This study defined optimized conditions for cell survival and seeding efficiency in the context of tissue engineering and cell-therapy strategies. An autologous stem cell therapy was used to successfully treat a patient with a debilitating craniofacial traumatic deficiency. This clinical report provides a foundation on which to develop more expanded studies using this approach for the treatment of larger numbers of patients with other debilitating conditions to further evaluate efficacy and feasibility.
Keywords: Bone regeneration, Bone marrow, Stem cells, Cell therapy, Implants, Scaffold
Abstract
Traumatic injuries involving the face are very common, yet the clinical management of the resulting craniofacial deficiencies is challenging. These injuries are commonly associated with missing teeth, for which replacement is compromised due to inadequate jawbone support. Using cell therapy, we report the upper jaw reconstruction of a patient who lost teeth and 75% of the supporting jawbone following injury. A mixed population of bone marrow-derived autologous stem and progenitor cells was seeded onto β-tricalcium phosphate (β-TCP), which served as a scaffold to deliver cells directly to the defect. Conditions (temperature, incubation time) to achieve the highest cell survival and seeding efficiency were optimized. Four months after cell therapy, cone beam computed tomography and a bone biopsy were performed, and oral implants were placed to support an engineered dental prosthesis. Cell seeding efficiency (>81%) of the β-TCP and survival during the seeding process (94%) were highest when cells were incubated with β-TCP for 30 minutes, regardless of incubation temperature; however, at 1 hour, cell survival was highest when incubated at 4°C. Clinical, radiographic, and histological analyses confirmed that by 4 months, the cell therapy regenerated 80% of the original jawbone deficiency with vascularized, mineralized bone sufficient to stably place oral implants. Functional and aesthetic rehabilitation of the patient was successfully completed with installation of a dental prosthesis 6 months following implant placement. This proof-of-concept clinical report used an evidence-based approach for the cell transplantation protocol used and is the first to describe a cell therapy for craniofacial trauma reconstruction.
Introduction
In addition to bruises, hematomas, and lacerations, dentoalveolar injuries are the most common injuries that occur in the facial region, accounting for 50% of the injuries for those seeking emergency treatment for head and neck injuries [1–3]. The resulting functional and aesthetic deficiencies from the loss of teeth and associated jawbone support due to these injuries are debilitating and very difficult to treat. The current standard-of-care protocol for advanced craniofacial reconstruction involving the oral cavity involves the use of large autogenous “block” bone grafts, whereby the donor bone blocks of bone are harvested from intraoral sites (mandibular ramus or symphysis) or extraoral sites (iliac crest, tibia) [4–7]. Although advanced grafting procedures have historically demonstrated varying degrees of success, major limitations are that they require two surgical sites (donor and recipient) and are often associated with long postoperative healing periods, moderate to severe discomfort during healing, tissue morbidity in the donor site, and prolonged sensory disturbances in the donor site.
Stem cell therapy is an emerging strategy that can potentially be used for the reconstruction of craniofacial deficiencies [8, 9]. Because cell-therapy approaches often involve the use of a polymer material to deliver cells to the defect area, the success of these approaches is heavily dependent not only on the polymer and cells used but also the conditions under which they are used. Despite many in vitro and in vivo studies designed to evaluate and optimize the cell attachment and biocompatibility of different materials, there is no clinical evidence of efficacy to support these data. In contrast, in the limited clinical reports investigating a cell-transplantation approach to regenerating craniofacial tissue, the clinical protocols and conditions used to deliver the cells are either not well described or not well justified [10–14].
In a randomized controlled clinical trial, our group recently reported the use of a gelatin sponge to deliver stem cells into small, localized, oral bone defects created following tooth removal [15]. Although results were favorable, the use of this sponge material as a cell carrier is not suitable for regeneration of large oral and craniofacial defects. β-Tricalcium phosphate (β-TCP) has more ideal properties as a cell carrier for addressing larger, more severe bone defects because it has rigid structural properties and is osteoconductive, which facilitates bone growth [16, 17]. Clinically, it has been used as a bone-graft substitute material in very limited orthopedic indications and in small, localized bone deficiencies around teeth [18, 19]. Recently, its use as a cell carrier for autologous adipose-derived stem cells has also been reported with the combined use of recombinant human bone morphogenetic protein-2 to treat craniofacial defects [14]. Nonetheless, to date, there has been no reported clinical investigation of its use as a scaffold for a stand-alone cell therapy in the treatment of large craniofacial deficiencies.
We report a stem cell therapy to reconstruct the upper jaw of a patient who lost front teeth and associated bone tissue following a severe traumatic injury to the face. β-TCP was used as a scaffold to deliver the cells to the jawbone defect, and following 4 months of healing, sufficient bone was regenerated to insert oral implants and restore them with dental prosthetics. In addition, the clinical conditions for cell attachment and survival were optimized for this cell-transplantation approach.
Materials and Methods
Patient
Following U.S. Food and Drug Administration and University of Michigan institutional review board approval to conduct a cell-therapy study for the oral reconstruction of traumatic craniofacial injuries, a 45-year-old woman presented to the clinic following an injury in which she suffered a traumatic blow to the face. The injury occurred 5 years prior to her initial presentation. and as a result of the injury, seven teeth (four in the anterior segment of the upper jaw and three in the anterior segment of the lower jaw) were avulsed and lost. Moreover, 75% of the supporting jawbone and soft tissue surrounding these teeth were also lost as a result of the injury. Consequently, the patient had severe oral-facial functional and aesthetic deficiency. Due to inadequate alveolar bone as a result of the injury, the patient was not a candidate for rehabilitation with oral implant therapy without advanced reconstructive bone-grafting procedures being performed. The patient was wearing an ill-fitting removable dental prosthesis on initial presentation and was deemed eligible for participation in the study.
Cell-Seeding Efficiency and Viability Studies
The production of ixmyelocel-T (tissue repair cells or ixmyelocel-T; Aastrom Biosciences, Ann Arbor, MI, http://www.aastrom.com) has been described previously [20]. Briefly, a bone marrow aspiration of the posterior ilium was performed under conscious sedation and local anesthetic. Collected marrow was transferred to a sterile blood bag, and bone marrow mononuclear cells (BMMNCs) were purified by Ficoll density gradient centrifugation. BMMNCs were then inoculated into a bioreactor, which is a proprietary computer-controlled, automated cell processing unit (Aastrom Replicell system; Aastrom Biosciences). This system incorporates single-pass perfusion in which fresh medium flows slowly over cells without retention of waste metabolites or differentiating cytokines. The culture medium consists of Iscove’s modified Dulbecco’s medium, 10% fetal bovine serum, 10% horse serum, and 5 μM hydrocortisone. After cultivation for 12 days at 37°C in 5% CO2 with a ramped continuous medium perfusion schedule, the ixmyelocel-T product was harvested by trypsinization, washed in a physiologic buffer, and collected into a sterile bag for storage until the time of transplantation. The cells were composed of a mixture of bone marrow-derived cells including expanded CD90+ mesenchymal stem cells, CD14+ monocytes/macrophages, and mononuclear cells from the original bone marrow aspirate [21, 22]. The cell population from this patient consisted of 26% CD90+ cells and 15% CD14+ monocytes and had a final concentration of 14.1 million cells per milliliter with cell viability of 91%. The primary purpose for obtaining these cells was their use in the clinical treatment of the bone defect; however, a specific section of the informed consent document obtained the patient’s permission to use and/or store “excess” cells and/or bone marrow (if available) for additional laboratory or preclinical studies.
For the cell-seeding and viability studies, T -150 flasks containing 90% confluent cell populations of ixmyelocel-T were trypsinized and counted. Cells were seeded onto equal volumes of β-TCP (Cerasorb; Curasan AG, Kleinostheim, Germany, http://www.curasan.de) particles (1:1 ratio of cell suspension to volume β-TCP) and allowed to incubate at either room temperature (RT) or on ice (4°C). After 15, 30, and 60 minutes, the residual cell suspension from the respective condition was collected, and the number of cells remaining was counted. The cell-seeding efficiency was an indirect measure of the number of cells that attached to the β-TCP particles. It was calculated through an assumption of a constant number of cells for seeding and deduction of the floating cells from this constant number to get the number of seeded cells (efficiency). Cell viability was measured as cell survival, determined through the dye exclusion method of trypan blue staining of the remaining or floating cells following incubation with the β-TCP and counting this proportion of cells relative to the total number of floating cells during the three respective time frames at RT and at 4°C.
Cell Therapy, Regenerative Analyses, and Oral Reconstruction
A cone beam computed tomography (CBCT) radiographic scan was performed to volumetrically evaluate the upper jawbone deficiency and generate three-dimensional reconstructions of the upper jaw. Under conscious sedation and local anesthesia, an intraoral full-thickness mucoperiosteal flap was elevated to expose the margins of the bony defect in the upper jaw. In the operating room, approximately 107 cells in suspension were incubated with β-TCP at RT 30 minutes prior to being administered to the defect site. Following clinical open bone measurements of the width of the alveolar bone, the defect site was prepared to receive the graft by creating small osteotomies penetrating through the outer cortical layer of bone to facilitate vascular infusion of the graft during healing. Four 8-mm “tenting” screws were used to help stabilize the β-TCP particles, and the β-TCP was then placed and covered with a resorbable collagen membrane (Conform collagen; Ace Surgical Supply, Inc., Brockton, MA, http://www.acesurgical.com) to help contain the grafted β-TCP/cell construct. In addition, 4-0 sutures were used to approximate the tissues, and the area was allowed to heal for 4 months. A second CBCT scan was performed immediately following grafting. Postoperative medications included an antibiotic, a corticosteroid, and an analgesic for pain management.
Four months following placement of the graft, a third CBCT was performed to evaluate the presence of mineralized tissue in the grafted area prior to implant placement. Following the imaging, the area was surgically re-entered for the placement of oral implants. During re-entry, clinical bone measurements were recorded, and a bone biopsy was performed in an area of the previously grafted region. This tissue biopsy was processed for micro-computed tomography (micro-CT) and histological analysis, as described previously [23]. Oral implants were placed in the grafted sites and allowed to heal underneath the gingival tissue for 6 months. At 6 months, the implants were uncovered, and a dental prosthesis was engineered and installed to connect to the oral implants. This study is registered with ClinicalTrials.gov identifier CT00755911.
Statistical Analysis
Statistical analysis was performed with the use of InStat software (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com). All data were plotted as mean ± SEM unless otherwise noted. One-way analysis of variance was performed for the cell-seeding efficiency and cell-viability studies with Bonferroni-corrected, post hoc, two-tailed t tests to determine statistically significant differences between groups. Statistical significance was defined as p < .05.
Results
Cell-Seeding Efficiency
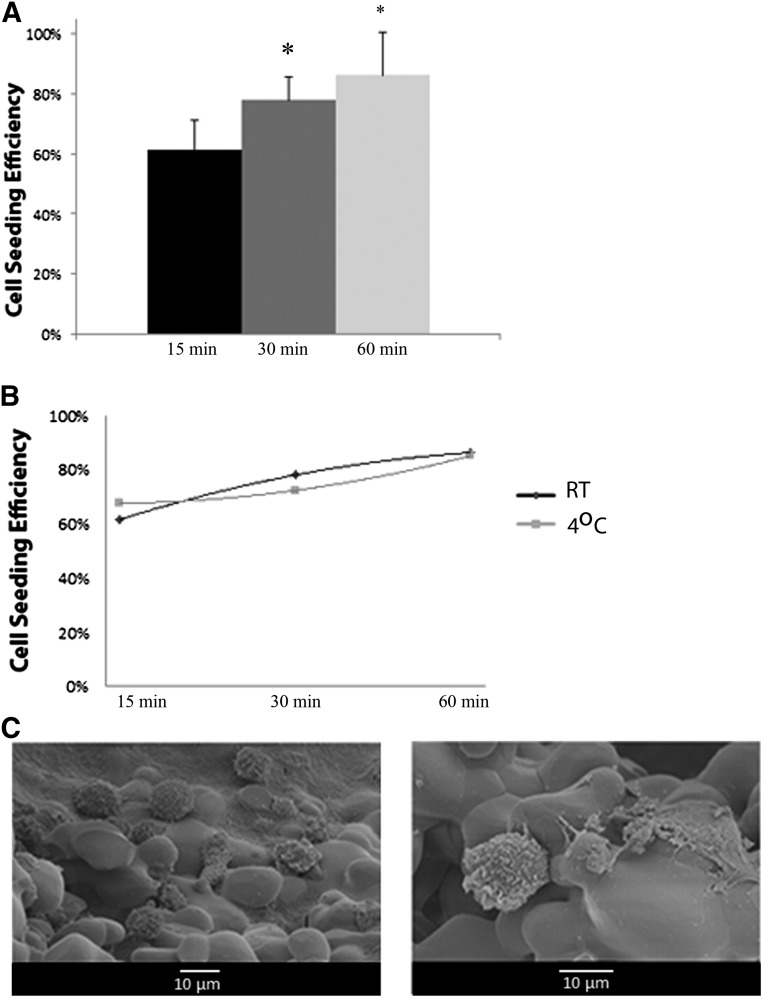
The time frame needed to achieve the highest cell attachment to β-TCP was determined in our cell-seeding efficiency studies (Fig. 1A). Cell-seeding efficiency of β-TCP following 15 minutes of incubation with cells was 60%, with a significant increase to 81% following 30 minutes of incubation (p < .05). There was no difference in the seeding efficiency between 30 minutes and 1 hour of incubation. In addition, when evaluating the effect of temperature on cell-seeding efficiency, there was no difference in seeding efficiency at 4°C relative to room temperature at the three time points evaluated (Fig. 1B). SEM images show diffuse distribution and attachment of the cells to one particle (500- to 1,000-μm particle sizes) of the graft material following 30 minutes of incubation at room temperature (Fig. 1C).
Figure 1.
Cell-seeding efficiency of β-tricalcium phosphate (β-TCP). (A): The overall cell-seeding efficiency is shown at different time intervals (15, 30, and 60 minutes) following seeding of the scaffold with cells. (B): The cell-seeding efficiency at the different time intervals was stratified by the temperature at which the cells were allowed to incubate with the scaffold. (C): SEM images at low and high magnification showing the distribution of cells and cell attachment to one β-TCP particle (individual particle sizes ranged from 500 to 1,000 μm). ∗, p < .05 relative to the 15-minute condition. Abbreviation: RT, room temperature.
Cell Viability During Cell Seeding
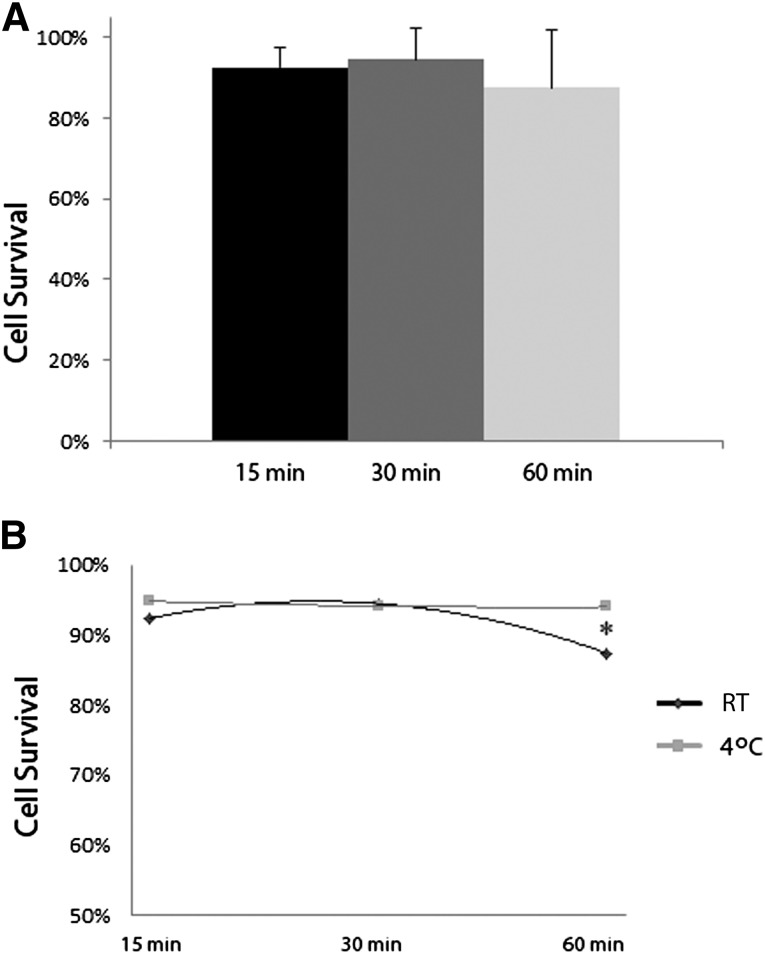
Another important variable in the context of cell therapy is the cell viability throughout the process of cell seeding and transplantation. Cell viability was evaluated in a similar manner to cell-seeding efficiency, at three different time points (15, 30, and 60 minutes) and two temperatures (RT, 4°C). Between the three time points evaluated, cell survival was no different, between 88% and 94% (Fig. 2A). However, when stratifying for temperature, there was a significant decrease (p < .05) in cell survival when incubated at RT for 1 hour relative to incubation at RT for 30 minutes or when incubated at 4°C for 1 hour (Fig. 2B). When at 4°C, the time frame of incubation did not affect cell survival. Overall, the optimum conditions for cell survival were 30-minute incubations at RT or 4°C or a 60-minute incubation period at 4°C.
Figure 2.
Cell viability following seeding on β-tricalcium phosphate. (A): Cell survival at different time intervals following loading of the scaffold is shown. (B): Cell survival at the different time intervals was stratified by the temperature at which the cells were maintained during the respective time intervals during which the cells were allowed to incubate with the scaffold. ∗, p < .05 between conditions. Abbreviation: RT, room temperature.
Clinical Cell Transplantation
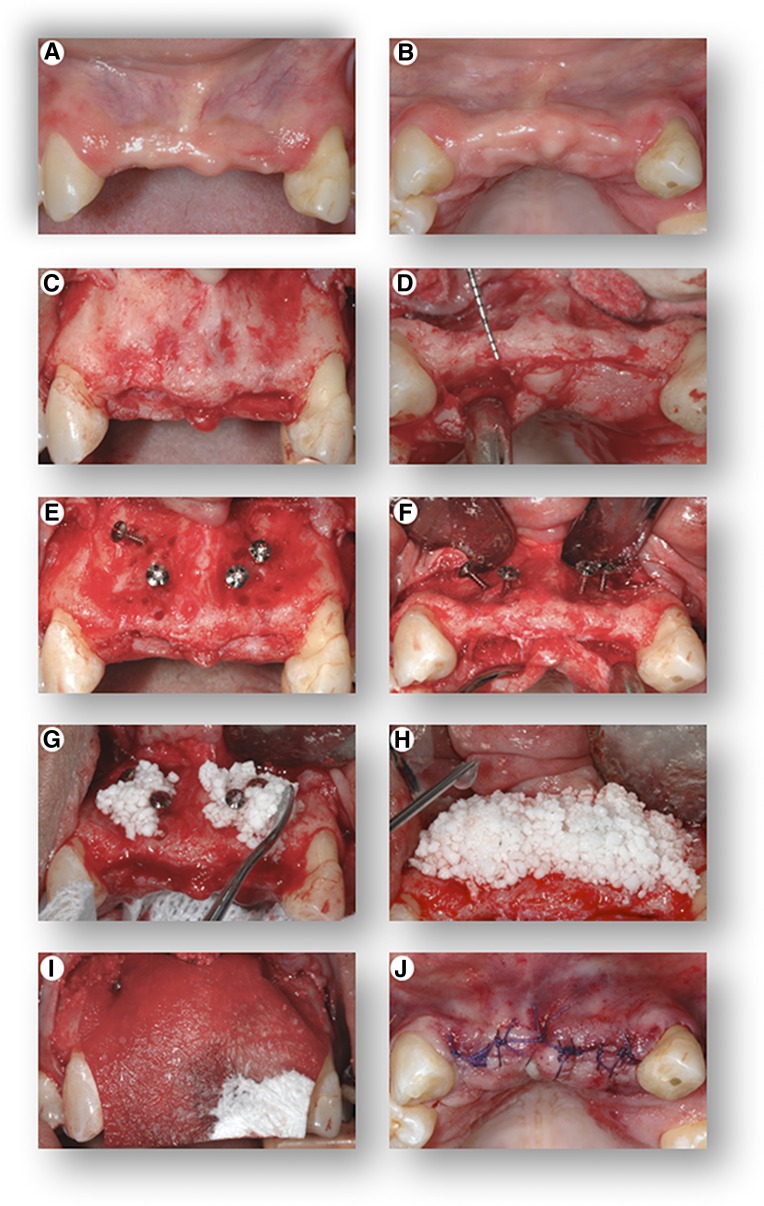
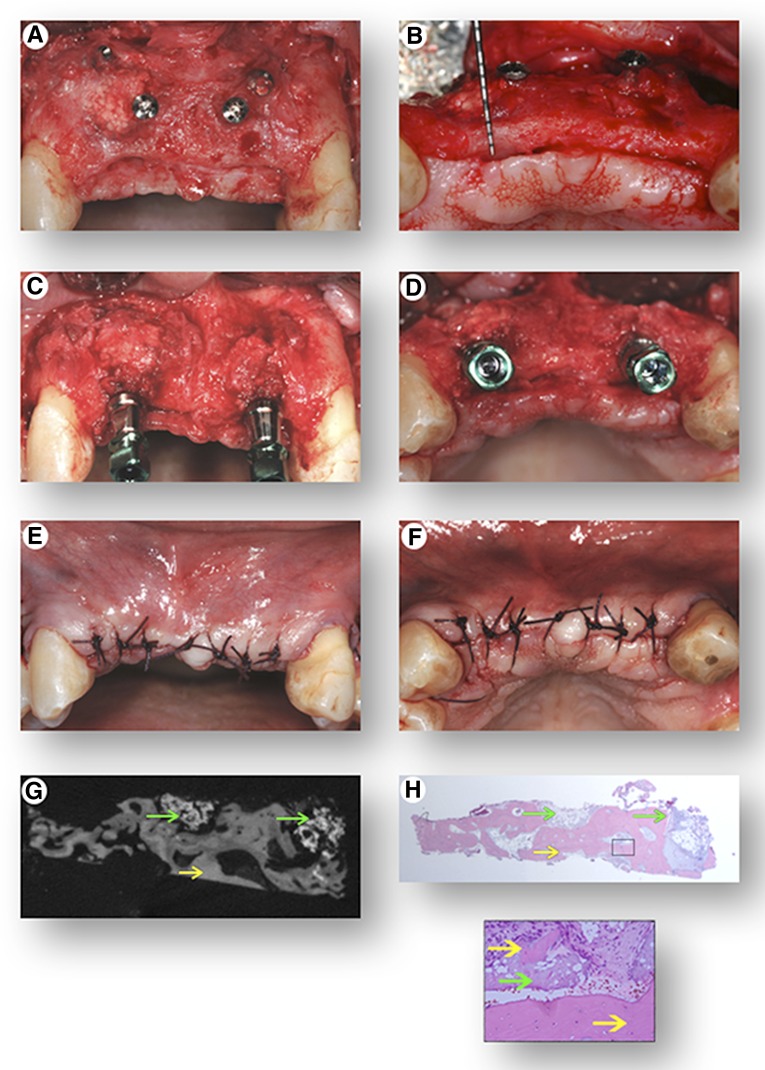
The protocol for transplantation of the cells used the optimized attachment and survival conditions, which were to maintain the cells on ice (4°C) until 30 minutes prior to transplantation, at which time they were incubated with the β-TCP at RT. During this period in which the cells were incubating, the gingival flap was reflected to expose the underlying bone, and measuring instruments were used to measure the horizontal dimension of the alveolar ridge, which was 3 mm (Fig. 3A–3D). In a healthy dentition, horizontal ridge width of this area of the maxilla normally ranges from 8 to 12 mm, and to securely place and stabilize a dental implant, 7–8 mm is the minimum width required. Tenting screws were placed in the area to receive the graft and were used to help consolidate the graft material and prevent collapse of the overlying collagen membrane and soft tissue following closure of the flap (Fig. 3E, 3F). The graft was applied to the deficient area, and an additional 0.5 mL of the cell suspension was added following placement of the graft into the site (Fig. 3G, 3H). A barrier membrane was placed over the graft to prevent soft tissue infiltration into the graft during the early stage of healing (Fig. 3I), and the tissues were approximated completely (Fig. 3J).
Figure 3.
Cell transplantation procedure. Front view (A) and top view (B) of the initial clinical presentation showing severe hard and soft tissue alveolar ridge defects of the upper jaw. Following elevation of a full-thickness gingival flap, the images show front view (C) and top view (D) of the severely deficient alveolar ridge, clinically measuring a width of only 2–4 mm. Front view (E) and top view (F) of the placement of “tenting” screws in preparation of the bony site to receive the graft. Placement of the β-tricalcium phosphate (seeded with the cells 30 minutes prior to placement at room temperature) into the defect (G), with additional application of the cell suspension following placement of the graft in the recipient site (H). Placement of a resorbable barrier membrane (I) to stabilize and contain the graft within the recipient site, and top view (J) of primary closure of the flap.
Radiographic, Clinical, and Histological Analyses of Jawbone Reconstruction
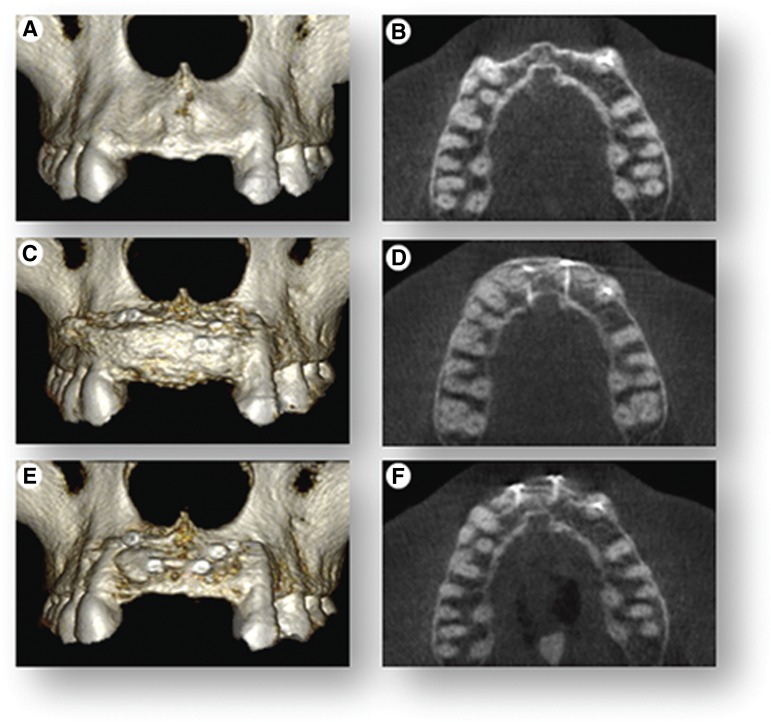
The 75% horizontal bone deficiency in the upper jaw in the area of the missing teeth was clearly evident radiographically and using volumetric evaluation of three-dimensional reconstructed CBCT images prior to treatment (Fig. 4A). Immediately after grafting, a second CBCT was performed and showed a 10- to 12-mm increase in horizontal width of the jawbone (Fig. 4B). Four months after grafting and immediately before implant placement, a third CBCT was performed and showed that, compared with immediately following grafting, there was an overall 25% reduction of the initial grafted width (Fig. 4B, 4C). However, relative to the original jawbone deficiency, there was a net 5- to 6-mm horizontal gain in width of the jawbone, resulting in 80% regeneration of the original jawbone deficiency (Fig. 4A, 4C).
Figure 4.
Cone-beam computed tomography (CBCT) scans. CBCT scans were used to render three-dimensional reconstructions of the anterior segment of the upper jaw and cross-sectional (top view) radiographic images to show volumetric changes of the upper jaw at three time points. (A, B): The initial clinical presentation shows 75% jawbone width deficiency. (C, D): Immediately following cell therapy grafting, there is full restoration of jawbone width. (E, F): Images show 25% resorption of graft at 4 months and overall net 80% regeneration of the original ridge-width deficiency.
Four months following healing, the grafted site was re-entered for oral implant placement, and there was clinically apparent evidence of bone regeneration with a new horizontal ridge width of 8–9 mm (Fig. 5A, 5B). Oral implants were then stably placed in the previously grafted sites and biomechanically torqued to standard-of-care guidelines of 35 newton centimeters (Fig. 5C, 5D). Implants were left submerged under the gingival tissue (Fig. 5E, 5F) for 6 months of healing. Micro-CT and histological evaluation of the bone biopsy harvested from the area of the grafted region revealed highly vascularized, mineralized tissue indicative of bone formation and 80% of the β-TCP matrix resorbed (Fig. 5G, 5H). Full functional and aesthetic restoration of the area was completed 6 months following implant placement, with the engineering and placement of an oral implant-supported dental prosthesis (Fig. 6).
Figure 5.
Surgical re-entry of the grafted site and implant placement. Following elevation of a full-thickness gingival flap, front view (A) and top view (B) of the treated site reveal regenerated tissue and a reconstructed alveolar ridge clinically measuring a width of 8–10 mm. Front view (C) and top view (D) of the placement of dental implants in the regenerated sites. (E, F): Primary closure of the site. A bone core biopsy was retrieved from one of the regenerated sites to determine the presence of mineralized tissue with micro-computed tomography analysis (G) and to confirm the histomorphometric appearance of bone tissue histologically (H) with hematoxylin and eosin staining (green arrows highlight residual β-tricalcium phosphate, yellow arrows highlight bone tissue; magnification: ×40 and ×100).
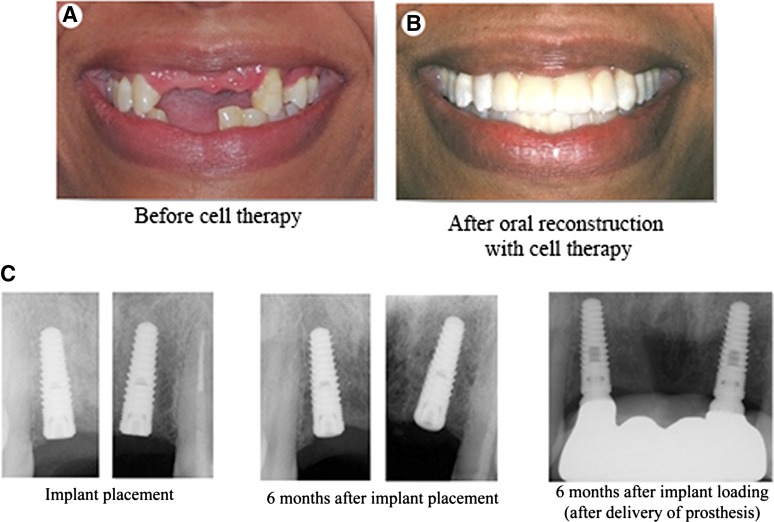
Figure 6.
Complete oral rehabilitation. Clinical presentation of the patient prior to initiation of treatment (A) and following completed oral reconstruction (B). (C): Periapical radiographs of oral implants showing osseointegration of implants and stable bone levels at the time of placement, 6 months following placement, and 6 months following functional restoration and biomechanical loading of implants with a dental prosthesis.
Discussion
Regenerative medicine aims to use tissue engineering and biomimetic strategies to functionally restore and replace damaged and lost tissue [24]. In this report, we describe a cell therapy for the oral reconstruction of a patient who lost teeth and supporting jawbone tissue as a result of a traumatic injury to the face. In addition, optimized parameters for cell attachment and survival were defined for the cell transplantation protocol used in this approach. To date, this study represents the most advanced craniofacial trauma reconstruction using a stem cell-based therapy for oral rehabilitation involving oral implants.
Important considerations in regenerative medicine involving cell-transplantation protocols are the conditions under which cells are delivered [9, 25]. These parameters are of even greater importance if biomaterials are used for delivery of cells. β-TCP has been used as a bone graft substitute material to fill in small, localized bone deficiencies around teeth and in very limited orthopedic indications. However, there have been no reported clinical investigations of its use as a scaffold for a stand-alone cell therapy to treat large craniofacial deficiencies. In a case series, Sandor and colleagues recently reported its use as a scaffold to deliver adipose-derived stem cells to jawbone defects in combination with large doses of BMP-2 [14]. These defects were secondary to tumor resective surgery, and although radiographic outcomes were deemed favorable, limited data were presented relative to the clinical, functional, and histological integrity of the regenerated jawbone tissue. We used β-TCP in our study as a carrier to deliver the cells because tricalcium phosphates are highly biocompatible, have been shown to support osteogenic activity of mesenchymal stem cells, and have been used as a delivery vehicle in a number of animal studies in which cell transplantation has been used [16, 26–28]. Krebsbach and colleagues reported that relative to other biomaterials commonly used clinically, such as gelatin sponges and demineralized bone matrix, tricalcium phosphates most consistently yield bone formation in vivo when used as a delivery vehicle for mesenchymal stem cells [27]. Although this material has favorable characteristics for cell proliferation, differentiation, and in vivo bone formation, studies have not evaluated or reported the cell-seeding efficiency (i.e., how efficiently cells attach to β-TCP) of the cells when β-TCP is used as a delivery vehicle. Cell attachment and seeding efficiency can have significant influence on the regenerative response in determining the number of cells that reach the regenerative site [29]. Our study determined that the minimum time needed for incubation of the cells to allow the greatest cell attachment (>81%) was 30 minutes. We did not evaluate time points longer than 60 minutes because cell seeding occurs at the time of surgical application of the cells. Hence, incubation times greater than 60 minutes would affect the clinical protocol and prolong the surgical procedure, which could have adverse consequences on outcomes (e.g., increased risk of infection, increased bleeding, increased inflammation). Another important clinical consideration for cell transplantation, particularly if there is an incubation period prior to delivery of the cells, is the incubation temperature. During the incubation time frames of 15, 30, and 60 minutes, it was determined that the cell-seeding efficiency was no different if cells were incubated at 4°C or at room temperature.
Regardless of the material or modality used to deliver cells during the process of cell transplantation, it is clear that cell-seeding efficiency is an important determinant of the number of cells that reach a regenerative site. Despite the impact of seeding efficiency on successful regenerative outcomes, the viability of the cells that are delivered is even more critical to the outcomes achieved with this approach [30]. Although temperature did not affect cell-seeding efficiency in our study, it is well established that temperature can have a profound effect on cell viability. In various tissue-grafting and organ-transplantation protocols, it is often highly desirable to maintain tissue specimens at 4°C (on ice) until ready for application or placement in the recipient site [31–34]. In regenerative cell-transplantation strategies involving stem cells, although important, this parameter has not been thoroughly examined. In our study, we found that if cells were incubated with β-TCP for 30 minutes or less, survival was not affected by the incubation temperature (room temperature vs. 4°C). If cells were incubated for 1 hour, cell survival was significantly greater when the cells were incubated at 4°C relative to when incubation occurred at room temperature. Beyond 30 minutes, it was determined that cells should be maintained at 4°C prior to delivery to achieve the greatest cell viability.
Using a different biomaterial for cell delivery, our group recently completed a randomized controlled clinical trial investigating a similar cell-therapy approach in the treatment of small, localized, alveolar bone defects created following tooth removal [15]. The extraction socket defect created in alveolar bone following tooth removal heals, to an extent, without intervention and thus serves as a natural clinical model of bone healing [35]. In our previous study, ixmyelocel-T was administered to these extraction socket defects at the time of tooth removal, and delivery of the cells resulted in acceleration of the innate bone regeneration within the localized defect. Despite these promising results, because there is innate bone regeneration following tooth removal, this model does not serve as an ideal model for evaluating de novo bone regeneration. In addition, this study was performed as a proof of concept to demonstrate safety and efficacy of the approach; however, from a feasibility standpoint, cell therapy would not be used in such a localized defect following removal of a tooth. The more appropriate application for this cell therapy would be in more complex and severe craniofacial defects, as often occur following oral-facial trauma. A severe defect resulting from a traumatic injury would not naturally resolve without significant intervention and also results in significant functional and aesthetic deficiencies. As such, these defects typically require advanced bone-grafting procedures with autogenous blocks of bone or guided bone regenerative (GBR) procedures [36]. Similar to our surgical procedure, the GBR approach uses a protective barrier membrane to cover the allogeneic or alloplastic graft material during healing. However, following GBR for large reconstructions of alveolar bone, most protocols allow a healing period, minimally, of 6–8 months before re-entry for oral implant placement [37]. Through delivery of 100 million cells using a tissue engineering cell-therapy approach, in only 4 months we were able to regenerate 80% of the original jawbone deficiency, which was sufficient to stably place oral implants to biomechanically support a dental prosthesis.
Conclusion
Cell survival and seeding efficiency in the context of tissue engineering and cell-therapy strategies are critical parameters for success that have not been rigorously examined in a clinical context. This study defined optimized conditions for these parameters using an autologous stem cell therapy to successfully treat a patient who had a debilitating craniofacial traumatic deficiency. To our knowledge, there have been no other clinical reports of cell therapy for the treatment of craniofacial trauma defects. This clinical report serves as solid foundation on which to develop more expanded studies using this approach for the treatment of larger numbers of patients with other debilitating conditions (e.g., congenital disorders) to further evaluate efficacy and feasibility.
Acknowledgments
This study was funded by a Career Award for Medical Scientists from the Burroughs Wellcome Fund (D.K.), the Oral-Maxillofacial Surgery Foundation, the National Center for Advancing Translational Sciences/NIH (UL1TR000433), and the National Institute of Dental and Craniofacial Research/NIH (1R56DE23095-01A1). We thank Ronnda Bartel, Judy Douville, Andrew Eisenberg, Christina Huffman, and Susan Tarle for administrative and clinical support.
Author Contributions
A.R.: conception and design, provision of study materials or patients, data analysis and interpretation, manuscript writing, final approval of manuscript; E.E.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; S.E.: conception and design, financial support, data analysis and interpretation, final approval of manuscript; S.A.: conception and design, data analysis and interpretation, final approval of manuscript; S.T.: provision of study materials or patients, data analysis and interpretation, final approval of manuscript; I.R., F.W., and A.L.: collection and/or assembly of data, data analysis and interpretation, final approval of manuscript; D.K.: conception and design, financial support, collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Thorén H, Numminen L, Snäll J, et al. Occurrence and types of dental injuries among patients with maxillofacial fractures. Int J Oral Maxillofac Surg. 2010;39:774–778. doi: 10.1016/j.ijom.2010.03.024. [DOI] [PubMed] [Google Scholar]
- 2.Gassner R, Tuli T, Hachl O, et al. Cranio-maxillofacial trauma: A 10 year review of 9,543 cases with 21,067 injuries. J Craniomaxillofac Surg. 2003;31:51–61. doi: 10.1016/s1010-5182(02)00168-3. [DOI] [PubMed] [Google Scholar]
- 3.Allareddy V, Allareddy V, Nalliah RP. Epidemiology of facial fracture injuries. J Oral Maxillofac Surg. 2011;69:2613–2618. doi: 10.1016/j.joms.2011.02.057. [DOI] [PubMed] [Google Scholar]
- 4.Misch CM. Comparison of intraoral donor sites for onlay grafting prior to implant placement. Int J Oral Maxillofac Implants. 1997;12:767–776. [PubMed] [Google Scholar]
- 5.Myeroff C, Archdeacon M. Autogenous bone graft: Donor sites and techniques. J Bone Joint Surg Am. 2011;93:2227–2236. doi: 10.2106/JBJS.J.01513. [DOI] [PubMed] [Google Scholar]
- 6.Kirmeier R, Payer M, Lorenzoni M, et al. Harvesting of cancellous bone from the proximal tibia under local anesthesia: Donor site morbidity and patient experience. J Oral Maxillofac Surg. 2007;65:2235–2241. doi: 10.1016/j.joms.2006.11.038. [DOI] [PubMed] [Google Scholar]
- 7.Barone A, Ricci M, Mangano F, et al. Morbidity associated with iliac crest harvesting in the treatment of maxillary and mandibular atrophies: A 10-year analysis. J Oral Maxillofac Surg. 2011;69:2298–2304. doi: 10.1016/j.joms.2011.01.014. [DOI] [PubMed] [Google Scholar]
- 8.Krebsbach PH, Robey PG. Dental and skeletal stem cells: Potential cellular therapeutics for craniofacial regeneration. J Dent Educ. 2002;66:766–773. [PubMed] [Google Scholar]
- 9.Gamie Z, Tran GT, Vyzas G, et al. Stem cells combined with bone graft substitutes in skeletal tissue engineering. Expert Opin Biol Ther. 2012;12:713–729. doi: 10.1517/14712598.2012.679652. [DOI] [PubMed] [Google Scholar]
- 10.Delaere P, Vranckx J, Verleden G, et al. Tracheal allotransplantation after withdrawal of immunosuppressive therapy. N Engl J Med. 2010;362:138–145. doi: 10.1056/NEJMoa0810653. [DOI] [PubMed] [Google Scholar]
- 11.Macchiarini P, Jungebluth P, Go T, et al. Clinical transplantation of a tissue-engineered airway. Lancet. 2008;372:2023–2030. doi: 10.1016/S0140-6736(08)61598-6. [DOI] [PubMed] [Google Scholar]
- 12.Marcacci M, Kon E, Moukhachev V, et al. Stem cells associated with macroporous bioceramics for long bone repair: 6- to 7-year outcome of a pilot clinical study. Tissue Eng. 2007;13:947–955. doi: 10.1089/ten.2006.0271. [DOI] [PubMed] [Google Scholar]
- 13.Soltan M, Smiler D, Soltan C, et al. Bone grafting by means of a tunnel dissection: Predictable results using stem cells and matrix. Implant Dent. 2010;19:280–287. doi: 10.1097/ID.0b013e3181e40166. [DOI] [PubMed] [Google Scholar]
- 14.Sándor GK, Numminen J, Wolff J, et al. Adipose stem cells used to reconstruct 13 cases with cranio-maxillofacial hard-tissue defects. Stem Cells Translational Medicine. 2014;3:530–540. doi: 10.5966/sctm.2013-0173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kaigler D, Pagni G, Park CH, et al. Stem cell therapy for craniofacial bone regeneration: A randomized, controlled feasibility trial. Cell Transplant. 2013;22:767–777. doi: 10.3727/096368912X652968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Yeo A, Rai B, Sju E, et al. The degradation profile of novel, bioresorbable PCL-TCP scaffolds: An in vitro and in vivo study. J Biomed Mater Res A. 2008;84:208–218. doi: 10.1002/jbm.a.31454. [DOI] [PubMed] [Google Scholar]
- 17.Emerton KB, Drapeau SJ, Prasad H, et al. Regeneration of periodontal tissues in non-human primates with rhGDF-5 and beta-tricalcium phosphate. J Dent Res. 2011;90:1416–1421. doi: 10.1177/0022034511423665. [DOI] [PubMed] [Google Scholar]
- 18.Kim DM, Camelo M, Nevins M, et al. Alveolar ridge reconstruction with a composite alloplastic biomaterial. Int J Periodontics Restorative Dent. 2012;32:e204–e209. [PubMed] [Google Scholar]
- 19.Stavropoulos A, Windisch P, Gera I, et al. A phase IIa randomized controlled clinical and histological pilot study evaluating rhGDF-5/β-TCP for periodontal regeneration. J Clin Periodontol. 2011;38:1044–1054. doi: 10.1111/j.1600-051X.2011.01778.x. [DOI] [PubMed] [Google Scholar]
- 20.Dennis JE, Esterly K, Awadallah A, et al. Clinical-scale expansion of a mixed population of bone-marrow-derived stem and progenitor cells for potential use in bone-tissue regeneration. Stem Cells. 2007;25:2575–2582. doi: 10.1634/stemcells.2007-0204. [DOI] [PubMed] [Google Scholar]
- 21.Caldwell J, Palsson BO, Locey B, et al. Culture perfusion schedules influence the metabolic activity and granulocyte-macrophage colony-stimulating factor production rates of human bone marrow stromal cells. J Cell Physiol. 1991;147:344–353. doi: 10.1002/jcp.1041470221. [DOI] [PubMed] [Google Scholar]
- 22.Schwartz RM, Palsson BO, Emerson SG. Rapid medium perfusion rate significantly increases the productivity and longevity of human bone marrow cultures. Proc Natl Acad Sci USA. 1991;88:6760–6764. doi: 10.1073/pnas.88.15.6760. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kaigler D, Pagni G, Park CH, et al. Angiogenic and osteogenic potential of bone repair cells for craniofacial regeneration. Tissue Eng Part A. 2010;16:2809–2820. doi: 10.1089/ten.tea.2010.0079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Vacanti JP, Langer R. Tissue engineering: The design and fabrication of living replacement devices for surgical reconstruction and transplantation. Lancet. 1999;354(suppl 1):SI32–SI34. doi: 10.1016/s0140-6736(99)90247-7. [DOI] [PubMed] [Google Scholar]
- 25.Steinert AF, Rackwitz L, Gilbert F, et al. Concise review: The clinical application of mesenchymal stem cells for musculoskeletal regeneration: Current status and perspectives. Stem Cells Translational Medicine. 2012;1:237–247. doi: 10.5966/sctm.2011-0036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rai B, Lin JL, Lim ZX, et al. Differences between in vitro viability and differentiation and in vivo bone-forming efficacy of human mesenchymal stem cells cultured on PCL-TCP scaffolds. Biomaterials. 2010;31:7960–7970. doi: 10.1016/j.biomaterials.2010.07.001. [DOI] [PubMed] [Google Scholar]
- 27.Krebsbach PH, Kuznetsov SA, Satomura K, et al. Bone formation in vivo: Comparison of osteogenesis by transplanted mouse and human marrow stromal fibroblasts. Transplantation. 1997;63:1059–1069. doi: 10.1097/00007890-199704270-00003. [DOI] [PubMed] [Google Scholar]
- 28.Zhou J, Lin H, Fang T, et al. The repair of large segmental bone defects in the rabbit with vascularized tissue engineered bone. Biomaterials. 2010;31:1171–1179. doi: 10.1016/j.biomaterials.2009.10.043. [DOI] [PubMed] [Google Scholar]
- 29.Coquelin L, Fialaire-Legendre A, Roux S, et al. In vivo and in vitro comparison of three different allografts vitalized with human mesenchymal stromal cells. Tissue Eng Part A. 2012;18:1921–1931. doi: 10.1089/ten.TEA.2011.0645. [DOI] [PubMed] [Google Scholar]
- 30.Jungebluth P, Haag JC, Lim ML, et al. Verification of cell viability in bioengineered tissues and organs before clinical transplantation. Biomaterials. 2013;34:4057–4067. doi: 10.1016/j.biomaterials.2013.02.057. [DOI] [PubMed] [Google Scholar]
- 31.McLaren AJ, Friend PJ. Trends in organ preservation. Transplant Int. 2003;16:701–708. doi: 10.1007/s00147-003-0659-2. [DOI] [PubMed] [Google Scholar]
- 32.Kheirabadi BS, Fahy GM. Permanent life support by kidneys perfused with a vitrifiable (7.5 molar) cryoprotectant solution. Transplantation. 2000;70:51–57. [PubMed] [Google Scholar]
- 33.Garrity JT, Stoker AM, Sims HJ, et al. Improved osteochondral allograft preservation using serum-free media at body temperature. Am J Sports Med. 2012;40:2542–2548. doi: 10.1177/0363546512458575. [DOI] [PubMed] [Google Scholar]
- 34.Eves PC, Baran M, Bullett NA, et al. Establishing a transport protocol for the delivery of melanocytes and keratinocytes for the treatment of vitiligo. Tissue Eng Part C Methods. 2011;17:375–382. doi: 10.1089/ten.TEC.2010.0221. [DOI] [PubMed] [Google Scholar]
- 35.Schenk RK, Buser D, Hardwick WR, et al. Healing pattern of bone regeneration in membrane-protected defects: A histologic study in the canine mandible. Int J Oral Maxillofac Implants. 1994;9:13–29. [PubMed] [Google Scholar]
- 36.Melcher AH. On the repair potential of periodontal tissues. J Periodontol. 1976;47:256–260. doi: 10.1902/jop.1976.47.5.256. [DOI] [PubMed] [Google Scholar]
- 37.McAllister BS, Haghighat K. Bone augmentation techniques. J Periodontol. 2007;78:377–396. doi: 10.1902/jop.2007.060048. [DOI] [PubMed] [Google Scholar]