This study reviews the past, present, and future of cord transplantation, including the potential use of single- and double-unit cord blood transplantation in multiple hematological malignancies including leukemia and aggressive lymphomas in light of recent discoveries. Current excitement in the field revolves around the development of safer techniques to improve homing, engraftment, and immune reconstitution after cord blood transplantation.
Keywords: Umbilical cord blood transplantation, Lymphoma, Leukemia, Graft versus host disease, Hematopoietic stem cell transplantation, Transplant related mortality
Abstract
Allogeneic hematopoietic stem cell transplantation is an important treatment option for fit patients with poor-risk hematological malignancies; nevertheless, the lack of available fully matched donors limits the extent of its use. Umbilical cord blood has emerged as an effective alternate source of hematopoietic stem cell support. Transplantation with cord blood allows for faster availability of frozen sample and avoids invasive procedures for donors. In addition, this procedure has demonstrated reduced relapse rates and similar overall survival when compared with unrelated allogeneic hematopoietic stem cell transplantation. The limited dose of CD34-positive stem cells available with single-unit cord transplantation has been addressed by the development of double-unit cord transplantation. In combination with improved conditioning regimens, double-unit cord transplantation has allowed for the treatment of larger children, as well as adult patients with hematological malignancies. Current excitement in the field revolves around the development of safer techniques to improve homing, engraftment, and immune reconstitution after cord blood transplantation. Here the authors review the past, present, and future of cord transplantation.
Introduction
Stem cell therapy has the potential to treat several life-threatening and debilitating conditions including cancer, Alzheimer’s disease, and neurological injury. Although investigation is ongoing in developing areas such as human embryonic stem cells and inducible pluripotent stem cells, hematopoietic stem cells have been clinically applied for decades now. Although these hematopoietic stem cells are perhaps more differentiated than embryonic stem cells, autologous and allogeneic sources of hematopoietic stem cells can restore the hematopoietic system in a patient with a hematologic malignancy after high-dose chemotherapy.
Hematopoietic stem cells can be readily obtained from different adult tissues such as bone marrow and peripheral blood. More recently, umbilical cord blood has become recognized as yet another source of these valuable cells. Previously disposed after childbirth, cord blood has become a precious product and valuable tool for patients with malignancies that lack a stem cell donor. The National Marrow Donor Program has approximately 23 million volunteer adult donors [1]; nevertheless many patients, particularly minorities [2], who need an allogeneic stem cell transplant do not have a suitable matched unrelated donor. Thus possibilities include mismatched related, mismatched unrelated, or cord blood donors. It has been more than 20 years since the first human cord blood transplant was performed. Methods of collection, banking, cryopreservation of cord blood, and thus clinical outcomes continue to improve worldwide for both malignant and nonmalignant conditions.
History of Cord Blood Transplantation: The Past
During fetal development, hematopoiesis transitions from the fetal yolk sac to the liver and finally to the adult bone marrow. Fetal liver cells as a hematopoietic stem cell source were abandoned because of poor success rates. It was then hypothesized that cord blood might be a better provider of progenitor cells because of increased availability and long-term maintenance of a higher number of stem cells [3].
The first cord blood transplant recipient was a patient with Fanconi’s anemia who received a cord blood unit from his human leukocyte antigen (HLA)-identical sibling in 1988 [4]. A combination of factors triggered the use of this new technology in Fanconi’s anemia, including the recently acquired capability of prenatally diagnosing this condition via amniotic fluid sampling, improved HLA testing, and mastering the harvesting/cryopreservation/thawing of cord blood cells. The patient engrafted completely with donor cells and has remained in complete hematological remission for more than 20 years, without graft-versus-host disease (GVHD). It was hypothesized that fewer or less-developed T cells in the cord blood unit compared with bone marrow or peripheral blood would yield less GVHD. Less acute/chronic GVHD [5] and similar survival from HLA-identical sibling cord blood transplantation, albeit with delayed granulocyte/platelet engraftment, were observed in subsequent studies.
After encouraging outcomes in the matched related sibling arena [3], the first unrelated mismatched cord blood transplants followed in children and adults [5–7]. Subsequently, an international cooperative cord blood bank network, the Netcord group, was established in 1998 [8]. The availability and ease of cord blood collection and banking made cord blood searching and acquisition faster [7] than the search for bone marrow stem cells. Furthermore, the appeal of cord blood increased as it became apparent that less stringent HLA matching (in comparison with bone marrow or peripheral blood progenitor cells) was required [9], perhaps because fewer activated lymphocytes are present in cord blood [10]. All those advantages brought cord blood to its present role as a prime candidate for use in hematopoietic stem cell transplantation.
Current Applications of Cord Blood Transplantation: The Present
Much progress has occurred since the pioneering of the first cord blood transplant, with more than 35,000 transplants performed to date. The indication for transplantation has now transitioned from nonmalignant to malignant diseases, and the majority of recipients are now adults lacking an HLA-matched donor.
Pediatric Patients With Malignancies
The initial positive results of cord blood transplantation in pediatric patients [11, 12] prompted the Cord Blood Transplantation study: a pivotal prospective multicenter trial of cord blood transplantation (CBT) in 191 pediatric patients with hematologic malignancies [13]. In this study, the median time to neutrophil engraftment was 27 days; the rate of acute grade 3/4 GVHD by day 100 was 19.5%, and chronic GVHD at 2 years was 20.8%. The probability of 2-year survival was 49.5% [13], that faired favorably compared with previous reports. A larger study from the Center for International Blood and Marrow Transplant Research was conducted with 503 children with acute leukemia transplanted with cord blood versus 282 children transplanted with HLA-matched unrelated donors [14]. The 5-year leukemia-free survival was similar for allele-matched bone marrow transplants and cord blood units mismatched at either one or two antigens. These data suggested that the progression-free survival was similar to allogeneic bone marrow transplantation. The decreased risk of GVHD made CBT more attractive because it allowed greater donor-recipient HLA disparity. Despite the fact that GVHD was lower, a graft-versus-leukemia effect was observed.
Pediatric Patients With Nonmalignant Diseases
Nonmalignant conditions that have been treated by cord blood transplantation include severe combined immune deficiency [15], hemoglobinopathies [16], Krabbe’s disease [17], chronic granulomatous disease [18], and Hurler’s syndrome [19]. Boelens et al. [20] evaluated the outcomes of transplantation using various hematopoietic cell sources in 258 children with Hurler’s syndrome after myeloablative conditioning. Event-free survival after HLA-matched sibling donor and 6 of 6 matched unrelated cord blood donor was similar at 81% but lower at 68% after 5 of 6 matched cord blood donor and at 57% after 4 of 6 matched unrelated cord blood donor. Interestingly, full-donor chimerism was higher after cord blood transplantation (92% versus 69%, p = .039). A low progenitor cell dose is one of the major disadvantages of single-unit cord blood transplantation, resulting in slower engraftment and higher rates of graft failure. As such, CBT for other bone marrow syndromes, such as severe aplastic anemia and Fanconi’s anemia, remains uncertain because of higher graft failure in this population when compared with other indications [21].
Adult Patients
Encouraging pediatric results led to the first large study of cord blood transplantation in adults in 2001. This study enrolled 68 patients with hematologic malignancies who received myeloablative conditioning. At 40 months after single-cord blood transplantation, 26% of patients remained disease-free [22]. Compared with unrelated stem cell transplantation with myeloablative conditioning, cord blood transplantation displayed similar leukemia-free survival (LFS), chronic GVHD rates, transplant-related mortality, and relapse rate in patients with acute leukemia [23]. In a subsequent study of matched unrelated, mismatched related and one- or two-HLA-antigen-mismatched cord blood transplants, there were similar rates of treatment-related mortality, treatment failure, and overall mortality [24]. Similarly, a retrospective analysis comparing unrelated bone marrow (472 patients) or peripheral blood progenitor cells (888 patients) with cord blood (165 patients) transplantation in adults with acute leukemia found that LFS after CBT was comparable with outcomes seen with 8 or 8 or 7 of 8 allele-matched peripheral blood progenitor cells or bone marrow transplantation [25]. The incidence of chronic, but not acute, GVHD was lower after CBT compared with 8 of 8 allele-matched bone marrow transplantation (p = .01). However, transplant-related mortality was higher after CBT when compared with 8 of 8 allele-matched peripheral blood progenitor cell recipients (p = .003) or bone marrow transplantation (p = .003) [25]. Therefore, this study encouraged the use of cord blood transplantation if no HLA-matched unrelated adult donors are available.
Double Cord Blood Transplantation
In an effort to overcome the relatively low number of progenitor cells present in a single cord blood unit, double cord blood transplantation (DCBT) was developed. In a study of 23 adults with high-risk hematological malignancies undergoing DCBT, the median time to engraftment was 23 days [26]. Engraftment was derived from a single donor, in 76% of patients at day 21, with one predominating unit in all patients at day 100. Single-unit dominance after double-unit cord blood transplantation has been confirmed in subsequent studies [27, 28] and CD3+ cell dose is an independent factor associated with unit predominance [27]. Of note, despite higher incidence of grade 2 acute GVHD in recipients of two partially HLA-matched cord blood units, there were no detrimental effects on transplantation-related mortality at 1 year (24 versus 39%, p = .02) [29]. Incidence of relapse or progression was found to be 31% at 1 year with a significantly lower risk (p = .03) in recipients of double-unit cord blood in patients with non-Hodgkin’s lymphoma (n = 61), Hodgkin’s lymphoma (n = 29), and chronic lymphocytic leukemia (n = 14) [30]. Furthermore, a prospective study comparing single versus double cord blood transplantation confirmed a lower relapse risk after infusion of two units (30.4% versus 59.3%, p = .045) [31]. A study that compared double cord blood transplantation (n = 128) to matched-related (n = 204) and matched-unrelated donor transplantation (n = 152) for adult leukemia patients found a significantly lower risk of relapse in recipients of double cord blood (15%) compared with matched-related donor (43%) and matched-unrelated donor (37%). However, nonrelapse mortality was higher for double cord blood (34%) compared with matched-related donor (24%) and matched-unrelated donor (14%) [32].
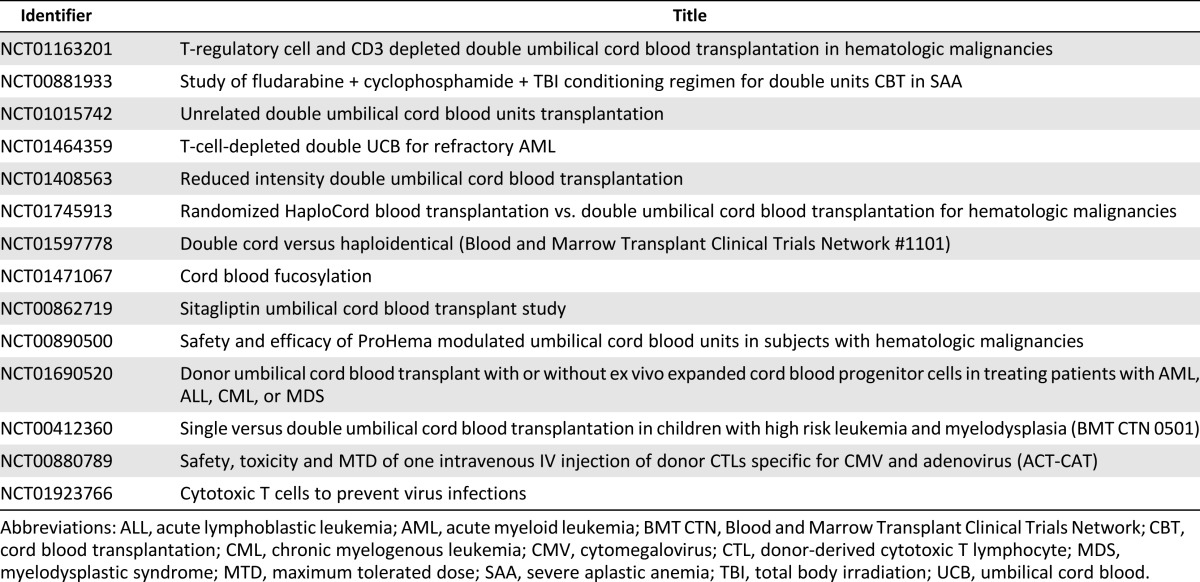
To improve these outcomes, cord blood transplantation has been explored after reduced intensity conditioning (RIC) regimens including the fludarabine, cyclophosphamide, and low-dose total body irradiation regimen [33] and the fludarabine, melphalan, and rabbit anti-thymocyte globulin [34] regimen. Adults with acute leukemia undergoing DCBT with the RIC regimen (n = 120), DCBT with alternative RIC regimens (including an alkylating agent plus fludarabine plus or minus total body irradiation) (n = 40), and 8 of 8 (n = 313) or 7 of 8 HLA-matched (n = 111) peripheral blood progenitor cells RIC transplants demonstrated a probability of survival of 38%, 19%, 44%, and 37%, respectively. All groups showed similar outcomes with the exception of recipients of double cord-treated patients with alternative RIC regimens, who displayed higher transplant-related mortality and higher overall mortality [35]. Similarly, the Blood and Marrow Transplant Clinical Trials Network conducted two parallel multicenter phase II trials for patients without a suitable related donor [36]. The outcomes of RIC with fludarabine, cyclophosphamide, and total body irradiation with subsequent unrelated double cord versus HLA-haploidentical related donor marrow were compared in both trials. The 1-year cumulative incidence of nonrelapse mortality was higher after cord blood (24% versus 7%), although the relapse rate was higher after haplomarrow transplantation (31% versus 45%) [36]. These phase II trials endorsed the value of double cord transplantation as an alternative donor source and set the stage for a multicenter, phase III, randomized trial of RIC and transplantation of double unrelated cord blood versus HLA-haploidentical related bone marrow for patients with hematologic malignancies (BMT CTN #1101, NCT01597778).
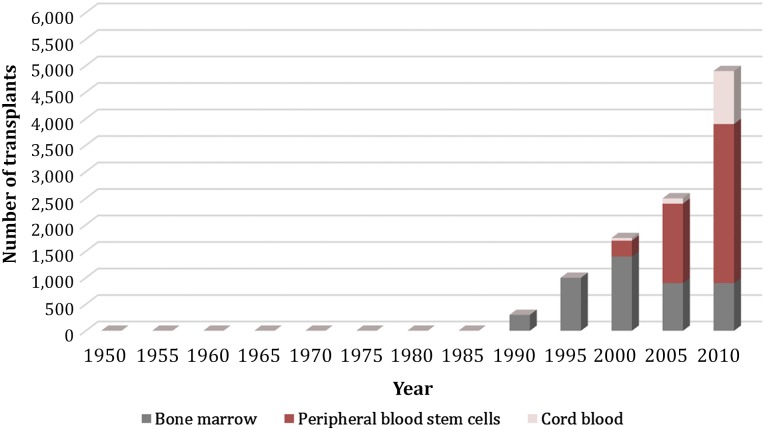
Additional preliminary data have recently been presented to further highlight cord blood as a viable transplant option (Table 1). Collins et al. [38] documented long-term durability of cord blood grafts in children with acute leukemia with an 8-year probability of overall survival of 78% compared with 81% with HLA-matched and 68% with HLA-mismatched bone transplantation. Of note, there were differences in transplant characteristics because the patients that received cord blood transplants were more likely to have received a non-irradiation-containing conditioning regimen [38]. Bachanova et al. [40] explored alternative donor transplantation for 1,593 adults with advanced non-Hodgkin and Hodgkin lymphoma and compared cord blood versus 8 of 8 HLA-matched unrelated donor versus 7 of 8 unrelated donor. They found similar results in a multivariate analysis among the 3 groups in 3-year relapse/progression, progression-free survival, and overall survival [40]. Although clinical outcomes continue to be optimized, the development of CBT has expanded the use of allogeneic transplantation to patients who were previously unable to find a suitable donor. Dahi et al. [39] recently reported on the decline in the percentage of non-Europeans with no available graft, in part because of the availability of cord blood as a source. Increasing experience with cord blood transplant has thus changed the landscape in hematopoietic cell sources for patients undergoing allogeneic hematopoietic stem cell transplantation (Fig. 1).
Table 1.
Selected cord blood clinical trials recently reported at meetings of the American Society of Hematology, American Society of Blood and Bone Marrow Transplantation, and the American Society of Clinical Oncology
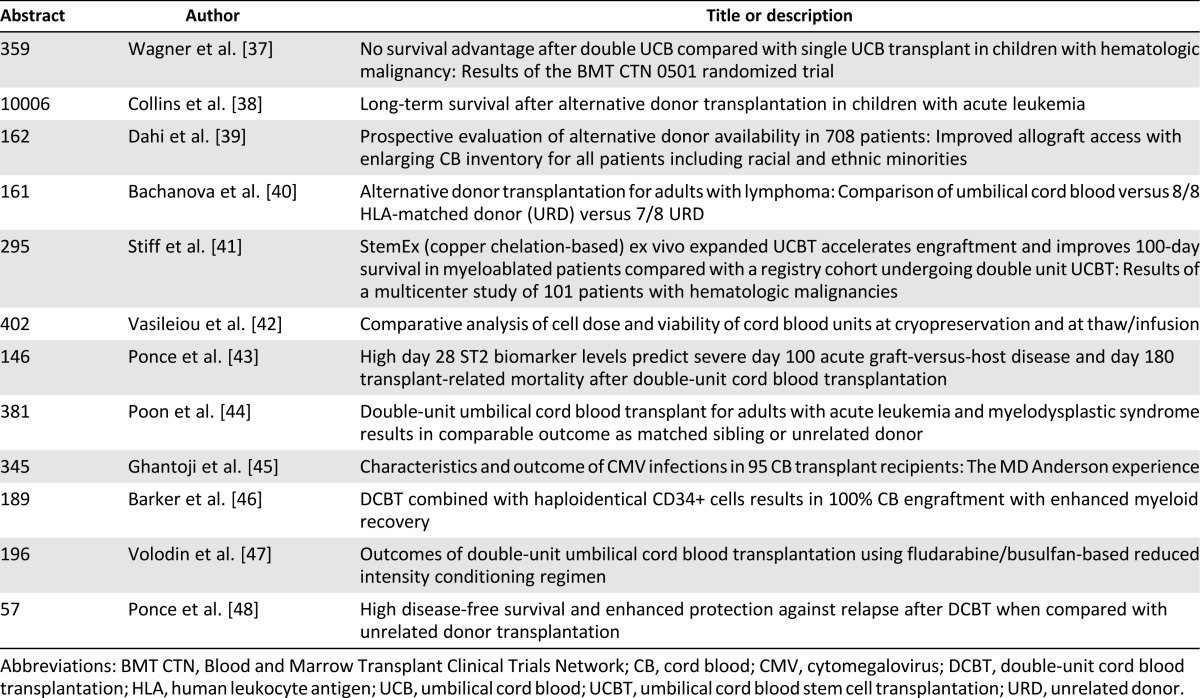
Figure 1.
Approximate trends regarding transplants by hematopoietic cell source and increasing numbers of cord blood transplantations [49, 50].
Single Versus Double CBT in Pediatric Patients
In the pediatric population, the use of two partially HLA-matched cord blood units has not been shown to be superior to a single unit, if the unit contains a sufficient number of hematopoietic stem cells. In a randomized study of 224 pediatric patients with hematologic malignancies by Wagner et al. [37], there was no difference between single-unit versus double-unit cord blood transplant in the overall rate of engraftment (89% versus 86%), chronic GVHD (28% versus 31%), risk of relapse at 1 year (12% versus 14%), or disease-free survival (68% versus 64%) [51]. Economically, the use of two units would be justifiable in the pediatric setting only when one unit does not contain enough number of progenitor cells. There are no randomized data of single versus double CBT in adult patients.
Novel Clinical Trials of Cord Blood Transplantation: The Future
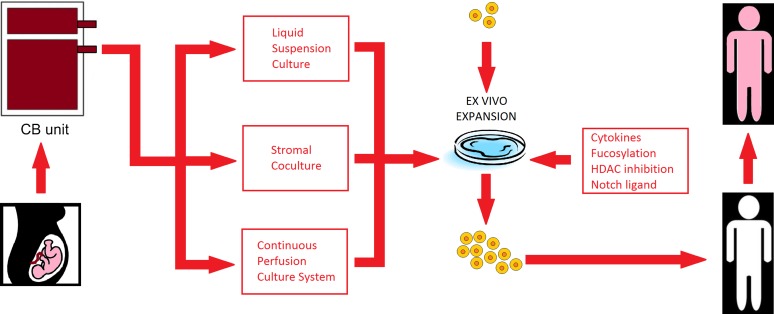
Novel strategies to improve cord blood transplantation outcomes include improving cord blood engraftment by the transplantation of ex vivo expanded cord blood cells. A potential advantage of expansion is the ability to use smaller cord blood units, which could in turn increase the number of available allografts. Expansion techniques currently reported include using the copper chelator tetraethylenepentamine [52], notch ligand-based cultures [53], and coculture of cord blood cells with bone marrow-derived mesenchymal stem cells [54] (Fig. 2). Expansion with notch ligand and the mesenchymal stem cell-based cocultures have resulted in rapid engraftment of neutrophils in a median of 15 days [53, 56]. Other strategies to improve engraftment include the direct intrabone injection of unrelated cord blood cells [57], supportive coinfusion from an HLA-haploidentical third party donor [58, 59], and the use of agents to enhance the homing of cord blood to the marrow via fucosylation [60, 61] or by prostaglandin E2 modulation [62, 63].
Figure 2.
Schematic of ex vivo expansion techniques for cord blood transplantation (based on [55]). Abbreviations: CB, cord blood; HDAC, histone deacetylase.
Ongoing clinical trials are also evaluating cord blood-derived immune cells to improve the rate of GVHD and antitumor efficacy. Expanding cord blood regulatory T cells, a subset of CD4+ T cells, may potentially represent a novel cell-based approach for reducing the risk of GVHD [64]. Antigen presenting cell-mediated expansion of human cord blood natural killer cells as an antitumor cellular therapy is being explored as well [65].
Delayed immune reconstitution after cord blood transplantation remains one of the most daunting obstacles to the widespread use of cord blood transplantation. As such, the expansion of cytotoxic T-cell lymphocytes from cord blood has been instituted to target the most common viral infections in this setting: cytomegalovirus, Epstein-Barr virus, and adenovirus [66]. It has also been suggested that combining haploidentical donors with cord blood transplantation can lead to faster immune reconstitution with rapid B-cell and delayed T-cell recovery [67].
Despite the numerous milestones achieved in hematopoietic cell transplantation (Table 2) and ex vivo cord blood stem cell expansion (Table 3), many questions remain unanswered in the field of cord blood transplantation. Fortunately, answers may be forthcoming from numerous ongoing clinical trials (Table 4): ex vivo expanded stem cells (NCT01221857), fucosylation prior to infusion (NCT01471067), and inhibition of CD26 peptidase using sitagliptin to enhance engraftment (NCT00862719). Other novel trials include ACTCAT (NCT00880789) and ACTCAT2 (NCT01923766), which use modified cytotoxic T-lymphocytes to prevent or treat cytomegalovirus, adenovirus, and/or Epstein Barr Virus reactivation or infection after cord blood transplant [113, 114].
Table 2.
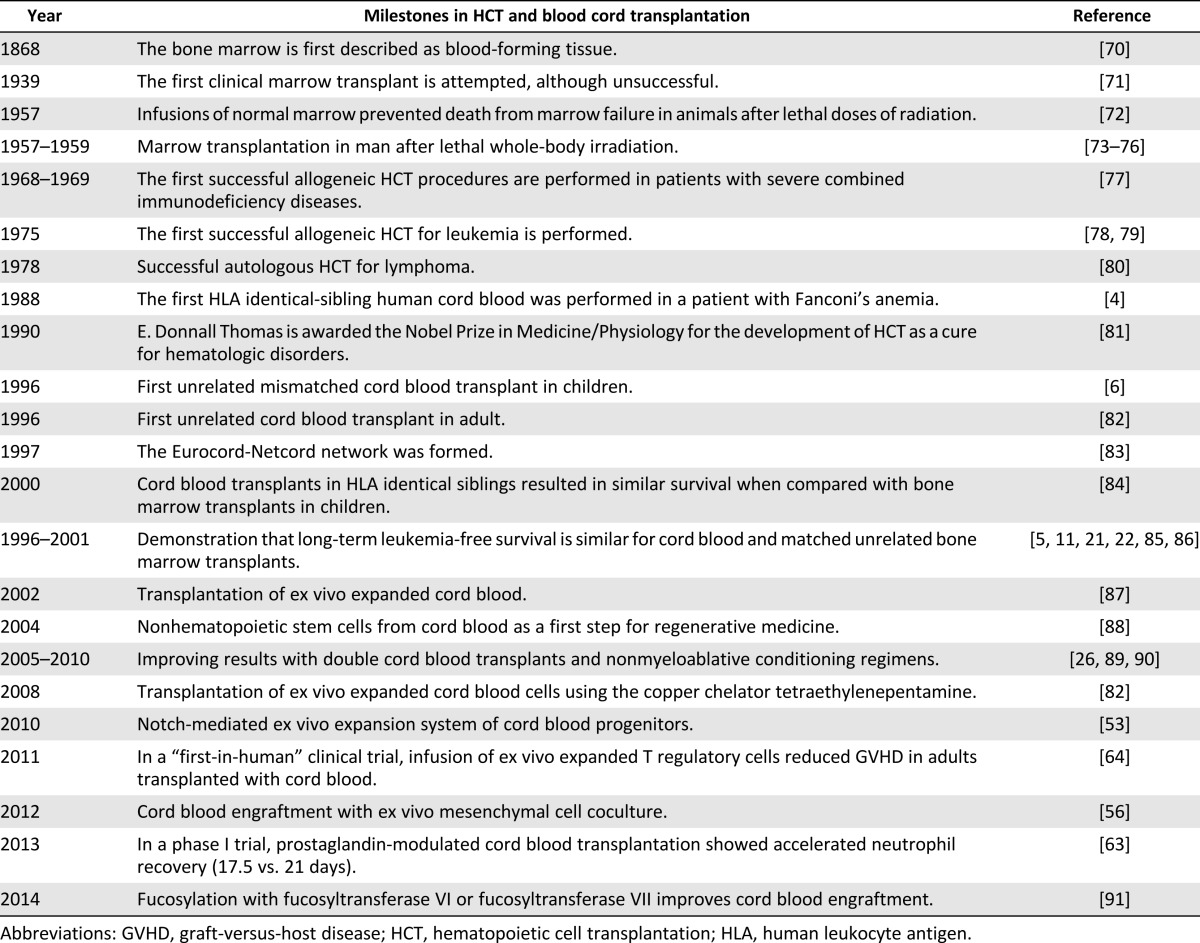
Table 3.
Selected published translational studies on cord blood-derived ex vivo stem cell expansion
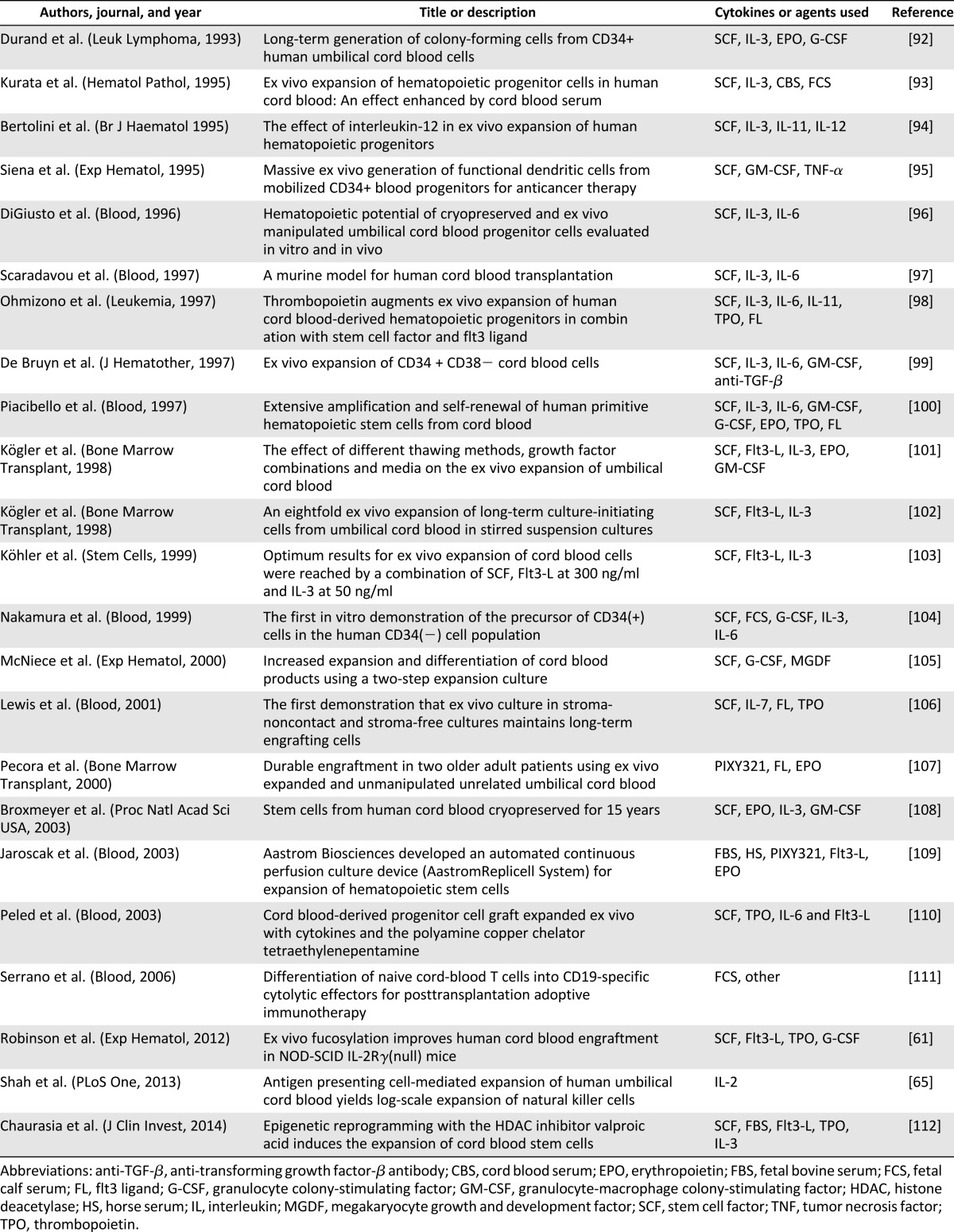
Table 4.
Selected single and double-unit cord blood transplantation studies at ClinicalTrials.gov (search included only open studies and excluded studies with unknown status; accessed December 30, 2013)
Conclusion: Umbilical Cord Transplantation Coming of Age
The future for stem cell transplantation forecasts a combination of supportive care optimization and advances in conditioning chemotherapy and immunotherapy to increase survival and decrease morbidity. Cord blood transplantation as a source of stem cells has the potential to fill the gap of a growing population of patients who do not have a fully matched donor but need allogeneic hematopoietic stem cell transplantation. Our experience in this field has evolved from initial single-unit cord blood transplantation for a few diseases in children to double-unit cord blood transplantation for multiple hematologic malignancies in adults. In addition, cord blood provides countless hematopoietic and nonhematopoietic stem cells whose full potential in stem cell biology and regenerative medicine has yet to be fully uncovered.
Acknowledgment
C.M.B. and E.J.S. have been funded by NIH Program Project Grant P01CA148600.
Author Contributions
J.M., N.S., and E.J.S.: collection and/or assembly of data, data analysis and interpretation, manuscript writing, final approval of manuscript; K.R., C.H., C.M.B., B.O., A.O., U.P., J.M., and I.M.: data analysis and interpretation; final approval of manuscript.
Disclosure of Potential Conflicts of Interest
N.S. is a compensated consultant with Sanofi and has compensated research funding from Celgene.
References
- 1.Ballen KK, King RJ, Chitphakdithai P, et al. The national marrow donor program 20 years of unrelated donor hematopoietic cell transplantation. Biol Blood Marrow Transplant. 2008;14(suppl):2–7. doi: 10.1016/j.bbmt.2008.05.017. [DOI] [PubMed] [Google Scholar]
- 2.Barker JN, Byam CE, Kernan NA, et al. Availability of cord blood extends allogeneic hematopoietic stem cell transplant access to racial and ethnic minorities. Biol Blood Marrow Transplant. 2010;16:1541–1548. doi: 10.1016/j.bbmt.2010.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Broxmeyer HE, Douglas GW, Hangoc G, et al. Human umbilical cord blood as a potential source of transplantable hematopoietic stem/progenitor cells. Proc Natl Acad Sci USA. 1989;86:3828–3832. doi: 10.1073/pnas.86.10.3828. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gluckman E, Broxmeyer HA, Auerbach AD, et al. Hematopoietic reconstitution in a patient with Fanconi’s anemia by means of umbilical-cord blood from an HLA-identical sibling. N Engl J Med. 1989;321:1174–1178. doi: 10.1056/NEJM198910263211707. [DOI] [PubMed] [Google Scholar]
- 5.Wagner JE, Rosenthal J, Sweetman R, et al. Successful transplantation of HLA-matched and HLA-mismatched umbilical cord blood from unrelated donors: Analysis of engraftment and acute graft-versus-host disease. Blood. 1996;88:795–802. [PubMed] [Google Scholar]
- 6.Kurtzberg J, Laughlin M, Graham ML, et al. Placental blood as a source of hematopoietic stem cells for transplantation into unrelated recipients. N Engl J Med. 1996;335:157–166. doi: 10.1056/NEJM199607183350303. [DOI] [PubMed] [Google Scholar]
- 7.Barker JN, Krepski TP, DeFor TE, et al. Searching for unrelated donor hematopoietic stem cells: Availability and speed of umbilical cord blood versus bone marrow. Biol Blood Marrow Transplant. 2002;8:257–260. doi: 10.1053/bbmt.2002.v8.pm12064362. [DOI] [PubMed] [Google Scholar]
- 8.Hakenberg P, Kögler G, Wernet P. NETCORD: A cord blood allocation network. Bone Marrow Transplant. 1998;22(suppl 1):S17–S18. [PubMed] [Google Scholar]
- 9.Petersdorf EW, Gooley TA, Anasetti C, et al. Optimizing outcome after unrelated marrow transplantation by comprehensive matching of HLA class I and II alleles in the donor and recipient. Blood. 1998;92:3515–3520. [PubMed] [Google Scholar]
- 10.Garderet L, Dulphy N, Douay C, et al. The umbilical cord blood alphabeta T-cell repertoire: Characteristics of a polyclonal and naive but completely formed repertoire. Blood. 1998;91:340–346. [PubMed] [Google Scholar]
- 11.Locatelli F, Rocha V, Chastang C, et al. Factors associated with outcome after cord blood transplantation in children with acute leukemia. Blood. 1999;93:3662–3671. [PubMed] [Google Scholar]
- 12.Michel G, Rocha V, Chevret S, et al. Unrelated cord blood transplantation for childhood acute myeloid leukemia: A Eurocord Group analysis. Blood. 2003;102:4290–4297. doi: 10.1182/blood-2003-04-1288. [DOI] [PubMed] [Google Scholar]
- 13.Kurtzberg J, Prasad VK, Carter SL, et al. Results of the Cord Blood Transplantation Study (COBLT): Clinical outcomes of unrelated donor umbilical cord blood transplantation in pediatric patients with hematologic malignancies. Blood. 2008;112:4318–4327. doi: 10.1182/blood-2007-06-098020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Eapen M, Rubinstein P, Zhang MJ, et al. Outcomes of transplantation of unrelated donor umbilical cord blood and bone marrow in children with acute leukaemia: A comparison study. Lancet. 2007;369:1947–1954. doi: 10.1016/S0140-6736(07)60915-5. [DOI] [PubMed] [Google Scholar]
- 15.Fernandes JF, Rocha V, Labopin M, et al. Transplantation in patients with SCID: Mismatched related stem cells or unrelated cord blood? Blood. 2012;119:2949–2955. doi: 10.1182/blood-2011-06-363572. [DOI] [PubMed] [Google Scholar]
- 16.Kamani NR, Walters MC, Carter S, et al. Unrelated donor cord blood transplantation for children with severe sickle cell disease: Results of one cohort from the phase II study from the Blood and Marrow Transplant Clinical Trials Network (BMT CTN) Biol Blood Marrow Transplant. 2012;18:1265–1272. doi: 10.1016/j.bbmt.2012.01.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Escolar ML, Poe MD, Provenzale JM, et al. Transplantation of umbilical-cord blood in babies with infantile Krabbe’s disease. N Engl J Med. 2005;352:2069–2081. doi: 10.1056/NEJMoa042604. [DOI] [PubMed] [Google Scholar]
- 18.Bhattacharya A, Slatter M, Curtis A, et al. Successful umbilical cord blood stem cell transplantation for chronic granulomatous disease. Bone Marrow Transplant. 2003;31:403–405. doi: 10.1038/sj.bmt.1703863. [DOI] [PubMed] [Google Scholar]
- 19.Staba SL, Escolar ML, Poe M, et al. Cord-blood transplants from unrelated donors in patients with Hurler’s syndrome. N Engl J Med. 2004;350:1960–1969. doi: 10.1056/NEJMoa032613. [DOI] [PubMed] [Google Scholar]
- 20.Boelens JJ, Aldenhoven M, Purtill D, et al. Outcomes of transplantation using various hematopoietic cell sources in children with Hurler syndrome after myeloablative conditioning. Blood. 2013;121:3981–3987. doi: 10.1182/blood-2012-09-455238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rubinstein P, Carrier C, Scaradavou A, et al. Outcomes among 562 recipients of placental-blood transplants from unrelated donors. N Engl J Med. 1998;339:1565–1577. doi: 10.1056/NEJM199811263392201. [DOI] [PubMed] [Google Scholar]
- 22.Laughlin MJ, Barker J, Bambach B, et al. Hematopoietic engraftment and survival in adult recipients of umbilical-cord blood from unrelated donors. N Engl J Med. 2001;344:1815–1822. doi: 10.1056/NEJM200106143442402. [DOI] [PubMed] [Google Scholar]
- 23.Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med. 2004;351:2276–2285. doi: 10.1056/NEJMoa041469. [DOI] [PubMed] [Google Scholar]
- 24.Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med. 2004;351:2265–2275. doi: 10.1056/NEJMoa041276. [DOI] [PubMed] [Google Scholar]
- 25.Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: A retrospective analysis. Lancet Oncol. 2010;11:653–660. doi: 10.1016/S1470-2045(10)70127-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood. 2005;105:1343–1347. doi: 10.1182/blood-2004-07-2717. [DOI] [PubMed] [Google Scholar]
- 27.Ramirez P, Wagner JE, DeFor TE, et al. Factors predicting single-unit predominance after double umbilical cord blood transplantation. Bone Marrow Transplant. 2012;47:799–803. doi: 10.1038/bmt.2011.184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Gutman JA, Turtle CJ, Manley TJ, et al. Single-unit dominance after double-unit umbilical cord blood transplantation coincides with a specific CD8+ T-cell response against the nonengrafted unit. Blood. 2010;115:757–765. doi: 10.1182/blood-2009-07-228999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.MacMillan ML, Weisdorf DJ, Brunstein CG, et al. Acute graft-versus-host disease after unrelated donor umbilical cord blood transplantation: Analysis of risk factors. Blood. 2009;113:2410–2415. doi: 10.1182/blood-2008-07-163238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Rodrigues CA, Sanz G, Brunstein CG, et al. Analysis of risk factors for outcomes after unrelated cord blood transplantation in adults with lymphoid malignancies: A study by the Eurocord-Netcord and lymphoma working party of the European group for blood and marrow transplantation. J Clin Oncol. 2009;27:256–263. doi: 10.1200/JCO.2007.15.8865. [DOI] [PubMed] [Google Scholar]
- 31.Kindwall-Keller TL, Hegerfeldt Y, Meyerson HJ, et al. Prospective study of one- vs two-unit umbilical cord blood transplantation following reduced intensity conditioning in adults with hematological malignancies. Bone Marrow Transplant. 2012;47:924–933. doi: 10.1038/bmt.2011.195. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: Relative risks and benefits of double umbilical cord blood. Blood. 2010;116:4693–4699. doi: 10.1182/blood-2010-05-285304. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Barker JN, Weisdorf DJ, DeFor TE, et al. Rapid and complete donor chimerism in adult recipients of unrelated donor umbilical cord blood transplantation after reduced-intensity conditioning. Blood. 2003;102:1915–1919. doi: 10.1182/blood-2002-11-3337. [DOI] [PubMed] [Google Scholar]
- 34.Ballen KK, Spitzer TR, Yeap BY, et al. Double unrelated reduced-intensity umbilical cord blood transplantation in adults. Biol Blood Marrow Transplant. 2007;13:82–89. doi: 10.1016/j.bbmt.2006.08.041. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Brunstein CG, Eapen M, Ahn KW, et al. Reduced-intensity conditioning transplantation in acute leukemia: The effect of source of unrelated donor stem cells on outcomes. Blood. 2012;119:5591–5598. doi: 10.1182/blood-2011-12-400630. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Brunstein CG, Fuchs EJ, Carter SL, et al. Alternative donor transplantation after reduced intensity conditioning: Results of parallel phase 2 trials using partially HLA-mismatched related bone marrow or unrelated double umbilical cord blood grafts. Blood. 2011;118:282–288. doi: 10.1182/blood-2011-03-344853. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Wagner JE, Eapen M, Carter SL et al. No survival advantage after double umbilical cord blood (UCB) compared to single UCB transplant in children with hematological malignancy: Results of the blood and marrow transplant clinical trials network (BMT CTN 0501) randomized trial. Available at https://ash.confex.com/ash/2012/webprogram/Paper48413.html. Accessed September 2, 2014.
- 38.Collins CL, Ahn KW, Wang Z et al. Long-term survival after alternative donor transplantation in children with acute leukemia. J Clin Oncol 2013;31(suppl):10006a. [Google Scholar]
- 39.Dahi PB, Ponce DM, Byam C et al. Prospective evaluation of alternative donor availability in 708 patients: Improved allograft access with enlarging CB inventory for all patients including racial and ethnic minorities. Available at https://ash.confex.com/ash/2013/webprogram/Paper60210.html. Accessed September 2, 2014.
- 40.Bachanova V, Brunstein C, Burns LJ et al. Alternative donor transplantation for adults with lymphoma: Comparison of umbilical cord blood versus 8/8 HLA-matched donor (URD) versus 7/8 URD. Available at https://ash.confex.com/ash/2013/webprogram/Paper57320.html. Accessed September 2, 2014.
- 41.Stiff PJ, Montesinos P, Peled T et al. StemEx (copper chelation based) ex vivo expanded umbilical cord blood stem cell transplantation (UCBT) accelerates engraftment and improves 100 day survival in myeloablated patients compared to a registry cohort undergoing double unit UCBT: Results of a multicenter study of 101 patients with hematologic malignancies. Available at https://ash.confex.com/ash/2013/webprogram/Paper61421.html. Accessed September 2, 2014.
- 42.Vasileiou S, Baltadakis I, Panitsas F et al. Comparative analysis of cell dose and viability of cord blood units at cryopreservation and at thaw/infusion for unrelated stem cell transplantation in adult recipients. Available at https://bmt.confex.com/tandem/2014/webprogram/Paper4069.html. Accessed September 2, 2014.
- 43.Ponce DM, Hilden P, Mumaw C et al. High day 28 ST2 biomarker levels predict severe day 100 acute graft-versus-host disease and day 180 transplant-related mortality after double-unit cord blood transplantation. Available at https://ash.confex.com/ash/2013/webprogram/Paper60713.html. Accessed September 2, 2014. [DOI] [PMC free article] [PubMed]
- 44.Poon ML, Linn YC, Lim Z, et al. Double unit umbilical cord blood transplant for adults with acute leukemia and myelodysplastic syndrome results in comparable outcome as matched sibling or unrelated donor transplant only after myeloablative conditioning but not reduced intensity conditio. Biol Blood Marrow Transplant. 2014;20(suppl):S247. [Google Scholar]
- 45.Ghantoji SS, Goddu S, Sreenivasula S, et al. Characteristics and outcome of cytomegalovirus (CMV) infections in 95 cord blood transplant (CBT) recipients: The MD Anderson experience. Biol Blood Marrow Transplant. 2014;20(suppl):S223–S224. [Google Scholar]
- 46.Barker JN, Ponce DM, Dahi P, et al. Double-unit cord blood (CB) transplantation combined with haplo-identical CD34+ cells results in 100% CB engraftment with enhanced myeloid CD34+ recovery. Biol Blood Marrow Transplant. 2014;20(suppl):S138–S139. [Google Scholar]
- 47.Volodin L, Beitinjaneh A, Salman H, et al. Outcomes of double unit umbilical cord blood transplantation using fludarabine/busulfan based reduced intensity conditioning regimen. Biol Blood Marrow Transplant. 2014;20(suppl):S142. [Google Scholar]
- 48.Ponce DM, Devlin S, Lubin M, et al. High disease-free survival and enhanced protection against relapse after double-unit cord blood transplantation (DCB-T) when compared to unrelated donor transplantation (URD-T) in patients with acute leukemia, MDS and CML. Biol Blood Marrow Transplant. 2014;20(suppl):S58–S59. doi: 10.1016/j.bbmt.2015.07.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.National Marrow Donor Program. Adult Allogeneic HCT, by Transplant Type, Cell Source. Available at https://bethematchclinical.org/resources-and-education/hct-presentation-slides/adult-allogeneic-hct,-by-transplant-type,-cell-source/. Accessed August 30, 2014.
- 50.Appelbaum FR. Hematopoietic-cell transplantation at 50. N Engl J Med. 2007;357:1472–1475. doi: 10.1056/NEJMp078166. [DOI] [PubMed] [Google Scholar]
- 51.Oran B, Shpall E. Umbilical cord blood transplantation: A maturing technology. Hematology (Am Soc Hematol Educ Program) 2012;2012:215–222. doi: 10.1182/asheducation-2012.1.215. [DOI] [PubMed] [Google Scholar]
- 52.de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: A phase I/II clinical trial. Bone Marrow Transplant. 2008;41:771–778. doi: 10.1038/sj.bmt.1705979. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med. 2010;16:232–236. doi: 10.1038/nm.2080. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Robinson SN, Ng J, Niu T, et al. Superior ex vivo cord blood expansion following co-culture with bone marrow-derived mesenchymal stem cells. Bone Marrow Transplant. 2006;37:359–366. doi: 10.1038/sj.bmt.1705258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Kelly SS, Sola CB, de Lima M, et al. Ex vivo expansion of cord blood. Bone Marrow Transplant. 2009;44:673–681. doi: 10.1038/bmt.2009.284. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med. 2012;367:2305–2315. doi: 10.1056/NEJMoa1207285. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Frassoni F, Gualandi F, Podestà M, et al. Direct intrabone transplant of unrelated cord-blood cells in acute leukaemia: A phase I/II study. Lancet Oncol. 2008;9:831–839. doi: 10.1016/S1470-2045(08)70180-3. [DOI] [PubMed] [Google Scholar]
- 58.Bautista G, Cabrera JR, Regidor C, et al. Cord blood transplants supported by co-infusion of mobilized hematopoietic stem cells from a third-party donor. Bone Marrow Transplant. 2009;43:365–373. doi: 10.1038/bmt.2008.329. [DOI] [PubMed] [Google Scholar]
- 59.Fernández MN, Regidor C, Cabrera R, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol. 2003;31:535–544. doi: 10.1016/s0301-472x(03)00067-5. [DOI] [PubMed] [Google Scholar]
- 60.Xia L, McDaniel JM, Yago T, et al. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood. 2004;104:3091–3096. doi: 10.1182/blood-2004-02-0650. [DOI] [PubMed] [Google Scholar]
- 61.Robinson SN, Simmons PJ, Thomas MW, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rγ(null) mice. Exp Hematol. 2012;40:445–456. doi: 10.1016/j.exphem.2012.01.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood. 2009;113:5444–5455. doi: 10.1182/blood-2009-01-201335. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood. 2013;122:3074–3081. doi: 10.1182/blood-2013-05-503177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: Safety profile and detection kinetics. Blood. 2011;117:1061–1070. doi: 10.1182/blood-2010-07-293795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Shah N, Martin-Antonio B, Yang H, et al. Antigen presenting cell-mediated expansion of human umbilical cord blood yields log-scale expansion of natural killer cells with anti-myeloma activity. PLoS One. 2013;8:e76781. doi: 10.1371/journal.pone.0076781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood. 2009;114:1958–1967. doi: 10.1182/blood-2009-03-213256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Jain N, Liu H, Artz AS, et al. Immune reconstitution after combined haploidentical and umbilical cord blood transplant. Leuk Lymphoma. 2013;54:1242–1249. doi: 10.3109/10428194.2012.739688. [DOI] [PubMed] [Google Scholar]
- 68.American Society of Hematology. 50 years in hematology: Research that revolutionized patient care. Available at http://www.hematology.org/About-ASH/50-Years.aspx. Accessed August 30, 2014.
- 69.Gluckman E, Ruggeri A, Volt F, et al. Milestones in umbilical cord blood transplantation. Br J Haematol. 2011;154:441–447. doi: 10.1111/j.1365-2141.2011.08598.x. [DOI] [PubMed] [Google Scholar]
- 70.Cooper B. The origins of bone marrow as the seedbed of our blood: From antiquity to the time of Osler. Proc (Bayl Univ Med Cent) 2011;24:115–118. doi: 10.1080/08998280.2011.11928697. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Osgood EE, Riddle MC, Mathews TJ. Aplastic anemia treated with daily transfusions and intravenous marrow: Case report. Ann Intern Med. 1939;13:357–367. [Google Scholar]
- 72.Congdon CC. Experimental treatment of total-body irradiation injury: A brief review. Blood. 1957;12:746–754. [PubMed] [Google Scholar]
- 73.Thomas ED, Lochte HL, Jr, Cannon JH, et al. Supralethal whole body irradiation and isologous marrow transplantation in man. J Clin Invest. 1959;38:1709–1716. doi: 10.1172/JCI103949. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Thomas ED, Lochte HL, Jr, Lu WC, et al. Intravenous infusion of bone marrow in patients receiving radiation and chemotherapy. N Engl J Med. 1957;257:491–496. doi: 10.1056/NEJM195709122571102. [DOI] [PubMed] [Google Scholar]
- 75.Thomas ED, Lochte HL, Jr, Ferrebee JW. Irradiation of the entire body and marrow transplantation: Some observations and comments. Blood. 1959;14:1–23. [PubMed] [Google Scholar]
- 76.Thomas E, Storb R, Clift RA, et al. Bone-marrow transplantation (first of two parts) N Engl J Med. 1975;292:832–843. doi: 10.1056/NEJM197504172921605. [DOI] [PubMed] [Google Scholar]
- 77.Gatti RA, Meuwissen HJ, Allen HD, et al. Immunological reconstitution of sex-linked lymphopenic immunological deficiency. Lancet. 1968;2:1366–1369. doi: 10.1016/s0140-6736(68)92673-1. [DOI] [PubMed] [Google Scholar]
- 78.Graw RG, Jr, Lohrmann HP, Bull MI, et al. Bone-marrow transplantation following combination chemotherapy immunosuppression (B.A.C.T.) in patients with acute leukemia. Transplant Proc. 1974;6:349–354. [PubMed] [Google Scholar]
- 79.Bleyer WA, Blaese RM, Bujak JS, et al. Long-term remission from acute myelogenous leukemia after bone marrow transplantation and recovery from acute graft-versus-host reaction and prolonged immunoincompetence. Blood. 1975;45:171–181. [PubMed] [Google Scholar]
- 80.Appelbaum FR, Herzig GP, Ziegler JL, et al. Successful engraftment of cryopreserved autologous bone marrow in patients with malignant lymphoma. Blood. 1978;52:85–95. [PubMed] [Google Scholar]
- 81.Thomas ED. The Nobel Lectures in Immunology: The Nobel Prize for Physiology or Medicine, 1990. Bone marrow transplantation: Past, present and future. Scand J Immunol. 1994;39:339–345. doi: 10.1111/j.1365-3083.1994.tb03383.x. [DOI] [PubMed] [Google Scholar]
- 82.Laporte JP, Gorin NC, Rubinstein P, et al. Cord-blood transplantation from an unrelated donor in an adult with chronic myelogenous leukemia. N Engl J Med. 1996;335:167–170. doi: 10.1056/NEJM199607183350304. [DOI] [PubMed] [Google Scholar]
- 83.Gluckman E, Rocha V, Boyer-Chammard A, et al. Outcome of cord-blood transplantation from related and unrelated donors. N Engl J Med. 1997;337:373–381. doi: 10.1056/NEJM199708073370602. [DOI] [PubMed] [Google Scholar]
- 84.Rocha V, Wagner JE, Jr, Sobocinski KA, et al. Graft-versus-host disease in children who have received a cord-blood or bone marrow transplant from an HLA-identical sibling. N Engl J Med. 2000;342:1846–1854. doi: 10.1056/NEJM200006223422501. [DOI] [PubMed] [Google Scholar]
- 85.Cairo MS, Wagner JE. Placental and/or umbilical cord blood: An alternative source of hematopoietic stem cells for transplantation. Blood. 1997;90:4665–4678. [PubMed] [Google Scholar]
- 86.Sanz GF, Saavedra S, Planelles D, et al. Standardized, unrelated donor cord blood transplantation in adults with hematologic malignancies. Blood. 2001;98:2332–2338. doi: 10.1182/blood.v98.8.2332. [DOI] [PubMed] [Google Scholar]
- 87.Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant. 2002;8:368–376. doi: 10.1053/bbmt.2002.v8.pm12171483. [DOI] [PubMed] [Google Scholar]
- 88.Kögler G, Sensken S, Airey JA, et al. A new human somatic stem cell from placental cord blood with intrinsic pluripotent differentiation potential. J Exp Med. 2004;200:123–135. doi: 10.1084/jem.20040440. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Brunstein CG, Barker JN, Weisdorf DJ, et al. Umbilical cord blood transplantation after nonmyeloablative conditioning: Impact on transplantation outcomes in 110 adults with hematologic disease. Blood. 2007;110:3064–3070. doi: 10.1182/blood-2007-04-067215. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Rocha V, Crotta A, Ruggeri A, et al. Double cord blood transplantation: Extending the use of unrelated umbilical cord blood cells for patients with hematological diseases. Best Pract Res Clin Haematol. 2010;23:223–229. doi: 10.1016/j.beha.2010.07.005. [DOI] [PubMed] [Google Scholar]
- 91.Robinson SN, Thomas MW, Simmons PJ, et al. Fucosylation with fucosyltransferase VI or fucosyltransferase VII improves cord blood engraftment. Cytotherapy. 2014;16:84–89. doi: 10.1016/j.jcyt.2013.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Durand B, Eddleman K, Migliaccio AR, et al. Long-term generation of colony-forming cells (CFC) from CD34+ human umbilical cord blood cells. Leuk Lymphoma. 1993;11:263–273. doi: 10.3109/10428199309087003. [DOI] [PubMed] [Google Scholar]
- 93.Kurata H, Takakuwa K, Tanaka K. Ex vivo expansion of hematopoietic progenitor cells in human cord blood: An effect enhanced by cord blood serum. Hematol Pathol. 1995;9:73–78. [PubMed] [Google Scholar]
- 94.Bertolini F, Soligo D, Lazzari L, et al. The effect of interleukin-12 in ex-vivo expansion of human haemopoietic progenitors. Br J Haematol. 1995;90:935–938. doi: 10.1111/j.1365-2141.1995.tb05219.x. [DOI] [PubMed] [Google Scholar]
- 95.Siena S, Di Nicola M, Bregni M, et al. Massive ex vivo generation of functional dendritic cells from mobilized CD34+ blood progenitors for anticancer therapy. Exp Hematol. 1995;23:1463–1471. [PubMed] [Google Scholar]
- 96.DiGiusto DL, Lee R, Moon J, et al. Hematopoietic potential of cryopreserved and ex vivo manipulated umbilical cord blood progenitor cells evaluated in vitro and in vivo. Blood. 1996;87:1261–1271. [PubMed] [Google Scholar]
- 97.Scaradavou A, Isola L, Rubinstein P, et al. A murine model for human cord blood transplantation: Near-term fetal and neonatal peripheral blood cells can achieve long-term bone marrow engraftment in sublethally irradiated adult recipients. Blood. 1997;89:1089–1099. [PubMed] [Google Scholar]
- 98.Ohmizono Y, Sakabe H, Kimura T, et al. Thrombopoietin augments ex vivo expansion of human cord blood-derived hematopoietic progenitors in combination with stem cell factor and flt3 ligand. Leukemia. 1997;11:524–530. doi: 10.1038/sj.leu.2400588. [DOI] [PubMed] [Google Scholar]
- 99.De Bruyn C, Delforge A, Bron D, et al. Ex vivo expansion of CD34 + CD38- cord blood cells. J Hematother. 1997;6:93–102. doi: 10.1089/scd.1.1997.6.93. [DOI] [PubMed] [Google Scholar]
- 100.Piacibello W, Sanavio F, Garetto L, et al. Extensive amplification and self-renewal of human primitive hematopoietic stem cells from cord blood. Blood. 1997;89:2644–2653. [PubMed] [Google Scholar]
- 101.Kögler G, Callejas J, Sorg RV, et al. The effect of different thawing methods, growth factor combinations and media on the ex vivo expansion of umbilical cord blood primitive and committed progenitors. Bone Marrow Transplant. 1998;21:233–241. doi: 10.1038/sj.bmt.1701088. [DOI] [PubMed] [Google Scholar]
- 102.Kögler G, Callejas J, Sorg RV, et al. An eight-fold ex vivo expansion of long-term culture-initiating cells from umbilical cord blood in stirred suspension cultures. Bone Marrow Transplant. 1998;21(suppl 3):S48–S53. [PubMed] [Google Scholar]
- 103.Köhler T, Plettig R, Wetzstein W, et al. Defining optimum conditions for the ex vivo expansion of human umbilical cord blood cells. Influences of progenitor enrichment, interference with feeder layers, early-acting cytokines and agitation of culture vessels. Stem Cells. 1999;17:19–24. doi: 10.1002/stem.170019. [DOI] [PubMed] [Google Scholar]
- 104.Nakamura Y, Ando K, Chargui J, et al. Ex vivo generation of CD34(+) cells from CD34(−) hematopoietic cells. Blood. 1999;94:4053–4059. [PubMed] [Google Scholar]
- 105.McNiece I, Kubegov D, Kerzic P, et al. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol. 2000;28:1181–1186. doi: 10.1016/s0301-472x(00)00520-8. [DOI] [PubMed] [Google Scholar]
- 106.Lewis ID, Almeida-Porada G, Du J, et al. Umbilical cord blood cells capable of engrafting in primary, secondary, and tertiary xenogeneic hosts are preserved after ex vivo culture in a noncontact system. Blood. 2001;97:3441–3449. doi: 10.1182/blood.v97.11.3441. [DOI] [PubMed] [Google Scholar]
- 107.Pecora AL, Stiff P, Jennis A, et al. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant. 2000;25:797–799. doi: 10.1038/sj.bmt.1702222. [DOI] [PubMed] [Google Scholar]
- 108.Broxmeyer HE, Srour EF, Hangoc G, et al. High-efficiency recovery of functional hematopoietic progenitor and stem cells from human cord blood cryopreserved for 15 years. Proc Natl Acad Sci USA. 2003;100:645–650. doi: 10.1073/pnas.0237086100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: Results of a phase 1 trial using the AastromReplicell System. Blood. 2003;101:5061–5067. doi: 10.1182/blood-2001-12-0290. [DOI] [PubMed] [Google Scholar]
- 110.Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy. 2004;6:344–355. doi: 10.1080/14653240410004916. [DOI] [PubMed] [Google Scholar]
- 111.Serrano LM, Pfeiffer T, Olivares S, et al. Differentiation of naive cord-blood T cells into CD19-specific cytolytic effectors for posttransplantation adoptive immunotherapy. Blood. 2006;107:2643–2652. doi: 10.1182/blood-2005-09-3904. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Chaurasia P, Gajzer DC, Schaniel C, et al. Epigenetic reprogramming induces the expansion of cord blood stem cells. J Clin Invest. 2014;124:2378–2395. doi: 10.1172/JCI70313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Bart T, Boo M, Balabanova S, et al. Impact of selection of cord blood units from the United States and swiss registries on the cost of banking operations. Transfus Med Hemother. 2013;40:14–20. doi: 10.1159/000345690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Delaney C, Bollard CM, Shpall EJ. Cord blood graft engineering. Biol Blood Marrow Transplant. 2013;19(suppl):S74–S78. doi: 10.1016/j.bbmt.2012.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]