The authors describe a feeder-free method of generating induced pluripotent stem cells by relying on the use of a chemically defined medium that overcomes the need for embryoid body formation and neuronal rosette isolation for neuronal precursors and terminally differentiated neuron production. This specific and efficient single-step strategy allows the production of mature neurons in 20–40 days with multiple applications, especially for modeling human pathologies.
Keywords: Neuronal progenitors, Neural induction, Neural differentiation, Human induced pluripotent cells, Patch clamp, Dopaminergic neuron, Voltage-gated currents, GABA and glycine receptors
Abstract
For years, our ability to study pathological changes in neurological diseases has been hampered by the lack of relevant models until the recent groundbreaking work from Yamanaka’s group showing that it is feasible to generate induced pluripotent stem cells (iPSCs) from human somatic cells and to redirect the fate of these iPSCs into differentiated cells. In particular, much interest has focused on the ability to differentiate human iPSCs into neuronal progenitors and functional neurons for relevance to a large number of pathologies including mental retardation and behavioral or degenerative syndromes. Current differentiation protocols are time-consuming and generate limited amounts of cells, hindering use on a large scale. We describe a feeder-free method relying on the use of a chemically defined medium that overcomes the need for embryoid body formation and neuronal rosette isolation for neuronal precursors and terminally differentiated neuron production. Four days after induction, expression of markers of the neurectoderm lineage is detectable. Between 4 and 7 days, neuronal precursors can be expanded, frozen, and thawed without loss of proliferation and differentiation capacities or further differentiated. Terminal differentiation into the different subtypes of mature neurons found in the human brain were observed. At 6–35 days after induction, cells express typical voltage-gated and ionotrophic receptors for GABA, glycine, and acetylcholine. This specific and efficient single-step strategy in a chemically defined medium allows the production of mature neurons in 20–40 days with multiple applications, especially for modeling human pathologies.
Introduction
The recent development of induced pluripotent stem cells (iPSCs) has shown promise for understanding and modeling a number of human pathologies. These cells, obtained after reprogramming of somatic cells, are characterized by their capacity to proliferate and differentiate into the three embryonic layers—endoderm, mesoderm, or ectoderm—and subsequent derivatives [1, 2].
The advent of iPSCs has transformed the prospects for disease modeling and our capacity to study pathological processes, especially in the central nervous system [3–5]. A growing number of reports describe models of constitutive disorders that are based on human iPSCs (hiPSCs). This approach remarkably enhances our understanding of the pathological mechanisms of these different diseases and allows their use in drug discovery, opening new grounds for testing new therapeutics such as pharmacological treatments or regenerative cell-based therapies [3, 6, 7]. Although the generation of disease-specific iPSCs has become routine, the potential of iPSCs in tissue modeling poses challenges on multiple fronts, including directing the fate of iPSCs into relevant cell populations.
Protocols are established for some lineage commitment, but in some cases, experimental development is required or needs to be optimized to reduce the cost of the process and obtain large amounts of well-characterized differentiated cells.
Materials and Methods
Materials and methods are included in the supplemental online data.
Results
We developed a simple procedure to induce differentiation of human pluripotent cells (supplemental online Fig. 1) into neurons (Fig. 1; supplemental online Fig. 2). The first step requires predifferentiation and expansion of precursor cells in a defined medium on plates coated with Matrigel. The second step requires plating on fibronectin and then on laminin-coated dishes for final differentiation into mature neurons (Fig. 1). Alternatively, the production of specialized neurons can be achieved in the presence of specific factors.
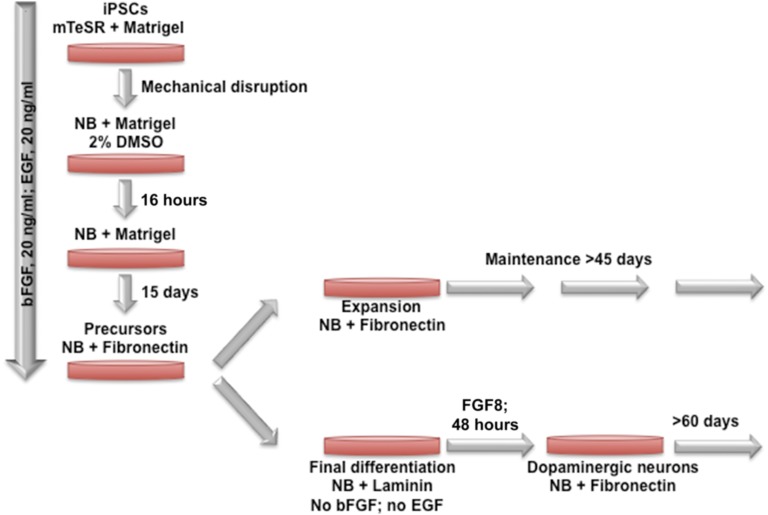
Figure 1.
Schematic representation of the differentiation procedure. Days 1–15: differentiation of human iPSCs (hiPSCs) into human neuronal stem cells (hNSCs). The hiPSCs were expanded, and mature hiPSCs cultured in mTeSR on Matrigel-coated plates were mechanically disrupted in 30–50 small clumps using a 23-gauge needle and plated onto a Matrigel-coated 35-mm culture dish in differentiation medium (DM) supplemented with 20 ng/ml bFGF, 20 ng/ml EGF (DM+). Optimal results were obtained with 2% (v/v) DMSO for 16 hours. After overnight incubation, medium was replaced with DM+. Differentiated cells progressively emerge as a monolayer in the periphery of the hiPSC colony and can be maintained and expanded for up to 15 days with medium replacement every day. After 10–15 days of differentiation, cells at 90%–100% confluence are dissociated with Dispase. Small clumps of hNSCs were plated onto fibronectin-coated 35-mm culture dishes, and 90% of cells adhered within a few minutes after plating. hNSCs can be maintained for several passages or expanded after splitting with Accutase or a cell scraper and replating at a density of 1 × 105 cells per 35-mm culture dish. For final differentiation, cells were mechanically separated with a 23-gauge needle and plated onto laminin-coated 6-well plates in DM without bFGF and EGF. Medium is replaced every day. Neurons develop in 5–7 days after plating. An example of final differentiation into dopaminergic neurons is presented after addition of specific cytokines, such as FGF8 and SHH. Abbreviations: bFGF, basic fibroblast growth factor; DMSO, dimethyl sulfoxide; EGF, epidermal growth factor; iPSC, induced pluripotent stem cell; NB, Neurobasal medium.
Following induction in the presence of dimethyl sulfoxide, hiPSC morphology is progressively modified (Fig. 2A, 2B). Immunofluorescence staining at different time points confirmed the progressive loss of the OCT4 pluripotency marker in flat spindle-like cells and the acquisition of a neuroepithelial phenotype, with expression of the NESTIN neural stem cell marker indicating a switch toward the neuronal lineage [8] as early as 4 days after induction (Fig. 2B). Expression of pluripotency markers becomes almost undetectable after 15 days (Fig. 2B). After plating on laminin, these neuronal progenitors self-renew, maintain their potency, and can be expanded for several weeks, as shown by the high percentage of cells expressing NESTIN, PAX6, or SOX1 at passages 1 and 6 (supplemental online Fig. 2). In the different population of neuronal progenitors, we also observed decreased expression of the NANOG and OCT4 pluripotency markers and an increase in PAX6 mRNA level but the absence of expression of the BRACHYURY or SOX17 mesodermal and endodermal markers (supplemental online Fig. 3A). Furthermore, this population of progenitors can be frozen and thawed without loss of capacity. This strategy is highly reproducible, and similar yields were obtained from different clones derived from different healthy human donor dermal fibroblasts (supplemental online Fig. 1).
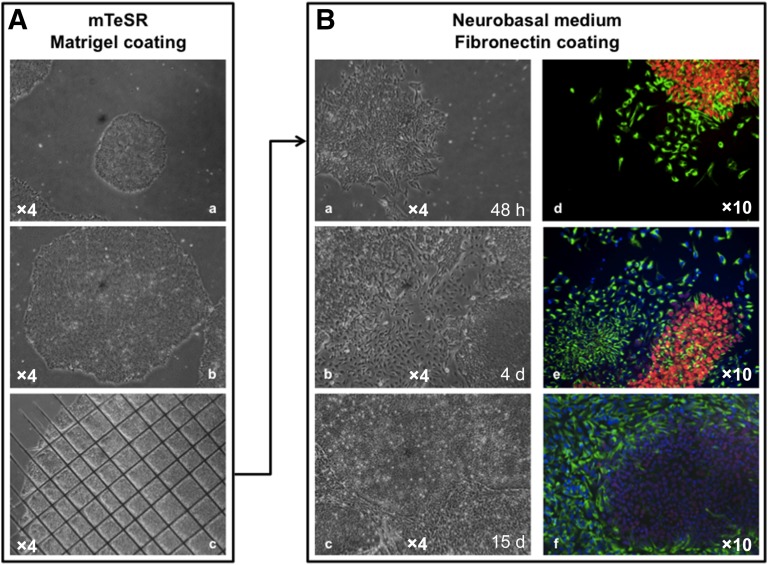
Figure 2.
Differentiation of human induced pluripotent stem cells (hiPSCs) into neuronal progenitors. (A): Bright-field images of small immature (Aa) and mature (Ab) hiPSC colonies grown on Matrigel-coated plates in mTeSR before mechanical disruption (Ac). (B): After mechanical disruption using a 23-gauge needle, clumps of cells were plated on Matrigel and grown in differentiation medium. Left, bright-field images of neuronal differentiation at 48 hours (Ba), 4 days (Bb), or 15 days (Bc) after induction; (Bd) at 48 hours after induction, differentiated cells expressing the Nestin neuronal marker (green) migrate out of the OCT4-positive hiPSC colony (red); (Be) neuronal precursors express NESTIN (green) and hiPSCs express OCT4 at day 4 after induction; after 15 days (Bf), OCT4 expression is barely detectable, and Nestin-positive neuronal precursors reach 90%–100% confluence. Abbreviations: d, days; h, hours.
After induction of the neuronal lineage, NESTIN-positive cells (Fig. 3B) can either be maintained and expanded in differentiation medium on fibronectin-coated plates or differentiated into mature neurons on laminin-coated plates after removal of basic fibroblast growth factor and epidermal growth factor and without additional factors. Final differentiation characterized by βIII-tubulin and NeuN staining, two markers of postmitotic neurons (Fig. 3C), and choline acetyltransferase (supplemental online Fig. 3B) is then achieved in 5–7 days after plating on laminin. In order to determine whether this protocol allows the production of specialized neurons, we tested differentiation toward the dopaminergic lineage by inducing mature neurons with FGF8 and SHH for 48 hours [9] (Fig. 3C). Functionality of dopaminergic neurons was assessed by immunofluorescence staining (expression of tyrosine hydroxylase [TH], a marker of functional dopaminergic neurons) (Fig. 3C) and quantitative reverse transcription polymerase chain reaction (expression of DDC) (supplemental online Fig. 3C). At 15 days after induction, we obtained high enrichment in functional dopaminergic neurons expressing tyrosine hydroxylase. Based on counting of TH-positive cells after immunofluorescence, we estimate that more than 80% of cells are positive for TH (Fig. 3C).
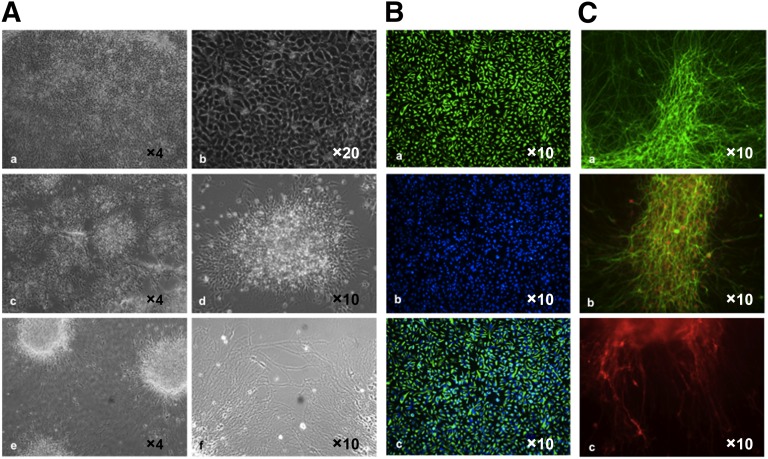
Figure 3.
Production of mature neurons. (A): Illustration of the different steps of neuronal maturation. Neuronal progenitors can be expanded on solid-coated plates to 90%–100% confluence (magnification ×5 [Aa] and ×10 [Ab]) or dissociated and plated at a lower density (magnification ×5 [Ac] and ×10 [Ad]) for further differentiation (magnification ×5 [Ae] and ×10 [Af]). (B): Immunofluoresence staining 2 days after plating of neuronal progenitors on laminin in Neurobasal medium but without basic fibroblast growth factor and epidermal growth factor. The majority of cells express Nestin (Ba); cells were counterstained with 4′,6-diamidino-2-phenylindole (Bb) and merged (Bc). (C): At 20–30 days, mature neurons derived from hiPSCs express βIII-tubulin (green [Ca, Cb]) and the marker of mature neurons, NeuN (red [Cb]). Dopaminergic differentiation was induced by addition of SHH and FGF8, as described. The production and functionality of dopaminergic neurons were assessed by immunofluorescence staining with antibodies against tyrosine hydroxylase 15 days after induction (red [Cc]).
To confirm the neuronal profile of hiPSC-derived neurons, we investigated their membrane properties, such as presence of neuronal voltage-gated and receptor-operated channels, by performing whole-cell patch clamp recordings [10] after maintenance for 7–35 days on laminin-coated 11-mm cover slips (Fig. 4). Cells had resting membrane potentials that ranged from −20 to −60 mV (mean ± SEM: −39 ± 3 mV; n = 17) and input resistance of 634 ± 136 MΩ (n = 19). To detect the presence of voltage-gated channels, cells were held at −70 mV and then at 10-mV incremental voltage from −80 mV to +30 mV with prepulse to −120 mV (Fig. 4B, insert). The vast majority of cells (32 of 37 tested) displayed voltage-gated currents. On depolarizing steps, increasing amplitude outward currents, typical for neuronal K+ ones, were recorded (Fig. 4A, 4B) with a mean current of 453 pA (n = 32) at 30 mV. At depolarization to +30-mV amplitude, currents increased in cells maintained in culture from 272 ± 96 pA (n = 5 at day 7 of differentiation) to 694 ± 52 (n = 3 at day 35 of differentiation). These currents were reversibly blocked by 20-mM tetraethylammonium (TEA) (Fig. 4A–4C), which confirmed its K+ nature.
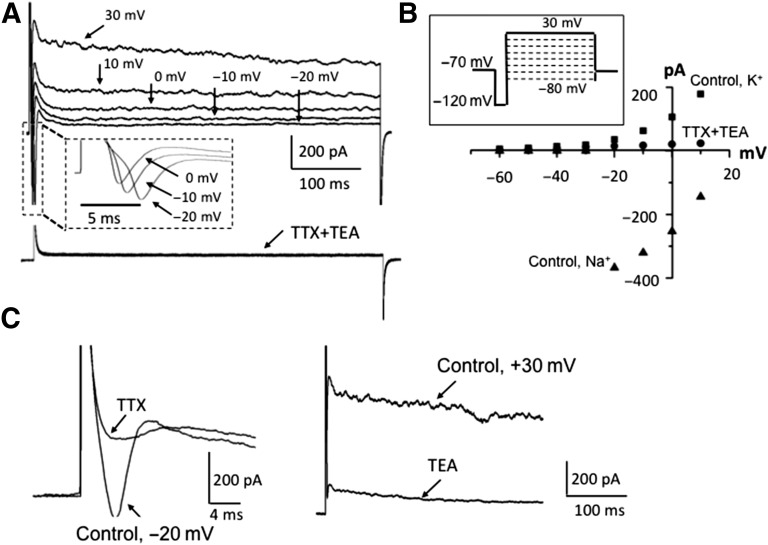
Figure 4.
Electrophysiological properties of human induced pluripotent stem cell (hiPSC) differentiated neurons. (A): Representative example (upper panel) of currents evoked by depolarizing voltage steps indicated above the traces (see scheme of the protocol in 4B, insert); expanded from dashed lines (insert), rectangular traces of fast-activating, fast-inactivating inward currents evoked by depolarizing voltage steps, as indicated; example (bottom traces) of currents evoked by the same set of depolarizing voltage steps as indicated above after 1-minute preapplication of TTX (1 μM) plus TEA (20 mM). Note the strong inhibition of both inward and outward components. (B): Current-voltage relations of outward K+ (▪) and inward Na+ (▴) in control conditions and after 1-minute application of 1 μM TTX + 20 mM TEA (●); scheme of depolarizing protocol (insert) for recording current-voltage relations. In the different tests, pulse protocol was the same. (C): Examples of single traces illustrating separate inhibition of voltage-gated Na+ currents by 1 μM TTX (left) and K currents by 20 mM TEA (right). Note different time scales. Abbreviations: TEA, tetraethylammonium; TTX, tetrodotoxin.
Fast-inactivating inward components, typical of neuronal Na+ currents were also observed in response to depolarizing pulses (Fig. 4A). Following a 100-ms hyperpolarizing prepulse to −120 mV to relieve Na+ channel inactivation, voltage steps ranging from −80 to +30 mV with 10-mV intervals (scheme of stimulation shown in Fig. 4B, insert) evoked inward currents; the threshold of activation was about −30 mV, and maximal inward amplitude was reached at about −20 mV (Fig. 4A, 4B). These currents were reversibly blocked by the specific antagonist of voltage-gated Na+ channels, tetrodotoxin (TTX) (Fig. 4A–4C). Simultaneous application of TTX and TEA suppressed both components of voltage-gated currents (Fig. 4A), indicating that these cells exhibit neuronal properties and are able to generate and propagate action potentials.
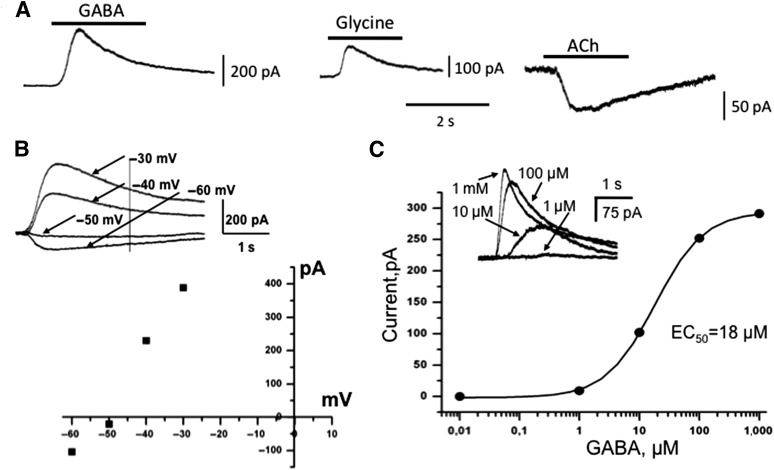
We investigated whether these cells express receptor-operated channels. Three agonists—glycine, acetylcholine (ACh), and GABA—were applied using a fast perfusion system. GABA- and glycine-evoked currents were recorded 7 days after differentiation (Fig. 5C, 5D). With 20-mM Cl− in the intracellular solution, reversal potential obtained from current voltage relations of GABA-induced currents was about −50 mV (Fig. 4B), which is close to the reversal potential for Cl− in similar conditions. Half maximal effective concentration (EC50) for GABA-induced currents was determined from dose-response relationships (Fig. 5C). It varied from 6 μM to 65 μM with a mean value of 22 ± 4 μM (n = 18). Sensitivity of GABA receptors to agonist varies strongly depending on subunit composition, localization, and developmental stage [11–14]. In our experiments, a tendency toward decreasing sensitivity with age in culture was observed. At the eighth day after induction of differentiation, for example, EC50 for GABA was 19 ± 4 (n = 6), whereas at the 21st day, we observed EC50 of 35 ± 19 (n = 3). This coincides with the developmental profile observed in thalamic nucleus neurons [13].
Figure 5.
Expression of neurotransmitter-activated channels in human induced pluripotent stem cell (hiPSC) differentiated neurons. (A): Representative examples of ionic currents induced by application of 1 mM GABA, 1 mM glycine, or 100 μM ACh in differentiated hiPSCs. Whole-cell recordings with intracellular solution containing 20 mM Cl− at holding potential 0 mV (for GABA and glycine) and at −40 mV for ACh. Bars above traces indicate the time of agonist application. (B): Current-voltage relationship of the GABA-evoked inward current. Example (top) of superimposed traces of GABA-evoked currents at different potentials. Reversal potential was approximately −50 mV, as predicted from Nernst equation for intracellular solution containing 20 mM KCl. (C): Dose-response dependency and superimposed traces (insert) of whole-cell currents induced by rapid application of different concentration of GABA. Recording at 0 mV. Abbreviations: ACh, acetylcholine; EC50, half maximal effective concentration; s, seconds.
As described previously, neuronal ionotropic GABA and glycine receptors exhibit similar Cl− selectivity [15]. In our study, from 25 cells on which GABA and glycine were simultaneously tested, 21 cells showed responses to GABA and glycine, whereas on 4 other cells, responses to both neurotransmitters were absent. First responses for ACh application were observed after 21 days following differentiation (Fig. 5A). At this stage, expression of ACh receptors coincided with those for glycine and GABA for all examined cells (n = 10). Reversal potential for acetylcholine-induced currents was about 0 mV (data not shown), indicating cation selectivity of these channels, as described previously [16]. Altogether, 82% of cells replied to GABA (37 of 45 cells), 71% responded to glycine (27 of 38 cells), and 25% responded to acetylcholine (10 of 40 cells).
Discussion
These data indicate that after plating on laminin, neuronal progenitors are differentiated into mature neurons that can be maintained for up to 35 days without loss of membrane property, as indicated by patch clamp recording. In addition, these cells can be differentiated toward specialized neurons (e.g., dopaminergic) in the presence of specific cytokines.
Self-renewing human embryonic stem cells and hiPSCs have the potential to differentiate into any cell type, representing an invaluable source of biological material, particularly for regenerative medicine. In numerous cases, however, the use of these cells in translational medicine is hampered by the efficiency and the low scale of the differentiation process.
We have described a novel and efficient protocol for the differentiation of hiPSCs into neuronal cells. Our protocol requires no feeder layers. Furthermore, compared with other published protocols, our procedure does not necessitate embryoid bodies followed by rosette (primitive neuroepithelial cells) and neurosphere formation [17–21], which might modify purity of the cell population; drug addition, which might perturb cellular homeostasis [20–24]; or cell sorting, limiting the quantity of differentiated cells available. Moreover, we believe that the method described reduces the overall cost of the differentiation process because our protocol does not require BDNF, Noggin, NT3, or GDNF, as described [18, 20, 21, 25], but only two cytokines at initial differentiation steps. Furthermore, it is not necessary to perform cell sorting, and our method yields large quantities of neuronal progenitors, which can be maintained and regularly expanded or further differentiated in only 10–15 days, limiting the quantity of reagents necessary for the production of neuronal progenitors or mature neurons.
These neuronal cells express different neuronal markers together with the Na+ and K+ voltage-operated channels and are able to generate and propagate action potentials. Moreover, these cells expressed different types of receptor-operated channels such as the Cl−-selective GABA receptors, responsible for the main inhibitory synaptic drive in the central nervous system.
Conclusion
A growing number of reports describe hiPSC-based models of constitutive disorders for understanding the pathological mechanisms of these different diseases and allowing their use in drug discovery and, potentially, cell therapy. Our method can be used to produce large amounts of mature neurons, including dopaminergic neurons. Our strategy provides a valuable tool for studying neuronal differentiation pathways and synaptic and postsynaptic responses and for testing pharmacological treatments. This approach could open the way to understanding a large number of pathologies including neurodevelopmental and neurodegenerative diseases.
Supplementary Material
Acknowledgments
We thank Dr. Jean Christophe Roux for helpful discussion. This study was funded by Fondation Lejeune (to F.M.) and the Association Française contre les Myopathies. C.B. is the recipient of a fellowship from the Algerian Ministry of Higher Education and Research. P.T. is the recipient of a fellowship from Fondation pour la Recherche Médicale. G.M. was supported by a fellowship from the French Ministry of Foreign Affairs.
Author Contributions
C.B.: conception and design, collection and assembly of the data, data analysis and interpretation; G.M.: collection and assembly of the data, data analysis and interpretation; C.E.-Y., E.B., and P.T.: provision of study materials; M.L.: collection and assembly of the data; B.B.: financial support, final approval of the manuscript; P.B.: conception and design, collection and assembly of the data, data analysis and interpretation, manuscript writing; F.M.: conception and design, collection and assembly of the data, data analysis and interpretation, manuscript writing, financial support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 2.Park IH, Zhao R, West JA, et al. Reprogramming of human somatic cells to pluripotency with defined factors. Nature. 2008;451:141–146. doi: 10.1038/nature06534. [DOI] [PubMed] [Google Scholar]
- 3.Lee G, Papapetrou EP, Kim H, et al. Modelling pathogenesis and treatment of familial dysautonomia using patient-specific iPSCs. Nature. 2009;461:402–406. doi: 10.1038/nature08320. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Brennand KJ, Simone A, Jou J, et al. Modelling schizophrenia using human induced pluripotent stem cells. Nature. 2011;473:221–225. doi: 10.1038/nature09915. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chamberlain SJ, Li XJ, Lalande M. Induced pluripotent stem (iPS) cells as in vitro models of human neurogenetic disorders. Neurogenetics. 2008;9:227–235. doi: 10.1007/s10048-008-0147-z. [DOI] [PubMed] [Google Scholar]
- 6.Viganò F, Möbius W, Götz M, et al. Transplantation reveals regional differences in oligodendrocyte differentiation in the adult brain. Nat Neurosci. 2013;16:1370–1372. doi: 10.1038/nn.3503. [DOI] [PubMed] [Google Scholar]
- 7.Espuny-Camacho I, Michelsen KA, Gall D, et al. Pyramidal neurons derived from human pluripotent stem cells integrate efficiently into mouse brain circuits in vivo. Neuron. 2013;77:440–456. doi: 10.1016/j.neuron.2012.12.011. [DOI] [PubMed] [Google Scholar]
- 8.Yang N, Ng YH, Pang ZP, et al. Induced neuronal cells: How to make and define a neuron. Cell Stem Cell. 2011;9:517–525. doi: 10.1016/j.stem.2011.11.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Carpenter M, Rao MS, Freed W, et al. Derivation and characterization of neuronal precursors and dopaminergic neurons from human embryonic stem cells in vitro. Methods Mol Biol. 2006;331:153–167. doi: 10.1385/1-59745-046-4:153. [DOI] [PubMed] [Google Scholar]
- 10.Fucile S, De Saint Jan D, de Carvalho LP, et al. Fast potentiation of glycine receptor channels of intracellular calcium in neurons and transfected cells. Neuron. 2000;28:571–583. doi: 10.1016/s0896-6273(00)00134-3. [DOI] [PubMed] [Google Scholar]
- 11.Mehta AK, Ticku MK. Developmental aspects of benzodiazepine receptors and GABA-gated chloride channels in primary cultures of spinal cord neurons. Brain Res. 1988;454:156–163. doi: 10.1016/0006-8993(88)90814-1. [DOI] [PubMed] [Google Scholar]
- 12.Hsiao SH, Mahoney JC, West JR, et al. Development of GABAA receptors on medial septum/diagonal band (MS/DB) neurons after postnatal ethanol exposure. Brain Res. 1998;810:100–113. doi: 10.1016/s0006-8993(98)00891-9. [DOI] [PubMed] [Google Scholar]
- 13.Gibbs JW, III, Schroder GB, Coulter DA. GABAA receptor function in developing rat thalamic reticular neurons: Whole cell recordings of GABA-mediated currents and modulation by clonazepam. J Neurophysiol. 1996;76:2568–2579. doi: 10.1152/jn.1996.76.4.2568. [DOI] [PubMed] [Google Scholar]
- 14.Krishek BJ, Smart TG. Proton sensitivity of rat cerebellar granule cell GABAA receptors: Dependence on neuronal development. J Physiol. 2001;530:219–233. doi: 10.1111/j.1469-7793.2001.0219l.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Bormann J, Hamill OP, Sakmann B. Mechanism of anion permeation through channels gated by glycine and gamma-aminobutyric acid in mouse cultured spinal neurones. J Physiol. 1987;385:243–286. doi: 10.1113/jphysiol.1987.sp016493. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fieber LA, Adams DJ. Acetylcholine-evoked currents in cultured neurones dissociated from rat parasympathetic cardiac ganglia. J Physiol. 1991;434:215–237. doi: 10.1113/jphysiol.1991.sp018466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hitoshi S, Seaberg RM, Koscik C, et al. Primitive neural stem cells from the mammalian epiblast differentiate to definitive neural stem cells under the control of Notch signaling. Genes Dev. 2004;18:1806–1811. doi: 10.1101/gad.1208404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Liu Y, Liu H, Sauvey C, et al. Directed differentiation of forebrain GABA interneurons from human pluripotent stem cells. Nat Protoc. 2013;8:1670–1679. doi: 10.1038/nprot.2013.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Lie KH, Chung HC, Sidhu KS. Derivation, propagation, and characterization of neuroprogenitors from pluripotent stem cells (hESCs and hiPSCs) Methods Mol Biol. 2012;873:237–246. doi: 10.1007/978-1-61779-794-1_15. [DOI] [PubMed] [Google Scholar]
- 20.Surmacz B, Fox H, Gutteridge A, et al. Directing differentiation of human embryonic stem cells toward anterior neural ectoderm using small molecules. Stem Cells. 2012;30:1875–1884. doi: 10.1002/stem.1166. [DOI] [PubMed] [Google Scholar]
- 21.Yan Y, Shin S, Jha BS, et al. Efficient and rapid derivation of primitive neural stem cells and generation of brain subtype neurons from human pluripotent stem cells. Stem Cells Translational Medicine. 2013;2:862–870. doi: 10.5966/sctm.2013-0080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Li W, Sun W, Zhang Y, et al. Rapid induction and long-term self-renewal of primitive neural precursors from human embryonic stem cells by small molecule inhibitors. Proc Natl Acad Sci USA. 2011;108:8299–8304. doi: 10.1073/pnas.1014041108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Menendez L, Yatskievych TA, Antin PB, et al. Wnt signaling and a Smad pathway blockade direct the differentiation of human pluripotent stem cells to multipotent neural crest cells. Proc Natl Acad Sci USA. 2011;108:19240–19245. doi: 10.1073/pnas.1113746108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Chambers SM, Fasano CA, Papapetrou EP, et al. Highly efficient neural conversion of human ES and iPS cells by dual inhibition of SMAD signaling. Nat Biotechnol. 2009;27:275–280. doi: 10.1038/nbt.1529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dottori M, Pera MF. Neural differentiation of human embryonic stem cells. Methods Mol Biol. 2008;438:19–30. doi: 10.1007/978-1-59745-133-8_3. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.