Multipotent mesenchymal stromal cells (MSCs) from C57BL/6 (H2b) mice were infused with fully major histocompatibility complex-mismatched Balb/c (H2d) allogeneic islets into the portal vein of diabetic C57BL/6 (H2b) mice, which were treated with costimulation blockade for 10 days. Their livers contained large numbers of islets surrounded by Foxp3+ regulatory T cells. MSC infusion and costimulation blockade have complementary immune-modulating effects that can be used for a broad number of applications.
Keywords: Mesenchymal stem cells, CTLA4Ig, Anti-CD40L, Costimulation blockade, Foxp3+ regulatory T cell, Rejection
Abstract
Multipotent mesenchymal stromal cell (MSC) therapy and costimulation blockade are two immunomodulatory strategies being developed concomitantly for the treatment of immunological diseases. Both of these strategies have the capacity to inhibit immune responses and induce regulatory T cells; however, their ability to synergize remains largely unexplored. In order to study this, MSCs from C57BL/6 (H2b) mice were infused together with fully major histocompatibility complex-mismatched Balb/c (H2d) allogeneic islets into the portal vein of diabetic C57BL/6 (H2b) mice, which were subsequently treated with costimulation blockade for the first 10 days after transplantation. Mice receiving both recipient-type MSCs, CTLA4Ig, and anti-CD40L demonstrated indefinite graft acceptance, just as did most of the recipients receiving MSCs and CTLA4Ig. Recipients of MSCs only rejected their grafts, and fewer than one half of the recipients treated with costimulation blockade alone achieved permanent engraftment. The livers of the recipients treated with MSCs plus costimulation blockade contained large numbers of islets surrounded by Foxp3+ regulatory T cells. These recipients showed reduced antidonor IgG levels and a glucose tolerance similar to that of naïve nondiabetic mice. Intrahepatic lymphocytes and splenocytes from these recipients displayed reduced proliferation and interferon-γ production when re-exposed to donor antigen. MSCs in the presence of costimulation blockade prevented dendritic cell maturation, inhibited T cell proliferation, increased Foxp3+ regulatory T cell numbers, and increased indoleamine 2,3-dioxygenase activity. These results indicate that MSC infusion and costimulation blockade have complementary immune-modulating effects that can be used for a broad number of applications in transplantation, autoimmunity, and regenerative medicine.
Introduction
Islet transplantation is a promising treatment for patients with insulin-dependent diabetes mellitus; however, lifelong immunosuppressive therapy is required to prevent rejection [1, 2]. Long-term administration of conventional immunosuppressive drugs can cause opportunistic infections, malignances, and drug toxicities, including hypertension, dyslipidemia, and diabetes mellitus [3]. To overcome this problem, cellular immunotherapy has been investigated as an alternative to conventional immunosuppressive drugs in transplantation [4, 5].
The successful use of mesenchymal stromal cells (MSCs) to treat steroid-resistant severe graft-versus-host disease (GVHD) was a milestone in regenerative medicine [6, 7]. Since those initial reports were published, multiple clinical trials have been initiated to test MSC treatment of GVHD, Crohn disease, ulcerative colitis, and multiple sclerosis. Currently, more than 400 registered clinical trials studying MSCs in different applications are ongoing [8]. Experimentally, MSCs are being studied in a plethora of immunological and degenerative disease models [9]. This broad applicability is subsequent partially to the ability of MSCs to modulate nearly every cellular component of the innate and adaptive immune system. This includes reducing inflammation and neutrophil activation [8], modulating macrophages toward an anti-inflammatory phenotype [9], inhibiting natural killer cell activation and cytotoxicity [10], modulating dendritic cell (DC) activation [11], and inhibiting CD4+ and CD8+ T cell responses [10, 12, 13]. This also includes the ability to convert conventional T cells into regulatory T cells [8, 9]. These capabilities have attracted a great amount of interest from the field of solid organ transplantation, and studies are being conducted to investigate MSC infusion in conjunction with organ transplantation in patients treated with standard immunosuppression [14].
Concurrently, costimulation blockade has emerged as a promising strategy for replacing immunity with long-term acceptance [15]. In a seminal article from 1996, it was demonstrated that by blocking B7 and CD40L with CTLA4Ig and anti-CD40L in the perioperative period, indefinite survival of vascularized allografts could be achieved [16]. Inhibition of costimulatory signals, while allowing for T cell receptor/major histocompatibility complex (MHC) interactions to remain intact, renders T cells anergic to donor antigen. In later studies, graft acceptance toward the transplants was shown to be in part due to the development of intragraft Foxp3+ regulatory T cells [17]. Later studies would show, however, that immune modulation by costimulation blockade was not sufficient in the most immunogenic transplant models [18]. Furthermore, heterologous immunity, the phenomenon of cross-reactivity of memory T cells to donor antigen, constitutes a major barrier in clinical settings [19].
Costimulation blockade and MSC treatment modulate many of the same components of the immune system and can induce the peripheral conversion of T cells into regulatory T cells [8, 19]. These two treatment strategies are being tested independently in clinical organ transplantation and in autoimmune diseases. Because these strategies share common goals and converge on some of the same target cells, it seems imperative to study their ability to synergise in downmodulating immune responses. In order to test this, we chose a robust model of transplant rejection using Balb/c insulin-producing islets injected through the portal vein into fully MHC-mismatched diabetic C57BL/6 mice. Recipient-type MSCs were injected with the Balb/c islets, and the recipients were treated with CTLA4Ig only or with CTLA4Ig and anti-CD40L every other day for the first 10 days. This combination of MSCs and costimulation blockade yielded superior islet graft survival and function in this highly immunogenic model.
Materials and Methods
Experimental Mice
Male Balb/c (H2d) and C57BL/6 (H2b) mice, aged 6–12 weeks (Taconic Biosciences, Ejby, Denmark, http://www.taconic.com), were used as donors and recipients, respectively. The mice were maintained under specific pathogen-free conditions and had free access to standard mice chow and water under a 12-hour light/dark cycle. The local Ethics Committee for Animal Research approved all animal experiments, which were performed in accordance with the local institutional and Swedish national rules and regulations.
Isolation, Culture, and Characterization of MSCs
C57BL/6 bone marrow (BM) cells were flushed from the femurs and tibias and cultured in mouse mesenchymal stem cell basal medium with supplements (Stemcell Technologies, Grenoble, France, http://www.stemcell.com). The cells were filtered through a 100-μm strainer and centrifuged at 1200 rpm for 5 minutes. After the viable cells were counted, the cells were plated in a 75-cm2 tissue culture flask in mouse mesenchymal stem cell basal media with supplements in a 5% CO2 humidified atmosphere at 37°C. Nonadherent cells were removed by rinsing and replacing the media after 48 hours, and the culture medium was replaced twice a week. Passages 3–10 of MSCs were used and phenotyped, and their ability to differentiate into adipocytes, osteocytes, and chondrocytes and to inhibit T cell activation was assessed (supplemental online Fig. 1) [20, 21].
Osteogenic and adipogenic differentiation was induced by culturing subconfluent cells in StemMACS OsteoDiff Media (Miltenyi Biotec GmbH, Bergisch Gladbach, Germany, http://www.miltenyibiotec.com) and Adipogenesis Differentiation Media (Life Technologies, Paisley, U.K., http://www.lifetechnologies.com), respectively. The media were changed every 2–3 days for 14 days. Subsequently, the cells were fixed in 4% formalin and stained with alizarin red (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) for osteogenesis and Oil Red O (Sigma-Aldrich) for adipogenesis according to the manufacturer’s protocols (supplemental online Fig. 1).
For differentiation toward chondrocytes, the cells were cultured in StemXVivo Chondrogenic Differentiation medium (R&D Systems, Abingdon, U.K., http://www.rndsystems.com) with StemXVivo Chondrogenic Differentiation Supplement (R&D Systems) in three-dimensional culture systems according to the manufacturer’s instructions. The media were changed every 3 days for 21 days; thereafter, the cell clusters were fixed in formalin and embedded in paraffin. After deparaffinization, the 5-μm sections were blocked with 5% rabbit serum (Life Technologies) in phosphate-buffered saline containing 0.2% Triton X-100. The monoclonal anti-aggrecan antibody (clone AF-28, Merck Millipore, Darmstadt, Germany, http://www.merckmillipore.com) was applied at 1:100 dilution, and the slides were incubated overnight. The secondary antibody, rabbit anti-mouse IgG conjugated with Alexa Fluor 488 (Life Technologies), was applied at 1:600 dilution, and the slides were incubated for 2 hours followed by washing and mounting with Vectashield (Vector Laboratories, Peterborough, U.K., http://www.vectorlabs.com) containing 4′6-diamidino-2-phenylindole (DAPI; Pierce, Rockford, IL, http://www.piercenet.com) (supplemental online Fig. 1). As a negative control, a proportion of the cells were kept in culture in mouse mesenchymal stem cell basal medium with supplements as described. Then, the cells were fixed in 4% formalin and stained with hematoxylin (supplemental online Fig. 1).
Isolation, Culture, and Phenotypic Characterization of DCs
C57BL/6 immature DCs were isolated according to methods previously described [22]. MSCs were added with 500 ng/ml lipopolysaccharide (LPS; L2654, Escherichia coli 026:B6, Sigma-Aldrich) to examine the inhibitory effect on DC maturation. CD11c+ DCs were separated from BM cells using CD11c MicroBeads (Miltenyi Biotec GmbH) according to the manufacturer’s instructions. The cell phenotype was analyzed using a FACScan flow cytometer (Becton, Dickinson and Company, Franklin Lakes, NJ, http://www.bd.com) using the CellQuest software (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com). DCs were stained with monoclonal antibodies (mAbs) against CD11c, CD80, CD86, MHC class II, and isotype-matched controls (eBioscience, Hatfield, U.K., http://www.ebioscience.com). The data are presented as the mean fluorescence intensity.
Islet Isolation and Transplantation
Balb/c islets were isolated by collagenase P (Roche Diagnostics GmbH, Mannheim, Germany, http://lifescience.roche.com), as described previously [23]. C57BL/6 recipient mice were rendered diabetic by intravenous injection of alloxan (75 mg/kg) (Sigma-Aldrich) 3 days before transplantation and transplanted with 250 Balb/c islet equivalents, either alone or together with C57BL/6 MSCs (2.5 × 105 cells per mouse) via the portal vein. The mice were treated with either CTLA4Ig and anti-CD40L (clone MR1) or CTLA4Ig only every other day until postoperative day 10 at doses of 0.5 mg on day 0 and 0.25 mg on days 2, 4, 6, 8, and 10 or with isotype control antibodies (human IgG and hamster IgG) at similar doses.
All antibodies and fusion proteins were purchased from BioXCell (West Lebanon, NH, http://www.bxcell.com). Nonfasting blood glucose levels of less than 11.1 mmol/l were considered to indicate a reversal of diabetes, and islet rejection was defined as >20 mmol/l nonfasting blood glucose for 2 consecutive days. The mice were followed up for 30 days or 100 days. Diabetic recipients were observed and treated with s.c. injection of insulin (Actrapid, Novo Nordisk, up to 2 U per day; this dose maintains the animal’s condition but does not affect the next day’s blood glucose level). All the mice were subjected to an intraperitoneal glucose tolerance test (IPGTT) 1 month after transplantation, and nontransplanted, age-matched male C57BL/6 mice served as controls. During the IPGTT, care was taken to minimize the stress of the mice, and they had free access to water.
Flow Cytometry
Donor-specific antibodies in mouse serum were detected by flow cytometry. Mouse serum was collected from naïve and transplanted recipients at euthanasia and kept frozen at −20°C until use. A total of 10 μl of mouse serum and 100 μl of T cells isolated from Balb/c splenocytes (1 × 106 cells per test) were incubated at 4°C for 30 minutes, labeled with fluorescein isothiocyanate (FITC)-conjugated goat anti-mouse IgG (1:100 diluted, Sigma-Aldrich) and analyzed by flow cytometry.
Immunohistochemistry
Livers from recipients surviving to 100 days after transplantation were snap-frozen, cut into 5-μm-thick sections, and fixed in 4% formaldehyde. For Foxp3 staining, 10% donkey serum (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, http://www.jacksonimmuno.com) was used as a blocking agent; for CD4 and CD8 staining, 5% donkey serum and 5% mouse serum was used (Dako, Glostrup, Denmark, http://www.dako.com). The sections were incubated with rat anti-mouse Foxp3 (eBioscience) or rat anti-mouse CD4 or CD8 (AbD Serotec, Oslo, Norway, http://www.abdserotec.com) overnight. Donkey anti-rat IgG (Alexa Fluor, Life Technologies) was added, and the slides were incubated for 1 hour and mounted with the antifading reagent containing DAPI. For insulin staining, the sections were incubated with rhodamine-conjugated anti-insulin antibody (Mabtech AB, Nacka, Sweden, http://www.mabtech.com) for 1 hour, followed by staining with DAPI.
Enzyme-Linked Immunospot Assay
To examine the graft-specific T cell activity, splenocytes and intrahepatic lymphocytes (IHLs) were isolated from the recipient livers and used as responder cells. Splenocytes (1 × 105 per well) or IHLs (1 × 104 per well) were seeded in triplicate with irradiated Balb/c splenocytes (4 × 105 per well) for 18 hours. Pretreated 96-well plates and the anti-rat interferon-γ (IFNγ) enzyme-linked immunospot (ELIspot) kit were gifts from Mabtech AB. The calculation of the number of spot forming units and cytokine activity were determined by the ELIspot counter software, version 3.5 (AID, Strasburg, Germany) and signifies a relative quantification of cytokine levels. The cytokine activities were calculated from spot numbers, intensities, and area size of the spots and analyzed by the ELIspot counter software, version 3.5 (AID).
Expression of Indoleamine 2,3-Dioxygenase, Transforming Growth Factor-β, Ins2, and Foxp3 Transcripts
Total RNA was isolated from recipient livers, as previously described [23]. Real-time polymerase chain reaction (PCR) was performed with 2× FAST SYBR Green Master Mix (Life Technologies) in triplicate using 7500 Fast Real-Time PCR System (Applied Biosystems, Foster City, CA, http://www.appliedbiosystems.com). Primer sequences (Life Technologies) and fragment sizes of the genes are shown in supplemental online Table 1. The level of sample mRNA was normalized by glyceraldehyde-3-phosphate dehydrogenase as an internal control.
Mixed Lymphocyte Reaction
Splenocytes isolated from recipients of each treatment group or naïve C57BL/6 and irradiated splenocytes prepared from naïve Balb/c mice were used as responders (2 ×105 per well) and stimulator cells (4 ×105 per well), respectively. After 3 days of culture in 96-well round-bottom plates (BD Biosciences) in a total volume of 0.2 ml Roswell Park Memorial Institute 1640 supplemented with 2 mmol/l glutamine, 100 U/ml penicillin, 100 IU/ml streptomycin, 5 × 10−5 M β-mercaptoethanol, and 10% (vol/vol) fetal bovine serum, the cells were pulsed with 1 µCi [3H] thymidine (Perkin Elmer, Waltham MA, http://perkinelmer.com) for an additional 18 hours and counted using a MicroBeta TriLux β-counter (Perkin Elmer). The results are expressed as a percentage of the sample group response minus the syngeneic response divided by the naïve allogeneic response minus the syngeneic response.
CD4+ T cells (1 × 105 per well) from naïve C57BL/6 spleen and irradiated pan-T cells (2 × 105 per well) from naïve Balb/c spleens were used as the responder and stimulator cells, respectively. C57BL/6-derived mature DC (mDC) or MSC-cocultured DCs (MSC-DCs) were used as antigen presenting cells (2 × 104 per well). In some experiments, MSCs (2 × 104 per well) were seeded onto the wells with mDC (mDC plus MSCs). After 3 days of culture, the cells were pulsed with 1 µCi of [3H] thymidine (Perkin Elmer) for 18 hours. The results are expressed as the mean cpm after subtraction of the syngeneic response. The results are also expressed as a percentage of the sample group allogeneic response minus the syngeneic response divided by the allogeneic response minus the syngeneic response in the mDC group.
Analysis of Regulatory T Cells by Flow Cytometry
Splenocytes from recipients were harvested on postoperative day 100 and labeled for surface CD4-FITC and CD25-phycoerythrin (PE), and intracellular Foxp3-PerCP (eBioscience). Naïve C57BL/6 CD4+ T cells (1 × 106 per milliliter) and C57BL/6-derived CD11c+ DCs (2 × 105 per milliliter) were cocultured with Balb/c pan-T cells (2 × 106 per milliliter) with or without MSCs (MSC-DCs) (2 × 105 per milliliter) at a ratio of 5:1:1 (CD4+ T/DC/MSC). In some experiments, MSCs (2 × 104 per well) were added to the wells with mDC (mDC plus MSCs). After 96 hours of incubation, CD4+ T cells were purified again and labeled for CD4, CD25, and Foxp3. L-kynurenine concentrations from the supernatants were determined using enzyme-linked immunosorbent assay (MyBioSource Inc., San Diego, CA, http://www.mybiosource.com).
Statistical Analysis
Data are reported as the mean ± SD. Allograft survival data were evaluated using the Kaplan-Meier method and compared using the log-rank test. All other data comparisons were analyzed using one-way analysis of variance followed by the Tukey-Kramer post hoc test. A value of p < .05 was considered significant.
Results
Islet Transplant Survival
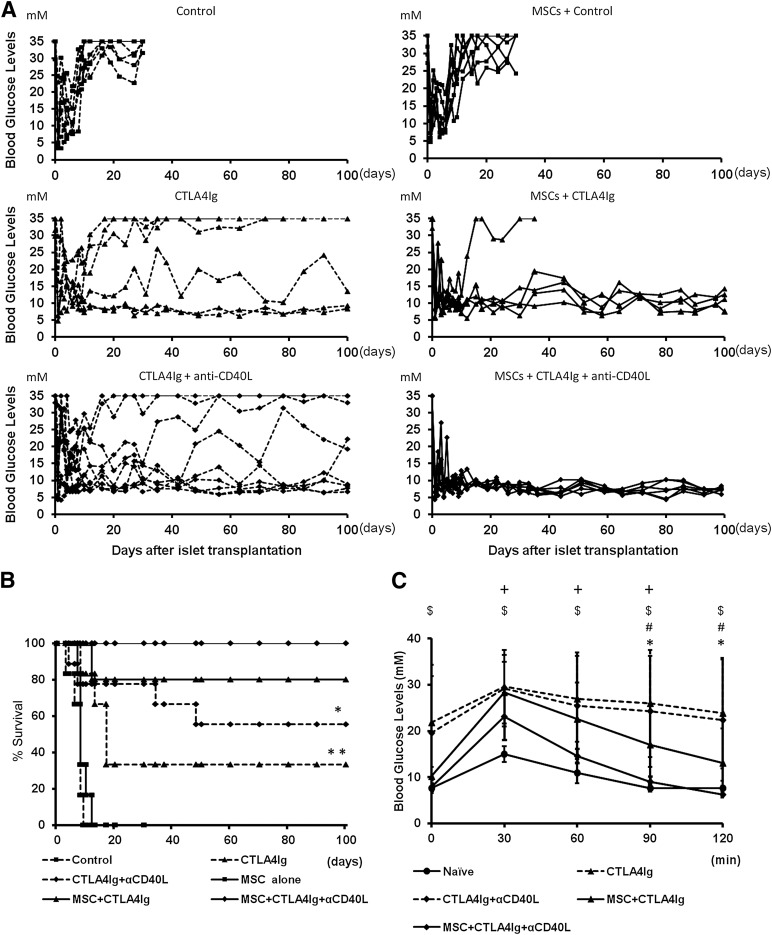
Mice receiving islet cell transplants and treated with isotype control antibodies exhibited a mean islet graft survival time (MST) of 7 days, demonstrating the robust immune response to fully MHC-mismatched islets after intraportal injection. Cotransplantation with recipient-type MSCs yielded a MST of 9 days (Table 1; Fig. 1A). In the group treated with CTLA4Ig only, 2 of 6 mice were normoglycemic for more than 100 days (MST 45.8 days). In the group treated with CTLA4Ig and anti-CD40L, 5 of 9 recipients had long-term graft survival of 100 days (MST 65.9 days). When treated with MSCs and CTLA4Ig, the MST was 82 days, with 4 of 5 surviving to 100 days. MSC cotransplantation prolonged islet graft survival in all recipients when they also were treated with costimulation blockade in the form of CTLA4Ig and anti-CD40L (MST ≥100 days). This increase in graft survival was significant compared with all other treatment groups (p < .05; Fig. 1B). In order to test graft function, IPGTTs were performed 1 month after transplantation. Recipients treated with MSCs, CTLA4Ig, and anti-CD40L had significantly lower blood glucose levels at 90 minutes than did groups receiving costimulation blockade only (p < .05; Fig. 1C). The blood glucose levels in the mice treated with MSCs plus CTLA4Ig and anti-CD40L were equivalent to the levels in nondiabetic nontransplanted mice at 90 minutes after glucose administration.
Table 1.
Experimental groups and islet graft survival
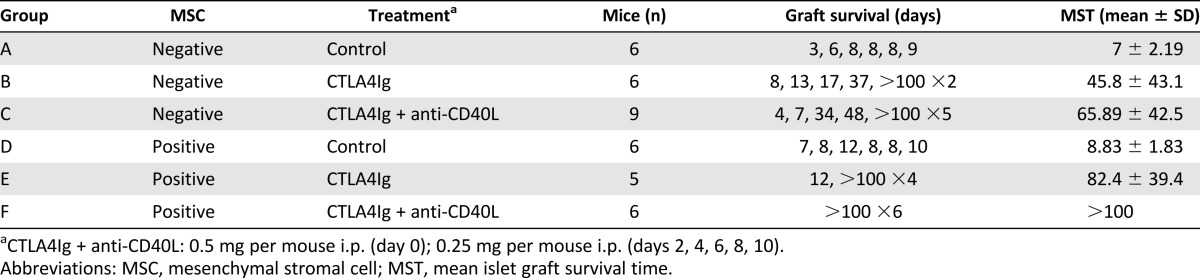
Figure 1.
Islet allograft survival and function. (A): Eight- to 12-week-old diabetic C57BL/6 mice received 250 islets from fully major histocompatibility complex-mismatched Balb/c donors by portal vein injection. Recipients were randomized to cotransplantation with 2.5 × 105 MSCs originating from recipient bone marrow and treatment with CTLA4Ig only or CTLA4Ig and anti-CD40L or isotype control antibodies. Graft survival was followed by studying the fasting blood glucose levels. Blood glucose levels higher than 20 mM on more than 2 consecutive days was considered rejection. (B): Percentage of graft survival. Differences between groups were tested using log-rank statistics. ∗, p < .05: MSC + CTLA4Ig + anti-CD40L vs. CTLA4Ig + anti-CD40L; ∗∗, p < .05: MSC + CTLA4Ig + anti-CD40L vs. CTLA4Ig. (C): Intraperitoneal glucose tolerance test was performed 4 weeks after transplantation. Nontransplanted age-matched C57BL/6 naïve mice were tested simultaneously. Data are shown as mean ± SD. ∗, p < .05: MSC + CTLA4Ig + anti-CD40L vs. CTLA4Ig + anti-CD40L; #, p < .05: MSC + CTLA4Ig + anti-CD40L vs. CTLA4Ig; $, p < .05: naïve vs. CTLA4Ig; +, p < .05: naïve vs. CTLA4Ig + anti-CD40L. Statistical analysis was performed by analysis of variance with the Tukey-Kramer post hoc test, n = 5–9. Abbreviations: αCD40L, anti-CD40L; MSC, mesenchymal stromal cell.
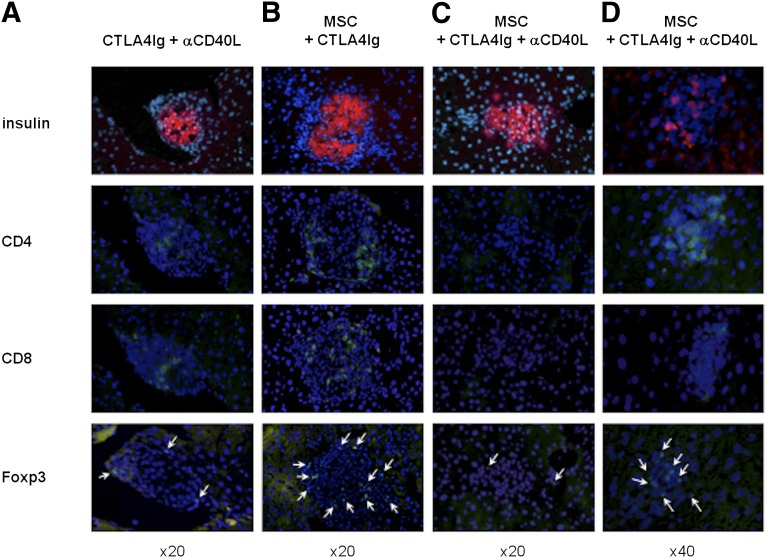
Insulin-producing islets could be found in all the livers of the different recipient groups with normal glucose levels at 100 days after transplantation (Fig. 2). Recipients treated with CTLA4Ig and anti-CD40L, having survived to 100 days after transplantation, had infiltrating CD4+ and CD8+ lymphocytes in the liver, but only a few stained positively for Foxp3 (Fig. 2A). Mice treated with MSCs and CTLA4Ig displayed a similar infiltration of CD4+ and CD8+ T cells, with some cells staining positively for Foxp3 (Fig. 2B). In the group receiving MSCs, CTLA4Ig, and anti-CD40L treatment, little or no CD8 staining could be found and the lymphocytic infiltrate around the islets stained mostly for CD4 and Foxp3 (Fig. 2C, 2D).
Figure 2.
Islet histological findings. Histological evaluation of serial sections of islet grafts in the liver on postoperative day 100. Immunofluorescent staining for insulin (red), Foxp3, CD4, and CD8 (green) was performed on recipient livers treated with (A): CTLA4Ig plus anti-CD40L, (B): MSCs plus CTLA4Ig, and (C, D): MSCs plus CTLA4Ig plus anti-CD40L. 4′6-Diamidino-2-phenylindole (blue) was used for nuclear staining. Foxp3 stained cells are indicated by arrows. Original magnification, ×20 (A–C), ×40 (D). Abbreviations: αCD40L, anti-CD40L; MSC, mesenchymal stromal cell.
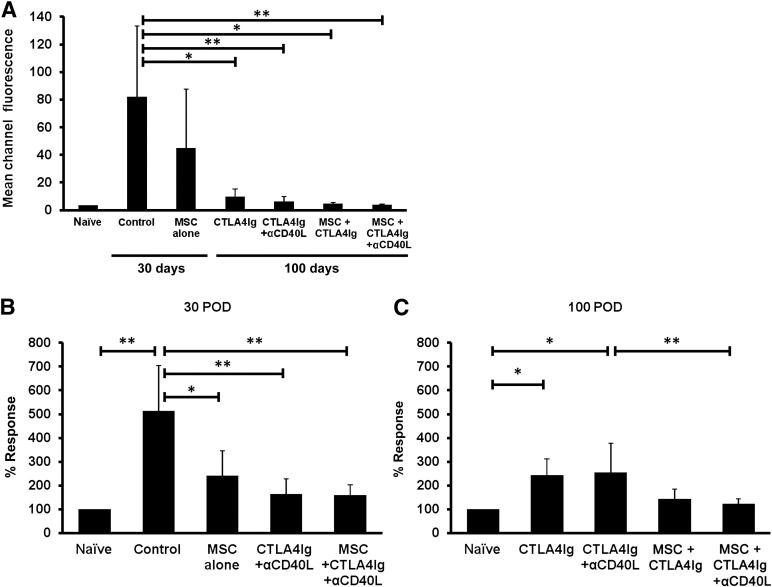
In order to study the presence of antidonor antibody responses, Balb/c pan-T cells were incubated with recipient serum and then stained with anti-mouse anti-IgG. The costimulation blockade-treated recipients and recipients treated with MSCs plus costimulation blockade had significantly lower levels of antidonor antibodies compared with the control (Fig. 3A). The mean channel fluorescence was equivalent to that found in naïve mice, indicating that no substantial donor antigen-specific B cell activation had occurred in these recipients. The recipients treated with MSCs plus CTLA4Ig and anti-CD40L showed a tendency to reduced staining of Balb/c cells compared with the group treated with costimulation blockade alone; however, this difference did not reach statistical significance (Fig. 3A).
Figure 3.
Presence of donor specific antibodies (DSAs) and suppression of T-cell alloreactive response. (A): DSAs in mouse serum were examined against Balb/c T cells by flow cytometry. The mean channel fluorescence (MCF) of each group was compared. The control group showed significantly higher MCF compared with the costimulation treated group with and without MSC coinjection. Coinjection of recipient MSCs did not significantly reduce MCF. ∗, p < .05; ∗∗, p < .01. (B, C): Suppression of T-cell proliferative response in recipients treated with MSC and costimulation blockade. Splenocytes (2 × 105) from C57BL/6 recipients were harvested and cocultured with Balb/c splenocytes (4 × 105) in 96-well round-bottom plates for 4 days and the proliferation was measured by [3H] thymidine incorporation. Results are shown as a percentage of the response calculated from the mean cpm of the allogeneic reaction after subtraction of the mean syngeneic response in each group divided by that of naïve mice in each experiment at postoperative (B): 30 days or (C): 100 days. ∗, p < .05; ∗∗, p < .01. Splenocyte proliferation in the group treated with MSCs, CTLA4Ig, and anti-CD40L was significantly reduced compared with the group treated with CTLA4Ig and anti-CD40L only, indicating complementary immune inhibition when combining MSCs with CTLA4Ig and anti-CD40L. Abbreviations: αCD40L, anti-CD40L; MSC, mesenchymal stromal cell; POD, postoperative day.
T Cell Responses and Increase in CD4+CD25+Foxp3+ T Cells
In order to study T-cell reactivity in recipients, splenocytes were isolated and exposed to donor antigen, and the proliferation was measured. Splenocytes from mice receiving CTLA4Ig and anti-CD40L with or without MSCs (159.9% ± 43.8% or 164.1% ± 63.2%) had significantly reduced alloreactive responses compared with the control mice (513.5% ± 190%) at 30 days (Fig. 3B). This effect was observed even if the recipients had been treated with MSCs alone (240.7% ± 105.8%, p < .05). At 100 days, the CTLA4Ig and anti-CD40L treated recipients induced significantly more alloreactive responses than did the naïve mice (CTLA4Ig alone: 242.6% ± 70.3%, CTLA4Ig plus anti-CD40L mAb: 253.3% ± 124.5%, p < .05). Groups treated with MSCs plus costimulation blockade showed reduced alloreactive responses and no significant differences were found compared with naïve mice (Fig. 3C). Moreover, recipients treated with MSCs combined with CTLA4Ig and anti-CD40L (122.3% ± 22.8%) showed a significantly reduced alloreactive T cell proliferation compared with the CTLA4Ig and anti-CD40L treated recipients (p < .01; Fig. 3C).
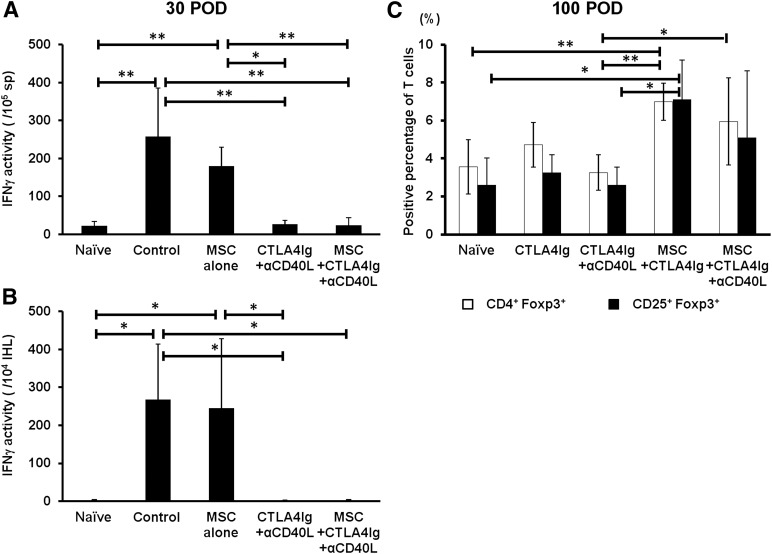
In order to further characterize the recall immune responses, the IFNγ spot number and activities were measured at donor antigen exposure at 30 and 100 days after transplantation. Splenocytes from recipients treated with CTLA4Ig and anti-CD40L with or without MSCs (24.3 ± 19.7 or 26.8 ± 9.8 activities per 105 cells, respectively) showed significantly lower production of IFNγ compared with isotype control-treated recipients or recipients treated with MSCs only (257.6 ± 128.2 or 180.0 ± 49.7 activities per 105 cells, respectively; p < .01 or p < .05; Fig. 4A). The activity of IFNγ-producing IHLs from the recipients treated with CTLA4Ig and anti-CD40L with or without MSCs (1.5 ± 2.6 or 0.6 ± 3.1 activities per 104 cells) was significantly lower than that of the control (268.8 ± 144.8 activities per 104 cells, p < .05) and MSC treated recipients (249 ± 176 activities per 104 cells; Fig. 4B). The frequency of CD4+CD25+Foxp3+ T cells in the spleen was evaluated at 100 days after transplantation in the different treatment groups. Recipients treated with MSCs, CTLA4Ig, and anti-CD40L (6.0% ± 2.3%) and MSCs and CTLA4Ig only (7.0% ± 1.0%) had significantly more CD4+CD25+Foxp3+ T cells than did mice that had received only CTLA4Ig and anti-CD40L (3.3% ± 0.9%, p < .05 or p < .01; Fig. 4C).
Figure 4.
Recipient T cell responses to donor antigen and quantification of CD4+CD25+Foxp3+ T cells in spleen. (A): Splenocytes (1 × 105) or (B): intrahepatic lymphocytes (1 × 104) from C57BL/6 recipients were cocultured with Balb/c splenocytes (4 × 105) in capture antibody-mounted 96-well plates for 20 hours and spot forming units and IFNγ activity were measured. Results are shown as mean ± SD. ∗, p < .05; ∗∗, p < .01. (C): Splenocytes from C57BL/6 recipients were harvested at POD 100 and fluorescence-activated cell sorting analysis was performed for the detection of CD4+CD25+Foxp3+ T cells. The percentage of CD4+Foxp3+ or CD25+Foxp3+ T cells is shown as mean ± SD. ∗, p < .05; ∗∗, p < .01. Abbreviations: αCD40L, anti-CD40L; IFNγ, interferon-γ; IHL, intrahepatic lymphocyte; MSC, mesenchymal stromal cell; POD, postoperative day; sp, spleen.
Intrahepatic Expression of Indoleamine 2,3-Dioxygenase, Transforming Growth Factor-β, Insulin, and Foxp3
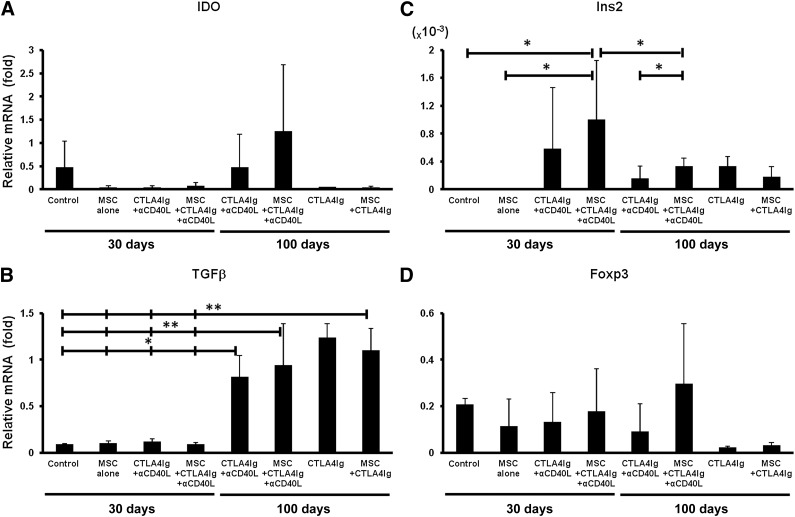
MSCs and tolerogenic DCs both use indoleamine 2,3-dioxygenase (IDO) to degrade tryptophan as an immune modulating pathway in the prevention of T cell activation. In order to study whether this pathway was activated in the transplanted recipients, the mRNA levels of IDO in the livers were quantified. The IDO mRNA levels in the islet grafted livers of mice treated with MSCs, CTLA4Ig, and anti-CD40L (1.25 ± 1.43) appeared to be higher than that of mice receiving CTLA4Ig and anti-CD40L without MSCs (0.48 ± 0.72) at 100 days after transplantation, but the difference was not statistically significant (Fig. 5A). IDO mRNA expression in the other groups was negligible. Transforming growth factor-β (TGFβ) mRNA expression levels were significantly upregulated in all long-term functioning graft recipients compared with the levels at 30 days after transplantation in all groups (Fig. 5B).
Figure 5.
Real-time polymerase chain reaction analysis of four genes from islet-transplanted livers. (A–D): Total RNA was isolated from the livers in all islet transplanted recipients at the time of sacrifice. The expression of each gene transcript was normalized to the expression level of the house keeping gene, glyceraldehyde-3-phosphate dehydrogenase, and a relative value is given. Results are shown as mean ± SD. ∗, p < .05; ∗∗, p < .01. Abbreviations: αCD40L, anti-CD40L; IDO, indoleamine 2,3-dioxygenase; MSC, mesenchymal stromal cell; POD, postoperative day.
In order to estimate the functional islet-graft mass, the Ins2 mRNA levels in the livers were measured. Only when CTLA4Ig and anti-CD40L were combined with MSCs (1.0 ± 0.85 × 10−3) did recipients maintain significantly higher Ins2 levels than those receiving control antibodies (0.0002 ± 3.79E−5 × 10−3) or MSCs alone (0.0002 ± 0.0002 × 10−3, p < .05). In the group treated with MSCs plus CTLA4Ig and anti-CD40L, Ins2 mRNA expression decreased gradually but was, at 100 days, significantly higher than in the group treated with CTLA4Ig and anti-CD40L (0.33 ± 0.11 × 10−3 vs. 0.16 ± 0.17 × 10−3, p < .05; Fig. 5C). The combination of MSCs, CTLA4Ig, and anti-CD40L (0.3 ± 0.26) seemed to upregulate Foxp3 transcription levels more than did CTLA4Ig and anti-CD40L (0.09 ± 0.12); however, statistical significance was not reached (Fig. 5D).
MSCs Attenuate DC Maturation
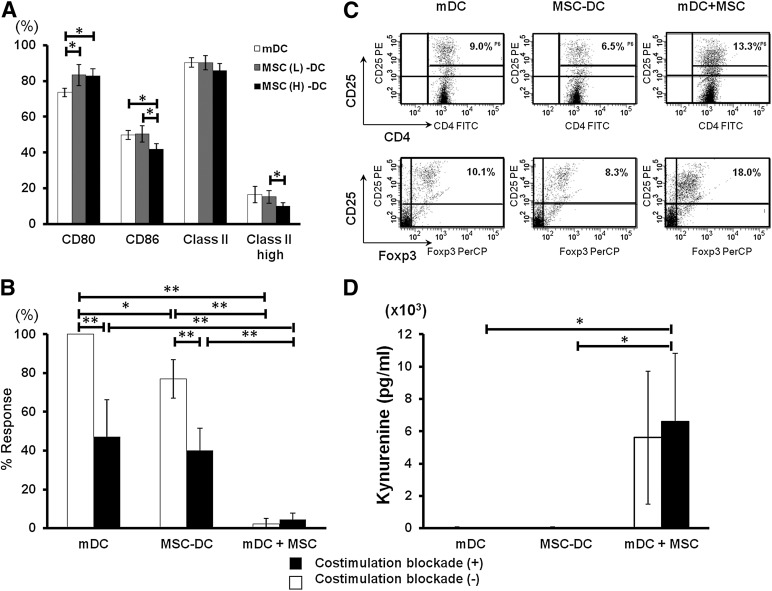
To determine the potential mechanisms underlying the immune modulating function of MSCs on DCs, the direct effect of MSCs on DC maturation was examined. Flow cytometric analysis revealed that CD86 and MHC class II high expression were significantly (p < .05) downregulated in a dose-dependent fashion on DCs cocultured with MSCs (MSC-DCs) compared with mature DCs (mDCs) (Fig. 6A). In contrast, CD80 expression by MSC-DCs was significantly upregulated compared with mDCs, suggesting that DC maturation was altered by MSCs.
Figure 6.
Suppression of dendritic cell (DC) maturation and alloreactive T cell proliferation or Foxp3 T cell induction by MSCs in vitro. (A): After a 24-hour stimulation with lipopolysaccharide, bone marrow-DCs with or without MSCs were isolated using anti-CD11c+ microbeads, and their surface markers were analyzed by fluorescence-activated cell sorting (FACS). The results are representative of four independent experiments. The percentage of CD11c+ cells expressing high levels of major histocompatibility complex class II decreased significantly when the amount of MSCs increased in the coculture. ∗, p < .05. (B): C57BL/6 CD4+ T cells (1 × 105) were cocultured with syngeneic DCs (2 × 104) and allogeneic Balb/c pan T cells (2 × 105) in the presence (black bar) or absence (white bar) of costimulation blockade for 4 days. The results are expressed as the mean cpm of relative increase after which syngeneic response was subtracted from and shown as mean ± SD of six independent experiments. ∗, p < .05; ∗∗, p < .01. (C): C57BL/6 CD4+ T cells (2 × 106) were cocultured with syngeneic DCs (4 × 105) and Balb/c pan T cells (4 × 106) in 12-well plates for 4 days. Nonadherent cells were harvested, followed by CD4+ isolation, and stained with CD4, CD25, and Foxp3 for FACS analysis. Results are one of the representatives of three independent experiments. (D): Supernatants in the presence (black bar) or absence (white bar) of costimulation blockade after 4 days of culture described in (C) were collected and the kynurenine concentrations were analyzed by enzyme-linked immunosorbent assay. Results are expressed as mean ± SD of three independent experiments. ∗, p < .05. Abbreviations: FITC, fluorescein isothiocyanate; mDC, mature dendritic cell; MSC, mesenchymal stromal cell; MSC (L)-DC, low dose (1 × 104; gray bar) mesenchymal stromal cell-treated dendritic cell; MSC (H)-DC, high dose (1 × 105; black bar) mesenchymal stromal cell-treated dendritic cell.
To assess DC function, mixed lymphocyte reactions (MLRs) were performed with naïve C57BL/6 CD4+ T cells and naïve Balb/c pan T cells in the presence of mature dendritic cells (mDCs) or MSC-DCs. T cell proliferation was significantly suppressed when mDCs were cocultured with MSCs (142.6 ± 176.1 cpm, 2.26% ± 2.87%) compared with mDCs (6960.7 ± 2276.9 cpm, 100%) or MSC-DCs (4613.1 ± 1268.1 cpm, 80.0% ± 9.9%; p < .01; Fig. 6B). The addition of CTLA4Ig and anti-CD40L significantly reduced the proliferation of T cells when added to mDCs (3236.2 ± 1545.7 cpm, 47.17% ± 19.18%) or MSC-DCs (2355.5 ± 683.2 cpm, 40.24% ± 11.37%). When mDCs were cultured with MSCs, T cell proliferation was completely abrogated, independently of the presence of costimulation blockade (269.8 ± 193.1 cpm, 4.45% ± 3.28%).
In addition, increased numbers of CD4+CD25+Foxp3+ T cells were observed in MLRs with CD4+ T cells cocultured with mDC and MSCs than in MLRs cocultured with mDCs or MSC-DCs, but the difference was not statistically significant (Fig. 6C). IDO activity in MLRs was also examined by quantifying the kynurenine levels in culture supernatant. The kynurenine levels were significantly higher in mDCs cultured with MSCs (6580 ± 4234 pg/ml) than in mDCs (17.3 ± 30.0 pg/ml) or MSC-DCs alone (19.9 ± 27.9 pg/ml) in the presence of CTLA4Ig and anti-CD40L (p < .05), suggesting a synergistic effect of combining costimulation blockade with MSC coculture at the level of IDO activity (Fig. 6D).
Discussion
This study demonstrates that by combining recipient-type MSC infusion with CTLA4Ig and anti-CD40L, a complementary and potentiated immunomodulatory effect can be obtained in a stringent model of immune reactivity. The addition of MSCs to conventional costimulation blockade led to normoglycemia and indefinite graft survival in all recipients. When graft function was tested with IPGTT, these recipients demonstrated better glucose control and had blood glucose levels similar to those of nondiabetic, nontransplanted mice at 90 minutes. Histological analysis of these recipients showed low numbers of infiltrating lymphocytes. The cells that were present were CD4+ and expressed Foxp3. No CD8+ infiltrates were found in the livers of these recipients. The antidonor IgG levels were lower in these mice than in the recipients receiving only costimulation blockade, indicating an increased inhibition of B cell responses. When cocultured with donor antigen, splenocytes from these recipients demonstrated reduced proliferation at both 30 and 100 days, implying that the inhibited T cell response was maintained in the long term. When MSCs were combined with CTLA4Ig and anti-CD40L, the proliferation was equivalent to that seen in naïve T cell responses and significantly reduced compared with CTLA4Ig and anti-CD40L treatment only, indicating an additive or synergistic effect of combining MSCs with CTLA4Ig and anti-CD40L. Splenocytes and IHLs from these donors expressed low levels of IFNγ when exposed to donor antigen, indicating an attenuated response by the lymphocytes. Flow cytometry of the splenocytes demonstrated a significant increase in the number of CD4+Foxp3+ and CD25+Foxp3+ lymphocytes, indicating that the number of regulatory T cells was increased in the recipients that received costimulation blockade and MSCs. MSCs as monotherapy had a negligible effect on graft survival. CTLA4Ig combined with anti-CD40L induced long-term normoglycemia in 5 of 9 recipients, but many had unstable glucose control. The combination of CTLA4Ig with MSCs yielded long-term graft survival in most recipients, along with the presence of Foxp3+ T cells around the islet grafts, decreased IFNγ on re-exposure to donor antigen, and elevated numbers of regulatory T cells in the spleen. Expression of TGFβ and insulin was significantly upregulated in the recipient liver.
In order to study the effect of our recipient-type MSCs on DCs, the cells were cocultured, and the expression of costimulatory molecules and MHC class II was studied. Exposure to high numbers of MSCs reduced the expression of CD86 and increased the levels of CD80, indicating that the DCs can be induced to differentiate toward a more immature or tolerogenic phenotype [22]. When MSCs were cultured with mature DCs, T cell proliferation was almost completely abrogated. The addition of costimulation blockade reduced T cell proliferation when exposed to DCs cocultured with MSCs, indicating an additional effect with this combination. When examining the supernatants of these MLRs, an increased level of kynurenine was found, implying substantial IDO activity and potential immune modulation by the MSCs and DCs.
Earlier studies have shown that MSCs transfected with vectors expressing CTLA4Ig could reduce immune responses in a model of autoimmune thyroiditis, with reduced lymphocytic infiltrates and decreased antibody responses toward thyroid antigen [24]. However, the presence of Foxp3+ T cells was not investigated. More recently, a similar study using allogeneic MSCs transduced with an adenoviral vector carrying the CTLA4Ig construct was shown to ameliorate collagen induced arthritis in mice [25]. However, studies to explore the effect on regulatory T cells were not performed and only cytokine profiles were investigated. MSCs have been shown to improve the outcomes in syngeneic islet transplants in rats by reducing inflammation and improving angiogenesis [26]. These processes are most likely at work in the experiments we have demonstrated, because inflammation and immunity are not two separate phenomena but are integrally linked. By exerting anti-inflammatory effects at transplantation, MSCs can reduce damage to the transplants. In the previous study with allogeneic mouse islet transplantation under kidney capsule, it was also shown that MSC coinjection could slow down allograft rejection [26]. A reduction of inflammation will subsequently lead to a reduction in DC activation and immune activation. The findings of the present study suggest that MSCs are also affecting DC maturation directly, which can complement the immune inhibition mediated by costimulation blockade. Costimulation blockade, by limiting the availability of costimulatory molecules, leads the reactive T cell population to interpret interactions with DCs as if it was in contact with tolerogenic or immature DCs [27]. This interaction leads to an anergic response and the peripheral conversion of T cells to regulatory T cells. MSCs have the ability to modulate DC differentiation into tolerogenic or immature DCs, with the subsequent effect that the DCs then induce regulatory T cells [28]. Thus, both costimulation blockade and MSCs are converging on the maturation of DCs and how they are perceived by T cells. MSCs are also capable of directly inducing regulatory T cells by tryptophan degradation and the production of kynurenine metabolites [29]. Tryptophan catabolism is an immune regulatory pathway that costimulation blockade and MSCs also have in common. One of the major pathways by which CTLA4Ig induces the generation of regulatory T cells is through the expression of IDO by DCs after binding to B7 on the DC surface [30]. In the present study, the synergy seen between MSC infusion and costimulation blockade could be partially due to the increased IDO activity by the MSCs and costimulation blockade-manipulated DCs working in concert.
The present study does not differentiate where in the chain of events the synergy of MSC infusion and costimulation blockade is occurring but does show that in this highly immunogenic model these two strategies, when combined, yield a superior outcome. CTLA4Ig recently received Food and Drug Administration approval as maintenance therapy for renal transplantation [31]. MSCs have been shown to decrease acute rejection rates and improve graft function in renal transplantation [32]. Agonistic anti-CD40 antibodies are effective in preventing rejection in islet, kidney, and liver transplants [33] and are currently in early clinical phase trials [34]. Antagonistic anti-CD40 is also an attractive reagent for treating autoimmune disease because of CD40s’ central role in dispersing “help” from T helper cells to the cellular components of the adaptive immune system [35]. These findings, together with the findings of the present study, imply that combining costimulation blockade with MSC infusion could improve outcomes even further in solid organ transplantation. However, the implications of the presented findings are not limited to transplantation. MSC infusion and costimulation blockade could potentiate each other’s immune-modulating effects in a number of autoimmune diseases for which these therapies are already being studied individually. In summary, the combination of costimulation blockade and MSC infusion is a potent strategy to inhibit immune responses and induce regulatory T cells. It could potentially treat many different immune-mediated diseases and, subsequently, have pivotal implications for the field of regenerative medicine.
Supplementary Material
Acknowledgments
Financial support was provided through the regional agreement on medical training and clinical research (Avtal om Läkarutbildning och Forskning [ALF]/ Agreement on Medical School Education and Research) between the Stockholm County Council and the Karolinska Institutet, the Strategic Research Program in Diabetes at the Karolinska Institutet (CLINTEC, H9), the Foundation for International Surgical Cooperation, the Swedish Child Diabetes Foundation, and the Swedish Research Council (Grant K2011-65X-3031-01-6), and the County Council of Västra Götaland (ALF).
Author Contributions
T.T. and M.K.-B.: conception and design, collection of data, data analysis and interpretation, manuscript writing, final approval of manuscript; Y.S., K.L., A.G., C.O., J.H., and M.C.: conception and design, collection of data, data analysis and interpretation, final approval of manuscript; T.L.: conception and design, financial support, administrative support, data analysis and interpretation, final approval of manuscript; B.-G.E. and A.T.: conception and design, financial support, administrative support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
M.C. is cofounder and owner of IsletOne AB (Stockholm, Sweden), holds an uncompensated employment/leadership position, and has compensated intellectual property rights and ownership interest.
References
- 1.McCall M, Shapiro AM. Update on islet transplantation. Cold Spring Harb Perspect Med. 2012;2:a007823. doi: 10.1101/cshperspect.a007823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Shapiro AMJ, Lakey JR, Ryan EA, et al. Islet transplantation in seven patients with type 1 diabetes mellitus using a glucocorticoid-free immunosuppressive regimen. N Engl J Med. 2000;343:230–238. doi: 10.1056/NEJM200007273430401. [DOI] [PubMed] [Google Scholar]
- 3.Miller LW. Cardiovascular toxicities of immunosuppressive agents. Am J Transplant. 2002;2:807–818. doi: 10.1034/j.1600-6143.2002.20902.x. [DOI] [PubMed] [Google Scholar]
- 4.Casiraghi F, Azzollini N, Cassis P, et al. Pretransplant infusion of mesenchymal stem cells prolongs the survival of a semiallogeneic heart transplant through the generation of regulatory T cells. J Immunol. 2008;181:3933–3946. doi: 10.4049/jimmunol.181.6.3933. [DOI] [PubMed] [Google Scholar]
- 5.Ildstad ST, Sachs DH. Reconstitution with syngeneic plus allogeneic or xenogeneic bone marrow leads to specific acceptance of allografts or xenografts. Nature. 1984;307:168–170. doi: 10.1038/307168a0. [DOI] [PubMed] [Google Scholar]
- 6.Le Blanc K, Rasmusson I, Sundberg B, et al. Treatment of severe acute graft-versus-host disease with third party haploidentical mesenchymal stem cells. Lancet. 2004;363:1439–1441. doi: 10.1016/S0140-6736(04)16104-7. [DOI] [PubMed] [Google Scholar]
- 7.Le Blanc K, Frassoni F, Ball L, et al. Mesenchymal stem cells for treatment of steroid-resistant, severe, acute graft-versus-host disease: A phase II study. Lancet. 2008;371:1579–1586. doi: 10.1016/S0140-6736(08)60690-X. [DOI] [PubMed] [Google Scholar]
- 8.Le Blanc K, Mougiakakos D. Multipotent mesenchymal stromal cells and the innate immune system. Nat Rev Immunol. 2012;12:383–396. doi: 10.1038/nri3209. [DOI] [PubMed] [Google Scholar]
- 9.Bernardo ME, Fibbe WE. Mesenchymal stromal cells: Sensors and switchers of inflammation. Cell Stem Cell. 2013;13:392–402. doi: 10.1016/j.stem.2013.09.006. [DOI] [PubMed] [Google Scholar]
- 10.Selmani Z, Naji A, Zidi I, et al. Human leukocyte antigen-G5 secretion by human mesenchymal stem cells is required to suppress T lymphocyte and natural killer function and to induce CD4+CD25highFOXP3+ regulatory T cells. Stem Cells. 2008;26:212–222. doi: 10.1634/stemcells.2007-0554. [DOI] [PubMed] [Google Scholar]
- 11.Chen HW, Chen HY, Wang LT, et al. Mesenchymal stem cells tune the development of monocyte-derived dendritic cells toward a myeloid-derived suppressive phenotype through growth-regulated oncogene chemokines. J Immunol. 2013;190:5065–5077. doi: 10.4049/jimmunol.1202775. [DOI] [PubMed] [Google Scholar]
- 12.Luz-Crawford P, Kurte M, Bravo-Alegría J, et al. Mesenchymal stem cells generate a CD4 +CD25+Foxp3+ regulatory T cell population during the differentiation process of Th1 and Th17 cells. Stem Cell Res Ther. 2013;4:65. doi: 10.1186/scrt216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Engela AU, Baan CC, Litjens NH, et al. Mesenchymal stem cells control alloreactive CD8(+) CD28(−) T cells. Clin Exp Immunol. 2013;174:449–458. doi: 10.1111/cei.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Dahlke MH, Hoogduijn M, Eggenhofer E, et al. Toward MSC in solid organ transplantation: 2008 Position paper of the MISOT Study Group. Transplantation. 2009;88:614–619. doi: 10.1097/TP.0b013e3181b4425a. [DOI] [PubMed] [Google Scholar]
- 15.Kinnear G, Jones ND, Wood KJ. Costimulation blockade: Current perspectives and implications for therapy. Transplantation. 2013;95:527–535. doi: 10.1097/TP.0b013e31826d4672. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Larsen CP, Elwood ET, Alexander DZ, et al. Long-term acceptance of skin and cardiac allografts after blocking CD40 and CD28 pathways. Nature. 1996;381:434–438. doi: 10.1038/381434a0. [DOI] [PubMed] [Google Scholar]
- 17.Oderup C, Malm H, Ekberg H, et al. Costimulation blockade-induced cardiac allograft tolerance: Inhibition of T cell expansion and accumulation of intragraft CD4(+)Foxp3(+) T cells. Transplantation. 2006;82:1493–1500. doi: 10.1097/01.tp.0000244064.66136.04. [DOI] [PubMed] [Google Scholar]
- 18.Trambley J, Bingaman AW, Lin A, et al. Asialo GM1(+) CD8(+) T cells play a critical role in costimulation blockade-resistant allograft rejection. J Clin Invest. 1999;104:1715–1722. doi: 10.1172/JCI8082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Kitchens WH, Haridas D, Wagener ME, et al. Combined costimulatory and leukocyte functional antigen-1 blockade prevents transplant rejection mediated by heterologous immune memory alloresponses. Transplantation. 2012;93:997–1005. doi: 10.1097/TP.0b013e31824e75d7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Kim YH, Wee YM, Choi MY, et al. Interleukin (IL)-10 induced by CD11b(+) cells and IL-10-activated regulatory T cells play a role in immune modulation of mesenchymal stem cells in rat islet allografts. Mol Med. 2011;17:697–708. doi: 10.2119/molmed.2010.00098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Park KS, Kim YS, Kim JH, et al. Trophic molecules derived from human mesenchymal stem cells enhance survival, function, and angiogenesis of isolated islets after transplantation. Transplantation. 2010;89:509–517. doi: 10.1097/TP.0b013e3181c7dc99. [DOI] [PubMed] [Google Scholar]
- 22.Choi YS, Jeong JA, Lim DS. Mesenchymal stem cell-mediated immature dendritic cells induce regulatory T cell-based immunosuppressive effect. Immunol Invest. 2012;41:214–229. doi: 10.3109/08820139.2011.619022. [DOI] [PubMed] [Google Scholar]
- 23.Takahashi T, Matsumoto S, Matsushita M, et al. Donor pretreatment with DHMEQ improves islet transplantation. J Surg Res. 2010;163:e23–e34. doi: 10.1016/j.jss.2010.04.044. [DOI] [PubMed] [Google Scholar]
- 24.Choi EW, Shin IS, Lee HW, et al. Transplantation of CTLA4Ig gene-transduced adipose tissue-derived mesenchymal stem cells reduces inflammatory immune response and improves Th1/Th2 balance in experimental autoimmune thyroiditis. J Gene Med. 2011;13:3–16. doi: 10.1002/jgm.1531. [DOI] [PubMed] [Google Scholar]
- 25.Sullivan C, Barry F, Ritter T, et al. Allogeneic murine mesenchymal stem cells: Migration to inflamed joints in vivo and amelioration of collagen induced arthritis when transduced to express CTLA4Ig. Stem Cells Dev. 2013;22:3203–3213. doi: 10.1089/scd.2013.0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ito T, Itakura S, Todorov I, et al. Mesenchymal stem cell and islet co-transplantation promotes graft revascularization and function. Transplantation. 2010;89:1438–1445. doi: 10.1097/tp.0b013e3181db09c4. [DOI] [PubMed] [Google Scholar]
- 27.Hubo M, Trinschek B, Kryczanowsky F, et al. Costimulatory molecules on immunogenic versus tolerogenic human dendritic cells. Front Immunol. 2013;4:82. doi: 10.3389/fimmu.2013.00082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Zhao ZG, Xu W, Sun L, et al. Immunomodulatory function of regulatory dendritic cells induced by mesenchymal stem cells. Immunol Invest. 2012;41:183–198. doi: 10.3109/08820139.2011.607877. [DOI] [PubMed] [Google Scholar]
- 29.Yagi H, Soto-Gutierrez A, Parekkadan B, et al. Mesenchymal stem cells: Mechanisms of immunomodulation and homing. Cell Transplant. 2010;19:667–679. doi: 10.3727/096368910X508762. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grohmann U, Orabona C, Fallarino F, et al. CTLA-4-Ig regulates tryptophan catabolism in vivo. Nat Immunol. 2002;3:1097–1101. doi: 10.1038/ni846. [DOI] [PubMed] [Google Scholar]
- 31.Charpentier B. Belatacept: A novel immunosuppressive agent for kidney transplant recipients. Expert Rev Clin Immunol. 2012;8:719–728. doi: 10.1586/eci.12.79. [DOI] [PubMed] [Google Scholar]
- 32.Tan J, Wu W, Xu X, et al. Induction therapy with autologous mesenchymal stem cells in living-related kidney transplants: A randomized controlled trial. JAMA. 2012;307:1169–1177. doi: 10.1001/jama.2012.316. [DOI] [PubMed] [Google Scholar]
- 33.Watanabe M, Yamashita K, Suzuki T, et al. ASKP1240, a fully human anti-CD40 monoclonal antibody, prolongs pancreatic islet allograft survival in nonhuman primates. Am J Transplant. 2013;13:1976–1988. doi: 10.1111/ajt.12330. [DOI] [PubMed] [Google Scholar]
- 34.Goldwater R, Keirns J, Blahunka P, et al. A phase 1, randomized ascending single-dose study of antagonist anti-human CD40 ASKP1240 in healthy subjects. Am J Transplant. 2013;13:1040–1046. doi: 10.1111/ajt.12082. [DOI] [PubMed] [Google Scholar]
- 35.Law CL, Grewal IS. Therapeutic interventions targeting CD40L (CD154) and CD40: The opportunities and challenges. Adv Exp Med Biol. 2009;647:8–36. doi: 10.1007/978-0-387-89520-8_2. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.