 when whole-mount images are compared with the control tissue (Fig. 1E).
Neonatal IBC/IPhC Ablation Does Not Impact Adult Spiral Ganglion Neuron Survival, Afferent Innervation, or Auditory Function.
To determine if the repopulation of IBCs/IPhCs had any impact on survival and function of spiral ganglion neurons (SGN), we examined the structure and function of the cochlea. Immunostaining for tubulin β3 (Tuj1) showed that neuronal projections in the organ of Corti were qualitatively normal in PlpDTA mice at P15 (Fig. S3A). Quantification of IHC synapses using immunostaining for the presynaptic ribbon [C-terminal binding protein 2 (Ctbp2)/Ribeye] and postsynaptic glutamate receptor 2 (GluR2) receptors showed no differences in synaptic density at P25 (Fig. S3 B–D). Moreover, sections at the level of Rosenthal’s canal showed that the SGN cell body density in PlpDTA cochleae was qualitatively comparable to that in controls (Fig. S3E, Upper), and tangential sections of the cochlea through the osseous spiral lamina near the habenula perforata showed no significant alteration of myelinated afferent axons that innervate the organ of Corti (Fig. S3E, Lower). Measurements of auditory brainstem response (ABR) thresholds from 7-wk-old mice showed that PlpDTA mice had normal hearing (Fig. S3F). Together, these results show that ablation of IBCs/IPhCs in neonatal mice is followed by efficient replacement that results in mature cochleae with normal structure and hearing sensitivity.
IBC/IPhC Repopulation in the Neonatal Organ of Corti Depends on GER Cells.
The rapid and complete repopulation of IBCs/IPhCs indicates that the organ of Corti contains a cell population that can regenerate these cells in the neonatal mouse. Because after neonatal ablation the organ of Corti of PlpTomDTA mice contained a very small number of Tomato+ IBCs/IPhCs by P7 in all regions of the cochlea (Fig. 1 A and B), we asked if some IBCs/IPhCs that have escaped ablation (i.e., that expressed the Tomato reporter but not DTA) could serve as the source for the repopulation. However, the number of Tomato+ IBCs/IPhCs in these mice at P30, after replacement had occurred, was still sparse and was noticeably smaller than in control PlpTom littermates similarly induced with tamoxifen at P0 and P1 (Fig. S4A). Furthermore, these Tomato+ cells accounted for very few of the GLAST+ and Sox2+ cells seen neighboring IHCs at P30 (Fig. S4A), suggesting that IBCs/IPhCs that escaped ablation were not the source of the repopulation.
To test if IBC/IPhC repopulation involved cell proliferation, we performed BrdU or EdU (5-ethynyl-2′-deoxyuridine) incorporation assays. Mice with neonatal IBC/IPhC ablation and control mice were injected with BrdU or EdU on one or multiple consecutive days between P3 and P20, and cochlear tissues were harvested and immunolabeled 24 h after the last injection. We found no evidence of cell proliferation in the organ of Corti or the GER region in PlpDTA mice (Fig. S4 B and C). BrdU+ or EdU+ cells were found only in the basal lamina underneath the GER (Fig. S4C) and the spiral ganglion region (medial to the organ of Corti) in both control and PlpDTA cochleae (Fig. S4B). These results indicate that repopulation of IBCs/IPhCs does not involve a proliferative response within the organ of Corti or the GER and led us to hypothesize that transdifferentiation of neighboring GER cells may be the mechanism of IBC/IPhC replacement.
GER cells are present in the organ of Corti until approximately P10 and are located medial to the IHCs, adjacent to IBCs (Fig. S1A). Because there are no mouse models available to trace GER cells specifically after IBC/IPhC ablation, we tested this hypothesis using two additional CreERT mouse lines in combination with the DTA allele. We used leucine-rich repeat-containing G-protein coupled receptor 5 (Lgr5)CreERT2;Rosa26DTA (Lgr5DTA) mice induced at P0 and P1 to ablate IBCs, IPhCs, a small population of GER cells (two or three rows of cells in the lateral GER close to the IHCs), and some inner PCs (IPCs) and DCs (Fig. S1C, targeted cells are shown in red) (34, 38–42). We also used GlastCreERT;Rosa26DTA (GlastDTA) mice to target IBCs, IPhCs, and a broader mosaic cell population of the entire GER after tamoxifen treatment at P0 and P1. The expression pattern of GlastCreERT was confirmed with the Tomato reporter allele (Figs. S1 D, F, and G and S5A). Analysis of Tomato expression in GlastTom cochleae also allowed us to quantify a base-to-apex gradient of GER area at P8 (Fig. S5 A and B).
To determine the pattern of recombination induced by Lgr5CreERT2 expression, we first injected Lgr5CreERT2;Ai14:Rosa26tdTom (Lgr5Tom) at P0 and P1 and analyzed reporter expression at P6. As shown in Fig. S6A, the Lgr5 promoter induced recombination in IBCs, IPhCs, IPCs, a small number of GER cells close to the IHC area, and some DCs in all cochlear turns. Lgr5DTA mice and littermate controls were induced with tamoxifen at P0 and P1 and were analyzed at P25. Cochleae in Lgr5DTA mice showed significant IPC, DC, and outer hair cell (OHC) loss in all cochlear turns at P25 (Fig. S6 B–D). The OHC loss likely was caused by the ablation of DCs (31). ABR thresholds were increased (20–40 dB) in Lgr5DTA mice as compared with controls (Fig. S6E), likely because of OHC loss (31). Reduced numbers of IPCs and DCs in P25 Lgr5DTA mice show that DTA-mediated cell death effectively occurred in Lgr5DTA cochleae, supporting previous findings that PCs and DCs are not replaceable after ablation at P0 and P1 (31). In contrast, GLAST+ and Sox2+ IBCs/IPhCs around the IHCs were normal in number in Lgr5DTA cochleae at P25 (Fig. S6 B–D). These results support the notion that IBC/IPhC replacement occurs after ablation in the early postnatal organ of Corti and suggest that ablation of Lgr5+ cells (Fig. S1C) in the area close to the IHCs concomitant with ablation of IBCs/IPhCs does not significantly affect IBC/IPhC repopulation and that other cell types contribute to SC replacement in the neonatal organ of Corti.
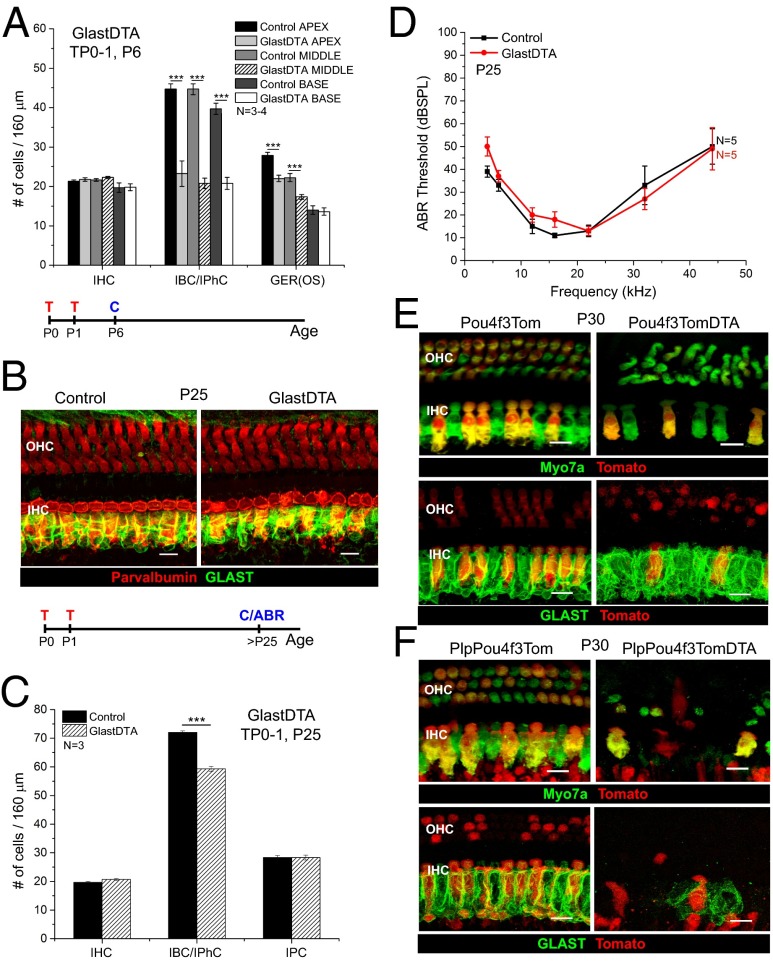
Tamoxifen induction of GlastDTA mice at P0 and P1 and cochlear analysis at P6 showed that ∼45% of Sox2+ IBCs/IPhCs had been ablated in all regions of the cochleae at this early age (Fig. 2A). The reduction is very similar to that seen in PlpDTA mice at P5 (∼43%) (Fig. 1C). In addition, the number of Sox2+ nuclei (Fig. 2A) and the area that they occupy in the GER (Fig. S5 C and D) were reduced significantly in the apex and middle of the cochleae of P6 GlastDTA mice. Consistent with reporter expression, IHCs (Fig. 2A) and PCs and DCs (Fig. S5E) were not affected in GlastDTA mice at P6. When GlastDTA mice (treated with tamoxifen at P0 and P1) were analyzed at P25, GLAST immunostaining around IHCs was similar to that observed in controls (Fig. 2B), but quantitative analysis of Sox2+ IBCs/IPhCs revealed that their numbers still were reduced to 75–85% of those in control mice in all cochlear regions (Fig. 2C and Fig. S5F). These mice retained normal ABR thresholds (Fig. 2D). As expected, the number of IPCs, which are not targeted by the GlastCreERT (Fig. S1 D–G), was not changed significantly (Fig. 2C and Fig. S5F). After comparing GER cells differentially labeled by GlastCreERT (Fig. S1F) and Lgr5CreERT2 (Fig. S6A), we conclude that ablation of a significant number of GER cells at early postnatal ages reduces the efficiency of IBC/IPhC replacement after damage in the neonatal organ of Corti. Nonetheless, the reduced number of IBCs/IPhCs is sufficient to support the survival and function of IHCs to preserve hearing function.
Fig. 2.
GER cells and HCs are important for IBC/IPhC replacement after neonatal ablation. (A) Quantification of the number of IHC, IBC/IPhC, and GER cells in 160-μm regions of each turn of the cochleae in GlastDTA and control mice at P6 (mean ± SEM, n = 3–4). GER(OS), GER cells were counted in optical cross-sections taken along the 160-μm regions of each turn. (B) Representative optical projections of whole mounts from the apical turn of the cochleae of GlastDTA and control mice induced with tamoxifen at P0 and P1; cochleae were analyzed at P25 showing parvalbumin+ HCs (red) and GLAST+ IBCs/IPhCs (green). The timelines below A and B show the protocol of tamoxifen induction (T), cochlea collection (C), and ABR measurement (ABR) used in each figure of these mouse models. (C) Quantification of the number of cells of each subtype present in the IHC area of the apical organ of Corti in GlastDTA and control mice at P25 (mean ± SEM, n = 3). IHCs and SCs were counted in 160-μm sections in the apical turn of the organ of Corti. SCs were counted as the number of Sox2+ nuclei in the specific locations of the cell type in control samples. (D) ABR thresholds from P25 GlastDTA and control mice (mean ± SEM, n = 5). No statistical differences in ABR thresholds were observed in GlastDTA and control mice. (E) Representative optical projections of whole mounts from the apical turn of the cochleae of P30 Pou4f3Tom and Pou4f3TomDTA mice showing IHC and OHC loss when the DTA allele is present (Upper) and preservation of GLAST+ IBCs/IPhCs after HC ablation in Pou4f3TomDTA mice (Lower). (F) Representative optical projections of whole mounts from the apical turn of the cochleae of PlpPou4f3Tom and PlpPou4f3TomDTA mice at P30 showing loss of HCs and absence of GLAST+ IBC/IPhC replacement after neonatal ablation. (Scale bars: 10 μm.) ***P < 0.001.
IBC/IPhC Replacement Depends on HCs.
IBCs/IPhCs are in close contact with IHCs, and cell–cell interactions (e.g., via Notch signaling) between these cells during embryonic development are known to regulate their differentiation (43, 44). Therefore, we tested if IBC/IPhC repopulation in the postnatal cochlea depends on HCs using a mouse line that targets both IHCs and OHCs of the organ of Corti (Pou4f3CreERT) mouse line (Fig. 2E) (45). By P30, Pou4f3CreERT;Ai14:Rosa26tdTom;Rosa26DTA triple transgenics (Pou4f3TomDTA) injected with tamoxifen at P0 andP1 displayed significant loss of IHCs and OHCs compared with their littermate controls (Pou4f3Tom) (Fig. 2E, Upper). Remarkably, GLAST immunofluorescence in these tissues showed that IBCs/IPhCs appeared to be unaffected by the IHC loss (Fig. 2E, Lower). In addition, at P30 the numbers of Sox2+ IBCs/IPhCs in Pou4f3CreERT;Rosa26DTA (Pou4f3DTA) mice injected with tamoxifen at P0 and P1 were similar to those in control mice (Fig. S7). In contrast, when we induced neonatal ablation of both IBCs/IPhCs and HCs in PlpCreERT;Pou4f3CreERT;Ai14:Rosa26tdTom;Rosa26DTA quadruple transgenic (PlpPou4f3TomDTA) mice, the mature organ of Corti showed a dramatic loss of GLAST+ IBCs/IPhCs (Fig. 2F). Together, these results indicate that HCs are not required for the survival of IBCs/IPhCs but are essential for their replenishment after targeted neonatal ablation.
IBC/IPhC Ablation After the Onset of Hearing Results in Profound Hearing Loss.
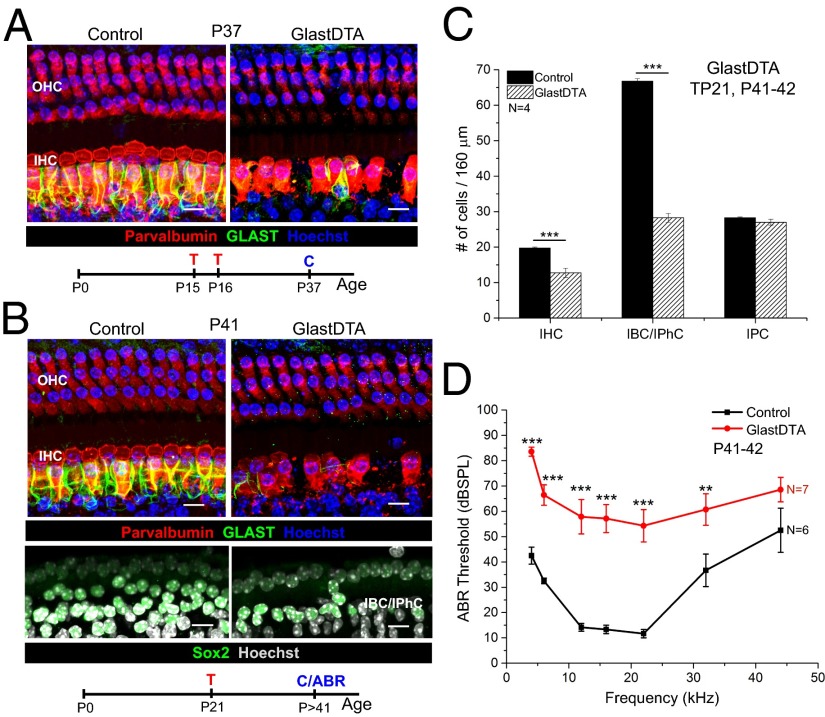
The remarkably mild effect of neonatal IBC/IPhC ablation on the adult cochlea was surprising, because a number of studies suggest that these cells play important roles in cochlear development (1, 2–6). Therefore, we thought it was necessary to test whether IBC/IPhC ablation at later stages had a different effect. We found that the PlpCreERT line did not produce effective gene recombination in IBCs/IPhCs beyond P10. Therefore, we used the GlastCreERT line (46, 47), which allows efficient and specific recombination in IBCs/IPhCs induced by tamoxifen injection at later ages (P15 and P16 or P21) (Fig. S1 E, H, and I). Mice with IBC/IPhC ablation at P15 and P16 (see Fig. S1 E and H for reporter expression) had almost complete absence of GLAST+ IBCs/IPhCs and reduced numbers of IHCs 3 wk later (Fig. 3A). This time frame is longer than the 2 wk required for complete repopulation of IBC/IPhC at neonatal ages. Similar results were obtained when IBC/IPhC ablation was induced at P21 (Fig. 3B). Quantitative analysis showed that GlastDTA mice induced with tamoxifen at P21 showed significant loss of IHCs and IBCs/IPhCs 3 wk later, but the number of IPCs remained intact (Fig. 3 B and C). Consistent with cell loss, these mice displayed profound ABR threshold shifts (40–50 dB) (Fig. 3D). Thus, the capacity to replace IBCs/IPhCs is lost after the first 2 wk of postnatal life, and loss of this SC subpopulation at ages after hearing onset (>P10) severely compromises IHC survival and hearing function.
Fig. 3.
IBC/IPhC repopulation fails after ablation in juvenile and adult mice and leads to IHC loss and hearing impairment. (A) Representative optical projections of whole mounts from the apical turn of the cochleae of GlastDTA and control mice induced with tamoxifen at P15 and P16; cochleae were analyzed 3 wk later at P37 (parvalbumin+ HC are shown in red; GLAST+ IBCs/IPhCs are shown in green; nuclei labeled with Hoechst 33342 in blue). The timeline of tamoxifen induction (T) and cochlea collection (C) for analysis is shown below the image. (B) Representative optical projection of whole mounts from the apical turn of the cochleae of GlastDTA and control mice induced with tamoxifen at P21; cochleae were collected 3 wk later at P41. The timeline of tamoxifen induction and cochlea collection for analysis is shown below the image. (C) Quantification of the number of IHCs, IBCs/IPhCs, and IPCs in 160 μm of the apical turn of cochleae of GlastDTA and control mice at P41–42 after P21 induction (mean ± SEM, n = 4). SCs were counted as number of Sox2+ nuclei in the specific location of the cell type in control samples. Statistical differences in cell numbers were determined by two-way ANOVA followed by Student t test with Bonferroni correction. (D) ABR thresholds measured from GlastDTA and control mice at ages P41–42 after P21 induction (mean ± SEM, n = 6–7). Statistical differences in thresholds at each frequency analyzed (4, 6, 12, 16, 22, 32, and 44 kHz) were determined by two-way ANOVA followed by Student t test with Bonferroni correction. (Scale bars: 10 um.) **P < 0.01, ***P < 0.001.
Discussion
Cell loss in the mammalian organ of Corti is a frequent cause of deafness. Therefore, defining the regenerative capacity of this sensory epithelium and the mechanisms that underlie cellular replacement are central questions in auditory biology. We have uncovered a previously unrecognized capacity of the neonatal organ of Corti to replace a subpopulation of SCs (IBCs and IPhCs that surround IHCs) rapidly and effectively. Furthermore, this regenerative response to cell loss supports preservation of hearing in the adult. However, the capacity for cell replacement disappears after the organ of Corti becomes functional at P15. These findings provide previously unidentified evidence that the window for spontaneous and effective SC replacement in the mammalian cochlea in vivo is limited to the early postnatal period and highlight the importance of understanding the mechanisms of these processes to develop regenerative approaches to treat hearing loss.
In contrast to the neonatal period, SC loss in the functional cochlea leads to IHC loss and significant hearing impairment, suggesting that in some cases hearing loss might be caused primarily by IBC and/or IPhC dysfunction or loss. In accordance with this possibility, mutations in the connexin 26 (Cx26) gene (GJB2 mutations), which forms gap junctions between SCs (48, 49), causes deafness. Mice with Cx26 loss show HC loss only after the onset of hearing (50, 51), providing evidence that SCs are key regulators of homeostasis during sensory processing in the functional organ of Corti. We also found that HCs must be present in the neonatal organ of Corti for IBC/IPhC replacement to occur after ablation. This result suggests either that HCs provide crucial signals for IBC/IPhC replacement (e.g., via Notch) or that concurrent loss of these cells damages the organ of Corti to an extent that impedes IBC/IPhC regeneration.
Remarkably, IBC/IPhC replacement after neonatal ablation does not involve proliferation but rather transdifferentiation of cells that respond to the damage, move toward IHCs, and adopt an IBC/IPhC fate. This regenerative process is different from the spontaneous regeneration of HCs and SCs seen in nonmammalian vertebrates after damage, which happens via both proliferation and transdifferentiation of SCs (21–23). It also differs from the limited HC regeneration that occurs in the neonatal organ of Corti after DTA-mediated HC ablation, which also involves proliferation (42). Our studies indicate that the most likely source of new IBCs and IPhCs after neonatal ablation is Sox2+ GER cells, which migrate into the IHC area and transdifferentiate into cells that morphologically and molecularly resemble IBCs/IPhCs (Fig. S8A). GER cells are immediate neighbors of IBCs, IPhCs, and IHCs and may be less differentiated than SCs in the organ of Corti. GER cells exhibit higher levels of Wnt signaling (e.g., β-catenin and Lgr5) (38–40, 52) and lower levels of Notch signaling (53) and respond readily to Atoh1 (atonal homolog 1) ectopic expression (54–57). GER cells also express Nestin, a stem/progenitor cell marker in the central nervous system (58).
The present findings also provide a framework for interpreting our previous observation that ectopic Atoh1 overexpression in IBCs and IPhCs in the neonatal cochlea converts them into HCs without altering organ of Corti morphology (54, 55). Based on our current findings, we propose that, after the original IBCs/IPhCs convert to HCs due to Atoh1 expression, they are replenished rapidly by transdifferentiation of GER cells.
Lgr5+ cells in the GER do not contribute significantly to the regeneration of IBCs and IPhCs, a surprising result given that Lgr5 is a marker of stem cells in other tissues (59–62) and that Lgr5+ cells proliferate and transdifferentiate into HCs after genetic manipulations in the neonatal organ of Corti (34, 38, 40). However, we found that other cells in the GER, targeted with GlastCreERT induced with tamoxifen at P0 and P1, are required for the replacement of at least a fraction of IBCs/IPhCs. Because the targeting of a significant number of GER cells in our studies did not completely abolished IBC/IPhC replacement at neonatal ages, we cannot rule out the possibility that other cell populations, such as tympanic border cells, also may contribute to this process (63). In contrast, when IBC/IPhC ablation was performed at P15 or P21, when the Sox2+ GER cell pool has receded completely, there was no regenerative response (Fig. S8B). Therefore, an effective way to trigger regenerative capacity in the adult auditory organ might be to prolong the survival time of GER cells or to revert existing IBCs/IPhCs to a more primitive phenotype.
In summary, we found that genetic ablation of specific cells in the auditory sensory epithelium through inducible cell-autonomous apoptosis provides an approach to study the tissue responses to cellular loss in the neonate, a stage at which neither ototoxic drugs or noise exposure have significant effects. This approach has allowed us to discover an intrinsic capacity of the young auditory organ to respond to damage that previously was unknown for mammals.
Materials and Methods
Mouse Models.
PlpCreERT (stock no. 005975) (26), Lgr5CreERT2 (stock no. 008875) (64), GLASTCreERT (Slc1a3 CreERT; stock no. 012586) (46, 47), Ai14:Rosa26tdTom (stock no. 007908), and Rosa26DTA transgenic mice of both genders were obtained from The Jackson Laboratory. Plp(eGFP) mice were obtained from Wendy Macklin at the University of Colorado, Denver (37). We generated Pou4f3CreERT mice. Specific induction protocols can be found in SI Materials and Methods. All mice used in this work are on a mixed background containing C57Bl6, Sv129, and FVB/N strains. All animal procedures were approved by the Institutional Animal Care and Use Committee of St. Jude Children’s Research Hospital and Boston Children’s Hospital.
Immunohistochemistry.
Methods for immunofluorescence have been published previously elsewhere (31). Details are described in SI Materials and Methods.
Plastic Sections.
Adult (P25) mice were perfused intracardially with histological fixative, consisting of 1.5% (wt/vol) paraformaldehyde and 2.5% (wt/vol) glutaraldehyde in 0.1 M phosphate buffer. Neonatal (P5) mice were decapitated, and the inner ears were dissected directly before immersion into the histological fixative. Inner ears were postfixed with the fixative overnight and then were osmicated in 1% osmium tetroxide, decalcified in 5% EDTA, dehydrated, and embedded in araldite. Serial 20-µm sections were cut using a Leica RM2165 microtome. These plastic sections were mounted on microscope slides in Permount (Fisher Scientific). Cochlear morphology and axonal density were examined under light microscope.
ABR.
ABRs were measured in mice 4 wk old and older using a Tucker Davis Technologies (TDT) System III as previously described (31). A detailed description is given in SI Materials and Methods.
Data Analysis.
Statistical analysis of ABR results and cell counts was performed using GraphPad Prism 5.0. Two-way ANOVA followed by Student t test with Bonferroni correction was used to compare ABR thresholds in experimental and control mice (*P < 0.05, **P < 0.01, ***P < 0.001). Student t test or two-way ANOVA was used to compare the number of cells of specific subtypes in experimental and control mice.
Supplementary Material
Acknowledgments
We thank Jie Fang (St. Jude Children’s Research Hospital) for contributions to the characterization of the GlastCreERT mouse line; Brandon Cox (Southern Illinois University) for helpful comments during the initiation of the project; Bryan Kuo (St. Jude Children’s Research Hospital) for characterization of the Lgr5CreERT2 mouse line; and Niels C. Danbolt (University of Oslo) for providing anti-GLAST antibodies. This work was supported by Sir Henry Wellcome Fellowship 08915 (to M.M.M.L.); a Hearing Health Foundation Emerging Research Grant (to G.W.); Boston Children's Hospital Otolaryngology Foundation (G.C.); National Institutes of Health Grants R01DC004820 (to G.C.), P30 HD18655 (Boston Children’s Hospital), DC006471 (to J.Z.), and P30CA21765 (St. Jude Children’s Research Hospital); Office of Naval Research Grants N000140911014, N000141210191, and N000141210775 (all to J.Z.); and by the American Lebanese Syrian Associated Charities of St. Jude Children’s Research Hospital. J.Z. is a recipient of The Hartwell Individual Biomedical Research Award.
Footnotes
The authors declare no conflict of interest.
*This Direct Submission article had a prearranged editor.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1408064111/-/DCSupplemental.
References
- 1.Oesterle EC. Changes in the adult vertebrate auditory sensory epithelium after trauma. Hear Res. 2013;297:91–98. doi: 10.1016/j.heares.2012.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Taylor RR, Jagger DJ, Forge A. Defining the cellular environment in the organ of Corti following extensive hair cell loss: A basis for future sensory cell replacement in the Cochlea. PLoS ONE. 2012;7(1):e30577. doi: 10.1371/journal.pone.0030577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Wan G, Corfas G, Stone JS. Inner ear supporting cells: Rethinking the silent majority. Semin Cell Dev Biol. 2013;24(5):448–459. doi: 10.1016/j.semcdb.2013.03.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Monzack EL, Cunningham LL. Lead roles for supporting actors: Critical functions of inner ear supporting cells. Hear Res. 2013;303:20–29. doi: 10.1016/j.heares.2013.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Fritzsch B, Tessarollo L, Coppola E, Reichardt LF. Neurotrophins in the ear: Their roles in sensory neuron survival and fiber guidance. Prog Brain Res. 2004;146:265–278. doi: 10.1016/S0079-6123(03)46017-2. [DOI] [PubMed] [Google Scholar]
- 6.Tritsch NX, Bergles DE. Developmental regulation of spontaneous activity in the Mammalian cochlea. J Neurosci. 2010;30(4):1539–1550. doi: 10.1523/JNEUROSCI.3875-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tritsch NX, Yi E, Gale JE, Glowatzki E, Bergles DE. The origin of spontaneous activity in the developing auditory system. Nature. 2007;450(7166):50–55. doi: 10.1038/nature06233. [DOI] [PubMed] [Google Scholar]
- 8.Nayak G, et al. Tricellulin deficiency affects tight junction architecture and cochlear hair cells. J Clin Invest. 2013;123(9):4036–4049. doi: 10.1172/JCI69031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Boettger T, et al. Deafness and renal tubular acidosis in mice lacking the K-Cl co-transporter Kcc4. Nature. 2002;416(6883):874–878. doi: 10.1038/416874a. [DOI] [PubMed] [Google Scholar]
- 10.Jin Z, Liang GH, Cooper EC, Jarlebark L. Expression and localization of K channels KCNQ2 and KCNQ3 in the mammalian cochlea. Audiol Neurootol. 2009;14(2):98–105. doi: 10.1159/000158538. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Glowatzki E, et al. The glutamate-aspartate transporter GLAST mediates glutamate uptake at inner hair cell afferent synapses in the mammalian cochlea. J Neurosci. 2006;26(29):7659–7664. doi: 10.1523/JNEUROSCI.1545-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sugawara M, Murtie JC, Stankovic KM, Liberman MC, Corfas G. Dynamic patterns of neurotrophin 3 expression in the postnatal mouse inner ear. J Comp Neurol. 2007;501(1):30–37. doi: 10.1002/cne.21227. [DOI] [PubMed] [Google Scholar]
- 13.Sobkowicz HM, August BK, Slapnick SM. Influence of neurotrophins on the synaptogenesis of inner hair cells in the deaf Bronx waltzer (bv) mouse organ of Corti in culture. Int J Dev Neurosci. 2002;20(7):537–554. doi: 10.1016/s0736-5748(02)00084-9. [DOI] [PubMed] [Google Scholar]
- 14.Stankovic K, et al. Survival of adult spiral ganglion neurons requires erbB receptor signaling in the inner ear. J Neurosci. 2004;24(40):8651–8661. doi: 10.1523/JNEUROSCI.0733-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zilberstein Y, Liberman MC, Corfas G. Inner hair cells are not required for survival of spiral ganglion neurons in the adult cochlea. J Neurosci. 2012;32(2):405–410. doi: 10.1523/JNEUROSCI.4678-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Lahne M, Gale JE. Damage-induced activation of ERK1/2 in cochlear supporting cells is a hair cell death-promoting signal that depends on extracellular ATP and calcium. J Neurosci. 2008;28(19):4918–4928. doi: 10.1523/JNEUROSCI.4914-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Giffard RG, Macario AJ, de Macario EC. The future of molecular chaperones and beyond. J Clin Invest. 2013;123(8):3206–3208. doi: 10.1172/JCI70799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Abrashkin KA, et al. The fate of outer hair cells after acoustic or ototoxic insults. Hear Res. 2006;218(1-2):20–29. doi: 10.1016/j.heares.2006.04.001. [DOI] [PubMed] [Google Scholar]
- 19.White PM, Doetzlhofer A, Lee YS, Groves AK, Segil N. Mammalian cochlear supporting cells can divide and trans-differentiate into hair cells. Nature. 2006;441(7096):984–987. doi: 10.1038/nature04849. [DOI] [PubMed] [Google Scholar]
- 20.Lin V, et al. Inhibition of Notch activity promotes nonmitotic regeneration of hair cells in the adult mouse utricles. J Neurosci. 2011;31(43):15329–15339. doi: 10.1523/JNEUROSCI.2057-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ronaghi M, Nasr M, Heller S. Concise review: Inner ear stem cells—an oxymoron, but why? Stem Cells. 2012;30(1):69–74. doi: 10.1002/stem.785. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Rubel EW, Furrer SA, Stone JS. A brief history of hair cell regeneration research and speculations on the future. Hear Res. 2013;297:42–51. doi: 10.1016/j.heares.2012.12.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Stone JS, Cotanche DA. Hair cell regeneration in the avian auditory epithelium. Int J Dev Biol. 2007;51(6-7):633–647. doi: 10.1387/ijdb.072408js. [DOI] [PubMed] [Google Scholar]
- 24.Slattery EL, Warchol ME. Cisplatin ototoxicity blocks sensory regeneration in the avian inner ear. J Neurosci. 2010;30(9):3473–3481. doi: 10.1523/JNEUROSCI.4316-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Ivanova A, et al. In vivo genetic ablation by Cre-mediated expression of diphtheria toxin fragment A. Genesis. 2005;43(3):129–135. doi: 10.1002/gene.20162. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Doerflinger NH, Macklin WB, Popko B. Inducible site-specific recombination in myelinating cells. Genesis. 2003;35(1):63–72. doi: 10.1002/gene.10154. [DOI] [PubMed] [Google Scholar]
- 27.Gómez-Casati ME, Murtie J, Taylor B, Corfas G. Cell-specific inducible gene recombination in postnatal inner ear supporting cells and glia. J Assoc Res Otolaryngol. 2010;11(1):19–26. doi: 10.1007/s10162-009-0191-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Madisen L, et al. A robust and high-throughput Cre reporting and characterization system for the whole mouse brain. Nat Neurosci. 2010;13(1):133–140. doi: 10.1038/nn.2467. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Abrahamsen B, et al. The cell and molecular basis of mechanical, cold, and inflammatory pain. Science. 2008;321(5889):702–705. doi: 10.1126/science.1156916. [DOI] [PubMed] [Google Scholar]
- 30.Burns JC, Cox BC, Thiede BR, Zuo J, Corwin JT. In vivo proliferative regeneration of balance hair cells in newborn mice. J Neurosci. 2012;32(19):6570–6577. doi: 10.1523/JNEUROSCI.6274-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Mellado Lagarde MM, et al. Selective ablation of pillar and deiters’ cells severely affects cochlear postnatal development and hearing in mice. J Neurosci. 2013;33(4):1564–1576. doi: 10.1523/JNEUROSCI.3088-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Dabdoub A, et al. Sox2 signaling in prosensory domain specification and subsequent hair cell differentiation in the developing cochlea. Proc Natl Acad Sci USA. 2008;105(47):18396–18401. doi: 10.1073/pnas.0808175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smeti I, et al. Expression of candidate markers for stem/progenitor cells in the inner ears of developing and adult GFAP and nestin promoter-GFP transgenic mice. Gene Expr Patterns. 2011;11(1-2):22–32. doi: 10.1016/j.gep.2010.08.008. [DOI] [PubMed] [Google Scholar]
- 34.Shi F, Kempfle JS, Edge AS. Wnt-responsive Lgr5-expressing stem cells are hair cell progenitors in the cochlea. J Neurosci. 2012;32(28):9639–9648. doi: 10.1523/JNEUROSCI.1064-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Morris JK, et al. A disorganized innervation of the inner ear persists in the absence of ErbB2. Brain Res. 2006;1091(1):186–199. doi: 10.1016/j.brainres.2006.02.090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Jin ZH, Kikuchi T, Tanaka K, Kobayashi T. Expression of glutamate transporter GLAST in the developing mouse cochlea. Tohoku J Exp Med. 2003;200(3):137–144. doi: 10.1620/tjem.200.137. [DOI] [PubMed] [Google Scholar]
- 37.Mallon BS, Shick HE, Kidd GJ, Macklin WB. Proteolipid promoter activity distinguishes two populations of NG2-positive cells throughout neonatal cortical development. J Neurosci. 2002;22(3):876–885. doi: 10.1523/JNEUROSCI.22-03-00876.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Chai R, et al. Wnt signaling induces proliferation of sensory precursors in the postnatal mouse cochlea. Proc Natl Acad Sci USA. 2012;109(21):8167–8172. doi: 10.1073/pnas.1202774109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Chai R, et al. Dynamic expression of Lgr5, a Wnt target gene, in the developing and mature mouse cochlea. J Assoc Res Otolaryngol. 2011;12(4):455–469. doi: 10.1007/s10162-011-0267-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Shi F, Hu L, Edge AS. Generation of hair cells in neonatal mice by β-catenin overexpression in Lgr5-positive cochlear progenitors. Proc Natl Acad Sci USA. 2013;110(34):13851–13856. doi: 10.1073/pnas.1219952110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Bramhall NF, Shi F, Arnold K, Hochedlinger K, Edge AS. Lgr5-positive supporting cells generate new hair cells in the postnatal cochlea. Stem Cell Rev. 2014;2(3):311–322. doi: 10.1016/j.stemcr.2014.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Cox BC, et al. Spontaneous hair cell regeneration in the neonatal mouse cochlea in vivo. Development. 2014;141(4):816–829. doi: 10.1242/dev.103036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Lanford PJ, et al. Notch signalling pathway mediates hair cell development in mammalian cochlea. Nat Genet. 1999;21(3):289–292. doi: 10.1038/6804. [DOI] [PubMed] [Google Scholar]
- 44.Zine A, Van De Water TR, de Ribaupierre F. Notch signaling regulates the pattern of auditory hair cell differentiation in mammals. Development. 2000;127(15):3373–3383. doi: 10.1242/dev.127.15.3373. [DOI] [PubMed] [Google Scholar]
- 45.Wan G, Gomez-Casati ME, Gigliello AR, Liberman C, Corfas G. Neurotrophin-3 regulates ribbon synapse density in the cochlea and induces synapse regeneration after acoustic trauma 2014 doi: 10.7554/eLife.03564. . eLife, 10.7554/eLife.03564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.de Melo J, et al. Injury-independent induction of reactive gliosis in retina by loss of function of the LIM homeodomain transcription factor Lhx2. Proc Natl Acad Sci USA. 2012;109(12):4657–4662. doi: 10.1073/pnas.1107488109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Wang Y, et al. Norrin/Frizzled4 signaling in retinal vascular development and blood brain barrier plasticity. Cell. 2012;151(6):1332–1344. doi: 10.1016/j.cell.2012.10.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Forge A, et al. Gap junctions and connexin expression in the inner ear. Novartis Found Symp. 1999;219:134–150, discussion 151–156. doi: 10.1002/9780470515587.ch9. [DOI] [PubMed] [Google Scholar]
- 49.Forge A, Marziano NK, Casalotti SO, Becker DL, Jagger D. The inner ear contains heteromeric channels composed of cx26 and cx30 and deafness-related mutations in cx26 have a dominant negative effect on cx30. Cell Commun Adhes. 2003;10(4-6):341–346. doi: 10.1080/cac.10.4-6.341.346. [DOI] [PubMed] [Google Scholar]
- 50.Lin L, et al. Ultrastructural pathological changes in the cochlear cells of connexin 26 conditional knockout mice. Mol Med Rep. 2013;8(4):1029–1036. doi: 10.3892/mmr.2013.1614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Takada Y, et al. Connexin 26 null mice exhibit spiral ganglion degeneration that can be blocked by BDNF gene therapy. Hear Res. 2014;309:124–135. doi: 10.1016/j.heares.2013.11.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Jacques BE, et al. A dual function for canonical Wnt/β-catenin signaling in the developing mammalian cochlea. Development. 2012;139(23):4395–4404. doi: 10.1242/dev.080358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Liu Z, et al. In vivo visualization of Notch1 proteolysis reveals the heterogeneity of Notch1 signaling activity in the mouse cochlea. PLoS ONE. 2013;8(5):e64903. doi: 10.1371/journal.pone.0064903. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Liu Z, Fang J, Dearman J, Zhang L, Zuo J. In vivo generation of immature inner hair cells in neonatal mouse cochleae by ectopic Atoh1 expression. PLoS ONE. 2014;9(2):e89377. doi: 10.1371/journal.pone.0089377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Liu Z, et al. Age-dependent in vivo conversion of mouse cochlear pillar and Deiters’ cells to immature hair cells by Atoh1 ectopic expression. J Neurosci. 2012;32(19):6600–6610. doi: 10.1523/JNEUROSCI.0818-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Zheng JL, Gao WQ. Overexpression of Math1 induces robust production of extra hair cells in postnatal rat inner ears. Nat Neurosci. 2000;3(6):580–586. doi: 10.1038/75753. [DOI] [PubMed] [Google Scholar]
- 57.Kelly MC, Chang Q, Pan A, Lin X, Chen P. Atoh1 directs the formation of sensory mosaics and induces cell proliferation in the postnatal mammalian cochlea in vivo. J Neurosci. 2012;32(19):6699–6710. doi: 10.1523/JNEUROSCI.5420-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Lopez IA, Zhao PM, Yamaguchi M, de Vellis J, Espinosa-Jeffrey A. Stem/progenitor cells in the postnatal inner ear of the GFP-nestin transgenic mouse. Int J Dev Neurosci. 2004;22(4):205–213. doi: 10.1016/j.ijdevneu.2004.04.006. [DOI] [PubMed] [Google Scholar]
- 59.Tan S, Barker N. Epithelial stem cells and intestinal cancer. Semin Cancer Biol. 2014 doi: 10.1016/j.semcancer.2014.02.005. [DOI] [PubMed] [Google Scholar]
- 60.de Lau W, Peng WC, Gros P, Clevers H. The R-spondin/Lgr5/Rnf43 module: Regulator of Wnt signal strength. Genes Dev. 2014;28(4):305–316. doi: 10.1101/gad.235473.113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Tian H, et al. A reserve stem cell population in small intestine renders Lgr5-positive cells dispensable. Nature. 2011;478(7368):255–259. doi: 10.1038/nature10408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Huch M, et al. Unlimited in vitro expansion of adult bi-potent pancreas progenitors through the Lgr5/R-spondin axis. EMBO J. 2013;32(20):2708–2721. doi: 10.1038/emboj.2013.204. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jan TA, et al. Tympanic border cells are Wnt-responsive and can act as progenitors for postnatal mouse cochlear cells. Development. 2013;140(6):1196–1206. doi: 10.1242/dev.087528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Barker N, et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature. 2007;449(7165):1003–1007. doi: 10.1038/nature06196. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.