Abstract
Contrast-enhanced magnetic resonance angiography (MRA) is a promising technique for coronary artery imaging. The blood signal changes during the contrast injection will result in image artifacts, blurring and relatively low signal-to-noise ratio, when the k-space segments from different cardiac cycles are combined to reconstruct the final image as “time averaged.” Thus, it is important to acquire data during maximal blood signal enhancement for first-pass, contrast-enhanced MRA, and relatively high temporal resolution is required. This work demonstrated the feasibility of highly constrained backprojection reconstruction for time-resolved, contrast-enhanced coronary MRA. With this method, the temporal resolution can be increased. In addition, coronary artery images around blood signal enhancement peak have significantly improved contrast-to-noise ratio and suppressed artifacts compared to the composite images which were collected during a much longer acquisition time during substantial blood signal changes.
Keywords: Magnetic resonance imaging, MRA, HYPR
1. Introduction
Coronary magnetic resonance angiography (MRA) has evolved as a promising noninvasive modality for the imaging of the coronary arteries and for the evaluation of coronary artery stenoses [1]. T1-shortening contrast agents have been used in coronary MRA to improve the coronary blood signal intensity and blood-myocardial contrast [2–4]. However, the blood signal changes during the contrast injection will result in image artifacts, blurring and relatively low signal-to-noise ratio (SNR), when the k-space segments from different cardiac cycles are combined to reconstruct the final image as “time averaged.” Therefore, it is important to acquire data during the maximal blood signal enhancement for first-pass, contrast-enhanced MRA, and relatively high temporal resolution is required. It is very hard to estimate the transit time for the contrast agent to flow to the coronary, so the time-resolved imaging method is needed to resolve the signal intensity change information. Due to the short data acquisition window per cardiac cycle and few cardiac cycles within the time during maximal blood signal enhancement, limited number of k-space lines or projections can be acquired and the k-space is highly undersampled, which in turn causes severe blurring and artifacts in the coronary images by conventional reconstruction methods, and relatively high temporal resolution is needed in contrast-enhanced MRA.
Many efforts have been made to increase the imaging speed. Parallel imaging methods [5–7] are commonly used to reduce the total acquisition time. However, the reduction factor is limited because of the SNR. Time-resolved techniques can be used to obtain the peak arterial enhancement by increasing the temporal resolution for contrast-enhanced MRA. Radial acquisitions can be used for accelerated sampling schemes. HighlY constrained back-PRojection (HYPR) has been proposed to reduce streak artifacts for time-resolved MRI and permit decreased numbers of projections for each image acquisition in the image time series [8,9]. With radial sampling, HYPR can provide a higher reduction factor compared to other imaging methods. The k-space is highly undersampled at each time point. HYPR images are multiplications of the “composite image,” which is reconstructed from full k-space data from different time points, and the “weighting maps,” which are low-resolution images from the undersampled k-space at each time point. With HYPR, time-resolved images can be reconstructed by combining the structural information from the fully sampled composite image and the dynamic signal intensity information from weighting maps.
For coronary MRA, the highly undersampled data acquisition with HYPR can reduce the data acquisition window, thus reducing the motion artifacts. Time-resolved (one image per cardiac cycle) HYPR images provide additional temporal information besides the conventionally reconstructed, time-averaged image. We hypothesize that the HYPR technique can improve the temporal resolution, contrast-to-noise ratio (CNR) for the coronary artery images around the blood signal intensity peak and suppress artifacts.
Computer simulations and healthy volunteer studies were performed to investigate the feasibility of HYPR reconstruction (HYPR PR) for time-resolved contrast-enhanced coronary MRA. Computer simulations were used to demonstrate the artifacts caused by blood signal changes when the k-space segments from different cardiac cycles are combined to reconstruct the final image as time averaged and the suppression effects of these artifacts by HYPR processing. In volunteer studies, the time-resolved HYPR images were compared with the single frame images and composite images. The behavior of CNR and artifacts was discussed, and the correlation of the signal intensity changes of the HYPR images and the reference images was calculated.
2. Methods
2.1. Segmented fast low-angle short imaging sequence with radial sampling
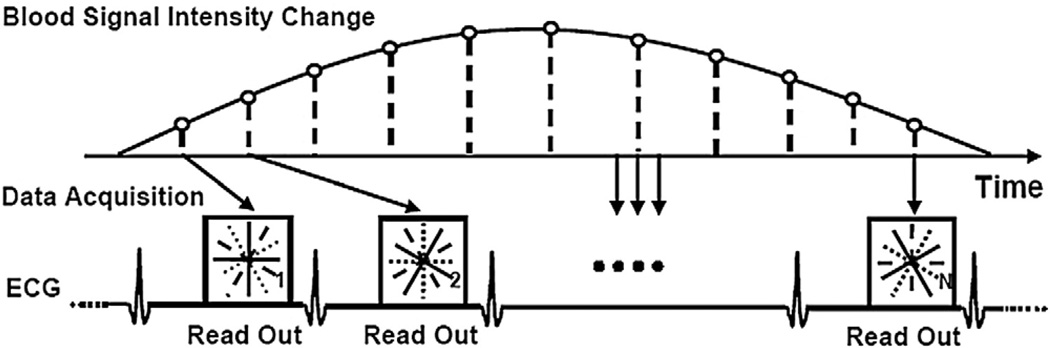
A segmented 2D fast low-angle short (FLASH) sequence with radial k-space sampling was used for HYPR acquisition in contrast-enhanced coronary imaging. As shown in Fig. 1, radial k-space was highly undersampled at each cardiac cycle to achieve high temporal resolution and short data acquisition window. Segments of interleaved and equally spaced k-space projections were acquired during mid-diastole at each cardiac cycle and finally combined to a full k-space to reconstruct the composite image. Blood signal intensity changes along with the infusion and washout of the contrast agents. HYPR method was used to reconstruct the time-resolved images for each cardiac cycle, so that the signal intensity changes along with the contrast injection can be recovered. ECG triggering was used to reduce the cardiac motion artifacts, and an inversion recovery prepulse followed by inversion time was used for the background suppression based on T1 weighting.
Fig. 1.
Schematic of the segmented 2D radial k-space acquisition, with highly undersampled, interleaved and equally spaced projections per cardiac cycle. Blood signal intensity varies in different cardiac cycles due to the flow in and out of the contrast agents. Full k-space was covered after multiple cardiac cycles, and high temporal resolution per cardiac cycle was achieved.
2.2. Simulations
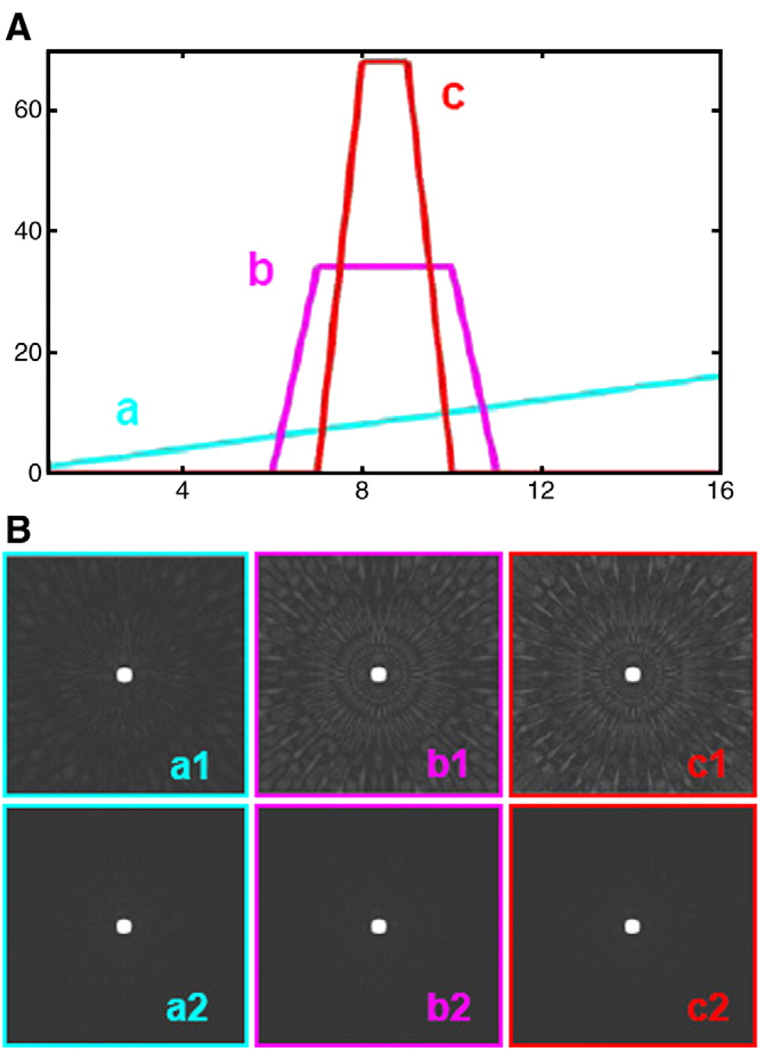
A 2D computer model was created by MATLAB to show the artifacts caused by blood signal changes, when the k-space segments from different cardiac cycles are combined to reconstruct the final image as time averaged. Acquisition of undersampled interleaved k-spaces with varying blood signal was simulated. As shown in Fig. 2A, three schemes of signal intensity changes were simulated to show different artifacts caused by different shapes of signal intensity changes when the integrations of the signal intensity are the same. The blue, pink and red lines show the signal intensity increases (a) from 1 to 16 proportionally, (b) equal to 34 in 7–10 heartbeats and 0 for 12 other heartbeats, and (c) equal to 68 in 8–9 heartbeats and 0 for 14 other heartbeats, respectively. HYPR postprocessing was used to reconstruct the time-resolved images to demonstrate the suppression effects for this kind of artifacts. The HYPR images at the blood signal peak were compared with the corresponding composite images.
Fig. 2.
Comparison of the simulation results of composite images and corresponding HYPR images with different signal change scheme: (a1) signal intensity increases from 1 to 16 proportionally; (b1) equal to 34 in 7–10 heartbeats, and 0 for 12 other heartbeats; (c1) equal to 68 in 8–9 heartbeats, and 0 for 14 other heartbeats. The corresponding HYPR images, (a2), (b2) and (c2), at the intensity peak are shown in the second line. The artifacts were successfully suppressed.
2.3. Contrast-enhanced MRA
Six healthy volunteers were scanned using a 1.5-T clinical whole-body scanner (MATNETOM Sonata; Siemens Medial Solutions, Erlangen, Germany; maximum slew rate=200 mT/m per millisecond; maximum gradient amplitude: 40 mT/m). Two cardiac phased array coils were used for signal reception. Written consent was obtained from all participants before imaging procedures were performed, in accordance with the Institutional Review Board at Northwestern University.
For every scan, contrast-enhanced images were acquired during the first-pass of the contrast agent within a single breath-hold. Three-point localization method was used to define 2D MRI plane, and the images were acquired targeting the left anterior descending (LAD) or right coronary artery (RCA). A cine acquisition was performed first to determine the trigger delay time for minimizing the cardiac motion-related artifacts. To estimate the approximate transit time for the contrast agent to flow to the root of the aorta, a test-bolus scan with a temporal resolution of one image per second was performed before the coronary MRI. Six milliliters of contrast material, gadopentetate dimeglumine (Magnevist, Berlex, Wayne, NJ, USA), chased by 9 ml of saline solution, was injected intravenously using a power injector (Spectris, Medrad, Indianola, PA, USA) at a rate of 3 ml/s. The blood signal changes of the coronary arteries were saved for further comparison and analysis.
After that, the coronary MRI with ECG-triggered, 2D radial FLASH sequence with radial sampling was performed with the following parameters: TR/TE/flip angle=5.02/2.51/20°, FOV=300 × 300 mm2, matrix=320×320, slice thickness=4 mm and breath-hold duration=10–16 s. The 2D k-space was acquired in an interleaved fashion with 20 projections per heartbeat over continuous 16 cardiac cycles. The contrast injection scheme was the same as the test-bolus scan.
2.4. Image reconstruction and data analysis
The HYPR method was used for the time-resolved contrast-enhanced image reconstruction. The datasets were reconstructed offline by MATLAB (MathWorks). A composite image was reconstructed by the conventional regridding method using all k-space views. One-dimensional Fourier transformation of the projections from each heartbeat, which provides the time-resolved information, was normalized by the back projection of the composite image to make the weighting maps. Multiplication of the weighting maps and the composite image gives rise to a series of time-resolved images (one image per cardiac cycle) after the contrast injection. Improved blood-myocardial CNR was expected for the images around the time of peak blood signal enhancement compared to the composite images.
As previously discussed, a test-bolus scan was performed before the contrast injection. The normalized blood signal-vs.-time curve from the test-bolus scan was used as a reference and compared to the curve from HYPR PR time-resolved images. The blood signal intensity for HYPR images was interpolated to the same time point as that of the test bolus. So the correlation coefficient of blood signal intensity changes between HYPR and bolus images can be calculated in MATLAB. CNR of the aorta at the level of the LAD or RCA was measured in the whole series of HYPR images and compared to that of the composite images. CNR was calculated by dividing the difference between the mean blood signal intensity and the mean myocardium signal intensity by the standard deviation of the background noise signal [8]. The improvement of the CNR for one method compared to another was tested by a paired one-tailed t test with a 95% confidence interval.
3. Results
3.1. Simulations
As shown in the top row of Fig. 2B, artifacts in the composite images are caused by combining the segmented k-space data with significantly different signal intensities. The obviousness of the artifacts is positively correlated with the intensity of the blood signal changes. With the increase of the intensity of the blood signal changes, the artifacts became more obvious in the composite images (from a1 to c1). After the HYPR postprocessing, the artifacts in the time-resolved HYPR images on the simulated signal intensity peak (a2, b2, c2) were dramatically suppressed compared to the original composite images.
3.2. In vivo studies
A series of contrast-enhanced HYPR images based on one full k-space data acquisition is illustrated in Fig. 3. The blood signal intensity changes were clearly observed in the images (top row) and their corresponding weighting maps (middle row). Compared with the single frame image based on the same 16 radial views (bottom row), HYPR images define the structure clearly with less striking artifacts.
Fig. 3.
Depiction of LAD with different contrast enhancement in different cardiac cycles: images in the top row show the HYPR images; the corresponding weighting map and the single frame images are respectively illustrated in the middle and bottom row. Note the signal intensity change of HYPR images was based on the weighting map. The image quality was improved much (with higher resolution and less striking artifacts) by the HYPR processing compared to the single frame images.
The blood signal intensity changes of the aorta at the LAD level after contrast injection for HYPR images were verified by the test-bolus scan, as shown in Fig. 4. For all the six volunteer studies, the blood signal-vs.-time curves of the aorta at the LAD/RCA level of HYPR images were highly correlated (average correlation was 0.9463) with those of the test bolus as reference. The time-resolved HYPR images track the true signal intensity changes approximately.
Fig. 4.
Typical example of the blood signal changes vs. time curve for comparison. The solid line describes the signal intensity curve of the test-bolus scan, and the dot line describes the signal intensity curve of the HYPR PR time-resolved images.
A full k-space-based composite image (A) and single frame undersampled images at the blood signal intensity peak without (B) and with HYPR postprocessing (C) from a volunteer during diastole are shown in Fig. 5. k-Space undersampling caused obvious streaking artifacts and blurring (B). HYPR image (C) gives much clearer structure with suppressed artifacts based on the same undersampled k-space projections as the single frame image (B). Compared to the composite image (A) by conventional reconstruction method, there were no apparent image artifacts in the HYPR images typically associated with vast undersampling of the PR k-space. The artifacts are successfully suppressed by HYPR algorithm. The signal in the region of interest of the HYPR image at the blood signal intensity peak is increased with similar background signal, and the background noise was suppressed which caused increased CNR in the HYPR images.
Fig. 5.
Contrast-enhanced diastolic images acquired with 2D radial MRA method from a healthy volunteer. (A) Composite image based on full k-space. (B) Single frame image at the blood signal intensity peak based on undersampled k-space. (C) HYPR image derived from the same under-sampled k-space used for (B).
With HYPR PR, time-resolved 2D coronary artery images were acquired for every cardiac cycle (with 16 radial views), resulting in a temporal resolution increase by a factor of 16 as compared to the conventional PR acquisition. Peak CNR of the coronary artery in the time-resolved HYPR images (53.83±16.89) was improved by 154% as compared to that of the composite image (21.17±7.4) (P=.001, paired t test), despite a data reduction factor of 16.
4. Discussion
This work demonstrated the feasibility of HYPR PR for time-resolved, contrast-enhanced coronary MRA. With this method, the temporal resolution can be increased. The blood signal intensity changes of the HYPR images were highly correlated to the reference images. In addition, coronary artery images around the time of peak blood signal enhancement have significantly improved CNR and fewer artifacts compared to the composite images collected by much longer acquisition times and during substantial blood signal changes.
With the HYPR method, time-resolved MRA images with a temporal resolution of one image per cardiac cycle are available. These images combine the signal intensity change information from the undersampled radial projections and the structural information from the composite image. Therefore, the time-resolved HYPR MRA images provide additional temporal information besides the conventionally reconstructed, time-averaged image, and the maximal blood signal enhancement when the CNR is higher than other time during the first-pass contrast injection can be caught.
There are certain limitations with HYPR MRA imaging. Breath-holding was used for 2D imaging. For the 3D whole-heart coronary MRA, navigator gating is needed to eliminate the respiratory motion artifacts because of the long acquisition time. Therefore, some cardiac cycles are neglected and the maximal blood signal enhancement could be missed. However, the quality of the time-resolved HYPR images with higher signal enhancement is still better than the conventional time-averaged image, especially when the data acquisition time is long.
In conclusion, HYPR is a promising method to improve the coronary MRA with higher CNR and reduced artifacts. This method will be applied in the 3D whole-heart coronary MRA technique, and the ability of this method to detect regional perfusion defects needs to be demonstrated in animals or patients with coronary artery disease.
References
- 1.Dhawan S, Dharmashankar KC, Tak T. Role of magnetic resonance imaging in visualizing coronary arteries. Clin Med Res. 2004;2(3):173–179. doi: 10.3121/cmr.2.3.173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Li D, Carr JC, Shea SM, Zheng J, Deshpande VS, et al. Coronary arteries: magnetization-prepared contrast-enhanced three-dimensional volume-targeted breath-hold MR angiography. Radiology. 2001;219(1):270–277. doi: 10.1148/radiology.219.1.r01ap37270. [DOI] [PubMed] [Google Scholar]
- 3.Paetsch I, Jahnke C, Barkhausen J, Spuentrup E, Cavagna F, et al. Detection of coronary stenoses with contrast enhanced, three-dimensional free breathing coronary MR angiography using the gadolinium-based intravascular contrast agent gadocoletic acid (B-22956) J Cardiovasc Magn Reson. 2006;8(3):509–516. doi: 10.1080/10976640600604922. [DOI] [PubMed] [Google Scholar]
- 4.Stuber M, Botnar RM, Danias PG, McConnell MV, Kissinger KV, et al. Contrast agent-enhanced, free-breathing, three-dimensional coronary magnetic resonance angiography. J Magn Reson Imaging. 1999;10(5):790–799. doi: 10.1002/(sici)1522-2586(199911)10:5<790::aid-jmri25>3.0.co;2-i. [DOI] [PubMed] [Google Scholar]
- 5.Griswold MA, Jakob PM, Heidemann RM, Nittka M, Jellus V, et al. Generalized autocalibrating partially parallel acquisitions (GRAPPA) Magn Reson Med. 2002;47(6):1202–1210. doi: 10.1002/mrm.10171. [DOI] [PubMed] [Google Scholar]
- 6.Pruessmann KP, Weiger M, Scheidegger MB, Boesiger P. SENSE: Sensitivity encoding for fast MRI. Magn Reson Med. 1999;42(5):952–962. [PubMed] [Google Scholar]
- 7.Park J, McCarthy R, Li D. Feasibility and performance of breath-hold 3D true-FISP coronary MRA using self-calibrating parallel acquisition. Magn Reson Med. 2004;52(1):7–13. doi: 10.1002/mrm.20153. [DOI] [PubMed] [Google Scholar]
- 8.Mistretta CA, Wieben O, Velikina J, Block W, Perry J, et al. Highly constrained backprojection for time-resolved MRI. Magn Reson Med. 2006;55(1):30–40. doi: 10.1002/mrm.20772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ge L, Kino A, Griswold M, Mistretta C, Carr JC, Li D. Myocardial perfusion MRI with sliding-window conjugate-gradient HYPR. Magn Reson Med. 2009 Aug 11; doi: 10.1002/mrm.22059. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]