The presence of angiogenic-stimulating factors in conditioned medium (CM) of peripheral blood-derived equine mesenchymal stromal cells (PB-MSCs) was examined, and their in vitro effect on angiogenesis-related endothelial cell (EC) behavior was studied. CM from PB-MSCs contained significant levels of several proangiogenic factors and induced angiogenesis in equine vascular ECs, with enhanced EC network formation by increasing vascular endothelial growth factor-A expression, an important angiogenesis stimulator.
Keywords: Angiogenesis, Mesenchymal stromal cells, Vascular endothelial growth factor, Secretome
Abstract
Mesenchymal stromal cells (MSCs) have received much attention as a potential treatment of ischemic diseases, including ischemic tissue injury and cardiac failure. The beneficial effects of MSCs are thought to be mediated by their ability to provide proangiogenic factors, creating a favorable microenvironment that results in neovascularization and tissue regeneration. To study this in more detail and to explore the potential of the horse as a valuable translational model, the objectives of the present study were to examine the presence of angiogenic stimulating factors in the conditioned medium (CM) of peripheral blood-derived equine mesenchymal stromal cells (PB-MSCs) and to study their in vitro effect on angiogenesis-related endothelial cell (EC) behavior, including proliferation and vessel formation. Our salient findings were that CM from PB-MSCs contained significant levels of several proangiogenic factors. Furthermore, we found that CM could induce angiogenesis in equine vascular ECs and confirmed that endothelin-1, insulin growth factor binding protein 2, interleukin-8, and platelet-derived growth factor-AA, but not urokinase-type plasminogen activator, were responsible for this enhanced EC network formation by increasing the expression level of vascular endothelial growth factor-A, an important angiogenesis stimulator.
Introduction
The sprouting of new capillaries from existing blood vessels, also termed “angiogenesis,” plays critical roles during development and tissue repair. Angiogenesis is a complex multistep process that is tightly regulated by “on-off switch signals” among angiogenic factors, extracellular matrix components, and endothelial cells. When this process becomes dysregulated, classified as either excessive or deficient angiogenesis, it can contribute to numerous malignant, ischemic, inflammatory, infectious, and immune disorders [1, 2]. In the case of deficient angiogenesis, a hallmark of many ischemic diseases, such as coronary artery disease, stroke, and chronic wounds, an angiogenesis stimulator can be used to induce therapeutic blood vessel growth. Conventional therapeutic angiogenic drugs have been shown to be useful for treating diseases of deficient angiogenesis; however, their success has been limited [3]. The observation that stem cells contribute to neovascularization has made stem cell therapy a highly active research area [4]. Numerous studies have been performed in rodent models to determine the potential of mesenchymal stromal cells (MSCs) to reverse the deleterious effects of ischemia [5–9]. Tögel et al. administered MSCs to rats after induction of ischemia-reperfusion acute renal failure, and the rats showed significantly improved renal function, with higher proliferative and lower apoptotic indexes immediately and 24 hours after renal ischemia, compared with the control rats that had received serum-free culture medium [8]. Another research group administered MSCs intramyocardially in rats 1 week after induction of myocardial infarction and found that the rats with MSC implantation showed significantly elevated vascular endothelial growth factor (VEGF) expression levels, accompanied by increased vascular density and regional blood flow in the infarct zone, 2 months after transplantation [7]. More recent studies have focused on the potential role of MSC-based therapies for critical limb ischemia, the worst form of peripheral arterial disease, defined as pain at rest or impending limb loss secondary to objectively proven arterial occlusive disease for more than 2 weeks [10]. No pharmacologic therapy is available, and the conditions often represent an unmet clinical need. Pilot human and animal studies and randomized controlled trials have shown that administration of MSCs from different sources is associated with better therapeutic outcomes by induced angiogenesis [10–16]. All these studies’ findings indicate that MSCs participate in the reconstruction of a favorable microenvironment, resulting in neovascularization and tissue regeneration that will eventually improve the physiological function of organs with ischemic damage. However, the exact underlying mechanisms leading to this improvement have not been fully elucidated [17]. An increasing body of evidence has shown that MSCs achieve their therapeutic effects mainly through secretion of different autocrine/paracrine factors, including growth factors, cytokines, chemokines, metabolites, and bioactive lipids [18]. Therefore, a thorough in vitro and in vivo examination of this spectrum of the regulatory and trophic factors secreted by MSCs, broadly defined as the MSC secretome, and their paracrine effects is warranted for rational therapy design and improvement of existing therapies [18].
Prospective clinical trials evaluating the beneficial effects of MSC therapies need to be well-designed by including appropriate control groups, sufficient similar cases, and a consistent and standardized panel of objective outcome measures [19]. In this regard, the horse represents a valuable nonrodent translational model for this type of clinical studies because horses (a) can be studied in their natural environment and share environmental stressors (e.g., work constraints, social restriction) with humans, (b) have numerous anatomical and biomechanical similarities to humans, and (c) have a wide spectrum of naturally occurring diseases with a pathogenic etiology similar to that in humans [20–23]. In addition, equine MSCs have already been used successfully for the clinical treatment of pathologic entities such as orthopedic injuries. Therefore, the horse could be a very valuable model to test the efficacy and safety of innovative MSC treatments with a beneficial outcome for both horses and humans [19, 24]. However, and despite their widespread use in equine regenerative medicine, fundamental studies on the basic functioning of equine MSCs are largely lacking. Thus, in order for the horse to be generally accepted as a valid translation model, research on the underlying mechanisms of equine MSCs are urgently needed.
The present study was conducted to examine the presence of angiogenic stimulating factors in the conditioned medium (CM) of equine peripheral blood-derived mesenchymal stromal cells (PB-MSCs) and to evaluate their in vitro effects on angiogenesis-related endothelial cell (EC) behavior, including proliferation and vessel formation. Our salient findings were that equine PB-MSCs could promote angiogenesis by stimulating the proliferation of ECs and their tubuli network formation in vitro. More specifically, these positive effects on angiogenesis were mediated by the PB-MSC secreted factors endothelin-1 (ET1), insulin growth factor binding protein 2 (IGFBP2), interleukin-8 (IL-8), and plasminogen-dependent growth factor AA (PDGF-AA) via upregulation of VEGF-A gene expression and VEGF-A secretion in ECs.
Materials and Methods
Cells
Equine PB-MSCs were isolated and characterized, exactly as previously described [25–27]. In brief, peripheral blood mononuclear cells (PBMCs) were isolated using density gradient centrifugation on Percoll (Sigma-Aldrich, St. Louis, MO, http://www.sigmaaldrich.com) and were seeded at a density of 16 × 104 cells per cm2 in a T75 flask in culture medium consisting of low glucose (LG) Dulbecco’s modified Eagle medium (DMEM) (Corning Life Sciences, Acton, MA, http://www.corning.com/lifesciences), supplemented with 30% fetal bovine serum (FBS) (Atlanta Biologicals, Lawrenceville, GA, http://www.atlantabio.com), 1% penicillin/streptomycin, 2 mM ultraglutamine (Invitrogen, Grand Island, NY, http://www.invitrogen.com), and 10−11 M low dexamethasone (Sigma-Aldrich). At 70% confluence, the cells were trypsinized with 0.25% trypsin-EDTA and were further cultured in expansion medium, which was identical to the culture medium but without dexamethasone. Equine vascular ECs were isolated from the carotid artery of healthy horses, euthanized for reasons not related to the present study, exactly as previously described [28]. Equine ECs were cultured in EC culture medium consisting of low-glucose DMEM supplemented with sodium pyruvate (Corning Life Sciences), 20% FBS (Atlanta Biologicals), 1% nonessential amino acids (Invitrogen), and 1% penicillin/streptomycin (Invitrogen). The equine dermal fibroblast cell line NBL6 (American Type Culture Collection, Manassas, VA, http://www.atcc.org) was cultured in standard medium consisting of minimal essential medium (Corning Life Sciences) supplemented with 10% FBS (Atlanta Biologicals) and 1% penicillin/streptomycin (Invitrogen).
CM and Pretreatments
CM were collected from PB-MSCs from 3 different horses after 2 days of culture and on 70% confluence of the cells. To this end, 6 × 105 PB-MSCs were seeded in a T75 flask in MSC medium. After 24 hours, cells were fed again with 8 ml of fresh MSC medium. Twenty-four hours later, the supernatant was collected and, after centrifugation twice for 7 minutes at 930g, used for additional experimentation.
For the pretreatment experiments, PB-MSCs were seeded in MSC medium supplemented with 20 ng/ml interferon-γ (IFNγ; R&D Systems Inc., Minneapolis, MN, http://www.rndsystems.com), 20 μM linoleic acid (LA) (Nu-Chek Prep, Elysian, MN, http://www.nu-chekprep.com) or 200 μM cobalt chloride (CoCl2; Sigma-Aldrich). After 24 hours of culturing, the cells were washed twice with phosphate-buffered saline (PBS) and fed again with 8 ml of fresh MSC medium. CM were collected 24 hours later, as described.
Recombinant Proteins
The recombinant proteins used in the present study included human ET1 (50 ng/ml), urokinase plasminogen activator (uPA; 50 ng/ml), IGFBP2 (100 ng/ml), and PDGF-AA (200 ng/ml), all from R&D Systems Inc. Recombinant equine IL-8 (50 ng/ml) was from AbD Serotec (Raleigh, NC, http://www.ab-direct.com) and equine VEGF (30 ng/ml) from Kingfisher Biotech (St. Paul, MN, http://www.kingfisherbiotech.com).
Flow Cytometry
In order to characterize equine MSCs immunophenotypically, the expression of several MSC markers was evaluated by flow cytometry, as previously described [25]. In brief, 2 × 105 cells were labeled using the following panel of primary antibodies: CD29 (clone TMD29; Chemicon International, Billerica, MA, http://www.chemicon.com), CD44 (clone TM7; Thermo Scientific, Waltham, MA, http://www.thermofisher.com), CD45 (clone F10-89-4; AbD Serotec), CD79α (clone HM57; AbD Serotec), CD90 (clone DH24A; VMRD, Pullman, WA, http://www.vmrd.com), CD105 (clone SN6; Abcam, Cambridge, MA, http://www.abcam.com), major histocompatibility complex (MHC) I (clone CZ3; D. Antczak’s laboratory, Cornell University, Ithaca, NY), MHC II (clone CZ11; D. Antczak’s laboratory, Cornell University), and a monocyte/macrophage marker (clone MAC387; AbD Serotec). For the detection of the CD79α and monocyte/macrophage markers, fixation and permeabilization pretreatment was performed using commercially available reagents (Invitrogen). In general, the cells were incubated for 15 minutes on ice in the dark with the primary antibodies and then washed twice in LG DMEM with 1% bovine serum albumin. Incubation for 15 minutes on ice in the dark with secondary goat anti-mouse, goat anti-rat, and goat anti-rabbit 488 linked antibodies (all Thermo Scientific) was performed to label the CD29-, CD45-, CD79α-, CD90-, CD105-, MHC I-, and MHC II-positive cells. In addition, the cells were incubated with or without (autofluorescence) isotype-specific murine IgG1 and IgM and rat IgG2a and IgG2b in parallel to establish the background signal. At least 10,000 cells were evaluated using a Beckman Coulter flow cytometer (Brea, CA, https://www.beckmancoulter.com) equipped with a 488-nm and a 638-nm laser. These data were analyzed further using the Kaluza Flow Analysis software (Beckman Coulter).
Differentiation Assay
To verify that the MSCs were capable of trilineage differentiation, adipogenic, osteogenic, and chondrogenic induction assays were used as previously described [29]. Adipogenic induction was performed using the commercially available StemPro Adipogenesis Differentiation Kit (Gibco, Life Technologies, Carlsbad, CA, http://www.lifetechnologies.com) according to the manufacturer’s instructions. The medium was exchanged every 3–4 days until day 14. At that time, the cells were fixed in 4% paraformaldehyde (PF), stained with Oil Red O for identification of lipid inclusions, and counterstained with hematoxylin. Osteogenic induction was performed using the commercially available StemPro Osteogenesis Differentiation Kit (Gibco) according to the manufacturer’s instructions. The medium was exchanged every 3−4 days until day 14, at which point, the cells were fixed in 4% PF, stained with 2% aqueous alizarin red for identification of calcium deposits, and counterstained with hematoxylin. Chondrogenic induction was performed on pellet cultures using the commercially available StemPro Chondrogenesis Differentiation Kit (Gibco) according to the manufacturer’s instructions. The medium was exchanged every 3–4 days until day 14. The pellets were fixed in 4% PF and stained with Alcian blue. All stained cells were imaged using standard microscopy.
Angiogenesis Assay
Endothelial tube-like formation assays were performed using an in vitro angiogenesis assay kit, according to the manufacturer’s instructions (Cayman Chemicals, Ann Arbor, MI, http://www.caymanchem.com). In brief, primary equine ECs were seeded in triplicate onto an extracellular matrix gel (1 × 104 cells per well) in 100 μl of CM and grown for 2 days in a 37°C incubator. ECs incubated with 100 μl of MSC medium were used to establish the baseline EC growth and ECs incubated with 1 μM JNJ-10198490 was used as a negative control, according to the manufacturer’s instructions. After 3 days of culture, the ECs were stained with 10 μl of 10× cell-based calcein and the presence of tree-like tubular networks was examined using an Eclipse TE2000-U inverted fluorescence microscope (Nikon, Melville, NY, http://www.nikon.com). To evaluate the effects of the individual proteins on endothelial tube-like formation, angiogenesis assays were repeated using single recombinant proteins instead of CM, exactly as described above.
Bromodeoxyuridine Proliferation Assay
The effect of CM on the proliferation of EC was evaluated using a bromodeoxyuridine (BrdU) proliferation assay kit (Abcam). To this end, ECs were seeded on a 96-well plate at a density of 4 × 104 cells per well and incubated with CM or regular MSC medium to establish the baseline EC proliferation. The BrdU proliferation assay kit was used according to the manufacturer’s instructions, and the resulting absorbance was read at 450 nm using a Multiskan EX microplate reader and Ascent software (Thermo Scientific). Empty wells and wells without BrdU were included as controls.
Angiogenesis Arrays
To screen for angiogenesis-related proteins in equine CM, a human angiogenesis proteome profiler antibody array, previously shown to cross-react with the horse was used, according to the manufacturer’s instructions (R&D Systems, Inc.) [30]. Positive signals were visualized using the ChemiDoc MP Imaging system (Bio-Rad, Hercules, CA, http://www.bio-rad.com). The array data were normalized to the background and quantified by measuring the sum of the intensities of the pixels within the spot boundary pixel area using image analysis software (Image Laboratory 4.1; Bio-Rad).
Enzyme-Linked Immunosorbent Assays
Interleukin-6
To detect the presence of interleukin-6 (IL-6) in CM, an equine-specific IL-6 enzyme-linked immunosorbent assay (ELISA) kit was used, according to the manufacturer’s instructions (Cloud-Clone Corp., Houston, TX, http://www.cloud-clone.us). The resulting yellow color was read at 450 nm on a Multiskan EX microplate reader using Ascent software (Thermo Scientific). The CM of equine PBMCs was included as a positive control.
Vascular Endothelial Growth Factor A
To detect the presence of VEGF-A in the CM of ECs and PB-MSCs, an equine-specific VEGF-A ELISA kit was used (Kingfisher Biotech). In brief, 100 μl of either standard VEGF-A or a CM sample was added to a well precoated with an anti-equine polyclonal VEGF-A antibody and then incubated for 2 hours at room temperature. The standard or samples were aspirated, the plates were washed four times with 0.1% PBS-Tween 20, and 100 μl of biotinylated anti-equine VEGF-A polyclonal antibody was added to each well for 1 hour at room temperature. The plate was washed four times to remove excess antibodies, and 100 μl of β-peroxidase (POD)-conjugated streptavidin was added for 1 hour at 37°C. Next, the plate was washed nine times, and 100 μl of ortho-phenylenediamine substrate solution was added for 30 minutes at room temperature. The POD-catalyzed substrate reaction was finalized with 50 μl of 4 M H2SO4, and the resulting yellow color was read at 490 nm on an Infinite M200 Pro microplate reader using i-control software (Tecan, Männedorf, Switzerland, http://www.tecan.com).
Traditional and Quantitative Reverse Transcription-Polymerase Chain Reaction
RNA was extracted from equine ECs using an RNeasy Mini kit (Qiagen, Valencia, CA, http://www.qiagen.com), followed by DNase digestion using DNase I (Invitrogen). cDNA was synthesized using Moloney murine leukemia virus reverse transcriptase (USB Corp., Cleveland, OH, http://www.usbweb.com), according to the manufacturer’s protocol.
Traditional reverse transcription-polymerase chain reaction (RT-PCR) using Taq DNA Polymerase (Invitrogen) was performed to evaluate the expression of EC receptors (Table 1). Equine β2-microglobulin (B2M) was used a housekeeping gene (Table 1), and primers were designed using primer 3. PCR products were run on a 1.5% agarose gel at 93 V for 1 hour.
Table 1.
Overview of primers used in polymerase chain reaction

For quantitative RT-PCR (qRT-PCR), primers were designed to correspond to different exons to avoid amplification of genomic DNA and are listed in Table 1. SYBR Green technology (Applied Biosystems, Carlsbad, CA, http://www.appliedbiosystems.com) was used, and the samples were run on an Applied Biosytems 7500 Fast Real Time PCR instrument. The comparative Ct method (2−ΔΔCt) was used to quantify gene expression levels, where ΔΔCt = ΔCt(sample) − ΔCt (reference). The reference consisted of ECs without incubation of recombinant proteins or conditioned medium. All samples and references were normalized to the endogenous housekeeping gene, B2M (Table 1), and the data are reported as the mRNA fold change.
Western Blot
The cells were lysed in RIPA buffer containing 20 mM Tris (pH 8.0), 137 mM NaCl, 10% glycerol, 1% Nonidet P40, 0.1% SDS, 0.5% deoxycholate, 0.2 mM phenylmethylsulfonyl fluoride, and 1× general protease inhibitor. Protein concentration in lysates was determined using a bicinchoninic acid protein assay (Thermo Scientific) before gel loading to ensure equal protein loading. A 6× sample buffer (300 mM Tris-HCl, pH 6.8, 60% glycerol, 30 mM dithiothreitol, 6% SDS) was added to yield a final concentration of 1×, and the lysates were boiled at 95°C for 10 minutes. The samples were subjected to SDS polyacrylamide gel electrophoresis on an 8% gel (acrylamide/bis-acrylamide ratio of 29:1) and transferred to Immobilon PVDF membranes (Millipore, Billerica, MA, http://www.millipore.com) using a Trans-Blot Turbo System (Bio-Rad). The membranes were blocked in 5% bovine serum albumin diluted in Tris-buffered saline (TBS) and incubated with anti-VEGF receptor 1+2, 1:500 (polyclonal; Abcam) for 2 hours at room temperature. The blots were washed and then incubated with a 1:20,000 dilution of anti-rabbit-conjugated horseradish peroxidase (Jackson ImmunoResearch Laboratories, Inc., West Grove, PA, http://www.jacksonimmuno.com) for 2 hours at room temperature. All blots were washed for 50 minutes (10 × 5 minutes) with TBS-Tween and then visualized with chemiluminescence using Clarity Western ECL (Bio-Rad). To confirm equal protein loading, the membranes were probed in parallel with anti-β-actin, 1:5,000 (polyclonal; Abcam).
Statistical Analysis
Student’s t test for paired data was used to test for statistically significant differences in VEGF and VEGF receptor (VEGFR) mRNA expression (qRT-PCR), BrdU incorporation, and IL-6 and VEGF protein expression (ELISA), between untreated and CM-stimulated ECs. The data given are the mean of 3 replicates, and the bars show the standard deviations.
Results
PB-MSCs Are Positive for MSC Markers, Negative for Differentiated Blood Cell Markers, and Capable of Differentiating In Vitro Toward Osteoblasts, Chondroblasts, and Adipocytes
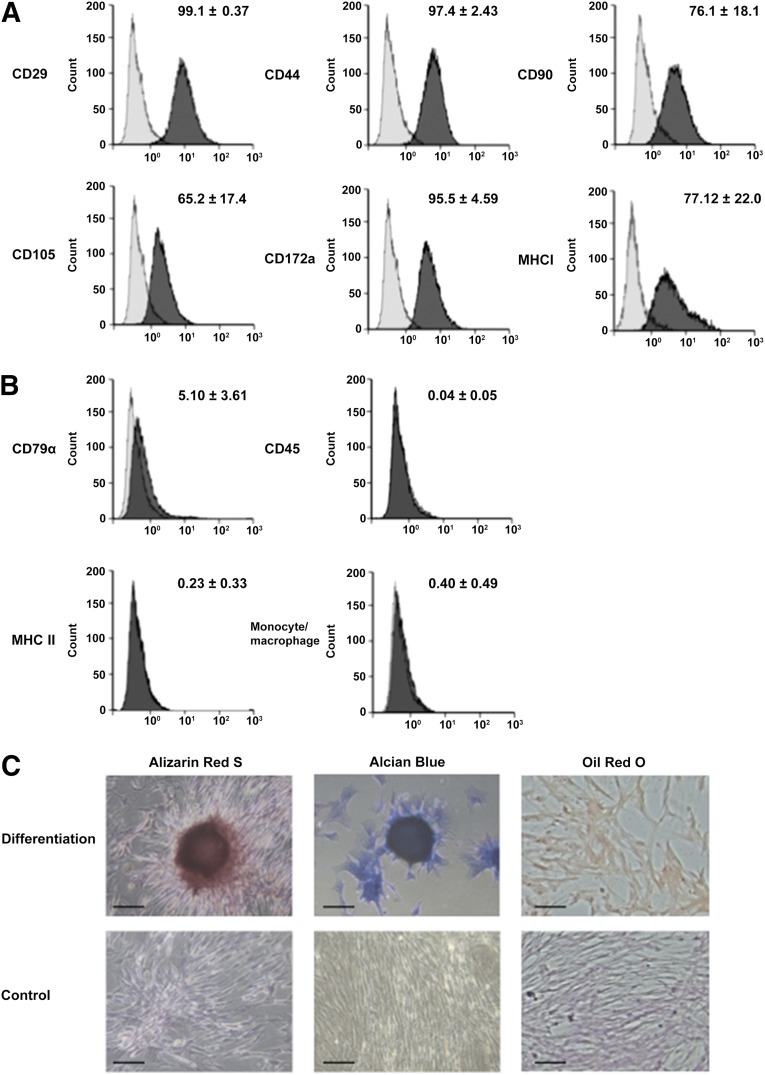
On flow cytometry, the putative equine MSCs were positive for the stem cell markers CD29, CD44, CD90, and CD105 (Fig. 1A) and negative for the pan-leukocyte marker CD45, B-lymphocyte marker CD79α, monocyte/macrophage marker, and an MHC II marker present on antigen-presenting cells (Fig. 1B). No signal was detected with relevant isotype controls for any of the cell markers (Fig. 1A, 1B). Furthermore, the trilineage differentiation capacity of the PB-MSCs was confirmed through in vitro adipogenic, osteogenic, and chondrogenic induction assays (Fig. 1C).
Figure 1.
Peripheral blood-derived equine mesenchymal stromal cells (MSCs) are positive for MSC markers, negative for differentiated blood cell markers, and capable of undergoing trilineage differentiation. Two-laser flow cytometry was performed with four MSC markers (CD29, CD44, CD90, and CD105) (A) and four negative markers (CD45, CD79α, major histocompatibility complex II, and a monocyte/macrophage marker) (B). Representative histograms illustrate relative numbers of cells versus mean fluorescence intensity. The light and dark gray histograms represent relevant isotype control and marker antibody staining, respectively, with the corresponding mean percentage of positive cells ± SD. (C): Representative microscopic images of alizarin red S, Alcian blue, and Oil Red O staining to confirm osteogenic, chondrogenic, and adipogenic differentiation, respectively. Negative control cells are also presented. Scale bars = 50 μm.
CM of Equine PB-MSCs Promotes Proliferation of Equine ECs and Stimulates Endothelial Tube-Like Formation
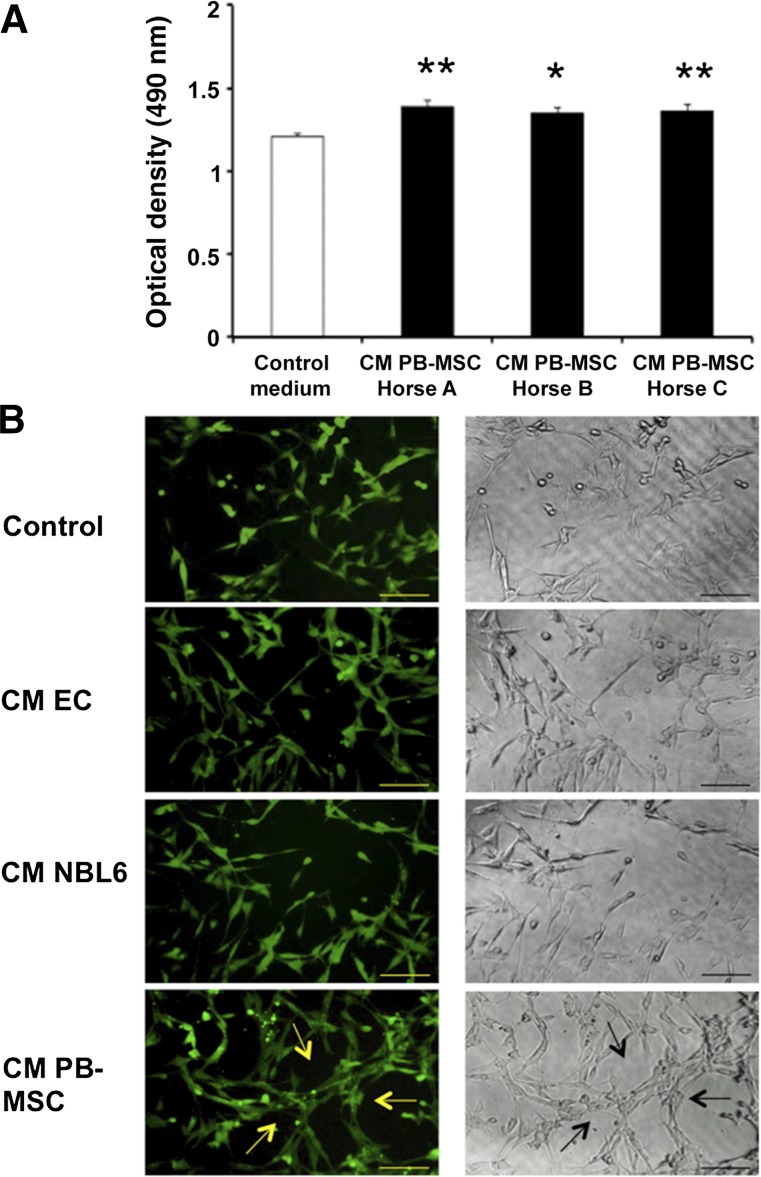
To investigate the effects of CM of PB-MSCs from three individual horses on EC proliferation, a BrdU proliferation assay was performed. The results showed that ECs incubated with PB-MSC-derived CM for 24 hours had a significantly greater proliferation rate than ECs incubated with regular medium, as indicated by a higher optical density (OD) (Fig. 2A).
Figure 2.
CM of equine PB-MSCs promotes the proliferation of equine ECs and stimulate endothelial tube-like formation in vitro. (A): A bromodeoxyuridine (BrdU) proliferation assay was performed to evaluate the proliferation activity of ECs after incubation with CM of PB-MSCs (black) or with MSC medium (white). BrdU incorporation was measured by determining the optical density at 450 nm on a Multiskan EX microplate reader using Ascent software (Thermo Scientific). (B): ECs were seeded on an extracellular matrix gel in the presence or absence of CM of PB-MSCs to evaluate the tube-like formation capacity in vitro. After 3 days of culture, ECs were stained with 10 μl of 10× cell-based calcein. JNJ inhibitor was used as a negative control. Fluorescent and bright-field photographs were taken using an Eclipse TE2000-U inverted fluorescence microscope (Nikon). Arrows indicate hollow tube-like structure formation. Scale bars = 100 μm. Abbreviations: CM, conditioned medium; ECs, endothelial cells; PB-MSCs, peripheral blood-derived equine mesenchymal stromal cells.
Next, an angiogenesis assay kit was used to evaluate endothelial tube-like formation, which mimics angiogenesis in vitro. To this end, equine ECs were cultured on a modified extracellular matrix in the presence or absence of PB-MSC-derived CM and visualized microscopically 24 hours later. When ECs were cultured with CM, the cells aligned and formed hollow tube-like structures, in contrast to culturing ECs in control medium (Fig. 2B). In addition, fewer networks of tube-like structures were observed when equine ECs were cultured in CM obtained from the ECs themselves or from the equine fibroblast cell line NBL6 (Fig. 2B), indicating that the observed effect was not a general CM effect but was mediated by CM from PB-MSCs specifically. When ECs were cultured with the JNJ inhibitor control, no tube-like networks formed (Fig. 2B).
CM of PB-MSCs Contains Multiple Proangiogenic Factors
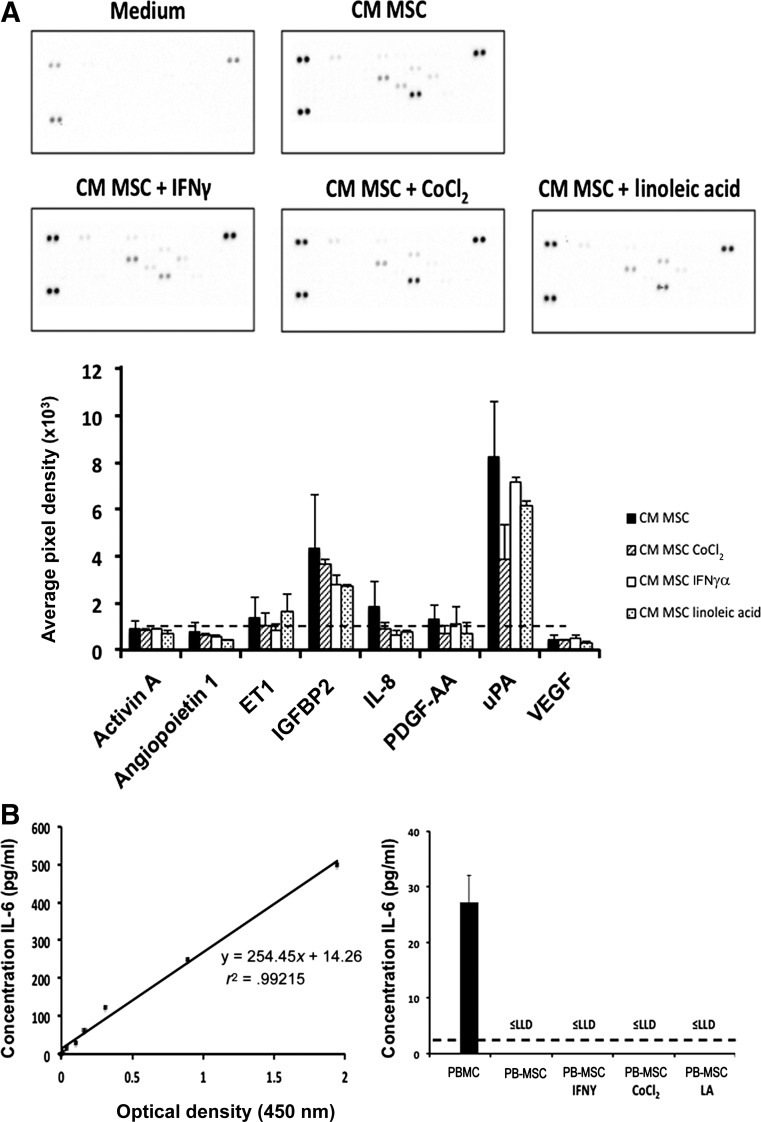
PB-MSC-derived CM was screened for the presence of angiogenic and anti-angiogenic factors using a human proteome profiler array, which can simultaneously detect the relative levels of 55 angiogenesis-related proteins and has been used previously for the detection of anti-angiogenic factors in CM of equine cells [30]. In total, 8 proteins (activin A, angiopoietin 1, ET1, IGFBP2, IL-8, PDGF-AA, uPA, and VEGF) were detected in the CM of PB-MSCs (Fig. 3A). Of those, 5 were detected at an average pixel density of 10,000 or more (Fig. 3A) and were studied in more detail. Because the angiogenesis-related protein IL-6, previously reported to be secreted by human MSCs [31], was not present in the proteome array, we decided to screen the CM of equine PB-MSCs for IL-6 using an equine-specific, commercially available, ELISA. IL-6 could not be detected in the CM of equine PB-MSCs, not even with a detection limit (lower limit of detection) as low as 2.6 pg/ml (Fig. 3B). Equine PBMCs were included as a positive control, and the IL-6 levels were readily detected in these samples, indicating that our negative result was not due to an experimental error (Fig. 3B).
Figure 3.
CM of equine PB-MSCs contains multiple proangiogenic factors. (A): A human angiogenesis proteome profiler array was used to evaluate the presence of angiogenic factors in the CM of PB-MSCs (black bars indicate untreated; dashed line bars, treated with CoCl2; white bars, treated with IFNγ; dotted bars, treated with linoleic acid). The average pixel density is proportional to the amount of phosphoprotein bound by each capture antibody and was calculated for each array spot. The dotted line indicates 10,000 pixel density (B). The presence of IL-6 in the CM of PB-MSCs was determined using an equine specific IL-6 enzyme-linked immunosorbent assay. PBMCs were included as a positive control. Absorbance was measured at 450 nm on a Multiskan EX microplate reader using Ascent software (Thermo Fisher Scientific). Abbreviations: CM, conditioned medium; IFNγ, interferon-γ; IGFBP2, insulin growth factor binding protein 2; IL, interleukin; LA, linoleic acid; LLD, lower limit of detection; PBMC, peripheral blood mononuclear cell; PB-MSC, peripheral blood-derived equine mesenchymal stromal cell; PDGF-AA, plasminogen dependent growth factor AA; uPA, urokinase plasminogen activator; VEGF, vascular endothelial growth factor.
Stimulation of PB-MSCs With IFNγ, LA, and CoCl2 Did Not Alter the Levels of Proangiogenic Factors in CM
It is well known that the in vivo microenvironment in which MSCs reside contains several factors that stimulate the secretion of angiogenic factors. Indeed, some of these factors have been reported to stimulate bone marrow-derived human and rodent MSCs to produce angiogenesis-related factors in vitro [32–34]. Therefore, the effects of these factors on the secretion of angiogenesis-related proteins in the CM of equine PB-MSCs were evaluated using the human proteome profiler array, as described above. Surprisingly, pretreatment of equine PB-MSCs with IFNγ, LA, or CoCl2 did not result in a significant increase in the secretion levels of the proangiogenic factors in the CM (Fig. 3A), nor did it induce IL-6 secretion in PB-MSC-derived MSCs (Fig. 3B).
ET1, IL-8, PDGF-AA, and IGFBP2, but Not uPA, Stimulate Endothelial Tube-Like Formation
As already mentioned, the functional role of ET1, IL-8, PDGF-AA, IGFBP2, and uPA were studied in more detail (Fig. 3A).
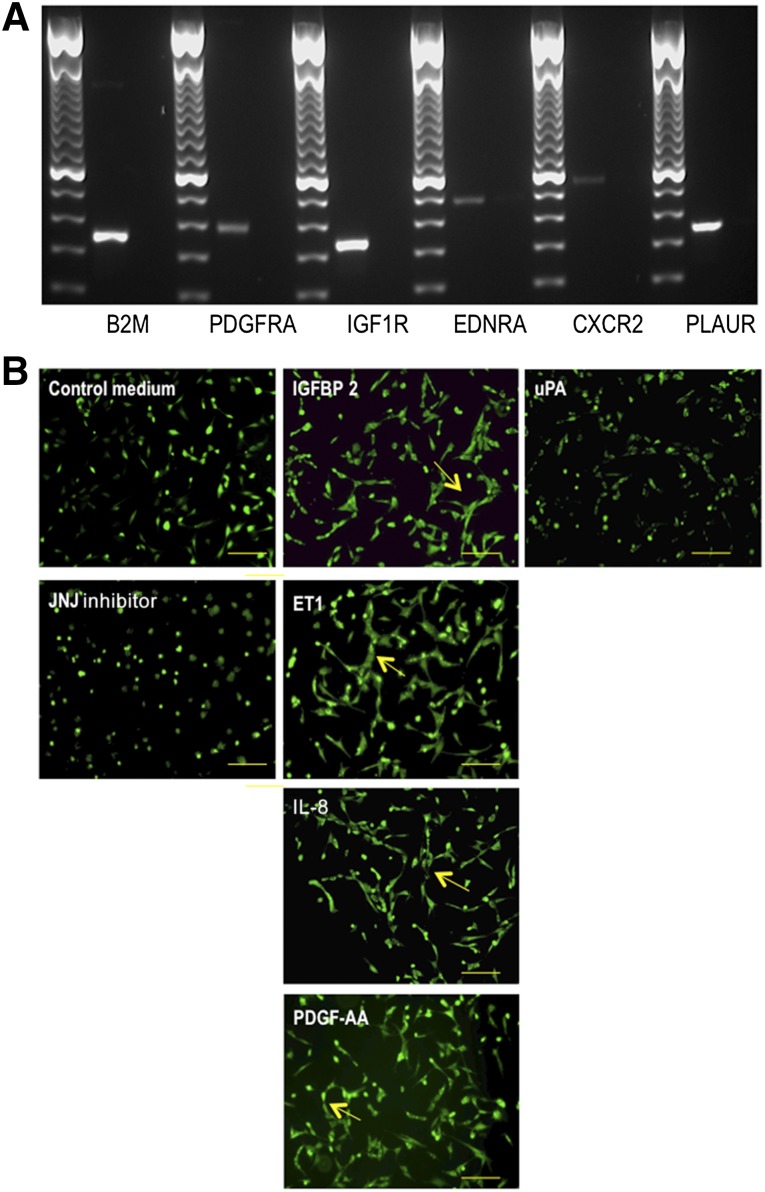
First, we evaluated whether vascular ECs express the appropriate receptors for these angiogenic factors using traditional RT-PCR. All receptors (i.e., endothelin receptor type A, insulin-like growth factor 1 receptor, C-X-C chemokine receptor type 2, platelet-derived growth factor receptor, α polypeptide, and plasminogen activator urokinase receptor) were expressed in equine ECs (Fig. 4A), indicating that their ligands could have the ability to regulate EC behavior.
Figure 4.
ET1, IL-8, PDGF-AA, and IGFBP2 stimulate endothelial tube-like formation in vitro. (A): Traditional reverse transcription-polymerase chain reaction (RT-PCR) was used to evaluate whether vascular endothelial cells (ECs) express receptors for the angiogenic factors detected in the peripheral blood-derived equine mesenchymal stromal cell-derived conditioned medium. RT-PCR products were run on a 1.5% agarose gel. (B): Equine ECs were seeded on an extracellular matrix gel in the presence of different recombinant proteins to evaluate the effect of these proteins on the tube-like formation capacity of ECs in vitro. After 1 day of culture, ECs were stained with 10 μl of 10× cell-based calcein. JNJ inhibitor was used as a negative control. Photographs were taken using an Eclipse TE2000-U inverted fluorescence microscope (Nikon). Arrows indicate hollow tube-like structure formation. Scale bars = 100 μm. Abbreviations: B2M, β2-microglobulin; CXCR2, C-X-C chemokine receptor type 2; EDNRA, endothelin receptor type A; ET1, endothelin-1; IGF1R, insulin-like growth factor 1 receptor; IL, interleukin; PDGFRA, platelet-derived growth factor receptor α; PDGF-AA, platelet-derived growth factor AA; PLAUR, plasminogen activator urokinase receptor; uPA, urokinase plasminogen activator.
Next, we aimed to determine the functional role of these proteins by studying their effects on the tubule network formation of ECs. To this end, equine ECs were cultured on a modified extracellular matrix in the presence of commercially available recombinants of these proteins. Four recombinant proteins (i.e., ET1, IL-8, IGFBP2, and PDGF-AA) clearly showed a positive effect on the formation of tube-like structures (Fig. 4B). In contrast, recombinant uPA did not stimulate the tubule network formation of ECs and even showed an inhibitory effect comparable to that of the JNJ inhibitor control (Fig. 4B).
ET1, IL-8, PDGF-AA, and IGFBP2 Upregulate the Expression Level of VEGF-A in Equine ECs, and PB-MSC-Derived CM Increases VEGF Secretion in These Cells
VEGF has been described as one of the most important enhancers of angiogenesis, and its modulation might create new therapeutic possibilities for angiogenic disorders [35]. Because ET1, IL-8, PDGF-AA, and IGFBP2 have all been shown to stimulate the expression of VEGF (also called VEGF-A), we hypothesized that the angiogenic effects of the PB-MSC-derived CM on ECs results from the paracrine effect of the mentioned secreted factors on the expression level of VEGF-A [36–40].
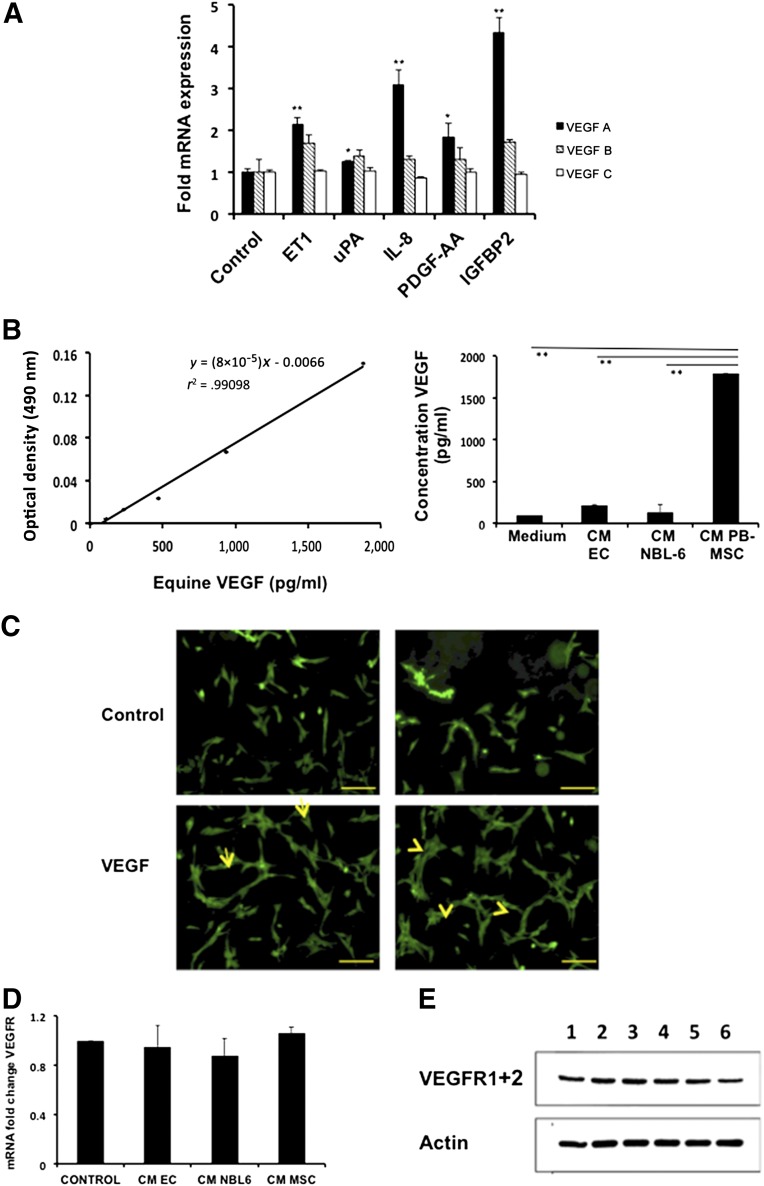
First, we incubated our ECs with the different recombinant proteins, and 48 hours later, the expression levels of VEGF-A, VEGF-B, and VEGF-C were evaluated using qRT-PCR. All recombinant proteins significantly increased the expression levels of VEGF-A, but not of VEGF-B or VEGF-C (Fig. 4A). IGFBP2 resulted in the greatest upregulation of VEGF-A in equine ECs (4.3-fold), followed by IL-8 (3.1-fold), ET1 (2.1-fold), and PDGF-AA (1.8-fold). As expected from the results of the angiogenesis assays, culturing ECs with recombinant uPA resulted in an almost negligible, nonsignificant increase in VGEF-A (1.2-fold) (Fig. 5A).
Figure 5.
CM of equine PB-MSCs stimulates angiogenesis in vitro through the stimulation of VEGF-A production via the secreted factors ET1, IL-8, PDGF-AA, and IGFBP2. (A): Quantitative reverse transcription-polymerase chain reaction (RT-PCR) was performed to evaluate whether recombinant uPA, ET1, IL-8, PDGF-AA, or IGFBP2 could change the expression levels of VEGF-A mRNA in ECs. (B): An equine-specific VEGF enzyme-linked immunosorbent assay was used to determine the levels of VEGF secreted by ECs when exposed to PB-MSC-derived CM. Control medium and EC-derived CM and NBL6-derived CM were included as controls. Absorbance was measured at 490 nm on an Infinite M200 Pro microplate reader using i-control software (Tecan). (C): ECs were seeded on an extracellular matrix gel in the presence of VEGF-A to confirm the positive effect of this recombinant protein on the tube-like formation capacity of ECs in vitro. After 1 day of culture, ECs were stained with 10 μl of 10× cell-based calcein. Arrows indicate hollow tube-like structure formation. Scale bars = 100 μm. (D): Quantitative RT-PCR was performed to evaluate whether CM of PB-MSCs changed the expression levels of VEGFR mRNA in ECs. (E): Western blotting was performed to evaluate whether CM of PB-MSCs changed the expression levels of VEGFR protein in ECs. Lane 1 represents ECs incubated in MSC expansion medium; lane 2, ECs in CM of ECs; lane 3, ECs in CM of NBL-6; and lanes 4-6, ECs in CM of PB-MSCs obtained from 3 different horses. Abbreviations: CM, conditioned medium; EC, endothelial cell; ET1, endothelin-1; IGFBP2, insulin-like growth factor binding protein 2; IL, interleukin; PB-MSC, peripheral blood-derived equine mesenchymal stromal cell; PDGF-AA, platelet-derived growth factor AA; VEGF, vascular endothelial growth factor; VEGFR, vascular endothelial growth factor receptor; uPA, urokinase plasminogen activator.
Second, we wished to study whether this increase in VGEF-A expression at the mRNA level could be translated into an actual increased secretion of VGEF-A by equine ECs. Thus, we used an equine VGEF-A-specific ELISA and evaluated the secretion levels of VEGF-A in equine ECs that were, or were not, preincubated with PB-MSC-derived CM. Preincubation of equine ECs with PB-MSC-derived CM resulted in a significantly greater level of VEGF-A (1,788 pg/ml) in the supernatants of ECs compared with ECs cultured in control medium (102 pg/ml; p < .01; Fig. 5B). In addition, preincubating ECs with CM from ECs themselves or CM from NBL6 resulted in VGEF-A levels of 219 pg/ml and 135 pg/ml, respectively (Fig. 5B). These levels were comparable to equine ECs cultured in control medium and were significantly lower than those in equine ECs preincubated with PB-MSC-derived CM, indicating that the observed effect was not a general CM effect but was mediated by CM from PB-MSCs specifically.
Next, we wished to evaluate whether the expression level of the VEGFR in equine ECs changes after preincubation with CM of PB-MSCs, at both the mRNA and the protein level. Preincubation of equine ECs with PB-MSC-derived CM did not result in a significantly higher level of VEGFR mRNA compared with ECs cultured in expansion medium, CM of equine ECs or CM of NBL-6 (p > .05; Fig. 5D). We also demonstrated, using Western blot analyses, that VEGFR is expressed by equine ECs and that no induction is visible after incubation with CM of PB-MSCs (Fig. 5E).
Finally, we determined whether VEGF-A also has a direct functional effect on angiogenesis by repeating the angiogenesis assays using recombinant VEGF-A. Compared with the baseline EC growth, a clear positive effect on the formation of tube-like structures was observed when ECs were cultured in the presence of VGEF-A (Fig. 5C).
Discussion
The present study is the first to identify bioactive factors secreted by equine MSCs that are able to induce angiogenesis in vitro through activation of VEGF-A expression and secretion in ECs (Fig. 5). MSC-induced enhancement of VEGF-A expression in muscle cells has already been described by other investigators; however, they did not identify the specific factors present in the CM of these cells responsible for this effect [41]. Furthermore, our study is the first to report a positive effect on angiogenesis by CM from PB-MSCs, because previous studies used CM from MSCs isolated from bone marrow (BM-MSCs) or adipose tissues [42–44]. It is also important to note that BM and adipose tissue, although more enriched in MSCs, are a more invasive source and, therefore, less accessible for regenerative therapy compared with PB as a source for MSCs [19, 27, 45]. PB-MSCs have been demonstrated to express the same markers and to have similar differentiation potential compared with those derived from BM. However, studies reviewed recently by Ranganath et al. have demonstrated that the MSC secretome, at least in vitro, is dependent on the cell source, purity, and preconditioning by microenvironmental factors, such as growth factors, small molecules, and hypoxia [18, 46, 47]. For example, Watt et al. compared the secretome of human MSCs derived from amnion fluid and BM using angiogenesis antibody arrays and found some significant differences in the presence of the angiogenic factors between the two sources [47]. These results indicate that data regarding the CM of BM-MSCs cannot be blindly extrapolated to CM derived from PB-MSCs. Future comparative studies will undoubtedly lead to a more tissue-specific approach to the use of the CM of these cells in regenerative medicine. However, in the present study, we decided to focus on the angiogenic in vitro potential of CM of PB-MSCs specifically.
After confirming the positive effects of equine PB-MSC-derived CM on the proliferation and tubule formation of ECs, we first aimed to identify the different angiogenic factors present in the CM of these cells using a human-specific proteome profiler array. Although this human proteome profiler array has been used previously to identify proteins secreted by equine cells [30], we acknowledge that we might have missed some bioactive factors in the equine CM owing to the lack of cross-reactivity. However, from all the factors we were able to identify with this array, VEGF-A, IL-8, PDGF-AA, ET1, and uPA have been described previously to be present in the secretome of human MSCs [18, 48, 49]. We identified one additional factor in the secretome of equine MSCs that has not been previously reported in the secretome of human MSCs (i.e., IGFBP2). This factor has been described to modulate different stages of neovascularization by enhancing VEGF gene promotor activity [39, 50]. In contrast to what has been described for human BM-MSCs, we were unable to detect IL-6 in the equine PB-MSC-derived CM [31, 51].
Next, we evaluated the effects of different factors, present in the in vivo microenvironment of MSCs, on the secretion of angiogenic factors by PB-MSCs. Inflammatory cytokines, such as IFNγ, have been demonstrated to stimulate mouse MSCs to express higher levels of VEGF via the hypoxia-inducible factor-1α signaling pathway [32]. Other factors of the metabolic environment (i.e., fatty acids) have been shown to have a positive effect on the secretion of growth factors and cytokines by MSCs. For example, LA has been demonstrated to increase secretion of IL-6, IL-8, VEGF, and nitric oxide by human MSCs [33]. Furthermore, microenvironment conditions, such as hypoxia, which can be mimicked by preconditioning with CoCl2, have been shown to result in improved angiogenesis [34]. When evaluating the effect of pretreatment of equine PB-MSCs with these different factors, no additional positive effects on the secretion levels of proangiogenic factors in CM could be observed. However, additional research is needed to determine whether this discrepancy results from (a) species differences (human vs. horse), (b) MSC source (bone marrow vs. peripheral blood), or (c) differences in the experimental setups.
From our findings using the proteome profiler array, we decided to evaluate the role of IGFBP2, IL-8, PDGF-AA, and ET1 in more detail. All these proteins have been described to stimulate VEGF-A, with the exception of IL-8, in the context of tumor cells. Therefore, we hypothesized that the secretome of equine PB-MSCs would stimulate angiogenesis through an upregulation of the expression and secretion of VEGF-A in ECs via its paracrine bioactive factors IGFBP2, IL-8, PDGF-AA, and ET-1 [37, 39, 52, 53]. To test this hypothesis, we started by analyzing the expression levels of VEGF-A, -B, and -C in ECs after incubation with the different recombinant factors. The latter two have never been studied in the horse, except in the context of tumors [54, 55], and we did not find any changes in the expression levels after incubation of ECs with the identified paracrine bioactive factors in the present study. In contrast, we found increased expression levels of VEGF-A in the ECs after CM stimulation using qRT-PCR. Importantly, we were also able to confirm a significantly greater secretion of VEGF-A using ELISA. Although no reports have been published on VEGF-A secretion by ECs after stimulation with CM of MSCs, a study by Katare et al. showed a similar effect in ischemic muscle cells [41].
When recapitulating the similarities and differences between the secretome of human MSCs (in accordance with available published data) and equine MSCs (from our present study), we found that most of the identified biofactors (i.e., IL-8, VEGF-A, PDGF-AA, and uPA) are present in the MSC CM of both species [18, 48]. However, we did find one additional secreted factor in the CM of equine MSCs (i.e., IGFBP2, which has not been reported in the CM of human MSCs). It will be interesting to determine whether this factor is also present in the CM of human MSCs and its functional role. Finally, we were unable to detect IL-6 in the CM of equine MSCs, although this factor has been reported to be present in high levels in the CM of human MSCs [31, 51]. When considering the functional effects of these secreted factors, we confirmed a positive effect on angiogenesis of IL-8, VEGF-A, IGFBP2, ET1, and PDGF-AA, in line with what has been reported in several human studies [37, 39, 52, 53]. However, we were unable to detect any positive effect on angiogenesis with uPA. This findings is in contrast to those from human studies, although this might be explained by the inefficiency of human recombinant uPA to stimulate angiogenesis in our equine system. However, overall, we can conclude that the factors secreted by equine PB-MSCs and their function in angiogenesis have many similarities with their human analogs, indicating the value of the horse as a translational model for MSC studies.
Conclusion
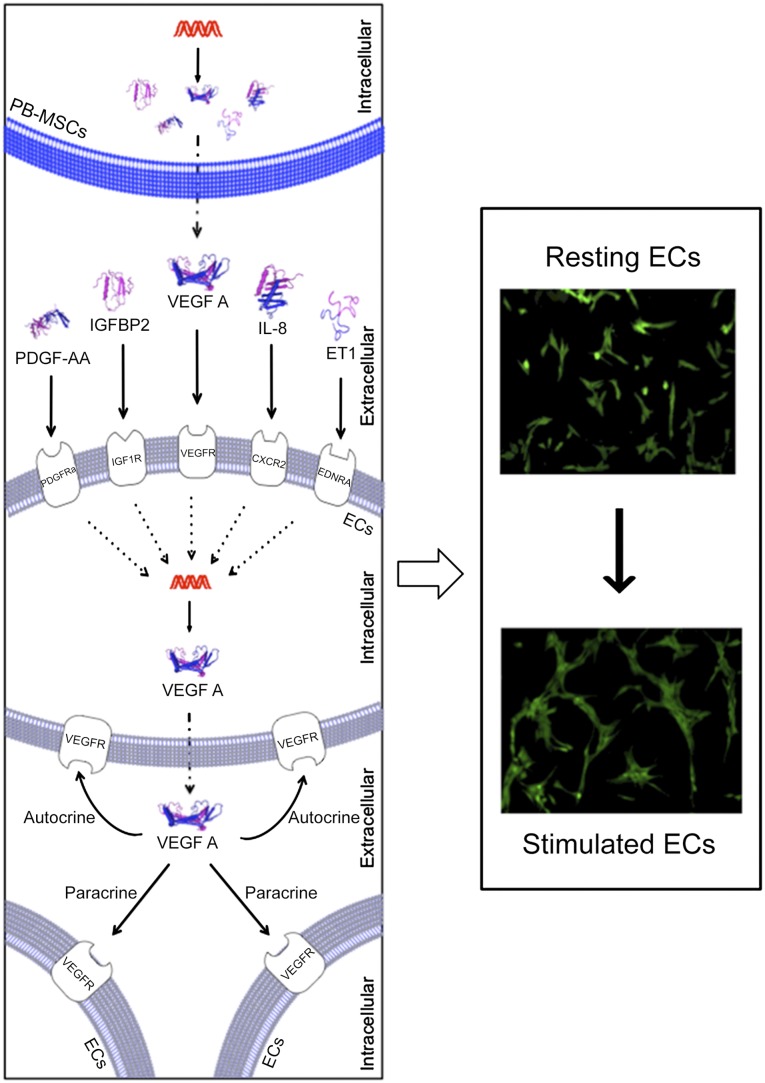
We have demonstrated for the first time that equine PB-MSCs stimulate angiogenesis in vitro through the secretion of different angiogenic factors that promote the secretion of VEGF-A by ECs (Fig. 6), a “mode of action” that can potentially be extrapolated to human MSCs.
Figure 6.
Schematic overview depicting how PB-MSC-derived CM stimulates angiogenesis of equine ECs in vitro. The secretome of equine PB-MSCs contains a variety of secreted bioactive factors, including the angiogenic factors ET1, IL-8, PDGF-AA, IGFBP2, and VEGF-A. Once secreted, these paracrine factors will bind to their receptors present on the cellular membrane of ECs. On receptor-ligand binding, ECs will increase the secretion of VEGF-A. This secreted VEGF-A will further enhance angiogenesis through autocrine and paracrine signaling. Abbreviations: CXCR2, C-X-C chemokine receptor type 2; EDNRA, endothelin receptor type A; ET1, endothelin-1; IGF1R, insulin-like growth factor 1 receptor; IL, interleukin; PDGFRA, platelet-derived growth factor receptor α; PDGF-AA, platelet-derived growth factor AA.
Acknowledgments
We gratefully acknowledge the Cornell Stem Cell program for providing financial support for our study. We also thank Donald Miller, Allison Seebald, and Rebecca Harman for technical assistance.
Author Contributions
L.B.: conception and design, collection and/or assembly of data, data analysis and interpretation, manuscript writing; G.R.V.d.W.: conception and design, data analysis and interpretation, manuscript writing, administrative support.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Carmeliet P. Angiogenesis in health and disease. Nat Med. 2003;9:653–660. doi: 10.1038/nm0603-653. [DOI] [PubMed] [Google Scholar]
- 2.Yoo SY, Kwon SM. Angiogenesis and its therapeutic opportunities. Mediators Inflamm. 2013;2013:e127170. doi: 10.1155/2013/127170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Potente M, Gerhardt H, Carmeliet P. Basic and therapeutic aspects of angiogenesis. Cell. 2011;146:873–887. doi: 10.1016/j.cell.2011.08.039. [DOI] [PubMed] [Google Scholar]
- 4.Sunkomat JNE, Gaballa MA. Stem cell therapy in ischemic heart disease. Cardiovasc Drug Rev. 2003;21:327–342. doi: 10.1111/j.1527-3466.2003.tb00125.x. [DOI] [PubMed] [Google Scholar]
- 5.Li Y, Chen J, Chen XG, et al. Human marrow stromal cell therapy for stroke in rat: Neurotrophins and functional recovery. Neurology. 2002;59:514–523. doi: 10.1212/wnl.59.4.514. [DOI] [PubMed] [Google Scholar]
- 6.Li Y, Chen J, Zhang CL, et al. Gliosis and brain remodeling after treatment of stroke in rats with marrow stromal cells. Glia. 2005;49:407–417. doi: 10.1002/glia.20126. [DOI] [PubMed] [Google Scholar]
- 7.Tang YL, Tang Y, Zhang YC, et al. Improved graft mesenchymal stem cell survival in ischemic heart with a hypoxia-regulated heme oxygenase-1 vector. J Am Coll Cardiol. 2005;46:1339–1350. doi: 10.1016/j.jacc.2005.05.079. [DOI] [PubMed] [Google Scholar]
- 8.Tögel F, Hu Z, Weiss K, et al. Administered mesenchymal stem cells protect against ischemic acute renal failure through differentiation-independent mechanisms. Am J Physiol Renal Physiol. 2005;289:F31–F42. doi: 10.1152/ajprenal.00007.2005. [DOI] [PubMed] [Google Scholar]
- 9.Zhu X-Y, Lerman A, Lerman LO. Concise review: Mesenchymal stem cell treatment for ischemic kidney disease. Stem Cells. 2013;31:1731–1736. doi: 10.1002/stem.1449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Liew A, O’Brien T. Therapeutic potential for mesenchymal stem cell transplantation in critical limb ischemia. Stem Cell Res Ther. 2012;3:28. doi: 10.1186/scrt119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Altaner C, Altanerova V, Cihova M, et al. Characterization of mesenchymal stem cells of “no-options” patients with critical limb ischemia treated by autologous bone marrow mononuclear cells. PLoS One. 2013;8:e73722. doi: 10.1371/journal.pone.0073722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bura A, Planat-Benard V, Bourin P, et al. Phase I trial: The use of autologous cultured adipose-derived stroma/stem cells to treat patients with non-revascularizable critical limb ischemia. Cytotherapy. 2014;16:245–257. doi: 10.1016/j.jcyt.2013.11.011. [DOI] [PubMed] [Google Scholar]
- 13.Das AK, Bin Abdullah BJ, Dhillon SS, et al. Intra-arterial allogeneic mesenchymal stem cells for critical limb ischemia are safe and efficacious: Report of a phase I study. World J Surg. 2013;37:915–922. doi: 10.1007/s00268-012-1892-6. [DOI] [PubMed] [Google Scholar]
- 14.Kaneko E, Izumimoto N, Toyoshima K, et al. [Therapeutic angiogenesis for critical limb ischemia] Nippon Ronen Igakkai Zasshi. 2013;50:366–368. doi: 10.3143/geriatrics.50.366. [DOI] [PubMed] [Google Scholar]
- 15.Zhi K, Gao Z, Bai J, et al. Application of adipose-derived stem cells in critical limb ischemia. Front Biosci (Landmark Ed) 2014;19:768–776. doi: 10.2741/4243. [DOI] [PubMed] [Google Scholar]
- 16.Lee EJ, Park H-W, Jeon H-J, et al. Potentiated therapeutic angiogenesis by primed human mesenchymal stem cells in a mouse model of hindlimb ischemia. Regen Med. 2013;8:283–293. doi: 10.2217/rme.13.17. [DOI] [PubMed] [Google Scholar]
- 17.Chen CP, Lee YJ, Chiu ST, et al. The application of stem cells in the treatment of ischemic diseases. Histol Histopathol. 2006;21:1209–1216. doi: 10.14670/HH-21.1209. [DOI] [PubMed] [Google Scholar]
- 18.Ranganath SH, Levy O, Inamdar MS, et al. Harnessing the mesenchymal stem cell secretome for the treatment of cardiovascular disease. Cell Stem Cell. 2012;10:244–258. doi: 10.1016/j.stem.2012.02.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.De Schauwer C, Van de Walle GR, Van Soom A, et al. Mesenchymal stem cell therapy in horses: Useful beyond orthopedic injuries? Vet Q. 2013;33:234–241. doi: 10.1080/01652176.2013.800250. [DOI] [PubMed] [Google Scholar]
- 20.Gershwin LJ. Veterinary autoimmunity: Autoimmune diseases in domestic animals. Ann N Y Acad Sci. 2007;1109:109–116. doi: 10.1196/annals.1398.013. [DOI] [PubMed] [Google Scholar]
- 21.Bähr A, Wolf E. Domestic animal models for biomedical research. Reprod Domest Anim. 2012;47(suppl 4):59–71. doi: 10.1111/j.1439-0531.2012.02056.x. [DOI] [PubMed] [Google Scholar]
- 22.Fureix C, Jego P, Henry S, et al. Towards an ethological animal model of depression? A study on horses. PLoS One. 2012;7:e39280. doi: 10.1371/journal.pone.0039280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gregory MH, Capito N, Kuroki K, et al. A review of translational animal models for knee osteoarthritis. Arthritis. 2012;2012:e764621. doi: 10.1155/2012/764621. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Spaas JH, Guest DJ, Van de Walle GR. Tendon regeneration in human and equine athletes: Ubi Sumus-Quo Vadimus (where are we and where are we going to)? Sports Med. 2012;42:871–890. doi: 10.1007/BF03262300. [DOI] [PubMed] [Google Scholar]
- 25.Spaas JH, Schauwer C, De, Cornillie P, et al. Culture and characterisation of equine peripheral blood mesenchymal stromal cells. Vet J. 2013;195:107–113. doi: 10.1016/j.tvjl.2012.05.006. [DOI] [PubMed] [Google Scholar]
- 26.De Schauwer C, van de Walle GR, Piepers S, et al. Successful isolation of equine mesenchymal stromal cells from cryopreserved umbilical cord blood-derived mononuclear cell fractions. Equine Vet J. 2013;45:518–522. doi: 10.1111/evj.12003. [DOI] [PubMed] [Google Scholar]
- 27.De Schauwer C, Goossens K, Piepers S, et al. Characterization and profiling of immunomodulatory genes of equine mesenchymal stromal cells from non-invasive sources. Stem Cell Res Ther. 2014;5:6. doi: 10.1186/scrt395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Van de Walle GR, Peters ST, VanderVen BC, et al. Equine herpesvirus 1 entry via endocytosis is facilitated by alphaV integrins and an RSD motif in glycoprotein D. J Virol. 2008;82:11859–11868. doi: 10.1128/JVI.00868-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Schnabel LV, Pezzanite LM, Antczak DF, et al. Equine bone marrow-derived mesenchymal stromal cells are heterogeneous in MHC class II expression and capable of inciting an immune response in vitro. Stem Cell Res Ther. 2014;5:13. doi: 10.1186/scrt402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bara JJ, McCarthy HE, Humphrey E, et al. Bone marrow-derived mesenchymal stem cells become antiangiogenic when chondrogenically or osteogenically differentiated: Implications for bone and cartilage tissue engineering. Tissue Eng Part A. 2014;20:147–159. doi: 10.1089/ten.tea.2013.0196. [DOI] [PubMed] [Google Scholar]
- 31.Yew T-L, Hung Y-T, Li H-Y, et al. Enhancement of wound healing by human multipotent stromal cell conditioned medium: The paracrine factors and p38 MAPK activation. Cell Transplant. 2011;20:693–706. doi: 10.3727/096368910X550198. [DOI] [PubMed] [Google Scholar]
- 32.Liu Y, Han ZP, Zhang SS, et al. Effects of inflammatory factors on mesenchymal stem cells and their role in the promotion of tumor angiogenesis in colon cancer. J Biol Chem. 2011;286:25007–25015. doi: 10.1074/jbc.M110.213108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Smith AN, Muffley LA, Bell AN, et al. Unsaturated fatty acids induce mesenchymal stem cells to increase secretion of angiogenic mediators. J Cell Physiol. 2012;227:3225–3233. doi: 10.1002/jcp.24013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Yu X, Lu C, Liu H, et al. Hypoxic preconditioning with cobalt of bone marrow mesenchymal stem cells improves cell migration and enhances therapy for treatment of ischemic acute kidney injury. PLoS One. 2013;8:e62703. doi: 10.1371/journal.pone.0062703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Hoeben A, Landuyt B, Highley MS, et al. Vascular endothelial growth factor and angiogenesis. Pharmacol Rev. 2004;56:549–580. doi: 10.1124/pr.56.4.3. [DOI] [PubMed] [Google Scholar]
- 36.Spinella F, Rosanò L, Di Castro V, et al. Endothelin-1 induces vascular endothelial growth factor by increasing hypoxia-inducible factor-1α in ovarian carcinoma cells. J Biol Chem. 2002;277:27850–27855. doi: 10.1074/jbc.M202421200. [DOI] [PubMed] [Google Scholar]
- 37.Shikada Y, Yonemitsu Y, Koga T, et al. Platelet-derived growth factor-AA is an essential and autocrine regulator of vascular endothelial growth factor expression in non-small cell lung carcinomas. Cancer Res. 2005;65:7241–7248. doi: 10.1158/0008-5472.CAN-04-4171. [DOI] [PubMed] [Google Scholar]
- 38.Martin D, Galisteo R, Gutkind JS. CXCL8/IL8 stimulates vascular endothelial growth factor (VEGF) expression and the autocrine activation of VEGFR2 in endothelial cells by activating NFkappaB through the CBM (Carma3/Bcl10/Malt1) complex. J Biol Chem. 2009;284:6038–6042. doi: 10.1074/jbc.C800207200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Azar WJ, Azar SHX, Higgins S, et al. IGFBP-2 enhances VEGF gene promoter activity and consequent promotion of angiogenesis by neuroblastoma cells. Endocrinology. 2011;152:3332–3342. doi: 10.1210/en.2011-1121. [DOI] [PubMed] [Google Scholar]
- 40.Das SK, Bhutia SK, Azab B, et al. MDA-9/syntenin and IGFBP-2 promote angiogenesis in human melanoma. Cancer Res. 2013;73:844–854. doi: 10.1158/0008-5472.CAN-12-1681. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Katare R, Riu F, Rowlinson J, et al. Perivascular delivery of encapsulated mesenchymal stem cells improves postischemic angiogenesis via paracrine activation of VEGF-A. Arterioscler Thromb Vasc Biol. 2013;33:1872–1880. doi: 10.1161/ATVBAHA.113.301217. [DOI] [PubMed] [Google Scholar]
- 42.Wang C-Y, Yang H-B, Hsu H-S, et al. Mesenchymal stem cell-conditioned medium facilitates angiogenesis and fracture healing in diabetic rats. J Tissue Eng Regen Med. 2012;6:559–569. doi: 10.1002/term.461. [DOI] [PubMed] [Google Scholar]
- 43.Bhang SH, Lee S, Shin J-Y, et al. Efficacious and clinically relevant conditioned medium of human adipose-derived stem cells for therapeutic angiogenesis. Mol Ther. 2014;22:862–872. doi: 10.1038/mt.2013.301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Boomsma RA, Geenen DL. Mesenchymal stem cells secrete multiple cytokines that promote angiogenesis and have contrasting effects on chemotaxis and apoptosis. PLoS One. 2012;7:e35685. doi: 10.1371/journal.pone.0035685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Kamal A, Iskandriati D, Dilogo I, et al. Comparison of cultured mesenchymal stem cells derived from bone marrow or peripheral blood of rats. J Exp Integr Med. 2014;4:17–22. [Google Scholar]
- 46.Chong P-P, Selvaratnam L, Abbas AA, et al. Human peripheral blood derived mesenchymal stem cells demonstrate similar characteristics and chondrogenic differentiation potential to bone marrow derived mesenchymal stem cells. J Orthop Res. 2012;30:634–642. doi: 10.1002/jor.21556. [DOI] [PubMed] [Google Scholar]
- 47.Watt SM, Gullo F, van der Garde M, et al. The angiogenic properties of mesenchymal stem/stromal cells and their therapeutic potential. Br Med Bull. 2013;108:25–53. doi: 10.1093/bmb/ldt031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Windmolders S, De Boeck A, Koninckx R, et al. Mesenchymal stem cell secreted platelet derived growth factor exerts a pro-migratory effect on resident cardiac atrial appendage stem Cells. J Mol Cell Cardiol. 2014;66:177–188. doi: 10.1016/j.yjmcc.2013.11.016. [DOI] [PubMed] [Google Scholar]
- 49.Salama M, Andrukhova O, Jaksch P, et al. Endothelin-1 governs proliferation and migration of bronchoalveolar lavage-derived lung mesenchymal stem cells in bronchiolitis obliterans syndrome. Transplantation. 2011;92:155–162. doi: 10.1097/TP.0b013e318222c9ea. [DOI] [PubMed] [Google Scholar]
- 50.Salani D, Taraboletti G, Rosanò L, et al. Endothelin-1 induces an angiogenic phenotype in cultured endothelial cells and stimulates neovascularization in vivo. Am J Pathol. 2000;157:1703–1711. doi: 10.1016/S0002-9440(10)64807-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Huang S-P, Wu M-S, Shun C-T, et al. Interleukin-6 increases vascular endothelial growth factor and angiogenesis in gastric carcinoma. J Biomed Sci. 2004;11:517–527. doi: 10.1007/BF02256101. [DOI] [PubMed] [Google Scholar]
- 52.Wu M-H, Huang C-Y, Lin J-A, et al. Endothelin-1 promotes vascular endothelial growth factor-dependent angiogenesis in human chondrosarcoma cells. Oncogene. 2014;33:1725–1735. doi: 10.1038/onc.2013.109. [DOI] [PubMed] [Google Scholar]
- 53.Hou Y, Ryu CH, Jun JA, et al. IL-8 enhances the angiogenic potential of human bone marrow mesenchymal stem cells by increasing vascular endothelial growth factor. Cell Biol Int. 2014;38:1050–1059. doi: 10.1002/cbin.10294. [DOI] [PubMed] [Google Scholar]
- 54.Müller K, Ellenberger C, Schoon H-A. Histomorphological and immunohistochemical study of angiogenesis and angiogenic factors in the ovary of the mare. Res Vet Sci. 2009;87:421–431. doi: 10.1016/j.rvsc.2009.04.011. [DOI] [PubMed] [Google Scholar]
- 55.Müller K, Ellenberger C, Hoppen H-O, et al. Immunohistochemical study of angiogenesis and angiogenic factors in equine granulosa cell tumours. Res Vet Sci. 2012;92:471–477. doi: 10.1016/j.rvsc.2011.02.016. [DOI] [PubMed] [Google Scholar]