The ability of adipose-derived stromal/stem cells (ASCs) to facilitate long-term allograft survival was investigated. ASC and bone marrow cell (BMC) coinfusion with minimal conditioning led to stable lymphoid and myeloid macrochimerism, deletion of alloreactive T cells, expansion of regulatory T cells, and long-term allograft survival (>200 days). Translational advanced vascular composite allotransplants with ASCs and low-dose donor BMCs in nonhuman primates can be developed to enhance functional outcomes and eliminate long-term immunosuppression.
Keywords: Adipose tissue-derived stromal cells, Allogeneic transplantation, Bone marrow, T regulatory cells, Chimerism, Tolerance induction
Abstract
Amputations and unsalvageable injuries with devastating tissue loss are common in the combat wounded. Reconstructive transplantation in the civilian setting using vascular composite allotransplants (VCAs) with multiple tissues (skin, muscle, nerve, bone) combined with long-term multidrug immunosuppression has been encouraging. However, skin rejection remains a critical complication. Adipose-derived stromal/stem cells (ASCs) are easily obtained from normal individuals in high numbers, precluding ex vivo expansion. The reparative function and paracrine immunomodulatory capacity of ASCs has gained considerable attention. The present study investigated whether ASCs facilitate long-term skin allograft survival. ASCs were isolated from fresh human subcutaneous adipose lipoaspirate. Full-thickness skin grafts from BALB/c mice were transplanted onto the dorsal flanks of C57BL/6 mice treated with five doses of anti-CD4/CD8 monoclonal antibodies (10 mg/kg) on days 0, +2, +5, +7, and +14 relative to skin grafting. A single nonmyeloablative low dose of busulfan (5 mg/kg) was given on day +5. Seven days after skin transplantation, ASCs (3 × 106) were infused i.v. with or without donor bone marrow cells (BMCs; 5 × 105). ASC+BMC coinfusion with minimal conditioning led to stable lymphoid and myeloid macrochimerism, deletion of alloreactive T cells, expansion of regulatory T cells, and long-term allograft survival (>200 days). ASCs constitutively produced high levels of anti-inflammatory/immunoregulatory factors such as prostaglandin E2, indoleamine 2,3-dioxygenase, APO-1/Fas (CD95), and programmed cell death-1 ligand-2. These findings serve as a foundation for developing a translational advanced VCA protocol, embodying both ASCs and low-dose donor BMCs, in nonhuman primates, with the goal of enhancing functional outcomes and eliminating the complications associated with long-term immunosuppression.
Introduction
Traumatic amputations, major burns, and unsalvageable injuries with devastating tissue loss are common in the combat wounded and victims of domestic terrorism and civilian trauma [1–3]. Reconstructive transplantation in the civilian setting using vascular composite allotransplants (VCAs) composed of multiple tissues, including skin, muscle, tendon, fat, blood vessels, nerve, and bone, combined with long-term multidrug immunosuppression, has been encouraging, especially in the context of hand and face transplantation [4, 5]. However, skin rejection remains a critical complication and one of the key immunologic challenges, along with the side effects of current immunosuppression [4, 6–8]. Modern transplantation has its origins with the understanding of immune responses in the context of skin coverage for burn wounds during World War II, and several strategies have had promise in the preclinical arena, including, most recently, costimulation alteration. However, long-term graft acceptance of VCAs with minimal or no immunosuppression has remained elusive [9–11].
Interest in multipotent tissue-derived mesenchymal stromal/stem cells (MSCs) as cell-based regenerative therapies for use in a variety of tissue repair and regeneration applications has gained much attention during the past decade [12, 13]. The intrinsic trophic and paracrine actions and anti-inflammatory and immunomodulatory properties of these cells have been shown to be key in stimulating the recruitment and mobilization of endogenous progenitor cells and tissue regeneration [14, 15], dampening immune-mediated responses [16, 17], inhibiting activated T-cell proliferation [18–20], and suppressing dendritic cell activation and differentiation [21, 22] within various inflammatory niches. Compared with bone marrow and cord blood-derived MSCs, adipose-derived stromal/stem cells (ASCs) have more potent inhibitory immunomodulatory effects on T and natural killer cell activation [23, 24].
The reparative function, paracrine immunomodulatory capacity, and immunosuppressive function of ASCs have gained considerable attention for clinical applications [25–28]. ASCs are an ideal source of allogeneic donor cells because they are well characterized phenotypically and functionally and can be easily obtained using relatively minimally invasive methods from normal individuals in high numbers, reducing the need for subsequent ex vivo expansion in culture [29]. Recently, we reported that human amnion-derived epithelial cells (AECs), when used in concert with immunological conditioning, support engraftment of limited numbers of donor bone marrow cells across major histocompatibility complex (MHC) barriers and lead to stable multilineage mixed-chimerism and skin allograft tolerance without the need for long-term immunosuppression [19]. In the present study, we investigated whether ASCs, with their favorable therapeutic profile, can facilitate long-term allograft survival in our well-established murine skin allograft transplantation model.
Materials and Methods
Animals
The Walter Reed Army Institute of Research/Naval Medical Research Center Institutional Animal Care and Use Committee reviewed and approved the study protocol, in compliance with all applicable Federal regulations governing the protection of animals in research. Pathogen-free female (10–12 weeks old) C57BL/6 (H-2b), BALB/c (H-2d), and C3H/HeJ (H-2k) mice were purchased from Jackson Laboratory (Bar Harbor, ME, http://www.jax.org). During the experiments, the mice were individually housed in microisolator cages on laminar flow racks and provided with standard laboratory food and water ad libitum.
Adipose Tissue-Derived Stromal/Stem Cells
Human ASCs were isolated from liposuction aspirates provided by healthy donors with informed consent under an institutional review board-approved protocol and obtained from LaCell, LLC (New Orleans, LA, http://www.lacell.wpengine.com) using previously published methods [30, 31] and stored in liquid nitrogen until infusion. For intravenous infusion, ASCs from multiple donors (≥2) were thawed, pooled, washed, and resuspended in Dulbecco’s phosphate-buffered saline containing 100 U/ml preservative-free heparin (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and 100 U/ml DNase containing 5 mM Mg2+ (Worthington Biochemical Corp., Lakewood Township, NJ, http://www.worthington-biochem.com) to minimize cellular aggregation and lethal pulmonary embolic events [19].
Skin Grafting
Full-thickness trunk skin grafts (4 cm2) from BALB/c were transplanted onto the dorsal flanks of C57BL/6 recipient mice and secured with an adhesive bandage for 7 days, as previously described [32]. Graft survival was monitored by daily visual inspection, and rejection was defined as the complete loss of viable epidermal tissue. Survival is expressed as the mean survival time (MST) ± SD.
Conditioning Regimen and Allogeneic Bone Marrow Plus Xenogeneic ASC Infusion Protocol
A total of 38 C57BL/6 mice underwent skin transplantation. Of the 38 mice, 10 with allografts were left untreated (controls). The remaining 28 skin transplanted mice were subjected to the previously reported nonmyeloablative conditioning regimen [19]. In brief, the regimen consisted of an intraperitoneal injection of anti-CD4 (YTS 191.1, 10 mg/kg) and anti-CD8 monoclonal antibodies (mAbs) (YTS 169.4, 10 mg/kg) on days 0, +2, +5, +7, and +14 and a nonmyeloablative low dose of busulfan (5 mg/kg i.p.; Sigma-Aldrich) on day +5. A cohort of 10 skin transplanted mice received conditioning therapy only. On day +7, 6 conditioned, skin-transplanted mice received an i.v. infusion of ASCs (3 × 106 cells). Another cohort of 12 conditioned, skin-transplanted mice received an i.v. coinfusion of ASCs (3 × 106 cells) and donor bone marrow cells (5 × 105).
Flow Cytometric Analysis of Chimerism and Quantification of T-Regulatory Cells and Alloreactive T-Cell Clonal Deletion
Multiple flow cytometric assays were conducted to assess the percentage of donor cell chimerism, the number of CD4+CD25+FoxP3+ T-regulatory cells, and the level of alloreactive T-cell depletion in the blood and spleen using a BD FACSAria II flow cytometer (BD Biosciences, San Jose, CA, http://www.bdbiosciences.com) and a panel of fluorochrome-labeled mAbs specific for mouse cell surface and intracellular proteins [19].
Mixed Lymphocyte Reaction, Suppressor-Regulatory Cell Assay, and Cytokine Measurements
A mixed lymphocyte reaction (MLR) was performed as previously described [19]. In brief, 5 × 105 responding C57BL/6 splenocytes were cultured with 5 ×105 BALB/c irradiated (30 Gy 137Cs) splenocytes in flat-bottom 96-well plates in complete culture medium consisting of Roswell Park Memorial Institute 1640 supplemented with 10% fetal bovine serum, 10 mM HEPES, 1% nonessential amino acids, 2 mM l-glutamine, 100 U/ml penicillin, 100 µg/ml streptomycin (all from Invitrogen, Carlsbad, CA, http://www.invitrogen.com), and 5 µg/ml 2-mercaptoethanol (Sigma-Aldrich) at 37°C in a humidified atmosphere of 5% CO2/95% air for 5 days. Irradiated ASCs were added at 5 × 105 per well for standard assays or in graded numbers in the titration experiments. Bromodeoxyuridine (BrdU) incorporation was detected by enzyme-linked immunosorbent assay (ELISA) (Roche, San Francisco, CA, http://www.roche.com). The results are expressed as the net absorbance of the stimulated cells minus the absorbance of the unstimulated cells. Irradiated splenocytes from tolerant recipients (putative regulatory cells) were added to allo-MLR cultures in graded numbers in titration experiments with a fixed number of responder cells (5 × 105). The cells and culture supernatants were collected from 48-hour allo-MLR cultures and ASC cultures (1 × 106 ASCs per milliliter) treated with or without interferon-γ (IFNγ) (10 ng/ml, Sigma-Aldrich). Total cellular mRNA was isolated using the TRIzol reagent (Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com) and extracted from cells using the Qiagen RNeasy Lipid Kit (Qiagen, Venlo, The Netherlands, http://www.qiagen.com). Reverse transcriptase-polymerase chain reaction (RT-PCR) was used to convert 1 µg of RNA to cDNA. In several experiments, responder cells were surface stained with fluorochrome-labeled mAbs to CD4 and CD25, followed by incubation with FACS permeabilizing solution (BD BioSciences), intracellular staining for Ki-67 (BioLegend, San Diego, CA, http://www.biolegend.com) and polychromatic flow cytometric analysis [33]. Quantitative real-time polymerase chain reaction (qRT-PCR) was used to detect mRNA transcripts for prostaglandin-endoperoxide synthase-2 (PTGS2-COX-2), transforming growth factor-β2 (TGFβ2), indole 2,3-dioxygenase (IDO), tumor necrosis factor (TNF) receptor superfamily member-6 (TNFRSF6, FAS), hepatocyte growth factor (HGF), programmed cell death-1 ligand-1 (PD-L1), and programmed cell death-1 ligand-2 (PD-L2). The ΔCt and 2−ΔCt methods to calculate the level of gene expression and relative fold changes in gene expression, respectively, using qRT-PCR [34]. Culture supernatants collected from 48-hour ASC cultures (1 × 106 ASCs per milliliter) with or without IFNγ (10 ng/ml) were analyzed for prostaglandin E2 (PGE2) and TGFβ2 using ELISA (Cayman Chemical, Ann Arbor, MI, http://www.caymanchem.com). Harvested ASCs were analyzed for cell surface expression of FAS, human leukocyte antigen-G (HLA-G), PD-L1 and PD-L2 (BioLegend), and intracellular staining of IDO (R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com).
Statistical Analysis
The interval to skin graft rejection is represented by Kaplan-Meier survival curves, and the comparison of graft survival was calculated using the log-rank test. Additional statistical analysis was performed using a two-tailed unpaired Student t test. A p value of <.05 was considered significant for all tests (GraphPad Software, Inc., San Diego, CA, http://www.graphpad.com).
Results
T-Cell Immunosuppressive Capacity of ASCs
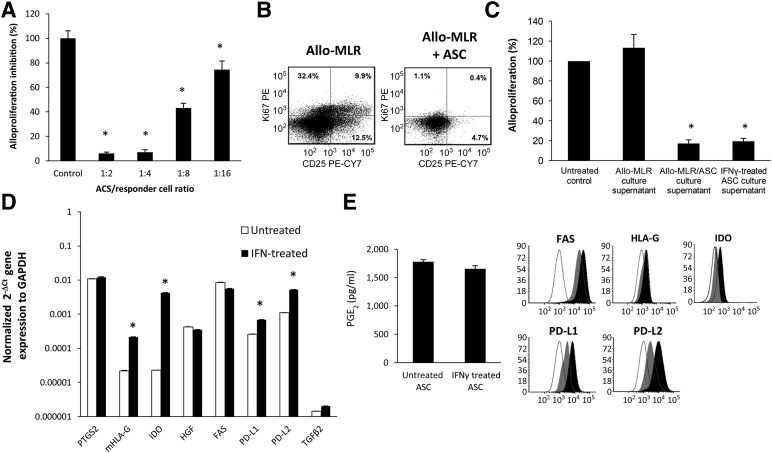
Human ASCs added to murine mixed lymphocyte (MLR) cultures at the initiation of culture suppressed murine T-cell proliferation in a dose-dependent manner (Fig. 1A). As shown in Figure 1B, CD4+ T cells from ASC MLR cocultures did not divide (with a low expression of Ki-67, a molecule expressed during cell cycle progression) [33] and remained phenotypically naïve, with low expression of CD25 compared with CD4+ T cells from control MLR cultures. Furthermore, soluble factors from day 3 ASC/MLR and ASC IFNγ-treated culture supernatants demonstrated potent inhibition of alloantigen-driven proliferation (Fig. 1C). We, and others, have demonstrated that ASCs express and produce PGE2 and indoleamine 2,3-dioxygenase (IDO) constitutively [28, 35–38]. In addition, the immunomodulatory effects of ASCs are associated with expressed mRNA transcripts for TNFRSF6 (FAS), HGF, PD-L1, and PD-L2 and lower transcript numbers for membrane HLA-G1 (mHLA-G1) (Fig. 1D). Consistent with the findings of Crop et al. [39], under MLR proinflammatory conditions and IFNγ stimulation, we determined that ASCs expressed marked increases of IDO (218.5-fold) and mHLA-G (11.2-fold) and modest increases of PD-L1 (4.1-fold) and PD-L2 (5.1-fold) gene transcripts. Protein expression validation of mRNA transcript results was performed using ELISA, cell surface staining, and intracellular staining for IDO using flow cytometric analysis (Fig. 2E). TGFβ2 in culture supernatants was not detected, as measured by ELISA (data not shown), consistent with the low gene transcript value we have reported. The HGF levels were not assayed.
Figure 1.
Human ASCs inhibit murine allo-MLR lymphocyte proliferation. (A): Naïve C57BL/6 splenocytes (as responder cells) were cultured 1:1 with irradiated (30 Gy) naïve BALB/c stimulatory cells. ASCs were added to the MLR at the onset of culture at the indicated responder/ASC ratios. After 4 days, the quadruplicate replicate cocultures were pulsed for 18 hours with bromodeoxyuridine (BrdU). A specific enzyme-linked immunosorbent assay (ELISA) kit was used to measure BrdU incorporation into newly synthesized DNA, expressed as the mean absorbance ± SD. The results are expressed as the mean percentage of alloproliferation of control cultures. ∗, p < .05, significant difference compared with control group. (B): Effect of ASC coculture on the expression of Ki-67 and CD25 on CD4+ responder T cells. A representative staining of gated CD4+ T cells from control MLR cultures and ASC-treated MLR cultures. (C): Supernatants from allo-MLR/ASC cocultures inhibited MLR lymphocyte proliferation. Naïve C57BL/6 splenocytes (as responders cells) were cultured 1:2 with irradiated (30 Gy) naïve BALB/c stimulatory cells. Supernatants (10% final plating dilution) from day 3 allo-MLR cultures, allo-MLR/ASC cocultures, or IFNγ treated (10 ng/ml) was added to the MLR at the onset of culture. Cell proliferation was analyzed by BrdU incorporation of quadruplicate cultures after a 5-day culture. Results are expressed as the mean percentage of the control MLR alloproliferation. (D): mRNA gene transcript expression patterns of immunoregulatory molecules PGE2, mHLA-G, HGF, FAS, PD-L1, PD-L2, and TGFβ2 by quantitative real-time polymerase chain reaction in unstimulated and IFNγ-treated ASC cultures after 48 hours of culture. Results are expressed as the relative expression compared with the housekeeping gene GAPDH. Results shown are representative of three separate experiments. ∗, p < .05, significant difference compared with the control group. (E): Effect of IFNγ treatment on ASC PGE2, FAS (CD95), HLA-G, PD-L1, PD-L2, and IDO production. PGE2 production was quantified by ELISA. Representative cell surface staining of FAS, HLA-G, PD-L1, and PD-L2 cell and intracellular staining of IDO was determined by flow cytometric analysis (black outlined histogram, isotype control; gray shaded histogram, untreated ASCs; and black shaded histogram, IFNγ treated). Abbreviations: ASC, adipose-derived stromal/stem cell; HGF, hepatocyte growth factor; IDO, indole 2,3-dioxygenase; IFNγ, interferon-γ; mHLA-G, membrane human leukocyte antigen-G; MLR, mixed lymphocyte reaction; PD-L1, programmed cell death-1 ligand-1; PD-L2, programmed cell death-1 ligand-2; PGE2, prostaglandin E2; TGFβ2, transforming growth factor-β2.
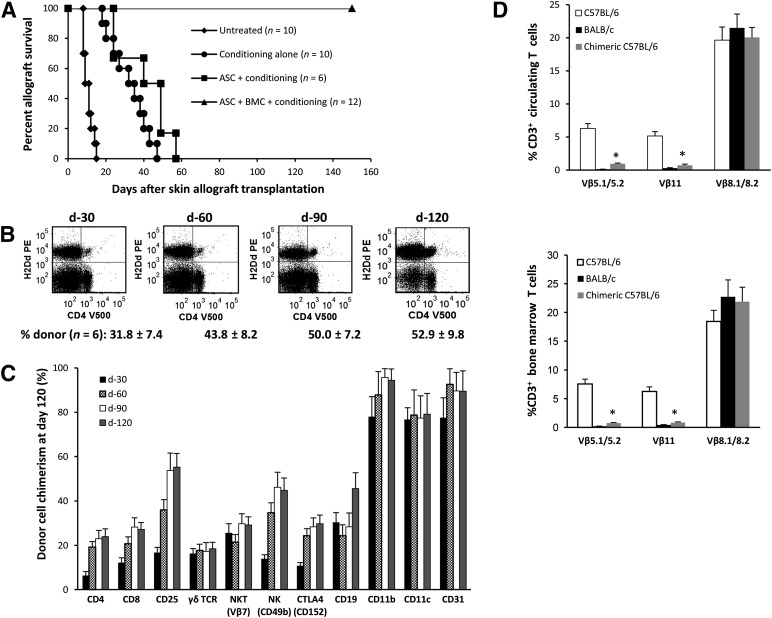
Figure 2.
A limited number of bone marrow cells (BMCs) plus adipose-derived stromal/stem cells (ASCs) induces indefinite skin allograft survival and mixed donor-recipient macrochimerism. (A): C57BL/6 (H-2b) recipients of BALB/c (H-2d) skin grafts received the following: (a) no treatment (n = 10); (b) cytoreduction condition only (n = 10; anti-CD4/CD8 monoclonal antibody [mAb] therapy plus 5 mg/kg busulfan); (c) ASC plus conditioning (n = 6); and (d) ASCs plus BMCs plus conditioning (n = 12). Treatment of mice with anti-CD4/CD8 mAbs (10 mg/kg) occurred on days 0, +2, +5, +7, and +14 relative to skin grafting on day 0. Mice were treated with a single dose of busulfan (5 mg/kg) at day 5 after grafting. (B, C): Percentage of donor-derived cells (H-2d) in the peripheral blood of allograft tolerant recipients measured 30, 60, 90, and 120 days after graft transplantation (n = 6 donor graft tolerant chimeric mice). Data points represent the mean ± SD for each lineage cell compartment (n = 6). (D): Deletion of alloreactive T-cell clones expressing specific TCR Vβ families. Peripheral blood and BMCs from chimeric and allograft recipient tolerant mice on day 200 after skin transplantation were stained with specific fluorochrome-conjugated antibodies (Abs) against CD3, Vβ5.1/5.2, Vβ8.1/8.2, and Vβ11 or isotype control Abs. The proportion of CD3+ T cells expressing each Vβ was determined by multicolor flow cytometry. Data points represent the mean ± SD for each group (n = 6). ∗, p < .05, significant difference compared with naïve C57BL/6 mice. Abbreviations: d, day; TCR, T cell receptor.
ASCs Support Skin Allograft Tolerance
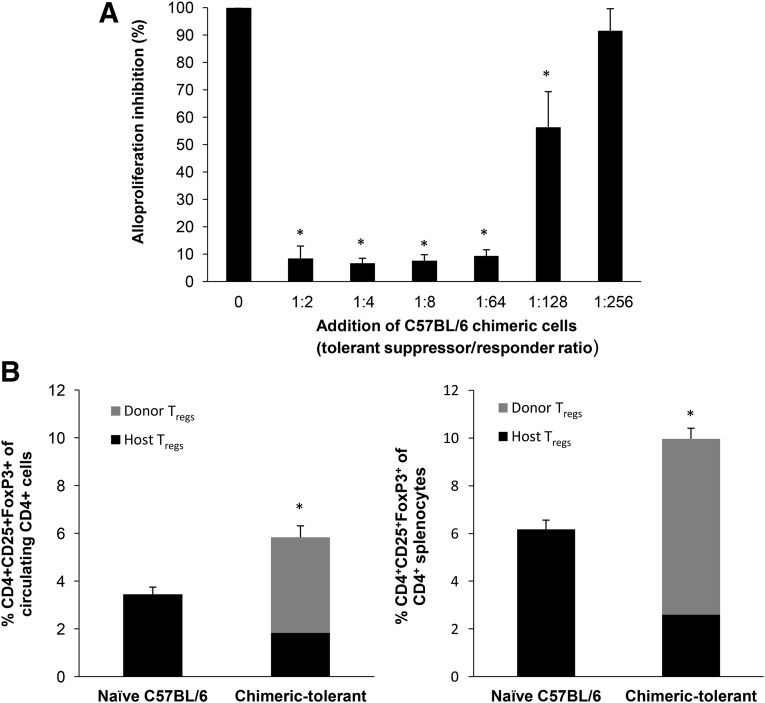
We have previously demonstrated that mice receiving limited numbers of donor bone marrow cells (BMCs) and AECs exhibited permanent tolerance to allogeneic and fully MHC-mismatched skin grafts [19]. As shown in Figure 2A, the coinfusion of human ASCs with limited numbers of donor BMCs effectively prevented allograft rejection (graft survival >200 days), with no evidence of graft-versus-host disease. Stable multilineage macrochimerism was clearly detected in the peripheral circulation and the spleen and bone marrow (data not shown) at 4 weeks (Fig. 2B, 2C), and both CD3+Vβ5.1/5.2+ and CD3+Vβ11+ alloreactive T cells were significantly depleted in both the periphery and the bone marrow (Fig. 2D). In contrast, the infusion of ASCs without donor BMCs showed no evidence of donor cell chimerism, with a partial, but not significant, effect in prolonging allograft survival (MST 40.5 ± 13.9 days, p = .1931) compared with the skin transplanted mice that had received only the conditioning regimen (MST 32.4 ± 9.9 days). We have previously reported that infusion of limited numbers of unfractionated donor BMCs alone in mice pretreated with full conditioning had no effect on prolonging graft survival or donor cell chimerism [19]. Consistent with the results from our previous study [19], most, if not all, of the administered ASCs became entrapped in the lungs and were undetectable using RT-PCR in any tissues after 7 days (data not shown). At 200 days, second-set skin grafting was performed on a cohort of chimeric mice to assess for allograft tolerance. Chimeric mice accepted a second BALB/C skin graft (>50 days) and rejected a third party C3H/HEJ skin graft within 2 weeks, indicating that the tolerance was antigen-specific (data not shown). Splenocytes isolated from chimeras at day 200 after skin grafting significantly inhibited allo-MLR antigen-specific T-cell responses in vitro in a dose-dependent manner (Fig. 3A), indicative of potent immunoregulatory suppressor cells. As shown in Figure 3B, chimeras had a significantly greater number of CD4+CD25+Foxp3+ regulatory T cells (Tregs) among circulating and splenic CD4+ T cells (3.5% ± 0.31% vs. 5.8% ± 0.48% and 6.18% ± 0.38% vs. 10.0% ± 0.44%, respectively, compared with age-matched naïve controls). Among the CD4+CD25+Foxp3+ Tregs in the blood and spleen, 69% and 74%, respectively, were of donor origin.
Figure 3.
Splenocytes from tolerant mice confer unresponsiveness to donor alloantigen in vitro. (A): Naïve C57BL/6 splenocytes (5 × 105) were cocultured with the same number of irradiated naïve allogeneic BALB/c spleen cells. To assess allospecific regulatory function in vitro, equal numbers of irradiated splenocytes from tolerant recipient C57BL/6 mice were cocultured as third-party regulatory cells at a suppressor/responder cell ratio ranging from 1:2 to 1:256. After 4 days, the cocultures were pulsed for 18 hours with bromodeoxyuridine. The data are expressed as the mean percentage of alloproliferation of control cultures. ∗, p < .05, significant difference compared with control group. Representative results from a single study using splenocytes from a single chimeric mouse (n = 6 mice). (B): Circulating leukocytes and splenocytes isolated from naïve and chimeric-tolerant C57BL/6 mice (day 200 after allograft transplantation) were stained with anti-CD4-V500, anti-CD25-PE-CY7, and anti-Foxp3-PB and analyzed by flow cytometry. The frequency of each T-regulatory cell population is expressed as the mean ± SD of six mice. ∗, p < .05, significant difference compared with naïve controls. Abbreviation: Tregs, regulatory T cells.
Discussion
In the present study, we have confirmed that ASCs have a potent immunosuppressive effect on T-cell activation in vitro and produce a number of key soluble factors involved in the immunomodulation of allogeneic T-cell proliferation and generation of regulatory T cells and have found that infusion of ASCs combined with limited numbers of donor bone marrow cells is effective in establishing permanent skin allograft survival in preconditioned mice under a nonmyeloablative regimen without the need for additional subsequent immunosuppression therapy. Indefinite allograft survival was associated with stable mixed lymphohematopoietic chimerism, central deletion of alloreactive T cells, and expansion of both donor and host CD4+CD25+FoxP3+ Tregs. These results are consistent with those from our previous studies using AECs as a source of protolerogenic immunoregulatory cells [19].
Immunological tolerance, defined as donor-specific hyporesponsiveness in the recipient in the absence of immunosuppression [40], has been achieved successfully in small animal models, with some success in preclinical nonhuman primate approaches and, rarely, in the clinical transplantation setting [41, 42]. The chronic inflammatory response and rejection of skin, a highly immunogenic tissue/organ, are major pathological challenges to establishing transplantation tolerance after VCA and reconstructive transplantation and avoiding the toxicity of long-term maintenance systemic immunosuppression [4, 6–8]. Clinically, the VCA graft rejection process is modulated by infiltrating activated proinflammatory cytokines producing CD4+ and CD8+ T cells [8]. In patients treated with nonspecific immune-depletion agents such as rapamycin, thymoglobulin, and alemtuzumab, suppression of donor-activated T cells, long-term graft acceptance, and stable graft function appear to correlate with increased numbers of immunosuppression-resistant recipient CD4+CD25highFoxP3+ Tregs [43, 44] within the graft and in circulation [44, 45]. The role of donor T-cell chimerism and the production and/or maintenance of donor Tregs in hyporesponsiveness have been clearly recognized in the prevention of allograft rejection [46–48]. Moreover, hyporesponsiveness has been shown to be reversible via Treg depletion [44]. Furthermore, Lee et al. [49] demonstrated in a mouse model that donor antigen reactive Treg therapy alone was insufficient to induce allograft tolerance in the absence of preconditioning to delete alloreactive T cells. In a more recent report using a rat hind limb osteomyocutaneous VCA transplant model, a single infusion of recipient (syngeneic) ASCs supported prolonged graft survival, elevated levels of circulating Tregs, and induced permanent tolerance in 60% of the transplant recipients in the absence of radiation conditioning and long-term immunosuppression [50]. However, the possibility of macro- or micro-donor cell chimerism was not assessed. More recently, Ramirez et al. [51] reported, using a full-thickness hemiabdominal wall and hind limb osteomyocutaneous allograft transplantation model in the rat, that 3 of 8 transplanted recipients that received a short course of cyclosporine-A and anti-lymphocyte serum and three doses of syngeneic ASCs on postoperative days 1, 8, and 15 became chimeric, which influenced long-term graft survival. We, and others, have shown that ASCs potently modulate immune responses in vitro and in vivo through suppression of dendritic cell (DC) differentiation and maturation and suppression of T-cell activation and proliferation [22, 24, 29, 36–38, 52]. Importantly, tolerance to both donor skin and bone marrow grafts was achieved using our protocol. Collectively, our findings indicate that a single systemic injection of human ASCs, combined with low numbers of donor BMCs, establishes in place the essential components thought to be important for support of indefinite allograft survival.
The mechanisms by which ASCs, AECs, and tissue-derived MSCs exert in vivo activation, inhibitory, and immunomodulatory effects on innate and adaptive immune responses are poorly defined. Although compelling evidence for the existence of ASC paracrine actions exists, much less is known about their nature, the stimuli for their release, and their mechanisms of action. Recent evidence has suggested that ASC-T-cell interactions and local microenvironmental factors (i.e., IFNγ) within inflammatory niches provide the differentiation induction signals for ASCs to acquire phenotypic and functional properties of regulatory nonprofessional antigen presenting cells (PD-L1+ and PD-L2+) resulting in the increased production of anti-inflammatory molecules, such as IDO, PGE2, TGFβ1, HGF, and interleukin-10 (IL-10), which induce or target immunoregulatory effector cells, such as Tregs and tolerogenic DCs [15, 53, 54]. However, other mechanisms cannot be discounted.
One of the most intriguing features of this work and that of others is that most, if not all, the tissue-derived stem cells infused intravenously become entrapped in the lungs, forming microemboli and resulting in secretion of anti-inflammatory protein TNFα-induced protein 6 (TSG-6), followed by apoptosis and necrosis (<7 days) [19, 55–57]. TSG-6 has been shown to decrease plasmin activity, neutrophil infiltration, and the synthesis of IL-1β, IL-6, macrophage inflammatory protein-1 (CCL2), chemokine ligand 1 (CXCL1), and matrix metallopeptidase 9 [56]. Furthermore, ASCs are CD95+ (FAS). Akiyama et al. [58] demonstrated in models of systemic sclerosis that the intravenous infusion of FAS+ bone marrow MSCs induces T-cell apoptosis and necrosis via the FAS/FASL pathway, which subsequently resulted in the upregulation of CD4+CD25+FoxP3+ Tregs, elevated levels of TGFβ, and tolerance induction. Collectively, these findings suggest that early ASC–immune cell interactions within an inflammatory niche, most likely owing to pulmonary inflammation caused by microemboli formation, could be critical for ASC/MSC-based therapeutic immunosuppression and tolerance induction.
Conclusion
We have demonstrated that a single infusion of ASCs combined with an extremely low dose of unfractionated BMCs results in stable long-term chimerism and subsequent skin graft tolerance without undo consequences such as graft-versus-host disease. The implications for this approach are broad and cross the entire field of allotransplantation, including bone marrow transfusion, solid organ transplantation, and vascularized composite allograft transplantation. The benefit in solid organ transplantation will be considerable, allowing for long-term graft survival without the need for chronic immunosuppression and the resulting multitude of adverse effects associated with such agents. The effect in VCA could be even more profound, in that patients who would be marginal candidates for allotransplantation of noncritical organs could have restoration of function with such an approach. Although additional work is needed to validate this approach in other animal models before clinical trials can be undertaken, the ability to use ASCs, which are non-donor-specific and clinically feasible, to induce tolerance opens a new horizon in transplantation.
Acknowledgments
We thank Doug Smoot for FACS support, Forum Shah for technical support, and LaCell, LLC, for graciously providing the ASCs used in this study. This work was supported by U.S. Navy Bureau of Medicine and Surgery Advanced Medical Development Program 604771N C165 001 A0812.
Author Contributions
T.A.D.: conception and design, data analysis and interpretation, manuscript writing, critical review, financial support, final approval of manuscript; K.A. and Y.L.: collection and/or assembly of data, data analysis and interpretation, critical review, final approval of manuscript; J.M.G.: provision of study material, data analysis and interpretation, critical review of manuscript, final approval of manuscript; E.A.E.: conception and design, data analysis and interpretation, critical review of manuscript, financial support, final approval of manuscript.
Disclosure of Potential Conflicts of Interest
J.M.G. and spouse are co-owners, employees, and stock holders of LaCell, LLC. J.M.G. is a holder of multiple patents related to adipose stem cell technology, consultant for multiple companies, and recipient of honoraria and research funding.
References
- 1.Elster EA, Butler FK, Rasmussen TE. Implications of combat casualty care for mass casualty events. JAMA. 2013;310:475–476. doi: 10.1001/jama.2013.167481. [DOI] [PubMed] [Google Scholar]
- 2.Melcer T, Sechriest VF, Walker J, et al. A comparison of health outcomes for combat amputee and limb salvage patients injured in Iraq and Afghanistan wars. J Trauma Acute Care Surg. 2013;75:S247–S254. doi: 10.1097/TA.0b013e318299d95e. [DOI] [PubMed] [Google Scholar]
- 3.Tintle SM, Baechler MF, Nanos GP, et al. Reoperations following combat-related upper-extremity amputations. J Bone Joint Surg Am. 2012;94:e1191–e1196. doi: 10.2106/JBJS.K.00197. [DOI] [PubMed] [Google Scholar]
- 4.Starzl R, Brandacher G, Lee WP, et al. Review of the early diagnoses and assessment of rejection in vascularized composite allotransplantation. Clin Dev Immunol. 2013;2013:402980. doi: 10.1155/2013/402980. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Schneeberger S, Landin L, Jableki J, et al. Achievements and challenges in composite tissue allotransplantation. Transplant Int. 2011;24:760–769. doi: 10.1111/j.1432-2277.2011.01261.x. [DOI] [PubMed] [Google Scholar]
- 6.Sarhane KA, Tuffaha SH, Broyles JM, et al. A critical analysis of rejection in vascularized composite allotransplantation: clinical, cellular and molecular aspects, current challenges, and novel concepts. Front Immunol. 2013;4:406. doi: 10.3389/fimmu.2013.00406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Sarhane KA, Khalifian S, Ibrahim Z, et al. Diagnosing skin rejection in vascularized composite allotransplantation: advances and challenges. Clin Transplant. 2014;28:277–285. doi: 10.1111/ctr.12316. [DOI] [PubMed] [Google Scholar]
- 8.Cendales LC, Kirk AD, Moresi JM, et al. Composite tissue allotransplantation: classification of clinical acute skin rejection. Transplantation. 2005;80:1676–1680. [PubMed] [Google Scholar]
- 9.Billingham RE, Brent L, Medawar PB. Actively acquired tolerance of foreign cells. Nature. 1953;172:603–606. doi: 10.1038/172603a0. [DOI] [PubMed] [Google Scholar]
- 10.Elster EA, Blair PJ, Kirk AD. Potential of costimulation-based therapies for composite tissue allotransplantation. Microsurgery. 2000;20:430–434. doi: 10.1002/1098-2752(2000)20:8<430::aid-micr14>3.0.co;2-k. [DOI] [PubMed] [Google Scholar]
- 11.Elster EA, Xu H, Tadaki DK, et al. Primate skin allotransplantation with anti-CD154 monotherapy. Transplant Proc. 2001;33:675–676. doi: 10.1016/s0041-1345(00)02197-7. [DOI] [PubMed] [Google Scholar]
- 12.Hoch AI, Leach JK. Concise review: optimizing expansion of bone marrow mesenchymal stem/stromal cells for clinical applications. Stem Cells Translational Medicine. 2014;3:643–652. doi: 10.5966/sctm.2013-0196. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Maumus M, Guerit D, Toupet K, et al. Mesenchymal stem cell-based therapies in regenerative medicine: Applications in rheumatology. Stem Cell Res Ther. 2011;2:14. doi: 10.1186/scrt55. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Caplan AI. Adult mesenchymal stem cells for tissue engineering versus regenerative medicine. J Cell Physiol. 2007;213:341–347. doi: 10.1002/jcp.21200. [DOI] [PubMed] [Google Scholar]
- 15.Ma S, Xie N, Li W, et al. Immunobiology of mesenchymal stem cells. Cell Death Differ. 2014;21:216–225. doi: 10.1038/cdd.2013.158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.De Miguel MP, Fuentes-Julián S, Blázquez-Martínez A, et al. Immunosuppressive properties of mesenchymal stem cells: Advances and applications. Curr Mol Med. 2012;12:574–591. doi: 10.2174/156652412800619950. [DOI] [PubMed] [Google Scholar]
- 17.Baron F, Storb R. Mesenchymal stromal cells: A new tool against graft-versus-host disease. Biol Blood Marrow Transplant. 2012;18:822–840. doi: 10.1016/j.bbmt.2011.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Duffy MM, Ritter T, Ceredig R, et al. Mesenchymal stem cell effects on T-cell effector pathways. Stem Cell Res Ther. 2011;2:34. doi: 10.1186/scrt75. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Anam K, Lazdun Y, Davis PM, et al. Amnion-derived multipotent progenitor cells support allograft tolerance induction. Am J Transplant. 2013;13:1416–1428. doi: 10.1111/ajt.12252. [DOI] [PubMed] [Google Scholar]
- 20.Bartholomew A, Sturgeon C, Siatskas M, et al. Mesenchymal stem cells suppress lymphocyte proliferation in vitro and prolong skin graft survival in vivo. Exp Hematol. 2002;30:42–48. doi: 10.1016/s0301-472x(01)00769-x. [DOI] [PubMed] [Google Scholar]
- 21.Ramasamy R, Fazekasova H, Lam EW, et al. Mesenchymal stem cells inhibit dendritic cell differentiation and function by preventing entry into the cell cycle. Transplantation. 2007;83:71–76. doi: 10.1097/01.tp.0000244572.24780.54. [DOI] [PubMed] [Google Scholar]
- 22.Ivanova-Todorova E, Bochev I, Mourdjeva M, et al. Adipose tissue-derived mesenchymal stem cells are more potent suppressors of dendritic cells differentiation compared to bone marrow-derived mesenchymal stem cells. Immunol Lett. 2009;126:37–42. doi: 10.1016/j.imlet.2009.07.010. [DOI] [PubMed] [Google Scholar]
- 23.Melief SM, Zwaginga JJ, Fibbe WE, et al. Adipose tissue-derived multipotent stromal cells have a higher immunomodulatory capacity than their bone marrow-derived counterparts. Stem Cells Translational Medicine. 2013;2:455–463. doi: 10.5966/sctm.2012-0184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Ribeiro A, Laranjeira P, Mendes S, et al. Mesenchymal stem cells from umbilical cord matrix, adipose tissue and bone marrow exhibit different capability to suppress peripheral blood B, natural killer and T cells. Stem Cell Res Ther. 2013;4:125. doi: 10.1186/scrt336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Gimble JM, Bunnell BA, Guilak F. Human adipose-derived cells: An update on the transition to clinical translation. Regen Med. 2012;7:225–235. doi: 10.2217/rme.11.119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Gimble JM, Nuttall ME. Adipose-derived stromal/stem cells (ASC) in regenerative medicine: Pharmaceutical applications. Curr Pharm Des. 2011;17:332–339. doi: 10.2174/138161211795164220. [DOI] [PubMed] [Google Scholar]
- 27.Gimble JM, Bunnell BA, Chiu ES, et al. Concise review: Adipose-derived stromal vascular fraction cells and stem cells: Let’s not get lost in translation. Stem Cells. 2011;29:749–754. doi: 10.1002/stem.629. [DOI] [PubMed] [Google Scholar]
- 28.McIntosh KR, Frazier T, Rowan BG, et al. Evolution and future prospects of adipose-derived immunomodulatory cell therapeutics. Expert Rev Clin Immunol. 2013;9:175–184. doi: 10.1586/eci.12.96. [DOI] [PubMed] [Google Scholar]
- 29.Kern S, Eichler H, Stoeve J, et al. Comparative analysis of mesenchymal stem cells from bone marrow, umbilical cord blood, or adipose tissue. Stem Cells. 2006;24:1294–1301. doi: 10.1634/stemcells.2005-0342. [DOI] [PubMed] [Google Scholar]
- 30.Aust L, Devlin B, Foster SJ, et al. Yield of human adipose-derived adult stem cells from liposuction aspirates. Cytotherapy. 2004;6:7–14. doi: 10.1080/14653240310004539. [DOI] [PubMed] [Google Scholar]
- 31.Yu G, Wu X, Dietrich MA, et al. Yield and characterization of subcutaneous human adipose-derived stem cells by flow cytometric and adipogenic mRNA analyzes. Cytotherapy. 2010;12:538–546. doi: 10.3109/14653241003649528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Anam K, Amare MF, Zins SR, et al. Infusion of Lin- bone marrow cells results in multilineage macrochimerism and skin allograft tolerance in minimally conditioned recipient mice. Transpl Immunol. 2010;24:69–75. doi: 10.1016/j.trim.2010.10.003. [DOI] [PubMed] [Google Scholar]
- 33.Soares A, Govender L, Hughes J, et al. Novel application of Ki67 to quantify antigen-specific in vitro lymphoproliferation. J Immunol Methods. 2010;362:43–50. doi: 10.1016/j.jim.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Livak KJ, Schmittgen TD. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-delta delta C(T)) method. Methods. 2001;25:402–408. doi: 10.1006/meth.2001.1262. [DOI] [PubMed] [Google Scholar]
- 35.DelaRosa O, Lombardo E, Beraza A, et al. Requirement of IFN-gamma-mediated indoleamine 2,3-dioxygenase expression in the modulation of lymphocyte proliferation by human adipose-derived stem cells. Tissue Eng Part A. 2009;15:2795–2806. doi: 10.1089/ten.TEA.2008.0630. [DOI] [PubMed] [Google Scholar]
- 36.Najar M, Raicevic G, Boufker HI, et al. Mesenchymal stromal cells use PGE2 to modulate activation and proliferation of lymphocyte subsets: Combined comparison of adipose tissue, Wharton’s jelly and bone marrow sources. Cell Immunol. 2010;264:171–179. doi: 10.1016/j.cellimm.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 37.Kang JW, Kang KS, Koo HC, et al. Soluble factors-mediated immunomodulatory effects of canine adipose tissue-derived mesenchymal stem cells. Stem Cells Dev. 2008;17:681–693. doi: 10.1089/scd.2007.0153. [DOI] [PubMed] [Google Scholar]
- 38.Roemeling-van Rhijn M, Mensah FK, Korevaar SS, et al. Effects of hypoxia on the immunomodulatory properties of adipose tissue-derived mesenchymal stem cells. Front Immunol. 2013;4:203. doi: 10.3389/fimmu.2013.00203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Crop MJ, Baan CC, Korevaar SS, et al. Inflammatory conditions affect gene expression and function of human adipose tissue-derived mesenchymal stem cells. Clin Exp Immunol. 2010;162:474–486. doi: 10.1111/j.1365-2249.2010.04256.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Wu S, Xu H, Ravindra K, et al. Composite tissue allotransplantation: Past, present and future—The history and expanding applications of CTA as a new frontier in transplantation. Transplant Proc. 2009;41:463–465. doi: 10.1016/j.transproceed.2009.01.027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Page EK, Dar WA, Knechtle SJ. Tolerogenic therapies in transplantation. Front Immunol. 2012;3:198. doi: 10.3389/fimmu.2012.00198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Sachs DH, Kawai T, Sykes M. Induction of tolerance through mixed chimerism. Cold Spring Harb Perspect Med. 2014;4:a015529. doi: 10.1101/cshperspect.a015529. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Boros P, Bromberg JS. Human FOXP3+ regulatory T cells in transplantation. Am J Transplant. 2009;9:1719–1724. doi: 10.1111/j.1600-6143.2009.02704.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Noris M, Casiraghi F, Todeschini M, et al. Regulatory T cells and T cell depletion: Role of immunosuppressive drugs. J Am Soc Nephrol. 2007;18:1007–1018. doi: 10.1681/ASN.2006101143. [DOI] [PubMed] [Google Scholar]
- 45.De Serres SA, Sayegh MH, Najafian N. Immunosuppressive drugs and Tregs: A critical evaluation. Clin J Am Soc Nephrol. 2009;4:1661–1669. doi: 10.2215/CJN.03180509. [DOI] [PubMed] [Google Scholar]
- 46.Domenig C, Sanchez-Fueyo A, Kurtz J, et al. Roles of deletion and regulation in creating mixed chimerism and allograft tolerance using a nonlymphoablative irradiation-free protocol. J Immunol. 2005;175:51–60. doi: 10.4049/jimmunol.175.1.51. [DOI] [PubMed] [Google Scholar]
- 47.Pilat N, Wekerle T. Transplantation tolerance through mixed chimerism. Nat Rev Nephrol. 2010;6:594–605. doi: 10.1038/nrneph.2010.110. [DOI] [PubMed] [Google Scholar]
- 48.Xu H, Chilton PM, Huang Y, et al. Production of donor T cells is critical for induction of donor-specific tolerance and maintenance of chimerism. J Immunol. 2004;172:1463–1471. doi: 10.4049/jimmunol.172.3.1463. [DOI] [PubMed] [Google Scholar]
- 49.Lee K, Nguyen V, Lee KM, et al. Attenuation of donor-reactive T cells allows effective control of allograft rejection using regulatory T cell therapy. Am J Transplant. 2014;14:27–38. doi: 10.1111/ajt.12509. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Cheng HY, Ghetu N, Huang WC, et al. Syngeneic adipose-derived stem cells with short-term immunosuppression induce vascularized composite allotransplantation tolerance in rats. Cytotherapy. 2014;16:369–380. doi: 10.1016/j.jcyt.2013.06.020. [DOI] [PubMed] [Google Scholar]
- 51.Ramirez AE, Cheng HY, Lao WW, et al. A novel rat full-thickness hemi-abdominal wall/hindlimb osteomyocutaneous combined flap: Influence of allograft mass and vascularized bone marrow content on vascularized composite allograft survival. Transplant Int. 2014;27:977–986. doi: 10.1111/tri.12364. [DOI] [PubMed] [Google Scholar]
- 52.Gimble JM, Bunnell BA, Frazier T, et al. Adipose-derived stromal/stem cells: A primer. Organogenesis. 2013;9:3–10. doi: 10.4161/org.24279. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kronsteiner B, Wolbank S, Peterbauer A, et al. Human mesenchymal stem cells from adipose tissue and amnion influence T-cells depending on stimulation method and presence of other immune cells. Stem Cells Dev. 2011;20:2115–2126. doi: 10.1089/scd.2011.0031. [DOI] [PubMed] [Google Scholar]
- 54.Ren G, Su J, Zhang L, et al. Species variation in the mechanisms of mesenchymal stem cell-mediated immunosuppression. Stem Cells. 2009;27:1954–1962. doi: 10.1002/stem.118. [DOI] [PubMed] [Google Scholar]
- 55.Danchuk S, Ylostalo JH, Hossain F, et al. Human multipotent stromal cells attenuate lipopolysaccharide-induced acute lung injury in mice via secretion of tumor necrosis factor-alpha-induced protein 6. Stem Cell Res Ther. 2011;2:27. doi: 10.1186/scrt68. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Doorn J, Moll G, Le Blanc K, et al. Therapeutic applications of mesenchymal stromal cells: paracrine effects and potential improvements. Tissue Eng Part B Rev. 2012;18:101–115. doi: 10.1089/ten.TEB.2011.0488. [DOI] [PubMed] [Google Scholar]
- 57.Lee RH, Pulin AA, Seo MJ, et al. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell. 2009;5:54–63. doi: 10.1016/j.stem.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Akiyama K, Chen C, Wang D, et al. Mesenchymal-stem-cell-induced immunoregulation involves FAS-ligand-/FAS-mediated T cell apoptosis. Cell Stem Cell. 2012;10:544–555. doi: 10.1016/j.stem.2012.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]