Debilitating eye diseases such as age-related macular degeneration and retinitis pigmentosa currently represent a large unmet medical need that could potentially be addressed by stem cell therapy. A number of novel stem cell-based cellular therapies are now under development to treat a variety of eye diseases. The approaches being taken by the California Institute for Regenerative Medicine, together with its grantees, are discussed.
SUMMARY
Debilitating eye diseases such as age-related macular degeneration and retinitis pigmentosa currently represent a large unmet medical need that could potentially be addressed by stem cell therapy. A number of novel stem cell-based cellular therapies are now under development to treat a variety of eye diseases. The approaches being taken by the California Institute for Regenerative Medicine, together with its grantees, are discussed.
Introduction
More than 10 million Americans suffer from debilitating eye diseases. These include age-related macular degeneration (AMD), retinitis pigmentosa (RP), and a spectrum of other retinal degenerative diseases [1]. AMD is a progressive disease leading to loss of central high-acuity vision and legal blindness and typically affects adults >55 years of age. Several of these diseases, including RP, are inherited genetic disorders that can manifest early in childhood and frequently lead to complete blindness. Corneal injuries and limbal stem cell deficiencies can lead to neovascularization, chronic inflammation, and scarring and can result in corneal opacity and loss of vision, often requiring extensive surgery.
Many of these debilitating visual impairments are classified as high unmet medical needs because there are either no approved treatments or the approved treatments are limited, inadequate, or associated with serious complications. The economic impact on the health care system, coupled with the loss of productivity from those suffering from these visual impairments, is profound. When combined, diseases of visual impairment represent an annual cost to the U.S. heath care system on an order of magnitude of $195 billion, and this remains a critical unmet medical need for both patients and society as a whole [2]. The loss of productivity related to absenteeism alone is estimated to be approximately $40 billion annually [2].
The promise of regenerative medicine, and stem cell therapy in particular, may hold the key to treating and potentially reversing the damage collectively caused by these various diseases. The California Institute for Regenerative Medicine (CIRM), the $3 billion state stem cell funding agency, is actively working (with grantees) to advance novel treatments into the clinic for several debilitating eye diseases, including the dry form of AMD, RP, corneal injury, and limbal cell deficiency.
Dry Age-Related Macular Degeneration
Age-related macular degeneration affects high-acuity central vision that is required for reading, driving, using a computer screen, and recognizing faces. AMD is the leading cause of visual impairment and blindness in people aged >65 years in the U.S. The National Eye Institute estimates the prevalence of AMD to be approximately 1.75 million in the U.S. population, with that number projected to increase dramatically because of aging of the U.S. population [3]. There are currently no approved therapies for the more common atrophic form, known as dry AMD (80%–90% of cases), and the prognosis for these patients remains poor. In a subset of patients, dry AMD converts to wet AMD, a condition in which new and abnormal blood vessels originating in the choroid layer beneath the retina invade the retina and infiltrate the overlying retinal layers. A number of therapeutics (primarily vascular endothelial growth factor inhibitors) have been approved for this exudative (wet) form of AMD.
Although the etiology of dry AMD is still unclear, a large body of evidence suggests that it is primarily a disease of the retinal pigment epithelium (RPE), a monolayer of polarized cells that underlies and supports the photoreceptors of the retina. It appears that dysfunction or loss of RPE in the macula region of the retina precedes and leads to loss of the photoreceptors (PRs), ultimately resulting in the loss of central high-acuity vision. Geographic atrophy, the advanced stage of dry AMD, is characterized by a pattern of well-demarcated RPE atrophy associated with PR atrophy.
Reversing vision loss in situations of advanced geographic atrophy will presumably require replacing both lost PRs and RPE, a formidable task because the transplanted PRs will need to integrate into the retina and establish the appropriate neural connections required to transmit visual information to the brain. Currently, a more tractable approach with regard to developing a cellular therapy for dry AMD is based on the premise that if the dysfunctional or lost RPE can be replaced with healthy RPE, the remaining PRs will be rescued and visual function will be maintained. Such an approach is likely to be most effective in earlier stages of this slow, progressive disease, in situations in which some PRs remain.
In developing an RPE replacement therapy, two key challenges must be addressed: where to get healthy RPE cells for transplantation and how to deliver them. A number of investigators have shown that human pluripotent stem cells can be differentiated into RPE cells that are structurally and functionally similar to human fetal RPE cells, thus providing a ready source of RPE cells for transplantation. Cell delivery presents some additional challenges. In a normal eye, the RPE is tightly bound to a layer of extracellular matrix known as Bruch’s membrane (BrM), which forms the blood-retina barrier separating the blood vessels of the underlying choroid from the RPE. BrM is highly permeable to fluid and small molecules and regulates the reciprocal exchange of nutrients and metabolic waste products between the retina and the choroid. BrM undergoes both structural and functional changes during aging, leading to decreased permeability. It is also the site of accumulation of protein and lipid deposits called drusen, one of the hallmarks of dry AMD. Although it is not known whether drusen are the cause of RPE degeneration in AMD, the pathogenesis of AMD involves thickening and damage to BrM and impairment of its transport function. Evidence suggests that in order for transplanted RPE to be able to attach to BrM and support the PRs, it may be necessary to reconstruct or replace the damaged BrM.
The approach that CIRM grantee the California Project to Cure Blindness (CPCB) is taking is designed to do just that. The product that CPCB is developing with CIRM support is a subretinal implant designed to replace lost or dysfunctional RPE with healthy RPE derived from human embryonic stem cells. The distinguishing feature of the CPCB approach is that the RPE cells are supplied in the form of a differentiated, polarized monolayer attached to a nondegradable synthetic membrane designed specifically to mimic the permeability properties of a healthy BrM. The cell-membrane implant will be surgically inserted into the subretinal space using simple procedures developed for the retina and positioned with the apical surface of the RPE monolayer facing toward the PRs to facilitate interdigitation with the PRs, as is necessary for correct functioning of the RPE. The synthetic membrane, in addition to supporting the RPE, enables transport of nutrients and waste products between the retina and choroid capillaries. Although the addition of the synthetic membrane adds complexity, making this a combination product with additional manufacturing and regulatory hurdles, this strategy may have significant advantages over competing strategies using cells alone. A major advantage is that the synthetic membrane provides a potential solution to the problem of coaxing transplanted RPE cells to attach to a damaged BrM and is likely to result in better survival and functioning of the transplanted RPE. In addition, this strategy allows the transplanted RPE to be placed in precisely the right location and in the correct orientation to provide a new polarized RPE monolayer resembling that in a normal healthy eye.
Use of a human embryonic stem cell line as the source from which to derive transplantable RPE cells has a number of advantages in that it allows for manufacture of a well-defined, well-characterized, readily available, off-the-shelf product. An important question is whether the eye is an immunoprivileged site, as some believe, or whether immunosuppression will be required to maintain the implanted allogeneic cells. If the answer to the latter question is yes, then how much immunosuppression is needed and for how long? These questions cannot be answered preclinically but rather will need to be addressed in the clinic.
Use of autologous induced pluripotent stem cells (iPSCs) to derive RPE might be an approach to circumvent the need for immunosuppression; however, although it might solve one problem, such an approach brings new challenges. Currently, the manufacturing process for autologous iPSC-derived RPE is long and labor intensive and would have to be performed for each patient treated. Using the current methodologies, this approach likely is not feasible for a large market and will require significant technology advances that speed up and simplify the manufacturing. Moreover, the AMD population is elderly, and it remains to be verified whether autologous cells from patients with AMD would support the derivation of healthy RPE for transplantation. Nevertheless, despite the many remaining challenges, RPE derived from pluripotent stem cells offers great promise in treating a disease that remains without an effective treatment.
Treatment Alternatives for AMD
Currently there is no approved treatment for dry AMD, and the standard of care is limited to multivitamins (e.g., antioxidants, vitamins, minerals), which serve only to slow the progression of the early form of the disease. A number of conventional drug strategies are currently under investigation for geographic atrophy, targeting various molecular pathways. These strategies are aimed at neuroprotection, prevention of oxidative damage, or suppression of inflammation, and there is uncertainty about the preferred molecular pathway to target. At best, these strategies slow disease progression, and none replaces the damaged RPE or BrM. An experimental procedure, macular translocation, has been shown to improve visual acuity in some patients [4]; however, the procedure is associated with clinical complications including cataract formation, retinal detachment, intraocular bleeding, and total vision loss.
The total addressable market for dry AMD is estimated to be $25 billion in the U.S. and Europe. Although there are currently no approved treatments, several therapeutic approaches are currently in clinical trials for dry AMD (Table 1).
Table 1.
Summary of clinical stage cell therapy approaches for dry age-related macular degeneration
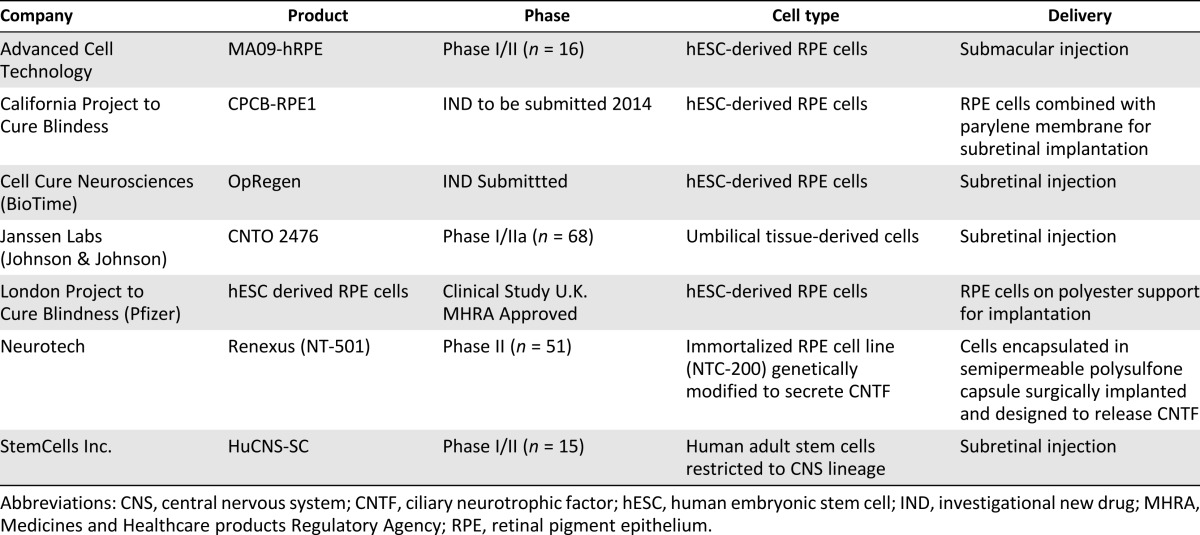
The approaches listed in Table 1 differ in their starting source of cells and delivery method but have common ground in their allogeneic approach. Although autologous approaches may prove a viable alternative for certain indications, diseases with large patient populations and multibillion-dollar market potential will likely be most efficiently served by an off-the-shelf allogeneic model, which provides for more cost-efficient manufacturing, scale-up, and distribution. The clinical trial landscape for dry AMD is still relatively early, but some promising results have been reported to date for a cell therapy approach, including a good safety profile, stabilization of the best corrected visual acuity test out to 1 year, and dose-dependent increases in retinal thickness involving photoreceptor layers (Neurotech phase II study, ClinicalTrials.gov identifier NCT00447954).
Although it will treat wet rather than dry AMD, it is worth noting that a landmark initial human clinical trial using autologous RPE derived from iPSCs has been cleared to begin at the institute for Biomedical Research and Innovation and the RIKEN Center in Kobe, Japan [5].
The key challenge for future development, licensure, and successful commercialization for all of these programs will be to demonstrate stabilization or improvement in a clinical meaningful endpoint such as visual acuity.
Retinitis Pigmentosa
RP is an inherited degenerative eye disease leading to a gradual decline of peripheral vision and, often, eventual blindness. RP is a retinal dystrophy initially involving loss of the rod photoreceptors, followed by loss of cone cells. Consequently, the earliest symptom associated with RP is loss of night vision, then a gradual loss of peripheral visual fields, followed by complete visual loss. This is a direct result of gradual degeneration of the cone cells that slowly progresses toward the macula, causing a loss of central vision and often complete blindness by middle age. The prevalence of RP is 1 in 4,000 people worldwide, resulting in >1.5 million visually impaired patients.
RP is a genetically heterogeneous disease that can be caused by diverse genetic alterations involving a number of different genes. Although approximately 3,000 mutations have been reported in 50 different genes known to cause nonsyndromic RP, most of the described mutations are clustered on the rhodopsin gene, accounting for 25% of the total RP patients. Moreover, the extensive variation in clinical manifestation among patients sharing the same mutation in the same gene exacerbates the challenges of managing and treating the condition.
Given the genetic heterogeneity associated with the disease and the lack of therapeutic options, cell therapy seems a promising therapeutic avenue for RP patients, but perhaps not initially as cell replacement therapy. In contrast to AMD, in which the degenerated area is limited to the macula region, a cell therapy that aims to replace a damaged tissue may not be suited for the correction of the relatively large area of the retina damaged in RP patients; however, cell therapy that uses cells to provide trophic support to the rod and cone cells may be effective to slow the progression of the disease [6].
CIRM’s RP project is focused on slowing the progression of RP by using retinal progenitor cells believed to provide neurotrophic factors that improve the survival and function of cone cells. The approach involves an intravitreal injection of progenitor stem cells obtained from the immature fetal retina and expanded in culture. These retinal progenitor cells secrete growth factors that diffuse within the host eye and enhance the survival and light sensitivity of photoreceptors in the dystrophic retina. Furthermore, in addition to their neurotropic support, these stem cells may differentiate and integrate in the retina and become functional photoreceptors. This approach may slow disease progression regardless of the underlying genetic mutation causing the disease.
As part of the current project, the retinal progenitor cells were tested in animals for safety and efficacy. Following approval by the U.S. Food and Drug Administration (FDA), a small number of patients with severe RP will be treated with this novel cell therapy to assess safety and efficacy in humans.
Treatment Alternatives for RP
Current medical therapies for retinitis pigmentosa are limited to vitamin supplements, such as vitamin A palmitate, that have been shown to slow the progression of the disease in some patients.
In February 2013, the FDA approved a device, the Argus II retinal prosthesis system (Second Sight Medical Products, Inc., Sylmar, CA, http://www.2-sight.eu/ee/), for adults with severe RP. The device consists of glasses with an attached video camera that captures images of the surrounding area. These images then become an electrical signal that is sent through a video-processing unit. The signal is wirelessly delivered to the eye, stimulating the retina. The electrical stimulation of the retina is recognized by the brain as spots of light. The system is designed to produce the sensation of light and may help patients identify the location or movement of objects and people; recognize large letters, words, or sentences; and aid in daily activities, such as detecting street curbs.
The landscape of cellular and gene therapies in clinical development for RP remains limited (Table 2).
Table 2.
Summary of cell/gene therapy approaches for the treatment of retinitis pigmentosa
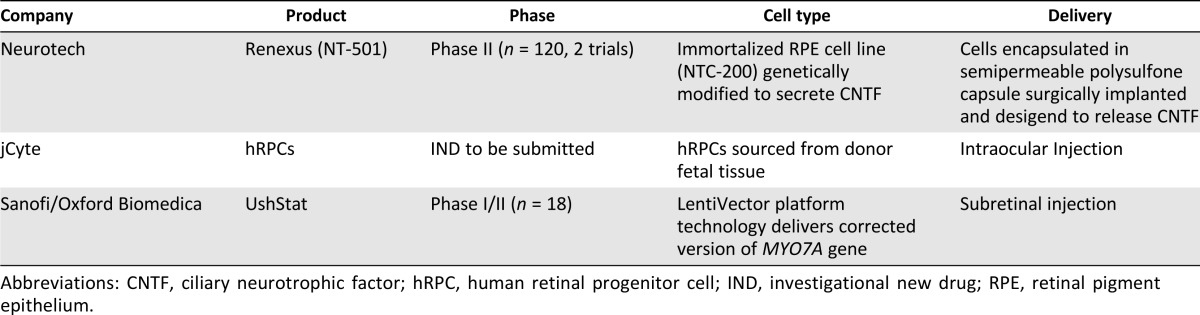
Clinical studies have demonstrated that the therapies appear to be safe and well tolerated, and the Neurotech studies (ClinicalTrials.gov identifiers NCT00447980, NCT00447993) showed a statistically significant dose-dependent increase in retinal thickness, as measured by optical coherence tomography. A challenge in the clinical evaluation of therapies for RP is that patients generally suffer from a gradual deterioration and loss of vision occurring over many years, making it difficult to measure the potential visual benefit of the therapies over a shorter time frame.
Beyond the approaches listed in Table 2, a handful of small molecules and biologics are being developed by biopharma companies and many teams engaged in early stage research at universities—many using gene therapy—targeting RP.
Limbal Cell Deficiency
The cornea is a transparent tissue in the front of the eye that refracts light and serves as a protective barrier for the eye. The clearness of the cornea is essential to visual acuity and depends on the integrity of the corneal epithelium and its ability to regenerate constantly. The area in the eye between the cornea and bulbar conjunctiva, known as the limbus, is populated with limbal stem cells (LSCs), which are responsible for the renewal of the corneal epithelium. Injury to the cornea by a burn or severe infection can damage the limbus, causing depletion of limbal stem cells. Without LSCs to restore the cornea, conjunctival cells migrate to the injured site and grow over the damaged cornea, creating an opacified surface that causes severe loss of vision [7]. Unfortunately, cornea transplantation does not work for this condition. Although allogeneic limbal tissue transplantation has been the main treatment option, it often provides only a temporary solution because rejection is common. The failure of the transplant eventually leads to more vascularized inflamed tissue and the formation of stroma scar.
An alternative approach was recently developed by De Luca and colleagues [8] that involved transplanting autologous ex vivo expanded limbal cells to regenerate the corneal epithelial cells. The group demonstrated permanent restoration of vision in 77% of treated eyes for up to 10 years. Although this promising approach has been used with great success in Europe and Asia, it relies on the autologous expansion of limbal cells on mouse feeder cells. A xenobiotic-free expansion method is required to bring this treatment to the U.S.
The CIRM-supported project aims to address this issue. This team is expanding human LSCs under various xenobiotic and feeder-free systems and comparing the resulting cells with those grown in the established feeder system using mouse cells. Rama et al. have demonstrated a direct correlation between graft success and a specific limbal cell marker [8]. The CIRM grantee is testing various growth conditions monitoring cell viability and expression of a limbal cell biomarker [9]. The system that supports the most efficient growth of LSCs will be a strong candidate for future clinical trials in the U.S.
Treatment Alternatives for Limbal Cell Deficiency
The standard of care for corneal injury and disease remains allogeneic limbal tissue transplantation (as described above); however, this approach is limited in effectiveness and restores transparency only temporarily because graft failure is common. Persistent inflammation and the production of scar tissue impairs visual acuity. Another approach is autologous limbal cells transplantation, which is used to regenerate the corneal epithelium, as described above. When no autologous limbal stem cells are available for expansion, as occurs in patients with bilateral limbal stem cell deficiency, an alternative approach is transplantation of cultivated oral mucosal epithelium. A problem with this cell therapy is that oral mucosal epithelium does not transdifferentiate into the corneal epithelial phenotype, and it is far from having an acceptable visual and clinical outcome.
According to ClinicalTrials.gov, 10 clinical trials are targeting limbal cell deficiency; only 5 are active, and all are being conducted outside the U.S.
Conclusion
The above-mentioned CIRM-funded programs are currently poised to move into the clinic and begin early clinical trials in dry AMD and RP. The eye offers a number of powerful advantages for advancing cell therapy approaches, namely, the availability of multiple sophisticated noninvasive imaging technologies to assess whether there is a signal of activity, the relatively small numbers of cells needed, and the possibility of using the nontreated eye as a control. Given the lack of available treatment options for dry AMD and RP, the poor prognoses of patients, and the tremendous quality-of-life impact of vision loss, these novel approaches have the potential to address a substantial unmet medical need.
Author Contributions
I.W.C., N.L., and A.A.: manuscript writing.
Disclosure of Potential Conflicts of Interest
The authors indicate no potential conflicts of interest.
References
- 1.Foundation Fighting Blindness Eye conditions. Available at http://www.blindness.org/eye-conditions. Accessed November 3, 2014.
- 2.The global economic cost of visual impairment Available at http://www.icoph.org/resources/146/The-Global-Economic-Cost-of-Visual-Impairment.html. Accessed October 30, 2014.
- 3.Friedman DS, O’Colmain BJ, Muñoz B, et al. Prevalence of age-related macular degeneration in the United States. Arch Ophthalmol. 2004;122:564–572. doi: 10.1001/archopht.122.4.564. [DOI] [PubMed] [Google Scholar]
- 4.Abdel-Meguid A, Lappas A, Hartmann K, et al. One year follow up of macular translocation with 360 degree retinotomy in patients with age related macular degeneration. Br J Ophthalmol. 2003;87:615–621. doi: 10.1136/bjo.87.5.615. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cyranoski D. Next-generation stem cells cleared for human trial. Nature News. 10.1038/nature.2014.15897.
- 6.Lin TC, Hsu CC, Chien KH, et al. Retinal stem cells and potential cell transplantation treatments. J Chin Med Assoc. 2014 doi: 10.1016/j.jcma.2014.08.001. [Epub ahead of print] [DOI] [PubMed] [Google Scholar]
- 7.Pellegrini G, De Luca M. Eyes on the prize: Limbal stem cells and corneal restoration. Cell Stem Cell. 2014;15:121–122. doi: 10.1016/j.stem.2014.07.011. [DOI] [PubMed] [Google Scholar]
- 8.Rama P, Matuska S, Paganoni G, et al. Limbal stem-cell therapy and long-term corneal regeneration. N Engl J Med. 2010;8:147–155. doi: 10.1056/NEJMoa0905955. [DOI] [PubMed] [Google Scholar]
- 9.Mei H, González S, Nakatsu MN, et al. A three-dimensional culture method to expand limbal stem/progenitor cells. Tissue Eng Part C Methods. 2014;20:393–400. doi: 10.1089/ten.tec.2013.0246. [DOI] [PMC free article] [PubMed] [Google Scholar]


