Significance
The existing literature on the long-term effects of marijuana on the brain provides an inconsistent picture (i.e., presence or absence of structural changes) due to methodological differences across studies. We overcame these methodological issues by collecting multimodal measures in a large group of chronic marijuana using adults with a wide age range that allows for characterization of changes across lifespan without developmental or maturational biases as in other studies. Our findings suggest that chronic marijuana use is associated with complex neuroadaptive processes and that onset and duration of use have unique effects on these processes.
Keywords: MRI, orbitofrontal cortex, functional connectivity, resting state fMRI, diffusion tensor imaging
Abstract
Questions surrounding the effects of chronic marijuana use on brain structure continue to increase. To date, however, findings remain inconclusive. In this comprehensive study that aimed to characterize brain alterations associated with chronic marijuana use, we measured gray matter (GM) volume via structural MRI across the whole brain by using voxel-based morphology, synchrony among abnormal GM regions during resting state via functional connectivity MRI, and white matter integrity (i.e., structural connectivity) between the abnormal GM regions via diffusion tensor imaging in 48 marijuana users and 62 age- and sex-matched nonusing controls. The results showed that compared with controls, marijuana users had significantly less bilateral orbitofrontal gyri volume, higher functional connectivity in the orbitofrontal cortex (OFC) network, and higher structural connectivity in tracts that innervate the OFC (forceps minor) as measured by fractional anisotropy (FA). Increased OFC functional connectivity in marijuana users was associated with earlier age of onset. Lastly, a quadratic trend was observed suggesting that the FA of the forceps minor tract initially increased following regular marijuana use but decreased with protracted regular use. This pattern may indicate differential effects of initial and chronic marijuana use that may reflect complex neuroadaptive processes in response to marijuana use. Despite the observed age of onset effects, longitudinal studies are needed to determine causality of these effects.
The rate of marijuana use has had a steady increase since 2007 (1). Among >400 chemical compounds, marijuana’s effects are primarily attributed to δ-9-tetrahydrocannabinol (THC), which is the main psychoactive ingredient in the cannabis plant. THC binds to cannabinoid receptors, which are ubiquitous in the brain. Consequently, exposure to THC leads to neural changes affecting diverse cognitive processes. These changes have been observed to be long-lasting, suggesting that neural changes due to marijuana use may affect neural architecture (2). However, to date, these brain changes as a result of marijuana use remains equivocal. Specifically, although functional changes have been widely reported across cognitive domains in both adult and adolescent cannabis users (3–6), structural changes associated with marijuana use have not been consistent. Although some have reported decreases in regional brain volume such as in the hippocampus, orbitofrontal cortex, amygdala, and striatum (7–12), others have reported increases in amygdala, nucleus accumbens, and cerebellar volumes in chronic marijuana users (13–15). However, others have reported no observable difference in global or regional gray or white matter volumes in chronic marijuana users (16, 17). These inconsistencies could be attributed to methodological differences across studies pertaining to study samples (e.g., severity of marijuana use, age, sex, comorbidity with other substance use or psychiatric disorders) and/or study design (e.g., study modality, regions of interest).
Because THC binds to cannabinoid 1 (CB1) receptors in the brain, when differences are observed, these morphological changes associated with marijuana use have been reported in CB1 receptor-enriched areas such as the orbitofrontal cortex, anterior cingulate, striatum, amygdala, insula, hippocampus, and cerebellum (2, 11, 13, 18). CB1 receptors are widely distributed in the neocortex, but more restricted in the hindbrain and the spinal cord (19). For example, in a recent study by Battistella et al. (18), they found significant brain volume reductions in the medial temporal cortex, temporal pole, parahippocampal gyrus, insula, and orbitofrontal cortex (OFC) in regular marijuana users compared with occasional users. Whether these reductions in brain volume lead to downstream changes in brain organization and function, however, is still unknown.
Nevertheless, emergent studies have demonstrated a link between brain structure and connectivity. For example, Van den Heuvel et al. and Greicius et al. demonstrated robust structural connections between white matter indexes and functional connectivity strength within the default mode network (20, 21). Similarly, others have reported correlated patterns of gray matter structure and connectivity that are in many ways reflective of the underlying intrinsic networks (22). Thus, given the literature suggesting a direct relationship between structural and functional connectivity, it is likely that connectivity changes would also be present where alterations in brain volume are observed as a result of marijuana use.
The goal of this study was to characterize alterations in brain morphometry and determine potential downstream effects in connectivity as a result of chronic marijuana use. To address the existing inconsistencies in the literature that may be in part due to methodological issues, we (i) used three different MRI techniques to investigate a large cohort of well-characterized chronic cannabis users with a wide age range (allowing for characterization without developmental or maturational biases) and compared them to age- and sex-matched nonusing controls; (ii) examined observable global (rather than select) gray matter differences between marijuana users and nonusing controls; and (iii) performed subsequent analyses to determine how these changes relate to functional and structural connectivity, as well as behavior. Given the existing literature on morphometric reductions associated with long-term marijuana use, we expected gray matter reductions in THC-enriched areas in chronic marijuana users that will be associated with changes in brain connectivity and marijuana-related behavior.
Methods
Participants.
A total of 110 participants consisting of 62 nonusing controls and 48 marijuana users were recruited through fliers and media advertisement in the Albuquerque, NM, metro area. We previously presented results on subgroups of these participants (8, 23, 24). Written informed consent was obtained from all participants in accordance with the Institutional Review Board (IRB) of The University of New Mexico. The inclusion criteria for all of the participants were as follows: (i) English as the primary language; and (ii) no current or history of psychosis, traumatic brain injury, or neurological disorder. Marijuana users (cannabis group) were included if they currently use marijuana regularly (at least four times per week) over the last 6 mo (confirmed via positive THC-COOH urinalysis). Nonusing controls (control group) had no self-reported regular use of marijuana and had a negative urine drug screen at baseline. Table 1 summarizes the demographic information, behavioral measures, and total number of participants per cohort.
Table 1.
Subject characteristics (mean ± SD)
| Demographic variable | Control | Cannabis users | Exclusively cannabis users |
| Participants (n) | 62 | 48 | 27 |
| Sex (M/F) | 39/23 | 33/15 | 17/10 |
| Age | 30.0 ± 7.4 | 28.3 ± 8.3 | 28.1 ± 8.9 |
| Race | |||
| White | 25 | 24 | 11 |
| Latino | 27 | 16 | 11 |
| Native American | 5 | 3 | 2 |
| Black | 4 | 3 | 3 |
| Asian | 1 | 0 | 0 |
| Education, y | 13.9 ± 1.7 | 14.2 ± 2.4 | 14.3 ± 2.5 |
| IQ* | 110.9 ± 11.6 | 105.8 ± 12.2 | 104.0 ± 1.4 |
| Age of onset | — | 18.1 ± 3.4 | 18.7 ± 2.9 |
| Years of use | — | 9.8 ± 8.0 | 8.7 ± 8.7 |
| Weekly use | — | 11.1 ± 1.4 | 11.2 ± 1.4 |
| Daily use | — | 3.1 ± 1.6 | 2.8 ± 1.4 |
| Marijuana-related problems (MPS) | — | 3.4 ± 4.1 | 2.5 ± 2.4 |
| Marijuana dependence (N) | — | 25 | 14 |
Control and cannabis users’ IQ were different (P < 0.05). IQ was assessed by using the Wechsler Abbreviated Scale of Intelligence (54).
Marijuana dependence was assessed via Structured Clinical Interview for DSM IV Disorders (55).
MRI Acquisition.
MRI scans were performed on a Siemens 3 Tesla Trio scanner by using the standard 12-channel phased array head coil. We used different MRI techniques to investigate brain changes between cannabis users and control groups: (i) a high resolution T1-weighted image to measure gray matter volume, (ii) a resting state functional MRI scan was collected to assess functional connectivity of the brain, and (iii) a diffusion tensor imaging scan was collected to provide an assessment of structural connectivity between brain regions via white matter tracts. The details of the imaging parameters and their processing techniques are provided below:
Whole brain high-resolution T1-weighted anatomical images were collected by using a multiecho magnetization prepared rapid gradient echo (MPRAGE) sequence with the following parameters: repetition time (TR)/echo time (TE)/inversion time (TI) = 2,530/1.64, 3.5, 5.36, 7.22, 9.08/1,200 ms, flip angle = 7°, field of view (FOV) = 256 × 256 × 192 mm3, voxel size = 1 × 1 × 1 mm3, and number of excitations (NEX) = 1. The sequence parameters for functional MRI (fcMRI) were: FOV = 240 × 240, matrix = 64 × 64, slice thickness = 4.55 mm, no gap between slices, voxel size = 3.75 × 3.75 × 4.55 mm2, 32 axial slices, TR/TE = 2,000/29 ms, flip angle = 60°, 158 image volumes, and scan duration = 5.5 min. The diffusion tensor imaging (DTI) MRI scans (b = 800 s/mm2) were acquired by using a twice-refocused spin echo sequence with 30 diffusion gradients and the b = 0 experiment repeated five times with the following parameters: TE/TR = 84/9,000 ms, flip angle = 90°, FOV = 256 × 256 × 144 mm3, voxel resolution = 2 × 2 × 2 mm3 and NEX = 1. The sequence parameters for fcMRI were FOV = 240 × 240 mm2, matrix = 64 × 64, slice thickness = 4.55 mm, voxel size = 3.75 × 3.75 × 4.55 mm3, 31 axial slices, TR/TE = 2,000/29 ms, flip angle = 90°, 158 image volumes, and scan duration = 5.3 min.
MRI Data Processing.
We used the voxel-based morphology (VBM) technique to investigate whole brain structural abnormalities. High-resolution T1 images were processed by using the Diffeomorphic Anatomical Registration Through Exponentiated Lie Algebra (DARTEL), an improved VBM method that can achieve intersubject brain images registration more accurately in SPM 8 (www.fil.ion.ucl.ac.uk/spm). Briefly, the following steps were performed on the T1 images: (i) MR images were segmented into gray matter (GM), white matter (WM), and cerebrospinal fluid; (ii) customized GM templates were created from the images of study by using DARTEL technique; (iii) after an initial affine registration of the GM DARTEL templates to the tissue probability maps in MNI space, nonlinear warping of GM images was performed to the DARTEL GM template and then used in the modulation step to ensure that relative volumes of GM were preserved following the spatial normalization procedure; (iv) the modulated, normalized GM images were smoothed with a 6-mm full width at half maximum (FWHM). Next, we conducted a two-sample t test with intelligence quotient (IQ) as a covariate. A voxel level threshold of P < 0.01 (FWE-corrected) and cluster size ≥ 15,936 mm3 was determined based on AFNI software’s 3dClustSim [National Institute of Mental Health (NIMH) Scientific and Statistical Computing Core]. For the analyses exploring relationships between activation maps and behavioral measures, 10-mm sphere masks were defined around the peak voxels of the significant gray matter clusters.
Resting state fMRI (rsfMRI) images were analyzed by using AFNI (NIMH Scientific and Statistical Computing Core) and in-house MATLAB scripts. The dataset was preprocessed with motion correction (realignment), slice timing correction, removal of the linear trend, transformation to standard MNI space (matrix = 53 × 63 × 46, resolution = 3 × 3 × 3 mm3), and smoothing by a Gaussian filter with a full width half maximum (FWHM) of 10 mm. Next, the images were band-pass filtered (0.01–0.1 Hz) on a voxel-by-voxel basis to keep only the appropriate frequency fluctuations. Next, the signals in white matter and cerebrospinal fluid were regressed out by using averaged signals from the white matter and the ventricles from each voxel time series. Functional connectivity was measured by using a seed-based approach by choosing bilateral orbitofrontal gyri cluster peaks from VBM analysis, [+26 +54 –8] and [−16 +58 –10] in MNI template. The cross-correlation coefficient between these seed voxels and all other voxels was calculated to generate a correlation map. Then, the correlation maps were transformed to a z-score map by using Fisher’s inverse hyperbolic tangent transformation. Next, a region of interest (ROI) analysis was performed. Each region’s anatomical region was defined based on automated anatomical labeling (AAL) database. Then, the orbitofrontal and temporal functional masks were defined as the top 200 voxels according to their z score in their functional connectivity maps as described by Chapman and coworkers (25).
Diffusion-weighted data were processed by using the University of Oxford’s Center for Functional Magnetic Resonance Imaging of the Brain Software Library release 4.0 (www.fmrib.ox.ac.uk/fsl). First, the data were corrected for head movement and eddy current distortions by using Eddycorrect, which aligns all of the volumes. Next, DTIfit was used to independently fit diffusion tensors to each voxel, with the brain mask limiting the fitting of tensors to brain space. The output of DTIfit yielded voxel-wise maps of fractional anisotropy (FA), axial diffusivity (AD) (λ1), radial diffusivity (RD) (average of λ2 and λ3) and mean diffusivity (MD) (average of λ1, λ2, and λ3) for each participant. Finally, in the tractography analysis, white matter tracts were constructed with minimum FA of 0.20 and maximum turning angle of 50°. Because the orbitofrontal cortex is innervated by the forceps minor, and, therefore, plays a role in decision-making processes, the forceps minor tract was delineated via two techniques—manual and automatic tractography—whereas the forceps major tract was delineated only manually as a control. In manual tractography, the forceps minor and forceps major tracts were delineated by drawing manual ROI per methods described in Wakana et al. (26). In automatic tractography, the VBM clusters (see Fig. 3) were coregistered to each participant’s native DTI space and used as an ROI to delineate the fiber tract. Specifically, these regions were dilated five times, using 3dAutomask in AFNI, to ensure the clusters were expanded into the white matter tissue. Last, an “AND” operation between the two clusters was performed and the resultant fiber was the forceps minor from the right middle orbitofrontal and left superior orbitofrontal gyri.
Fig. 3.
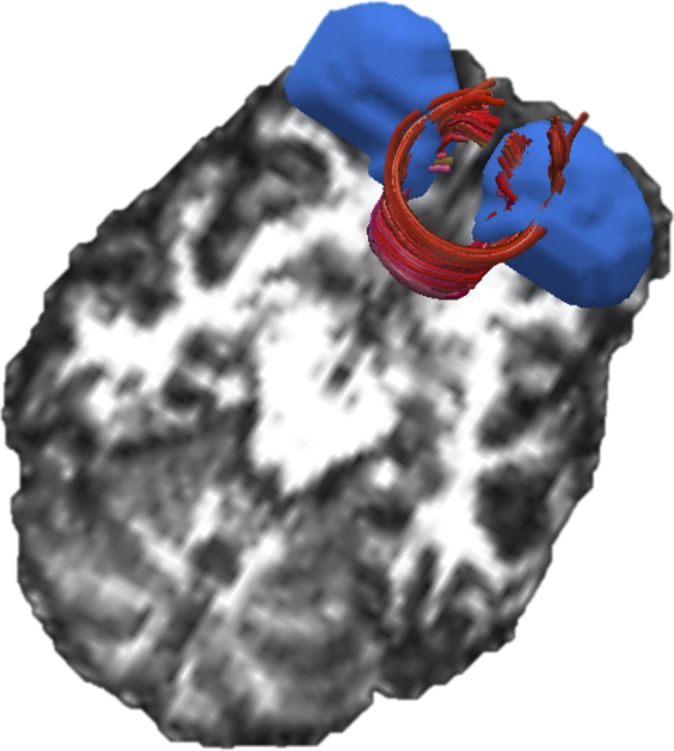
A representative participant’s forceps minor tract (in red) and gray matter nodes (in blue) is overlaid on its corresponding fractional anisotropy map.
Behavioral Measures.
Behavior related to marijuana use was captured by using the Marijuana Problem Survey (MPS) (27). The MPS is a 19-item measure that assesses the negative psychological, social, occupational, and legal consequences of marijuana use in the last 90 d (e.g., problems with family and significant others, missing work or losing a job, feeling bad about marijuana use). Each problem is rated from 0 (“no problem”) to 2 (“serious problem”), and the number of items endorsed as 1 or 2 is summed to create an index of the total number of problems (range = 0–19). Treatment-seeking marijuana users report an average of 9–10 problems.
Statistical Analysis.
A general statistical linear model was applied to assess the contribution of chronic marijuana use on cognition, gray matter volume, functional connectivity, and structural connectivity measures. The model included two groups (i.e., marijuana users and controls) and IQ as a covariate. Two sample t tests were performed to assess to identify how groups differed in the aforementioned measures, and we hypothesized that the cannabis group would show alterations in gray matter volume, functional connectivity, and structural connectivity. Last, parametric regression models were tested to examine the relationship among gray matter volume, functional connectivity, white matter integrity, and neurocognitive measures within the cannabis group. To ensure the best parametric regression fit, we performed Akaike information criterion (AIC) to provide a means for model selection (i.e., linear vs. quadratic).
Results
Sample Characteristics.
All MR images were visually inspected for possible artifacts. Of 110 participants, three control participants did not complete the functional connectivity MRI protocol. Nine participants (seven in the control group; two in the cannabis group) did not produce the forceps minor tract via the automated technique. No participant was excluded based on motion criteria of >3 mm and >3°. There was no significant difference in age or sex between the groups. However, the IQ of the marijuana users was significantly lower than the control group (P < 0.05). Table 1 summarizes participants’ demographic data.
MRI Measurements.
Voxelwise comparison of the high-resolution T1 images showed a significant lower gray matter volume in marijuana users in the right middle orbitofrontal (MNI coordinates: [+26 +54 –8]; t score = 3.37) and left superior orbitofrontal gyri (MNI coordinates: [−16 +58 –10]; t score = 3.19) [P < 0.01 (FWE corrected) and cluster ≥ 15,936 mm3] per anatomical automatic labeling (AAL), shown in Fig. 1. The reverse contrast, marijuana > control, did not yield any significant voxels.
Fig. 1.
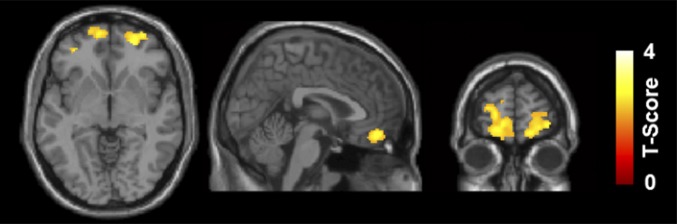
Group comparison of the gray matter volume by SPM8 plus DARTEL analysis demonstrates significant reduction of gray matter volume in bilateral orbitofrontal gyri (AAL atlas) in marijuana users compared with controls. Right side of the image represents the right hemisphere in axial view.
Following these observed structural alterations in the orbitofrontal region, we then characterized the functional connectivity of the orbitofrontal network. The components of this network consist of bilateral orbitofrontal and bilateral temporal gyri (28). Fig. 2A shows the average functional connectivity maps in the orbitofrontal network for the control and cannabis groups. These maps show qualitative differences between the groups such that the cannabis group had higher functional connectivity compared with the control group. Fig. 2B shows that, quantitatively, marijuana users had significantly higher connectivity in all four nodes (i.e., bilateral OFC and bilateral temporal lobe) compared to the control group.
Fig. 2.
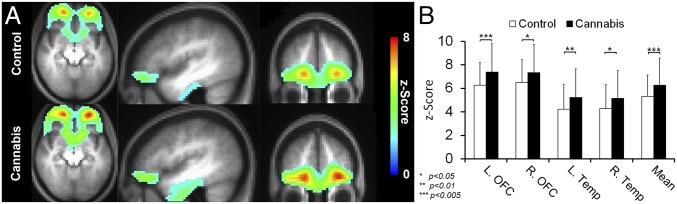
(A) The average functional connectivity maps (i.e., OFC network; bilateral OFC and temporal gyri) of the control and cannabis groups are superimposed on their average T1-weighted image. For illustration purposes, the z-score maps were arbitrarily thresholded (z score ≥ 2, k ≥ 50) to qualitatively visualize the difference in the intensity and cluster size. (B) Mean fcMRI z scores are shown for the orbitofrontal network for cannabis and controls groups. The cannabis group showed higher resting activity in the bilateral OFC and temporal gyri compared with the control group.
We also measured the structural connectivity of the forceps minor tract, which connects the orbitofrontal regions, both manually and automatically (shown in Fig. 3). We found that the forceps minor’s FA of the cannabis group was significantly higher than the control group in both automatic and manual methods, P = 0.003 and P < 0.001, respectively. As a manipulation check, we also measured the FA of the forceps major, which did not show any significant difference between control and cannabis groups. Additionally, we examined which component of FA may be driving this effect. To that end, we also carried out one-way ANOVA comparisons of mean diffusivity (MD), radial diffusivity (RD), and axial diffusivity (AD) between the groups. We found that RD of the cannabis users was significantly lower than that of the controls in both automatic and manual tractography, P = 0.05 and P = 0.004, respectively (shown in Table 2). Differences between AD and MD were not significant between the groups.
Table 2.
Forceps minor and forceps major tracts DTI parameters (mean ± SD)
| DTI measures | Control | Cannabis | P value |
| Forceps minor (automated) | |||
| FA | 0.551 ± 0.028 | 0.570 ± 0.032 | 0.003 |
| AD | 1.39e−03 ± 6.68e−05 | 1.40e−03 ± 4.27e−05 | 0.57 |
| RD | 5.32e−04 ± 4.60e−05 | 5.14e−04 ± 4.11e−05 | 0.05 |
| MD | 8.19e−04 ± 4.79e−05 | 8.09e−04 ± 3.45e−05 | 0.25 |
| Forceps minor (manual) | |||
| FA | 0.549 ± 0.025 | 0.569 ± 0.025 | <0.001 |
| AD | 1.34e−03 ± 5.36e−05 | 1.35e−03 ± 3.56e−05 | 0.77 |
| RD | 5.19e−04 ± 3.47e−05 | 4.99e−04 ± 3.14e−05 | 0.004 |
| MD | 7.94e−04 ± 3.53e−05 | 7.82e−02 ± 2.75e−05 | 0.051 |
| Forceps major (manual) | |||
| FA | 0.643 ± 0.029 | 0.651 ± 0.020 | 0.07 |
| AD | 1.59e−03 ± 5.75e−05 | 1.59e−03 ± 5.38e−05 | 0.88 |
| RD | 4.70e−04 ± 3.84e−05 | 4.60e−04 ± 3.14e−05 | 0.09 |
| MD | 8.41e−04 ± 3.27e−05 | 8.34e−04 ± 3.22e−05 | 0.17 |
Brain–Behavior Correlations.
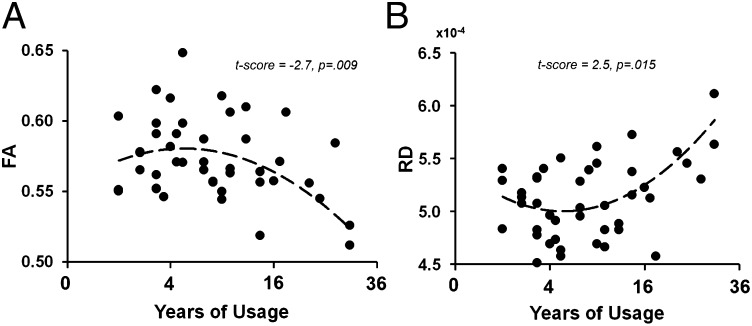
Our analyses showed significant correlations between forceps minor integrity/OFC functional connectivity and behavioral indicators of marijuana use (Table 3). The forceps minor’s FA and RD showed gains with initial heavy use but declined after chronic use, as shown in Fig. 4. The functional connectivity of the bilateral OFC showed similar patterns where there was an inverse correlation with age of onset of use such that earlier age of onset leads to higher functional connectivity of the bilateral OFC. Finally, there was an inverse correlation between the left temporal lobe functional connectivity and problems related with marijuana use, such that the greater the functional connectivity of the left temporal cortex to bilateral OFC, the lower the total score on MPS.
Table 3.
Associations between gray matter volume, functional connectivity, white matter integrity, and neurocognitive measures within the cannabis group
| Neuroimaging measures | Behavioral measures | Parametric model | t score | P value |
| DTI MRI | ||||
| Forceps minor—FA | Duration of use | Quadratic | −2.7 | 0.009 |
| Forceps minor—RD | Duration of use | Quadratic | 2.5 | 0.015 |
| fcMRI | ||||
| Left orbitofrontal cortex | Age of onset | Linear | −2.1 | 0.046 |
| Right orbitofrontal cortex | Age of onset | Linear | −2.1 | 0.042 |
| Left temporal cortex | MPS total | Linear | −2.7 | 0.010 |
The curve fitting for the general linear model was controlled by AIC.
Fig. 4.
The relationship between duration of marijuana use and forceps minor’s FA (A) and RD (B). The quadratic curve showed the best fit per AIC. The x axis has been transformed to ”square root of years of use” because of gap between participants’ years of use.
Post Hoc Analyses.
Because of the high comorbidity between marijuana, tobacco, and alcohol use, we performed additional analyses to control for potential confounding effects of tobacco and alcohol use. In these analyses, we excluded marijuana users who reported use of other substances. This resulted in 27 “exclusively” marijuana users as shown in Table 1. The association between the neuroimaging results and behavioral measures were also assessed as described previously. Similar to the main findings, the exclusively marijuana users showed significantly lower OFC gray matter density, and significantly higher OFC network functional connectivity and FA/RD of the forceps minor tract compared with the control group (Figs. S1 and S2 and Table S1). There was also a quadratic trend in the correlation between the forceps minor’s FA/RD and use duration, t score = −2.05, P = 0.05 and t score = 2.60, P = 0.016, respectively (Table 4). Additionally, the exclusively marijuana users showed an inverse relationship between bilateral OFC gray matter volume and problems related to marijuana use. That is, the lower the OFC gray matter volume in these participants, the higher their MPS total scores.
Table 4.
Brain–behavior correlations in exclusively marijuana users (n = 27)
| Neuroimaging measures | Behavioral measures | Parametric model | t score | P value |
| DTI MRI | ||||
| Forceps minor—FA | Duration of use in years | Quadratic | −2.05 | 0.05 |
| Forceps minor—RD | Duration of use in years | Quadratic | 2.60 | 0.016 |
| Gray matter volume | ||||
| Left middle OFC | MPS total score | Linear | 2.02 | 0.056 |
| Right superior OFC | MPS total score | Linear | 2.30 | 0.032 |
Lastly, to partially address how these abnormalities are related to cognitive processes, we conducted a mediation analysis to assess whether neural abnormalities (OFC gray matter volumes, OFC/temporal lobe functional connectivity, FA/RD of forceps minor) mediate lower scores on IQ in marijuana users. We did not find that the causal variable (i.e., marijuana use) was significantly correlated with the mediator variable (i.e., OFC gray matter volume, OFC/temporal functional connectivity, and FA/RD of forceps minor) and outcome variable (i.e., IQ). We, therefore, suggest that the path from marijuana use to neural abnormalities to decreases in IQ is more complex and, perhaps, include other mediators such as environmental (i.e., age of onset) and/or genetic factors.
Discussion
Unlike the animal literature, whether exposure to marijuana leads to long-term changes in human brain structure has been equivocal. To address this limitation, we evaluated brain structural changes associated with chronic marijuana use in a large group of well-characterized marijuana users relative to age- and sex-matched nonusing controls. Our findings provide evidence that heavy, chronic marijuana users have lower OFC gray matter volumes compared with nonusing controls. This finding remained even in the smaller sample of exclusively marijuana users (n = 27, i.e., no comorbid substance use), demonstrating that this effect (i) is robust and (ii) is greater than potential effects of comorbid substance use. Similar decreases in OFC volume have been reported in marijuana using adults (29) and adolescents (12) compared with nonusing controls. Interestingly, a prospective study also found that smaller OFC volumes at 12 y of age predicted initiation of marijuana use at 16 y of age (30). These effects on the OFC are not surprising given that the OFC is a primary region in the reward network, is enriched with CB1 receptors, and is highly implicated in addictive behaviors (23, 24, 31, 32) such as those related to disruptions in motivation (33) and decision making (34, 35). Whereas others have reported alterations in various CB1-enriched regions such as the amygdala, hippocampus, ventromedial prefrontal, OFC, insula, and striatum, our findings are specific to the OFC. Several animal and human studies have demonstrated greater THC-induced down-regulation of CB1 receptors in cortical areas relative to subcortical areas, which support our findings. Given that CB1 receptors are found on excitatory terminals of cortical projection neurons, this alteration in endocannabinoid signaling could affect the plasticity of OFC circuits (36). Unfortunately, the cross-sectional nature of the present study cannot directly address whether these reductions are the cause or the consequence of marijuana use. However, neurotoxic effects of cannabis have been widely reported in the animal literature. Based on the animal literature, potential mechanisms that may lead to OFC reductions due to cannabis neurotoxicity may, therefore, include neuronal loss, changes in cell size, or a reduction in CB1 density. It is possible, however, that these OFC abnormalities may reflect preexisting pathophysiology related to vulnerability to marijuana abuse and dependence.
To determine the potential downstream effects of OFC volume reduction, we evaluated OFC functional (fcMRI) and structural connectivity (DTI). Functional connectivity analysis revealed greater connectivity within the OFC network in marijuana users compared with controls, which is concordant with existing resting-state studies (37) and task-based studies (38, 39). This increased functional connectivity in users may suggest a compensatory mechanism whereby greater network recruitment is engaged to compensate for OFC liability (40). Tomasi et al. (41) illustrated how greater functional connectivity requires higher glucose consumption (∼70% of brain’s energy consumption), and, consequently, hubs of higher functional connectivity must be efficient. In their report, the OFC was described as having high glucose efficiency as measured by the ratio between the strength of functional integration (based on rsfMRI and the number of connections of the network nodes) and cerebral metabolic rate of glucose. Taken together, because the OFC is a network hub, observed increase in OFC functional connectivity concomitant with reductions in OFC gray matter may suggest neuroadaptive plasticity.
The findings of greater functional connectivity in OFC network in marijuana users were echoed by increased structural connectivity (i.e., FA) of the forceps minor in marijuana users relative to controls. Greater FA has been suggested to reflect better myelination and/or intact axons (42). Based on RD and AD measurements, it appears that the FA difference between the groups in the forceps minor was driven by lower RD, suggesting greater myelination in the marijuana users. Although not as widely reported, greater white matter microstructure in marijuana users has also been reported by DeLisi et al. (43) in adolescent moderate marijuana users; however, the difference from controls did not reach significance. Greater FA has also been reported in alcohol users (44, 45), which was posited to reflect a premorbid vulnerability for accelerated PFC myelin maturation in those at risk for alcohol use disorders. Among possible explanations for these findings of greater FA in marijuana users include differential effects of cannabis depending on the specific fiber tract. Specifically, because the forceps minor connects the OFC, which is enriched with CB1, it is possible that there are unique neural adaptations to the forceps minor that are unlike other white matter tracts in the brain (e.g., corpus callosum). Others have also reported antiinflammatory properties of cannabis constituents such as cannabidiol (CBD). DTI is sensitive to increased tissue water resulting in decreased FA as a result of inflammation; therefore, it is possible that any antiinflammatory effects of cannabis would lead to greater FA. Lastly, it is also possible that the effects of cannabis (i.e., CBD) may be beneficial to white matter in terms of regulation of mitochondrial activity, antioxidant processes, and modulation of clearance processes that protect neurons on the molecular level (46). Future studies are needed to examine these specific effects on white matter.
Altogether, if these effects are indeed due to neurotoxic effects of cannabis, the inverse relationship between OFC structure and connectivity suggests that OFC gray matter (vs. white matter) is more vulnerable to the effects of THC. Endogenous cannabinoids play an important role in synaptic pruning (47), therefore, introduction of exogenous cannabinoids such as THC might disrupt this system by competing for the receptors and, thereby, inhibiting synaptic pruning particularly in receptor-enriched areas such as the OFC (48). In other words, any premorbid developmental trajectory may be modified by exposure to cannabis, resulting in accelerated OFC myelin maturation. However, although the majority of the animal literature and emergent human studies illustrate the down-regulation of CB1 receptors as a result of THC, we acknowledge that longitudinal studies are needed to address causality of these neural abnormalities (49–51).
Our findings of negative correlations between connectivity indexes and measures of marijuana use suggest a cumulative deleterious effect of marijuana on OFC connectivity. There was a relationship between functional connectivity and onset of use that suggested that greater functional connectivity was associated with earlier onset of regular use, whereas chronic marijuana use showed lower structural connectivity (i.e., FA). This dissociation demonstrates the complexity of marijuana’s effects on the brain, particularly on marijuana’s interaction with neurodevelopmental periods. Along with the important findings by Cheetham et al. (30) suggesting that lower OFC volume predates the onset of marijuana use, we suggest that greater functional connectivity observed at the onset of marijuana use that then dissipates with chronic use may be a form of neural scaffolding. This comprehensive pattern of neural response to marijuana is of particular importance in terms of treatment and even policy. Future studies should focus on the nuances of these complex interactions.
To date, treatment and prognosis of cannabis use disorders is hampered by the inconclusive underlying pathophysiology associated with marijuana use. In this study, we found that chronic exposure to marijuana (i) reduces OFC gray matter volume, (ii) increases structural and functional connectivity, and (iii) leads to neural alterations that are modulated by age of onset and duration of use. All in all, these findings suggest that chronic marijuana use results in complex neuroadaptive processes. Future studies are needed to determine whether these changes revert back to normal following protracted abstinence from marijuana use. Existing literature shows that cognitive alterations and CB1 receptor down-regulation in regular marijuana users may return to normal values due to neuroadaptive phenomena occurring after periods of abstinence (51–53). Although our study cannot address whether the structural alterations observed are permanent or reversible, such an investigation would provide important information as to the trajectory of these effects. Given the indication that a quadratic trend may fit the trajectory of these alterations, it would be important to verify these findings with a longitudinal approach.
Supplementary Material
Acknowledgments
We thank Tim McQueeny for assistance with the behavioral data. This work was supported by National Institute on Drug Abuse Grant K01 DA021632.
Footnotes
The authors declare no conflict of interest.
This article is a PNAS Direct Submission. C.C. is a guest editor invited by the Editorial Board.
This article contains supporting information online at www.pnas.org/lookup/suppl/doi:10.1073/pnas.1415297111/-/DCSupplemental.
References
- 1. SAMHSA (2012) Results from the 2011 National Survey on Drug Use and Health: National Findings (Subst Abuse Ment Health Serv Admin, Rockville, MD), NSDUH Series H-30, DHHS Publication No. SMA 06-4194.
- 2.Lorenzetti V, Lubman DI, Whittle S, Solowij N, Yücel M. Structural MRI findings in long-term cannabis users: What do we know? Subst Use Misuse. 2010;45(11):1787–1808. doi: 10.3109/10826084.2010.482443. [DOI] [PubMed] [Google Scholar]
- 3.Sneider JT, Gruber SA, Rogowska J, Silveri MM, Yurgelun-Todd DA. A preliminary study of functional brain activation among marijuana users during performance of a virtual water maze task. J Addict. 2013;2013:461029. doi: 10.1155/2013/461029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Cousijn J, et al. Effect of baseline cannabis use and working-memory network function on changes in cannabis use in heavy cannabis users: A prospective fMRI study. Hum Brain Mapp. 2013;35(5):2470–2482. doi: 10.1002/hbm.22342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Smith AM, Longo CA, Fried PA, Hogan MJ, Cameron I. Effects of marijuana on visuospatial working memory: An fMRI study in young adults. Psychopharmacology (Berl) 2010;210(3):429–438. doi: 10.1007/s00213-010-1841-8. [DOI] [PubMed] [Google Scholar]
- 6.van Hell HH, et al. Chronic effects of cannabis use on the human reward system: An fMRI study. Euro Neuropsychopharmacol. 2010;20(3):153–163. doi: 10.1016/j.euroneuro.2009.11.010. [DOI] [PubMed] [Google Scholar]
- 7.Batalla A, et al. Modulation of brain structure by catechol-O-methyltransferase Val(158) Met polymorphism in chronic cannabis users. Addict Biol. 2013;19(4):722–32. doi: 10.1111/adb.12027. [DOI] [PubMed] [Google Scholar]
- 8.Schacht JP, Hutchison KE, Filbey FM. Associations between cannabinoid receptor-1 (CNR1) variation and hippocampus and amygdala volumes in heavy cannabis users. Neuropsychopharmacology. 2012;37(11):2368–2376. doi: 10.1038/npp.2012.92. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Demirakca T, et al. Diminished gray matter in the hippocampus of cannabis users: Possible protective effects of cannabidiol. Drug Alcohol Depend. 2011;114(2-3):242–245. doi: 10.1016/j.drugalcdep.2010.09.020. [DOI] [PubMed] [Google Scholar]
- 10.Ashtari M, et al. Medial temporal structures and memory functions in adolescents with heavy cannabis use. J Psychiatr Res. 2011;45(8):1055–1066. doi: 10.1016/j.jpsychires.2011.01.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yücel M, et al. Regional brain abnormalities associated with long-term heavy cannabis use. Arch Gen Psychiatry. 2008;65(6):694–701. doi: 10.1001/archpsyc.65.6.694. [DOI] [PubMed] [Google Scholar]
- 12.Churchwell JC, Lopez-Larson M, Yurgelun-Todd DA. Altered frontal cortical volume and decision making in adolescent cannabis users. Front Psychol. 2010;1:225. doi: 10.3389/fpsyg.2010.00225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cousijn J, et al. Grey matter alterations associated with cannabis use: Results of a VBM study in heavy cannabis users and healthy controls. Neuroimage. 2012;59(4):3845–3851. doi: 10.1016/j.neuroimage.2011.09.046. [DOI] [PubMed] [Google Scholar]
- 14.McQueeny T, et al. Gender effects on amygdala morphometry in adolescent marijuana users. Behav Brain Res. 2011;224(1):128–134. doi: 10.1016/j.bbr.2011.05.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Gilman JM, et al. Cannabis use is quantitatively associated with nucleus accumbens and amygdala abnormalities in young adult recreational users. J Neurosci. 2014;34(16):5529–5538. doi: 10.1523/JNEUROSCI.4745-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Block RI, et al. Effects of frequent marijuana use on brain tissue volume and composition. Neuroreport. 2000;11(3):491–496. doi: 10.1097/00001756-200002280-00013. [DOI] [PubMed] [Google Scholar]
- 17.Tzilos GK, et al. Lack of hippocampal volume change in long-term heavy cannabis users. Am J Addict. 2005;14(1):64–72. doi: 10.1080/10550490590899862. [DOI] [PubMed] [Google Scholar]
- 18.Battistella G, et al. Long-term effects of cannabis on brain structure. Neuropsychopharmacology. 2014;39(9):2041–2048. doi: 10.1038/npp.2014.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsou K, Brown S, Sañudo-Peña MC, Mackie K, Walker JM. Immunohistochemical distribution of cannabinoid CB1 receptors in the rat central nervous system. Neuroscience. 1998;83(2):393–411. doi: 10.1016/s0306-4522(97)00436-3. [DOI] [PubMed] [Google Scholar]
- 20.van den Heuvel M, Mandl R, Luigjes J, Hulshoff Pol H. Microstructural organization of the cingulum tract and the level of default mode functional connectivity. J Neurosci. 2008;28(43):10844–10851. doi: 10.1523/JNEUROSCI.2964-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-state functional connectivity reflects structural connectivity in the default mode network. Cereb Cortex. 2009;19(1):72–78. doi: 10.1093/cercor/bhn059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Segall JM, et al. Correspondence between structure and function in the human brain at rest. Front Neuroinform. 2012;6:10. doi: 10.3389/fninf.2012.00010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Marijuana craving in the brain. Proc Natl Acad Sci USA. 2009;106(31):13016–13021. doi: 10.1073/pnas.0903863106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Filbey FM, Schacht JP, Myers US, Chavez RS, Hutchison KE. Individual and additive effects of the CNR1 and FAAH genes on brain response to marijuana cues. Neuropsychopharmacology. 2010;35(4):967–975. doi: 10.1038/npp.2009.200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Tung KC, et al. Alterations in resting functional connectivity due to recent motor task. Neuroimage. 2013;78:316–324. doi: 10.1016/j.neuroimage.2013.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wakana S, et al. Reproducibility of quantitative tractography methods applied to cerebral white matter. Neuroimage. 2007;36(3):630–644. doi: 10.1016/j.neuroimage.2007.02.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Stephens RS, Babor TF, Kadden R, Miller M. Marijuana Treatment Project Research Group The Marijuana Treatment Project: Rationale, design and participant characteristics. Addiction. 2002;97(Suppl 1):109–124. doi: 10.1046/j.1360-0443.97.s01.6.x. [DOI] [PubMed] [Google Scholar]
- 28.Cohen MX, Heller AS, Ranganath C. Functional connectivity with anterior cingulate and orbitofrontal cortices during decision-making. Brain Res Cogn Brain Res. 2005;23(1):61–70. doi: 10.1016/j.cogbrainres.2005.01.010. [DOI] [PubMed] [Google Scholar]
- 29.Cousijn J, Goudriaan AE, Wiers RW. Reaching out towards cannabis: Approach-bias in heavy cannabis users predicts changes in cannabis use. Addiction. 2011;106(9):1667–1674. doi: 10.1111/j.1360-0443.2011.03475.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cheetham A, et al. Orbitofrontal volumes in early adolescence predict initiation of cannabis use: A 4-year longitudinal and prospective study. Biol Psychiatry. 2012;71(8):684–692. doi: 10.1016/j.biopsych.2011.10.029. [DOI] [PubMed] [Google Scholar]
- 31.Cousijn J, et al. Cannabis dependence, cognitive control and attentional bias for cannabis words. Addict Behav. 2013;38(12):2825–2832. doi: 10.1016/j.addbeh.2013.08.011. [DOI] [PubMed] [Google Scholar]
- 32.Cousijn J, et al. Individual differences in decision making and reward processing predict changes in cannabis use: A prospective functional magnetic resonance imaging study. Addict Biol. 2013;18(6):1013–1023. doi: 10.1111/j.1369-1600.2012.00498.x. [DOI] [PubMed] [Google Scholar]
- 33.Kringelbach ML, O’Doherty J, Rolls ET, Andrews C. Activation of the human orbitofrontal cortex to a liquid food stimulus is correlated with its subjective pleasantness. Cereb Cortex. 2003;13(10):1064–1071. doi: 10.1093/cercor/13.10.1064. [DOI] [PubMed] [Google Scholar]
- 34.Bolla KI, et al. Orbitofrontal cortex dysfunction in abstinent cocaine abusers performing a decision-making task. Neuroimage. 2003;19(3):1085–1094. doi: 10.1016/s1053-8119(03)00113-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bechara A, Damasio H, Damasio AR. Emotion, decision making and the orbitofrontal cortex. Cereb Cortex. 2000;10(3):295–307. doi: 10.1093/cercor/10.3.295. [DOI] [PubMed] [Google Scholar]
- 36.Gremel CM, Luo G, Lovinger D, Costa R. 2013. Endocannabinoid signaling in orbitofrontal cortex modulates habit formation. Society for Neuroscience Conference (Society for Neuroscience, San Francisco)
- 37.Orr C, et al. Altered resting-state connectivity in adolescent cannabis users. Am J Drug Alcohol Abuse. 2013;39(6):372–381. doi: 10.3109/00952990.2013.848213. [DOI] [PubMed] [Google Scholar]
- 38.Filbey F, Yezhuvath U. Functional connectivity in inhibitory control networks and severity of cannabis use disorder. Am J Drug Alcohol Abuse. 2013;39(6):382–391. doi: 10.3109/00952990.2013.841710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Harding IH, et al. Functional connectivity in brain networks underlying cognitive control in chronic cannabis users. Neuropsychopharmacology. 2012;37(8):1923–1933. doi: 10.1038/npp.2012.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Zhang D, Raichle ME. Disease and the brain’s dark energy. Nat Rev Neurol. 2010;6(1):15–28. doi: 10.1038/nrneurol.2009.198. [DOI] [PubMed] [Google Scholar]
- 41.Tomasi D, Wang GJ, Volkow ND. Energetic cost of brain functional connectivity. Proc Natl Acad Sci USA. 2013;110(33):13642–13647. doi: 10.1073/pnas.1303346110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Song SK, et al. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 43.Delisi LE, et al. A preliminary DTI study showing no brain structural change associated with adolescent cannabis use. Harm Reduction Journal. 2006 doi: 10.1186/1477-7517-3-17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Cardenas VA, et al. Not lesser but Greater fractional anisotropy in adolescents with alcohol use disorders. NeuroImage Clinical. 2013;2:804–809. doi: 10.1016/j.nicl.2013.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.De Bellis MD, et al. Diffusion tensor measures of the corpus callosum in adolescents with adolescent onset alcohol use disorders. Alcohol Clin Exp Res. 2008;32(3):395–404. doi: 10.1111/j.1530-0277.2007.00603.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bilkei-Gorzo A. The endocannabinoid system in normal and pathological brain ageing. Philos Trans R Soc Lond B Biol Sci. 2012;367(1607):3326–3341. doi: 10.1098/rstb.2011.0388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Kim HJ, Waataja JJ, Thayer SA. Cannabinoids inhibit network-driven synapse loss between hippocampal neurons in culture. J Pharmacol Exp Ther. 2008;325(3):850–858. doi: 10.1124/jpet.107.131607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Bossong MG, Niesink RJ. Adolescent brain maturation, the endogenous cannabinoid system and the neurobiology of cannabis-induced schizophrenia. Prog Neurobiol. 2010;92(3):370–385. doi: 10.1016/j.pneurobio.2010.06.010. [DOI] [PubMed] [Google Scholar]
- 49.Sim-Selley LJ. Regulation of cannabinoid CB1 receptors in the central nervous system by chronic cannabinoids. Crit Rev Neurobiol. 2003;15(2):91–119. doi: 10.1615/critrevneurobiol.v15.i2.10. [DOI] [PubMed] [Google Scholar]
- 50.Burston JJ, Wiley JL, Craig AA, Selley DE, Sim-Selley LJ. Regional enhancement of cannabinoid CB₁ receptor desensitization in female adolescent rats following repeated Delta-tetrahydrocannabinol exposure. Br J Pharmacol. 2010;161(1):103–112. doi: 10.1111/j.1476-5381.2010.00870.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Hirvonen J, et al. Reversible and regionally selective downregulation of brain cannabinoid CB1 receptors in chronic daily cannabis smokers. Mol Psychiatry. 2012;17(6):642–649. doi: 10.1038/mp.2011.82. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Bosker WM, et al. Medicinal Δ(9) -tetrahydrocannabinol (dronabinol) impairs on-the-road driving performance of occasional and heavy cannabis users but is not detected in Standard Field Sobriety Tests. Addiction. 2012;107(10):1837–1844. doi: 10.1111/j.1360-0443.2012.03928.x. [DOI] [PubMed] [Google Scholar]
- 53.Hanson KL, et al. Longitudinal study of cognition among adolescent marijuana users over three weeks of abstinence. Addict Behav. 2010;35(11):970–976. doi: 10.1016/j.addbeh.2010.06.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Wechsler D. 1999. Manual for the Wechsler Abbreviated Scale of Intelligence (The Psychological Corporation, San Antonio)
- 55.First MB, Spitzer RL, Gibbon M, Williams JBW. 1997. User's Guide for the Structured Clinical Interview for DSM-IV Axis I Disorders (SCID) (American Psychiatric Press, Washington, DC)
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.