In adults, a mesenchymal stromal/stem cell (MSC)-like population has been demonstrated in different compartments of human and murine nephrons. The evidence of an MSC presence within the adult kidney and the potential contribution of MSCs to the turnover of renal cells and injury repair is discussed.
Keywords: Kidney, Stem cells, Tissue resident mesenchymal stem cells, Glomeruli
Abstract
During fetal life, mesenchymal stromal/stem cells (MSCs) surround glomeruli and tubules and contribute to the development of the renal interstitium by secretion of growth factors that drive nephron differentiation. In the adult, an MSC-like population has been demonstrated in different compartments of human and murine nephrons. After injury, these cells might provide support for kidney regeneration by recapitulating the role they have in embryonic life. In this short review, we discuss the evidence of an MSC presence within the adult kidney and their potential contribution to the turnover of renal cells and injury repair.
Introduction
Multipotent mesenchymal stromal/stem cells (MSCs) are defined, according to the International Society for Cellular Therapy, on the basis of their ability to adhere to plastic, self-replicate, differentiate into mesodermal lineages (i.e., osteocytes, chondrocytes, adipocytes), and express specific surface markers (CD105, CD73, and CD90) [1] and by their immunomodulatory capacity and low immunogenicity [2]. MSCs might have potential therapeutic applications in regeneration after injury of different organs (e.g., heart, kidneys, lungs, and liver), and they are currently being used in clinical trials for treating a wide range of diseases (available at: http://www.clinicaltrials.gov). MSCs exhibit multidifferentiation capacity in vitro, and are able to migrate to injured sites in vivo after systemic administration [3]. Moreover, an increasing amount of evidence has indicated that the beneficial effects of MSC treatment on tissue/organ injury in different animal models can be mainly attributed to paracrine activities [4].
Historically, MSCs were first isolated from the bone marrow (BM), where they play a role in hematopoiesis [5, 6]. Subsequently, MSCs were also isolated from other organs and tissues [7–9]. The present review focused on MSCs identified in murine and human kidneys, in particular, from the glomeruli.
MSCs Resident in the Murine Kidney
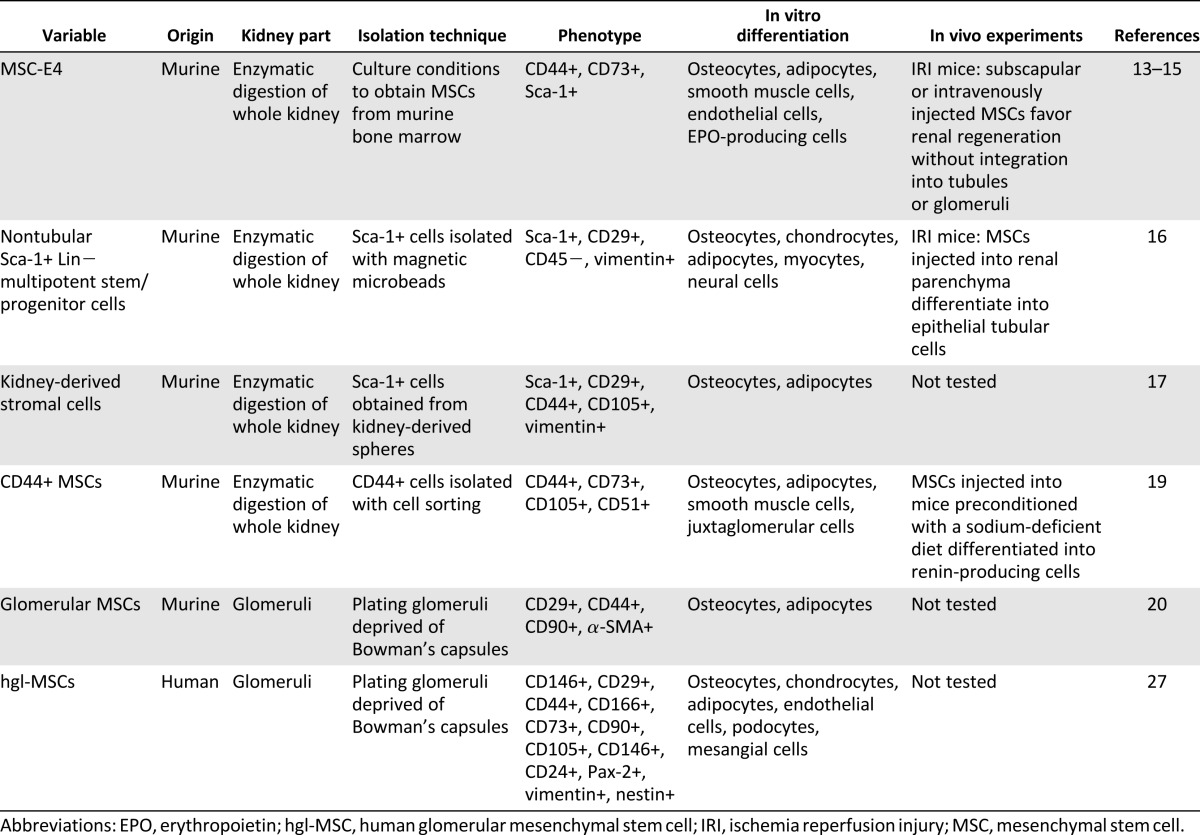
During embryogenesis, an MSC population can be detected in the kidneys. This population, which surrounds developing glomeruli and tubules, secretes growth factors that drive nephron differentiation [10]. Moreover, MSCs contribute to the development of the renal interstitium [11]. In the event of kidney injury, interstitial cells might provide support for kidney regeneration by assuming an immature phenotype and, thus, recapitulating the role performed during embryonic life [12]. Several years ago, using a method developed for culturing MSCs from the bone marrow, Plotkin and Goligorsky were able to isolate a clone of MSCs (termed “MSCs-E4”) from adult murine kidneys with characteristics similar to embryonic stromal cells [13]. This cell population was characterized by a phenotype (CD44, CD73, Sca-1 positivity) and a gene expression profile similar to that of mesenchymal renal embryonic cells. MSCs-E4 were shown to support angiogenesis and tubulogenesis in vitro and were able to differentiate along multiple mesodermal lineages, including adipocytes, osteocytes, smooth muscle cells, and endothelial cells. Moreover, in hypoxic conditions, MSCs-E4 differentiated into fibroblast-produced erythropoietin [13]. When MSCs-E4 were subcapsularly injected after ischemia and reperfusion renal injury (IRI), they were able to migrate throughout the cortex and medulla and reached the papillary compartment [13]. These adult renal MSCs had the propensity to lodge at perivascular locations in the cortex and medulla [14] and remained in the interstitium without evidence of integration into glomeruli or tubules. This type of stromal population capable of interacting with endothelial and epithelial cells could be important for renal homeostasis and repair after injury. When injected intravenously into IRI mice, MSCs-E4 engrafted the ischemic kidney and promoted tubular regeneration and functional recovery [15].
Another group of researchers described a population of stem cells, resident in the renal interstitial space in close proximity to tubules, that were specifically located at the renal papilla [16]. These so-called nontubular multipotent stem/progenitor cells were isolated by cell sorting using the Sca-1 antigen as a marker of stem cells and were CD45 negative. In addition, this cell population was positive for CD29 and vimentin and negative for cytokeratin. Although Sca-1+ cells showed minimal expression of the surface markers typically expressed by bone marrow MSCs, they were able to differentiate into mesodermal cell lineages, such as osteocytes, chondrocytes, adipocytes, and myocytes, and into neural cells. Moreover, when the Sca-1+ cells were directly injected into renal parenchyma after IRI, they differentiated into epithelial tubular cells, thus contributing to renal repair. Renal Sca-1+ cells displayed an immunomodulatory capacity in vitro, confirming their mesenchymal nature [16]. The presence of an MSC-like population with immunomodulatory capacity was confirmed by another study [17]. The MSC-like population described in that study, named “kidney-derived stromal cells” (KSCs), expressed Sca-1 antigen and MSC markers (i.e., CD29, CD44, CD105, vimentin) and were negative for hematopoietic (i.e., CD45, CD3, CD4, CD8) and tubular epithelial (i.e., cytokeratin, ZO-1, epithelial cell adhesion molecule) markers. In vitro, they had the capacity to differentiate into osteocytes and adipocytes [17]. Moreover, KSCs significantly reduced T cell proliferation, in a cell contact manner and were able to promote the differentiation of BM precursor into dendritic cells (DCs) refractory to maturation with lipopolysaccharide and producing increased interleukin-10 [17]. These cells were characterized by a phenotype with significant reduction of major histocompatibility complex class II and increased CD80 expression [17]. KSCs seem to act early during the differentiation of DCs from BM precursors to maintain DCs in an immature or “semimature” state associated with an immune-regulatory phenotype and self-tolerance induction [18].
More recently, Wang et al. reported the existence of renal MSC-like cells in mice [19]. This cell population was first detected by cytofluorimetric analyses of enzymatically digested murine kidneys. Renal MSC-like cells coexpressed CD44, CD117, CD105, and CD90 and represented about 5% of the total renal cells. After isolation of CD44+ cells by cell sorting and in vitro culture, they acquired the spindle-shape morphology typical of MSCs, lost the expression of CD117, maintained the expression of specific MSC markers (i.e., CD44, CD73, CD105, CD51) and expressed metanephric mesenchyme markers (i.e., Sox11, Foxd1, Id2, Eya4). Adult renal CD44+ MSCs were able to differentiate toward adipogenic, osteogenic, and smooth muscle cell lineages [19]. Remarkably, CD44+ MSCs were also able to differentiate into juxtaglomerular cells that contained renin in cytoplasmic granules. When CD44+ MSCs were injected into the renal artery of mice preconditioned with a sodium-deficient diet, they successfully engrafted in the kidneys, where they differentiated into renin-producing cells [19].
In the cited studies [13–17, 19], the murine renal resident cells with MSC characteristics were isolated by enzymatic digestion of the whole kidneys, with no distinction between the glomerular and tubular compartments. Investigations by da Silva Meirelles et al. [20] reported the isolation and characterization of an MSC population from isolated glomeruli deprived of Bowman’s capsules. Cells resembling the morphology of MSCs started outgrowth from plating glomeruli from day 3 or 4 after the beginning of culture. Similar to MSCs obtained from entire kidneys and from other organs and tissues, the murine glomerular MSC population (gl-MSCs) expressed specific MSC markers, such as CD29, CD44, and CD90, and were negative for hematopoietic markers (i.e., CD45, CD11b, CD13, CD19) and for the endothelial marker CD31. All the MSC populations obtained from different murine tissues and organs expressed α-smooth muscle actin (α-SMA). Moreover, the gl-MSC population described by da Silva Meirelles et al. [19] was able to undergo osteogenic and adipogenic differentiation and showed deposition of a calcium-rich mineralized matrix and acquisition of intracellular lipid droplets. In particular, gl-MSCs were able to differentiate more efficiently into the osteogenic lineage than were the whole kidney-derived MSCs. gl-MSCs deposited a rich mineralized matrix even after 1 week of culture with osteogenic medium.
MSCs Resident in the Human Kidney
Different populations of progenitors/stem cells have been identified in the adult kidney in humans. In particular, in human adult kidneys, the presence of resident progenitors/stem cells expressing the CD133 stem cell marker has been detected in the tubular compartment, in Bowman’s capsules, and, more recently, in the papilla [21–24]. A possible role exists for neural-cell adhesion molecule 1 (NCAM1) expression in the identification and function of human renal stem cells. NCAM1 has been shown to be expressed during embryonic kidney development [25] but not in the adult kidney. When human adult kidney epithelial cells were cultured, they all expressed CD133 and CD24, and a particular subpopulation (16% ± 9% of the total population) also showed re-expression of NCAM1 [26]. Sorted NCAM1-positive cells also expressed early nephron progenitor markers (e.g., Pax-2, WT1) and mesenchymal markers (e.g., vimentin) and displayed reduced E-cadherin expression. When NCAM1+ cells were cultured in the presence of conditioned medium produced by fetal renal cells, they differentiated into mesenchymal (adipogenic and osteogenic) lineages and retained the capacity to generate epithelial kidney spheres [26]. Depletion of NCAM1+ cells from kidney epithelial cells by specific anti-NCAM antibody treatment resulted in the loss of stemness properties both in vitro and in vivo [26], indicating the relevant role of NCAM1+ cells in renal regeneration.
To our knowledge, no research has described the isolation of MSCs from the whole human kidney or from the tubular compartment. Instead, the presence of an MSC population has been identified in the glomerular compartment of the human kidney [27]. We reported that adult human glomeruli deprived of Bowman’s capsule by enzymatic digestion and mechanical passages (to avoid contamination of Bowman’s capsule–associated CD133+ progenitors) contain a resident MSC population. In our culture system, we found that cells outgrowing from the decapsulated glomeruli at early passages were composed of two distinct populations of CD133+CD146+ and CD133−CD146+ cells. The population coexpressing CD133 and CD146 showed an endothelial commitment and were also positive for the expression of specific endothelial markers, such as CD31 and von Willebrand factor, and were not able to give rise to a clonal population in vitro or to differentiate into specific mesodermal lineages. The CD133−CD146+ cells were the only cells that were outgrown from the glomeruli that had survived after four cell culture passages, and a subpopulation of cells was able to generate clonal long-term cultures of human glomerular MSCs (hgl-MSCs). This population expressed typical MSC markers (i.e., CD29, CD44, CD166, CD73, CD90, CD105, CD146, vimentin, and nestin) and did not express hematopoietic markers (i.e., CD34, CD45). Moreover, hgl-MSC expressed typical markers of kidney stem cells, such as CD24 [22, 28] and Pax-2, a transcription factor expressed by stem cells present in the metanephric mesenchyme [29].
Because one of the major defining characteristics of MSCs is their ability to differentiate into multiple mesenchymal lineages, the capacity of hgl-MSCs to differentiate into specific connective tissue cells was evaluated. hgl-MSCs efficiently underwent osteogenic differentiation and, when cultured in adipocyte medium, were able to generate cells containing lipid droplets. In addition, hgl-MSCs were able to differentiate into chondrocytes. After 28 days of culture in specific nonadhesive conditions in the presence of transforming growth factor β3, hgl-MSC pellets were positive to staining with safranin O and Alcian blue, which is characteristic of chondrocytic differentiation [27].
The capacity of hgl-MSCs to differentiate, under appropriate culture conditions, into specific cell populations present in the glomeruli (i.e., endothelial cells, podocytes, mesangial cells) was also evaluated. Endothelial differentiation was obtained by culturing hgl-MSCs in the presence of vascular endothelial growth factor. After 3 weeks of culture in these conditions, hgl-MSCs had lost the expression of mesenchymal markers and had started to express specific endothelial markers, such as CD105, KDR, CD34, and CD31, and to acquire the capacity to form capillary-like structures in vitro in the presence of Matrigel (BD Biosciences, San Diego, CA, http://www.bdbiosciences.com). When hgl-MSCs were cultured in the presence of platelet-derived growth factor BB and transforming growth factor β1, the cells acquired a mesangial-like phenotype and expressed α-SMA and angiotensin-2 receptor 1. Moreover, in these conditions, hgl-MSCs acquired the ability to change their shape when stimulated with angiotensin-2, compatible with cell contraction. When hgl-MSCs were cultured for 3 weeks in the presence of transretinoic acid, they differentiated into podocyte-like cells and expressed typical markers, such as cytokeratin, podocin, nephrin, and synaptopodin (Table 1) [27].
Table 1.
Characteristics and in vitro and in vivo properties of MSC-like populations obtained from human and murine adult kidney
The mesenchymal nature of hgl-MSCs was also confirmed by a study that evaluated their immunomodulatory capacity [27]. In particular, hgl-MSCs were able to inhibit proliferation of phytohemagglutinin-stimulated peripheral blood mononuclear cells, similar to BM-MSCs.
Another important issue was demonstrating the renal origin of the isolated hgl-MSCs. Therefore, a population of hgl-MSCs was isolated from glomeruli of an explanted kidney from a male donor transplanted into a female recipient [27]. To determine whether the hgl-MSCs were derived from the bone marrow of the recipient or were resident in the transplanted kidney, the presence of the Y chromosome was detected at different culture passages. No female karyotype was found at the various culture passages, indicating that the hgl-MSCs were not derived from the bone marrow of the recipient (female) but, rather, represented a population resident in the glomeruli of the donor kidney (male) [27].
These data have demonstrated the presence of a population of resident multipotent progenitors in the adult human decapsulated glomeruli, with mesenchymal characteristics and the potential to contribute to the turnover of the different glomerular-specific cell types.
Pericytes: MSCs Resident in Capillary Walls?
Pericytes are vascular mural cells that stabilize vessels by modulating the endothelial phenotype and the extracellular matrix composition. A single marker for pericyte isolation and characterization has not been found. Similar to MSCs, pericytes are also currently characterized by coexpression of different markers, such as α-SMA, desmin, platelet-derived growth factor receptor-β, neuron-glial 2, CD146, CD73, and CD248 [30]. Some of these markers (i.e., CD146, CD73) are also expressed by MSCs and/or by endothelial cells (CD146). Pericytes and MSCs not only have common markers, but pericytes also exhibit mesodermal multilineage differentiation potential [31]. In particular, pericytes can differentiate into smooth muscle cells, adipocytes, chondrocytes, and osteoblasts [31, 32]. Within the glomeruli of the kidney, pericytes are known as mesangial cells and provide support for glomerular capillaries, control for glomerular filtration, and modulate the local injury response by proliferation and matrix remodeling. In addition, mesangial cells, similar to MSCs, are able to modulate innate and adaptive responses by acting as local immune modulators [33].
MSCs have been tested in different animal models of acute kidney injury (AKI) and chronic kidney disease (CKD). In AKI, MSCs have shown protective and regenerative effects [34–36]. In CKD, some experimental evidence has indicated that MSC treatment reduced fibrosis and ameliorated renal function [37–41]. However, MSC therapy for CKD remains to be investigated in more detail. In CKD rodent models, MSC treatment showed a beneficial effect [37–41], but in large animal models (e.g., sheep with ischemia and reperfusion renal injury), MSCs did not exhibit reparative or protective properties [42]. Pericytes/mesangial cells share some important characteristics with MSCs and could potentially be considered as resident renal stem/progenitor cells, although pericytes/mesangial cells have not yet been tested in animal models of kidney injuries. Moreover, pericytes were recently identified as a source of collagen I-producing cells in kidney disease [43], and pericytes can differentiate into myofibroblasts, causing scar tissue with progressive fibrosis and deterioration of renal function [44, 45]. These data indicate that pericytes might be a target for antifibrotic therapy. Before developing possible clinical interventions using pericytes as targets for therapy or as therapeutic agents, it is necessary to better understand the biology and behavior of these cells. In particular, it is necessary to study the capacity of pericytes to respond to tissue injury and to act as progenitor cells, facilitating the regenerative process and not the fibrotic response.
Conclusion
Different MSC-like populations have been demonstrated to be present in both human and murine kidneys. These cells have been found in the glomeruli and interstitium and have multipotent characteristics similar to bone marrow-resident MSCs. In particular, human and murine renal MSCs shared with bone marrow MSCs the multidifferentiation capacity toward specific mesenchymal lineages, such as the adipocytic, osteocytic, and chondrocytic lineages [13, 16, 19, 20] and immunomodulatory properties [16, 17, 27]. Moreover, renal resident MSCs were shown to differentiate, under appropriate culture conditions, into specific renal cell types, such as erythropoietin-producing fibroblasts [13], juxtaglomerular cells containing renin granules [19], podocytes, and mesangial cells [27]. Although renal resident MSCs have been shown to play a defined role in kidney development, little is known about their contribution to cell turnover and repair in the adult kidney.
Author Contributions
S.B. and G. Chiabotto: conception and design, manuscript writing; G. Camussi: conception and design, financial support, manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Dominici M, Le Blanc K, Mueller I, et al. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy. 2006;8:315–317. doi: 10.1080/14653240600855905. [DOI] [PubMed] [Google Scholar]
- 2.Krampera M, Galipeau J, Shi Y, et al. Immunological characterization of multipotent mesenchymal stromal cells–The International Society for Cellular Therapy (ISCT) working proposal. Cytotherapy. 2013;15:1054–1061. doi: 10.1016/j.jcyt.2013.02.010. [DOI] [PubMed] [Google Scholar]
- 3.Pittenger MF, Mackay AM, Beck SC, et al. Multilineage potential of adult human mesenchymal stem cells. Science. 1999;284:143–147. doi: 10.1126/science.284.5411.143. [DOI] [PubMed] [Google Scholar]
- 4.Caplan AI, Dennis JE. Mesenchymal stem cells as trophic mediators. J Cell Biochem. 2006;98:1076–1084. doi: 10.1002/jcb.20886. [DOI] [PubMed] [Google Scholar]
- 5.Prockop DJ. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science. 1997;276:71–74. doi: 10.1126/science.276.5309.71. [DOI] [PubMed] [Google Scholar]
- 6.Méndez-Ferrer S, Michurina TV, Ferraro F, et al. Mesenchymal and haematopoietic stem cells form a unique bone marrow niche. Nature. 2010;466:829–834. doi: 10.1038/nature09262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Tobita M, Mizuno H. Adipose-derived stem cells and periodontal tissue engineering. Int J Oral Maxillofac Implants. 2013;28:e487–e493. doi: 10.11607/jomi.te29. [DOI] [PubMed] [Google Scholar]
- 8.Hilkens P, Gervois P, Fanton Y, et al. Effect of isolation methodology on stem cell properties and multilineage differentiation potential of human dental pulp stem cells. Cell Tissue Res. 2013;353:65–78. doi: 10.1007/s00441-013-1630-x. [DOI] [PubMed] [Google Scholar]
- 9.Indumathi S, Harikrishnan R, Mishra R, et al. Comparison of feto-maternal organ derived stem cells in facets of immunophenotype, proliferation and differentiation. Tissue Cell. 2013;45:434–442. doi: 10.1016/j.tice.2013.07.007. [DOI] [PubMed] [Google Scholar]
- 10.Cullen-McEwen LA, Caruana G, Bertram JF. The where, what and why of the developing renal stroma. Nephron Exp Nephrol. 2005;99:e1–e8. doi: 10.1159/000081792. [DOI] [PubMed] [Google Scholar]
- 11.Alcorn D, Maric C, McCausland J. Development of the renal interstitium. Pediatr Nephrol. 1999;13:347–354. doi: 10.1007/s004670050624. [DOI] [PubMed] [Google Scholar]
- 12.Herzlinger D. Renal interstitial fibrosis: Remembrance of things past? J Clin Invest. 2002;110:305–306. doi: 10.1172/JCI16377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Plotkin MD, Goligorsky MS. Mesenchymal cells from adult kidney support angiogenesis and differentiate into multiple interstitial cell types including erythropoietin-producing fibroblasts. Am J Physiol Renal Physiol. 2006;291:F902–F912. doi: 10.1152/ajprenal.00396.2005. [DOI] [PubMed] [Google Scholar]
- 14.Ratliff BB, Singh N, Yasuda K, et al. Mesenchymal stem cells, used as bait, disclose tissue binding sites: A tool in the search for the niche? Am J Pathol. 2010;177:873–883. doi: 10.2353/ajpath.2010.090984. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Park HC, Addabbo F, et al. Kidney-derived mesenchymal stem cells contribute to vasculogenesis, angiogenesis and endothelial repair. Kidney Int. 2008;74:879–889. doi: 10.1038/ki.2008.304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Dekel B, Zangi L, Shezen E, et al. Isolation and characterization of nontubular Sca-1+Lin− multipotent stem/progenitor cells from adult mouse kidney. J Am Soc Nephrol. 2006;17:3300–3314. doi: 10.1681/ASN.2005020195. [DOI] [PubMed] [Google Scholar]
- 17.Huang Y, Johnston P, Zhang B, et al. Kidney-derived stromal cells modulate dendritic and T cell responses. J Am Soc Nephrol. 2009;20:831–841. doi: 10.1681/ASN.2008030310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lutz MB, Schuler G. Immature, semi-mature and fully mature dendritic cells: Which signals induce tolerance or immunity? Trends Immunol. 2002;23:445–449. doi: 10.1016/s1471-4906(02)02281-0. [DOI] [PubMed] [Google Scholar]
- 19.Wang H, Gomez JA, Klein S, et al. Adult renal mesenchymal stem cell-like cells contribute to juxtaglomerular cell recruitment. J Am Soc Nephrol. 2013;24:1263–1273. doi: 10.1681/ASN.2012060596. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.da Silva Meirelles L, Chagastelles PC, Nardi NB. Mesenchymal stem cells reside in virtually all post-natal organs and tissues. J Cell Sci. 2006;119:2204–2213. doi: 10.1242/jcs.02932. [DOI] [PubMed] [Google Scholar]
- 21.Bussolati B, Bruno S, Grange C, et al. Isolation of renal progenitor cells from adult human kidney. Am J Pathol. 2005;166:545–555. doi: 10.1016/S0002-9440(10)62276-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sagrinati C, Netti GS, Mazzinghi B, et al. Isolation and characterization of multipotent progenitor cells from the Bowman’s capsule of adult human kidneys. J Am Soc Nephrol. 2006;17:2443–2456. doi: 10.1681/ASN.2006010089. [DOI] [PubMed] [Google Scholar]
- 23.Ward HH, Romero E, Welford A, et al. Adult human CD133/1(+) kidney cells isolated from papilla integrate into developing kidney tubules. Biochim Biophys Acta. 2011;1812:1344–1357. doi: 10.1016/j.bbadis.2011.01.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Bussolati B, Moggio A, Collino F, et al. Hypoxia modulates the undifferentiated phenotype of human renal inner medullary CD133+ progenitors through Oct4/miR-145 balance. Am J Physiol Renal Physiol. 2012;302:F116–F128. doi: 10.1152/ajprenal.00184.2011. [DOI] [PubMed] [Google Scholar]
- 25.Harari-Steinberg O, Metsuyanim S, Omer D, et al. Identification of human nephron progenitors capable of generation of kidney structures and functional repair of chronic renal disease. EMBO Mol Med. 2013;5:1556–1568. doi: 10.1002/emmm.201201584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Buzhor E, Omer D, Harari-Steinberg O, et al. Reactivation of NCAM1 defines a subpopulation of human adult kidney epithelial cells with clonogenic and stem/progenitor properties. Am J Pathol. 2013;183:1621–1633. doi: 10.1016/j.ajpath.2013.07.034. [DOI] [PubMed] [Google Scholar]
- 27.Bruno S, Bussolati B, Grange C, et al. Isolation and characterization of resident mesenchymal stem cells in human glomeruli. Stem Cells Dev. 2009;18:867–880. doi: 10.1089/scd.2008.0320. [DOI] [PubMed] [Google Scholar]
- 28.Challen GA, Martinez G, Davis MJ, et al. Identifying the molecular phenotype of renal progenitor cells. J Am Soc Nephrol. 2004;15:2344–2357. doi: 10.1097/01.ASN.0000136779.17837.8F. [DOI] [PubMed] [Google Scholar]
- 29.Oliver JA, Barasch J, Yang J, et al. Metanephric mesenchyme contains embryonic renal stem cells. Am J Physiol Renal Physiol. 2002;283:F799–F809. doi: 10.1152/ajprenal.00375.2001. [DOI] [PubMed] [Google Scholar]
- 30.Stefanska AM, Peault B, Mullins JJ. Renal pericytes: Multifunctional cells of the kidneys. Pflugers Arch. 2013;465:767–773. doi: 10.1007/s00424-013-1263-7. [DOI] [PubMed] [Google Scholar]
- 31.Crisan M, Yap S, Casteilla L, et al. A perivascular origin for mesenchymal stem cells in multiple human organs. Cell Stem Cell. 2008;3:301–313. doi: 10.1016/j.stem.2008.07.003. [DOI] [PubMed] [Google Scholar]
- 32.Covas DT, Panepucci RA, Fontes AM, et al. Multipotent mesenchymal stromal cells obtained from diverse human tissues share functional properties and gene-expression profile with CD146+ perivascular cells and fibroblasts. Exp Hematol. 2008;36:642–654. doi: 10.1016/j.exphem.2007.12.015. [DOI] [PubMed] [Google Scholar]
- 33.Schlöndorff D, Banas B. The mesangial cell revisited: No cell is an island. J Am Soc Nephrol. 2009;20:1179–1187. doi: 10.1681/ASN.2008050549. [DOI] [PubMed] [Google Scholar]
- 34.Morigi M, Imberti B, Zoja C, et al. Mesenchymal stem cells are renotropic, helping to repair the kidney and improve function in acute renal failure. J Am Soc Nephrol. 2004;15:1794–1804. doi: 10.1097/01.asn.0000128974.07460.34. [DOI] [PubMed] [Google Scholar]
- 35.Herrera MB, Bussolati B, Bruno S, et al. Mesenchymal stem cells contribute to the renal repair of acute tubular epithelial injury. Int J Mol Med. 2004;14:1035–1041. [PubMed] [Google Scholar]
- 36.Duffield JS, Bonventre JV. Kidney tubular epithelium is restored without replacement with bone marrow-derived cells during repair after ischemic injury. Kidney Int. 2005;68:1956–1961. doi: 10.1111/j.1523-1755.2005.00629.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Semedo P, Correa-Costa M, Antonio Cenedeze M, et al. Mesenchymal stem cells attenuate renal fibrosis through immune modulation and remodeling properties in a rat remnant kidney model. Stem Cells. 2009;27:3063–3073. doi: 10.1002/stem.214. [DOI] [PubMed] [Google Scholar]
- 38.Choi S, Park M, Kim J, et al. The role of mesenchymal stem cells in the functional improvement of chronic renal failure. Stem Cells Dev. 2009;18:521–529. doi: 10.1089/scd.2008.0097. [DOI] [PubMed] [Google Scholar]
- 39.Villanueva S, Carreño JE, Salazar L, et al. Human mesenchymal stem cells derived from adipose tissue reduce functional and tissue damage in a rat model of chronic renal failure. Clin Sci (Lond) 2013;125:199–210. doi: 10.1042/CS20120644. [DOI] [PubMed] [Google Scholar]
- 40.Alfarano C, Roubeix C, Chaaya R, et al. Intraparenchymal injection of bone marrow mesenchymal stem cells reduces kidney fibrosis after ischemia-reperfusion in cyclosporine-immunosuppressed rats. Cell Transplant. 2012;21:2009–2019. doi: 10.3727/096368912X640448. [DOI] [PubMed] [Google Scholar]
- 41.Villanueva S, Ewertz E, Carrión F, et al. Mesenchymal stem cell injection ameliorates chronic renal failure in a rat model. Clin Sci (Lond) 2011;121:489–499. doi: 10.1042/CS20110108. [DOI] [PubMed] [Google Scholar]
- 42.Behr L, Hekmati M, Lucchini A, et al. Evaluation of the effect of autologous mesenchymal stem cell injection in a large-animal model of bilateral kidney ischaemia reperfusion injury. Cell Prolif. 2009;42:284–297. doi: 10.1111/j.1365-2184.2009.00591.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Stefańska A, Péault B, Mullins JJ. Renal pericytes: Multifunctional cells of the kidneys. Pflugers Arch. 2013;465:767–773. doi: 10.1007/s00424-013-1263-7. [DOI] [PubMed] [Google Scholar]
- 44.Humphreys BD, Lin S-L, Kobayashi A, et al. Fate tracing reveals the pericyte and not epithelial origin of myofibroblasts in kidney fibrosis. Am J Pathol. 2010;176:85–97. doi: 10.2353/ajpath.2010.090517. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Lin SL, Kisseleva T, Brenner DA, et al. Pericytes and perivascular fibroblasts are the primary source of collagen-producing cells in obstructive fibrosis of the kidney. Am J Pathol. 2008;173:1617–1627. doi: 10.2353/ajpath.2008.080433. [DOI] [PMC free article] [PubMed] [Google Scholar]


