Abstract
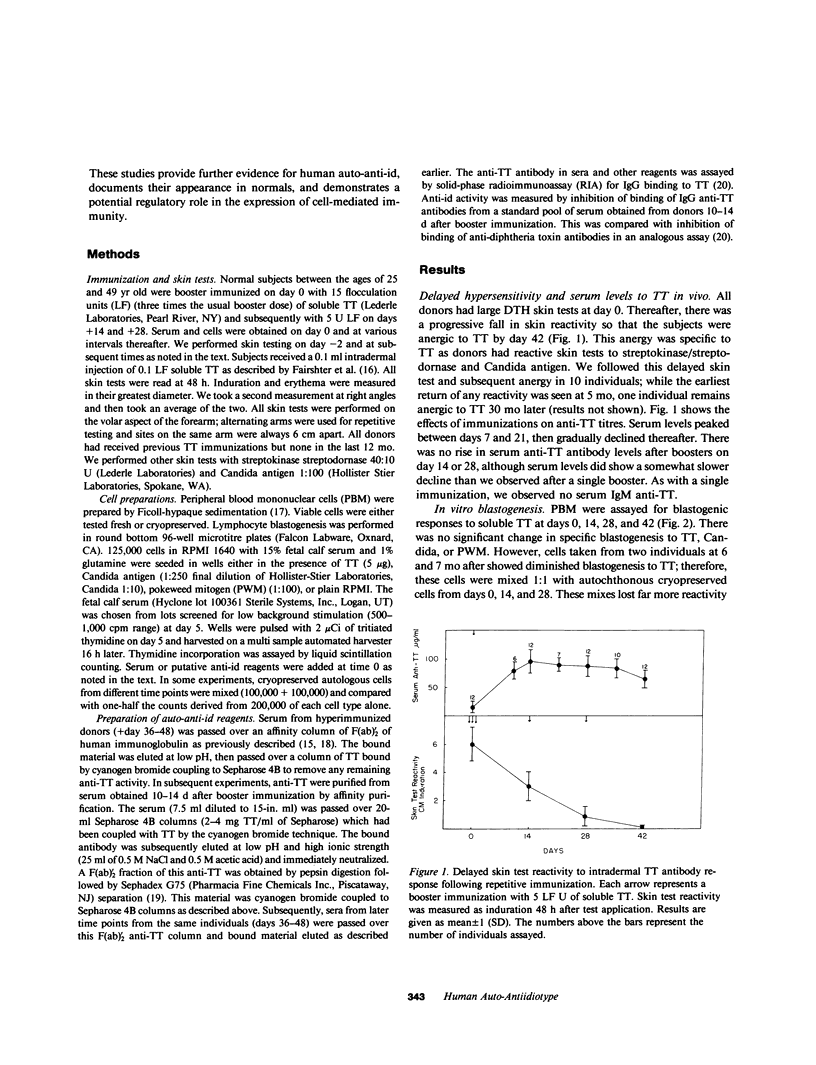
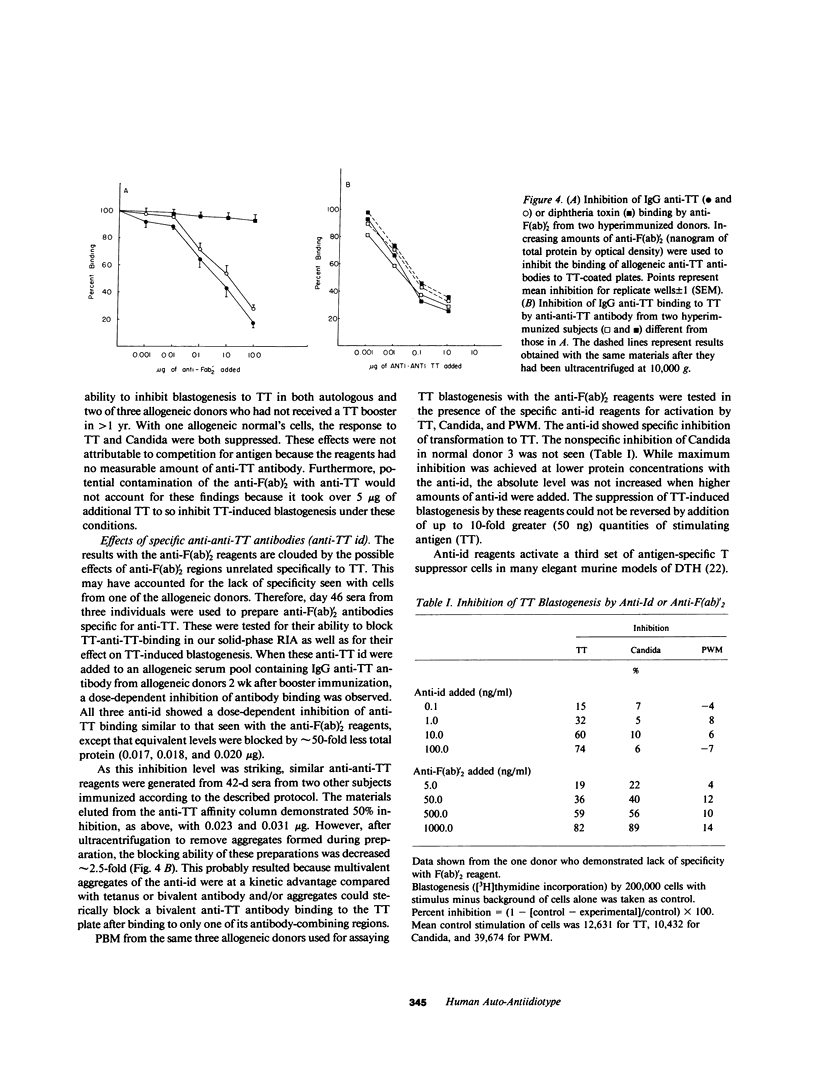
While investigating the effect on B cells of repetitive in vivo immunization with tetanus toxoid (TT), we observed the subsequent development of specific anergy for T cell delayed hypersensitivity (DTH) to TT. This appeared approximately 35 d after a series of five booster immunizations. Concurrently, in vitro T cell blastogenic responses were preserved. Serum obtained when the skin tests were nonreactive demonstrated a profound inhibitory activity on T cell reactivity. This activity was shown to be anti-antibody activity that was both anti-F(ab)'2 and, specifically, anti-TT F(ab)'2. It blocked binding of TT to a pool of allogeneic antibodies and also inhibited allogeneic antigen-specific T cell blastogenesis. Thus, we could identify activity in the serum of hyperimmunized individuals that appeared auto-anti-idiotypic (anti-id) and represented a single or family of major crossreacting idiotypes (id) for TT. The expression of the auto-anti-id correlated with the loss of T cell reactivity in vivo and in vitro. Subsequent examinations revealed persistent, specific cutaneous anergy beyond six months, which was then associated with a failure of T cells to react with antigen in vitro. Mixing experiments with cells from these later times and cryopreserved autologous cells obtained prior to hyperimmunization revealed there had been the development of antigen-specific T suppressor cells. Thus, in vivo DTH tolerance following hyperimmunization was associated with an inhibitory serum activity that appeared to be anti-id. Persistence of tolerance (greater than 6 mo) occurred with the development of T suppressor cells.
Full text
PDF




Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abdou N. I., Wall H., Lindsley H. B., Halsey J. F., Suzuki T. Network theory in autoimmunity. In vitro suppression of serum anti-DNA antibody binding to DNA by anti-idiotypic antibody in systemic lupus erythematosus. J Clin Invest. 1981 May;67(5):1297–1304. doi: 10.1172/JCI110158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benjamin C. D., Miller A., Sercarz E. E., Harvey M. A. A predominant idiotype on anti-hen egg white lysozyme antibodies from diverse mouses strains. J Immunol. 1980 Sep;125(3):1017–1025. [PubMed] [Google Scholar]
- Binz H., Kimura A., Wigzell H. Idiotype-positive T lymphocytes. Scand J Immunol. 1975 Sep;4(5-6):413–420. doi: 10.1111/j.1365-3083.1975.tb02646.x. [DOI] [PubMed] [Google Scholar]
- Blaser K., Nakagawa T., de Weck A. L. Investigation of a syngeneic murine model for the study of IgE antibody regulation with isologous antiidiotypic antibodies. Int Arch Allergy Appl Immunol. 1981;64(1):42–50. doi: 10.1159/000232672. [DOI] [PubMed] [Google Scholar]
- Bona C., Kearney J. Anti-immunoglobulin antibodies. II. Expression of individual and cross-reactive idiotypes on syngeneic and homologous anti-idiotype antibodies. J Immunol. 1981 Aug;127(2):491–495. [PubMed] [Google Scholar]
- Conger J. D., Lewis G. K., Goodman J. W. Idiotype profile of an immune response. I. Contrasts in idiotypic dominance between primary and secondary responses and between IgM and IgG plaque-forming cells. J Exp Med. 1981 May 1;153(5):1173–1186. doi: 10.1084/jem.153.5.1173. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cowdery J. S., Steinberg A. D. Serum antibody-binding antibodies produced during a primary antibody response. J Immunol. 1981 Jun;126(6):2136–2138. [PubMed] [Google Scholar]
- Fairshter R. D., Thornton D. B., Gottschalk H. R., Slater L. M., Galant S. P. In vivo and in vitro cell-mediated immunity to tetanus toxoid in adults. J Allergy Clin Immunol. 1980 Dec;66(6):452–457. doi: 10.1016/0091-6749(80)90005-6. [DOI] [PubMed] [Google Scholar]
- Geha R. S. Presence of auto-anti-idiotypic antibody during the normal human immune response to tetanus toxoid antigen. J Immunol. 1982 Jul;129(1):139–144. [PubMed] [Google Scholar]
- Geha R. S. Presence of circulating anti-idiotype-bearing cells after booster immunization with tetanus toxoid (TT) and inhibition of anti-TT antibody synthesis by auto-anti-idiotypic antibody. J Immunol. 1983 Apr;130(4):1634–1639. [PubMed] [Google Scholar]
- Geha R. S., Weinberg R. P. Anti-idiotypic antisera in man. I. Production and immunochemical characterization of anti-idiotypic antisera to human antitetanus antibodies. J Immunol. 1978 Oct;121(4):1518–1523. [PubMed] [Google Scholar]
- Gleason K., Köhler H. Regulatory idiotypes. T helper cells recognize a shared VH idiotope on phosphorylcholine-specific antibodies. J Exp Med. 1982 Aug 1;156(2):539–549. doi: 10.1084/jem.156.2.539. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Graf M. W., Uhr J. W. Regulation of antibody formation by serum antibody. I. Removal of specific antibody by means of immunoadsorption. J Exp Med. 1969 Nov 1;130(5):1175–1186. doi: 10.1084/jem.130.5.1175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiernaux J. Antiidiotypic networks. Fed Proc. 1981 Apr;40(5):1484–1488. [PubMed] [Google Scholar]
- Hirai Y., Lamoyi E., Dohi Y., Nisonoff A. Regulation of expression of a family of cross-reactive idiotypes. J Immunol. 1981 Jan;126(1):71–74. [PubMed] [Google Scholar]
- Infante A. J., Infante P. D., Gillis S., Fathman C. G. Definition of T cell idiotypes using anti-idiotypic antisera produced by immunization with T cell clones. J Exp Med. 1982 Apr 1;155(4):1100–1107. doi: 10.1084/jem.155.4.1100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ju S. T., Pierres M., Germain R., Benacerraf B., Dorf M. E. Idiotypic analysis of anti-GAT antibodies. VIII. Comparison of interstrain and allotype-associated idiotypic specificities. J Immunol. 1981 Jan;126(1):177–182. [PubMed] [Google Scholar]
- Kennedy R. C., Dreesman G. R. Common idiotypic determinant associated with human antibodies to hepatitis B surface antigen. J Immunol. 1983 Jan;130(1):385–389. [PubMed] [Google Scholar]
- Klaus G. G. Antigen-antibody complexes elicit anti-idiotypic antibodies to self-idiotopes. Nature. 1978 Mar 16;272(5650):265–266. doi: 10.1038/272265a0. [DOI] [PubMed] [Google Scholar]
- Metzger D. W., Furman A., Miller A., Sercarz E. E. Idiotypic repertoire of anti-hen eggwhite lysozyme antibodies probed with hybridomas. Selection after immunization of an IdX marker common to antibodies of distinct epitope specificity. J Exp Med. 1981 Sep 1;154(3):701–712. doi: 10.1084/jem.154.3.701. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W. Antigen receptors on murine T lymphocytes in contact sensitivity. I. Functional inhibition of effector T cells by monovalent 2,4-dinitrophenol: implication for a two-receptor model. J Exp Med. 1981 Dec 1;154(6):1811–1826. doi: 10.1084/jem.154.6.1811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moorhead J. W., Sy M. S. Antigen receptors on murine T lymphocytes in contact sensitivity. II. Presentation and characterization of syngeneic anti-idiotype serum against DNFB-sensitized T cells. J Immunol. 1982 Jun;128(6):2533–2538. [PubMed] [Google Scholar]
- NISONOFF A., WISSLER F. C., LIPMAN L. N., WOERNLEY D. L. Separation of univalent fragments from the bivalent rabbit antibody molecule by reduction of disulfide bonds. Arch Biochem Biophys. 1960 Aug;89:230–244. doi: 10.1016/0003-9861(60)90049-7. [DOI] [PubMed] [Google Scholar]
- Nasu H., Chia D. S., Knutson D. W., Barnett E. V. Naturally occurring human antibodies to the F(ab')2 portion of IgG. Clin Exp Immunol. 1980 Nov;42(2):378–386. [PMC free article] [PubMed] [Google Scholar]
- Okuda K., Minami M., Furusawa M., Dorf M. E. Analysis of T cell hybridomas. II. Comparisons among three distinct types of monoclonal suppressor factors. J Exp Med. 1981 Dec 1;154(6):1838–1851. doi: 10.1084/jem.154.6.1838. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rocklin R. E., Sheffer A. L., Greineder D. K., Melmon K. L. Generation of antigen-specific suppressor cells during allergy desensitization. N Engl J Med. 1980 May 29;302(22):1213–1219. doi: 10.1056/NEJM198005293022201. [DOI] [PubMed] [Google Scholar]
- Rowley D. A., Griffith P., Lorbach I. Regulation by complementary idiotypes. Ig protects the clone producing it. J Exp Med. 1981 Jun 1;153(6):1377–1390. doi: 10.1084/jem.153.6.1377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Saxon A., Feldhaus J., Robins R. A. Single step separation of human T and B cells using AET treated srbc rosettes. J Immunol Methods. 1976;12(3-4):285–288. doi: 10.1016/0022-1759(76)90050-8. [DOI] [PubMed] [Google Scholar]
- Schrater A. F., Goidl E. A., Thorbecke G. J., Siskind G. W. Production of auto-anti-idiotypic antibody during the normal immune response to TNP-ficoll. I. Occurrence in AKR/J and BALB/c mice of hapten-augmentable, anti-TNP plaque-forming cells and their accelerated appearance in recipients of immune spleen cells. J Exp Med. 1979 Jul 1;150(1):138–153. doi: 10.1084/jem.150.1.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuler W., Weiler E., Weiler I. J. Biological and serological comparison of syngeneic and allogeneic anti-idiotypic antibodies. Mol Immunol. 1981 Dec;18(12):1095–1105. doi: 10.1016/0161-5890(81)90025-0. [DOI] [PubMed] [Google Scholar]
- Scott M. G., Fleischman J. B. Preferential idiotype-isotype associations in antibodies to dinitrophenyl antigens. J Immunol. 1982 Jun;128(6):2622–2628. [PubMed] [Google Scholar]
- Stevens R. H., Saxon A. Reduced in vitro production of anti-tetanus toxoid antibody after repeated in vivo immunization with tetanus toxoid. J Immunol. 1979 Feb;122(2):592–598. [PubMed] [Google Scholar]
- Sunday M. E., Benacerraf B., Dorf M. E. Hapten-specific T cell responses to 4-hydroxy-3-nitrophenyl acetyl. VIII. Suppressor cell pathways in cutaneous sensitivity responses. J Exp Med. 1981 Apr 1;153(4):811–822. doi: 10.1084/jem.153.4.811. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Dietz M. H., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. IV. Idiotype-bearing I-J+ suppressor T cell factors induce second-order suppressor T cells which express anti-idiotypic receptors. J Exp Med. 1980 May 1;151(5):1183–1195. doi: 10.1084/jem.151.5.1183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sy M. S., Nisonoff A., Germain R. N., Benacerraf B., Greene M. I. Antigen- and receptor-driven regulatory mechanisms. VIII. Suppression of idiotype-negative, p-azobenzenearsonate-specific T cells results from the interaction of an anti-idiotypic second-order T suppressor cell with a cross-reactive-idiotype-positive, p-azobenzenearsonate-primed T cell target. J Exp Med. 1981 Jun 1;153(6):1415–1425. doi: 10.1084/jem.153.6.1415. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takaoki M., Sy M. S., Whitaker B., Nepom J., Finberg R., Germain R. N., Nisonoff A., Benacerraf B., Greene M. I. Biologic activity of an idiotype-bearing suppressor T cell factor produced by a long-term T cell hybridoma. J Immunol. 1982 Jan;128(1):49–53. [PubMed] [Google Scholar]
- Wuilmart C., Wikler M., Urbain J. Induction of autoanti-idiotypic antibodies and effects on the subsequent immune response. Mol Immunol. 1979 Dec;16(12):1085–1092. doi: 10.1016/0161-5890(79)90042-7. [DOI] [PubMed] [Google Scholar]
- Yamagata J., Barnett E. V., Knutson D. W., Nasu H., Chia D. Characterization and measurement of anti-IgG antibodies in human sera by radioimmunoassay (RIA). J Immunol Methods. 1979;29(1):43–56. doi: 10.1016/0022-1759(79)90124-8. [DOI] [PubMed] [Google Scholar]
- Zanetti M., Bigazzi P. E. Anti-idiotypic immunity and autoimmunity. I. In vitro and in vivo effects of anti-idiotypic antibodies to spontaneously occurring autoantibodies to rat thyroglobulin. Eur J Immunol. 1981 Mar;11(3):187–195. doi: 10.1002/eji.1830110306. [DOI] [PubMed] [Google Scholar]




