Abstract
The afferent arteriole (Af-Art) controls glomerular capillary pressure, an important determinant of glomerular injury. Af-Art myogenic response is mediated by ATP, and ATP signaling is in turn mediated by 20-HETE. Dahl salt-sensitive rats (Dahl SS) have decreased renal 20-HETE production. We hypothesized that Dahl SS have an impaired myogenic response and constrictor response to ATP, due to decreased 20-HETE. Af-Arts from Dahl SS or Dahl salt-resistant rats (Dahl SR) were microdissected and perfused. When myogenic response was induced by increasing Af-Art perfusion pressure from 60 to 140 mmHg, luminal Af-Art diameter decreased in Dahl SR but not in Dahl SS (−3.1 ± 0.8 vs. 0.5 ± 0.8 μm, P < 0.01). The 20-HETE antagonist 20-HEDE (10−6 M) blocked the myogenic response in Dahl SR but had no effect in Dahl SS. Addition of a subconstrictor concentration of 20-HETE (but not a subconstrictor concentration of norepinephrine) restored the myogenic response in Dahl SS. We then perfused Af-Arts at 60 mmHg and tested the effects of the ATP analog α,β-methylene-ATP (10−6 M). Maximum ATP-induced constriction was attenuated in Dahl SS compared with Dahl SR (1.5 ± 0.5 vs. 7.4 ± 0.8 μm, P < 0.001). 20-HEDE attenuated ATP-induced Af-Art constriction in Dahl SR but not in Dahl SS, and consequently, ATP-induced constriction was no longer different between strains. In conclusion, Dahl SS have an impaired myogenic response and ATP-induced Af-Art constriction due to a decrease in Af-Art 20-HETE. The impaired myogenic responses may contribute to the nephrosclerosis that develops in Dahl SS.
Keywords: myogenic response, salt-sensitive hypertension, afferent arteriole, 20-HETE, ATP
renal blood flow is highly regulated, with at least three intrinsic renal mechanisms contributing to its fine control: myogenic response, tubuloglomerular feedback (TGF), and connecting tubule glomerular feedback (CTGF) (5, 13, 27). In the myogenic response, the afferent arteriole (Af-Art) constricts in response to increases in perfusion pressure. Because of its very short activation delay, the myogenic response is particularly well-suited for isolating glomerular capillaries from rapidly changing renal perfusion pressure (21). Increased glomerular capillary pressure leads to endothelial, mesangial, and podocyte injury, which ultimately results in glomerulosclerosis (2). Myogenic response is an important component of the overall renal autoregulation (17). Therefore, the myogenic response plays an important role in preventing excessive glomerular capillary pressure, and consequent glomerular barotrauma.
Salt-sensitive hypertension, defined as an increase in blood pressure (BP) in response to excess sodium intake, is common in hypertensive patients, particularly in the elderly, diabetics, African-Americans, and obese patients (7). Salt sensitivity is associated with a higher risk of developing glomerulosclerosis and renal failure (19), possibly due to higher glomerular capillary pressures. In a comparison between salt-resistant and salt-sensitive patients, calculated intraglomerular pressure fell in the former but increased in the latter in response to high-NaCl intake (6).
Although the myogenic response has been studied extensively in normotensive animals, less is known of how the myogenic response is affected in hypertensive models. We recently found that spontaneously hypertensive rats (SHR) have an enhanced myogenic response (25), a fact that may help explain the remarkably little glomerular injury seen in this hypertensive strain. On the other hand, it has been proposed that in Dahl salt-sensitive (Dahl SS) rats a defect in myogenic autoregulation can lead to glomerular barotrauma, and it explains their susceptibility to hypertensive nephropathy (23).
In normotensive animals, the myogenic response is mediated by extracellular ATP acting on P2X receptors (14). And the effect of P2X receptor activation is in turn mediated by 20-HETE (13). Furthermore, inhibitors of 20-HETE formation and action blunt the effect of and the myogenic response in vitro and renal autoregulation in vivo (18, 36), suggesting that 20-HETE may be a second messenger of the myogenic response downstream from ATP.
The role of 20-HETE in the impairment of Af-Art myogenic response in Dahl SS rats is unknown, but Dahl SS rats are known to have a decreased 20-HETE production in renal microvessels (32). Thus, we hypothesize that a decrease in 20-HETE in Dahl SS rats causes an impairment in myogenic response and ATP-induced Af-Art constriction.
METHODS
We used methods similar to those previously described to isolate the Af-Art with glomerulus intact (16, 26). Young male Dahl SS and age-matched salt-resistant (Dahl SR) and Sprague-Dawley (SD) rats (Harlan Laboratories, Indianapolis, IN) were used (body wt 100–150 g). Both kidneys were removed under ketamine/xylazine anesthesia and sliced longitudinally along the corticomedullary axis. This protocol was approved by the Henry Ford Hospital Institutional Animal Care and Use Committee and adhered to APS's Guiding Principles in the Care and Use of Animals, including the provision to comply with the National Institutes of Health Guide for the Care and Use of Laboratory Animals.
Microdissection and Microperfusion of Af-Arts
The kidney slices were placed in ice-cold minimum essential medium (MEM; GIBCO) containing 5% BSA (Intergen), and a single superficial Af-Art with glomerulus intact was microdissected under a stereomicroscope. With the use of a micropipette, the arteriole was transferred to a temperature-controlled chamber mounted on an inverted microscope (IMT-2; Olympus) with Hoffmann modulation and cannulated with an array of glass pipettes. Af-Arts were perfused from the proximal end in an orthograde direction. Intraluminal pressure was measured by Landis' technique using a fine pipette introduced into the arteriole through the perfusion pipette (20). Microdissection and cannulation were completed within 90 min at 2–8°C, after which the bath was gradually warmed to 37°C for the rest of the experiment. Once temperature was stable, a 30-min equilibration period was allowed before any measurements were taken. Images of the Af-Art were displayed at magnifications up to ×1,980, recorded at 5-s intervals with a video camera, and measured with a computer equipped with Metavue image analysis system (MDS Analytical Technologies, Toronto, Canada). For the purpose of standardizing our measurements, each data point resulted from averaging three individual measurements. One measurement was taken at the site of maximal constriction, one 5 μm upstream from it, and one 5 μm downstream from it. Maximal constriction usually occurred in the proximal or mid parts of the Af-Art. To examine the myogenic response in Af-Arts, we monitored their luminal diameter for 5 min after raising intraluminal pressure in 20-mmHg increments from 60 to 140 mmHg. Then, pressure was returned to 60 mmHg, and a second curve was constructed with the addition of various pharmacological agents in the bath and lumen of the Af-Art. This is based on our previous experience showing that the myogenic response is stable and reproducible (25).
Experimental Protocols
Response of the Af-Art to increased intraluminal pressure.
Myogenic response experiments were conducted as described above. Here, we compared the myogenic response of Dahl SS and Dahl SR rats.
Effect of 20-HEDE on the myogenic response.
Using Dahl SS, Dahl SR, and SD rats, we constructed two consecutive myogenic response curves, the second in the presence of 20-HEDE (a 20-HETE antagonist; 10−6 M).
Effect of a subconstrictor concentration of exogenous 20-HETE or norepinephrine on the myogenic response in Dahl SS rats.
We evaluated whether a subconstrictor concentration of exogenous 20-HETE (5 × 10−8 M) could restore the myogenic response in Dahl SS. As a control, we repeated this experiment using a subconstrictor concentration of norepinephrine (NE; 5 × 10−8 M, which is the highest concentration of NE that did not induce Af-Art constriction in our previous experiments).
Effect of exogenous ATP on Af-Art constriction.
We induced Af-Art constriction by using the ectonucleotidase-resistant ATP analog α,β-methylene-ATP (10−6 M) in Dahl SS and Dahl SR rats.
Effect of 20-HEDE on ATP-induced Af-Art constriction in Dahl SR and Dahl SS rats.
We induced Af-Art constriction with α,β-methylene-ATP as above, two consecutive times, the second in the presence 20-HEDE.
Statistics
Baseline Af-Art diameters were not significantly different between Dahl SS and Dahl SR. Experiments comparing curves obtained from different groups of animals were analyzed using repeated-measures ANOVA, followed by pairwise comparisons using the pooled variance from the ANOVA. Experiments comparing two curves obtained sequentially in the same preparation (i.e., control and treated) were analyzed using a paired t-test. Data are expressed as means ± SE. When multiple comparisons were performed, Hochberg's adjustment was used to determine significance.
RESULTS
Response of the Af-Art to Increased Intraluminal Pressure
As expected, Dahl SR Af-Arts displayed myogenic response by constricting in response to increasing perfusion pressure. However, Dahl SS Af-Arts remained essentially unchanged; i.e., they were neither distended by increasing intraluminal pressure, nor did they exhibit any measurable constriction (Fig. 1). Thus, Dahl SS displayed impaired myogenic response compared with Dahl SR.
Fig. 1.
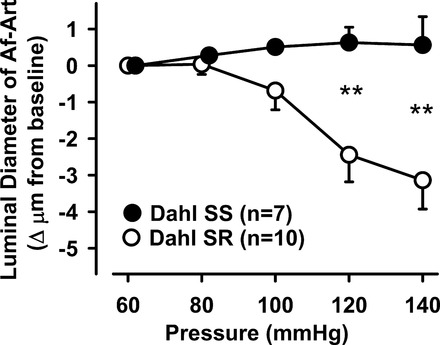
Afferent arteriole (Af-Art) response to a stepwise increase in perfusion pressure in Dahl salt-sensitive (SS) and Dahl salt-resistant (SR) rats. When Af-Art pressure was increased from 60 to 140 mmHg, diameter decreased only in Dahl SR but not in Dahl SS rats (**P < 0.01 Dahl SS vs. Dahl SR).
Effect of 20-HEDE on the Myogenic Response
To test whether 20-HETE contributes to myogenic response, and whether its contribution is different in Dahl SS and Dahl SR, we used the 20-HETE antagonist 20-HEDE. As an additional control, we also studied SD Af-Arts. 20-HEDE blocked the myogenic response in both Dahl SR (Fig. 2A) and SD rats (Fig. 2B). In contrast, adding 20-HEDE to Dahl SS Af-Arts had no effect on luminal diameter (Fig. 2C). These data suggest that a decrease in 20-HETE may explain the impairment seen in myogenic response in Dahl SS.
Fig. 2.
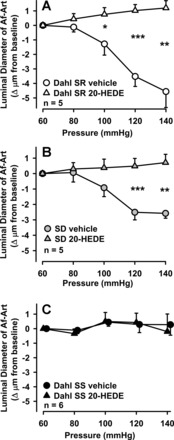
A: effect of 20-HEDE on the myogenic response in Dahl SR Af-Arts. 20-HEDE significantly blocked pressure-induced constriction (*P < 0.05, **P < 0.01, ***P < 0.001 control vs. 20-HEDE). B: effect of 20-HEDE on the myogenic response in Sprague-Dawley (SD) rat. 20-HEDE significantly blocked pressure-induced constriction (**P < 0.01, ***P < 0.001 control vs. 20-HEDE). C: effect of 20-HEDE on the myogenic response in Dahl SS Af-Arts. 20-HEDE has no effect on Af-Art diameter.
Effect of a Subconstrictor Concentration of Exogenous 20-HETE or NE on the Myogenic Response in Dahl SS Rats
To confirm that a decrease in 20-HETE participates in the attenuation of myogenic response seen in Dahl SS, we added a subconstrictor concentration of exogenous 20-HETE. As summarized in Fig. 3A, 20-HETE restored the myogenic response. As a control, we repeated this experiment but adding NE instead of 20-HETE, at the highest concentration that does not cause constriction, based on previous experiments. As shown in Fig. 3B, a subconstrictor concentration of NE did not affect the myogenic response. These data support the hypothesis that the attenuation of myogenic response seen in Dahl SS rats is due to lack of 20-HETE, rather than a nonspecific defect in vascular smooth muscle contraction.
Fig. 3.
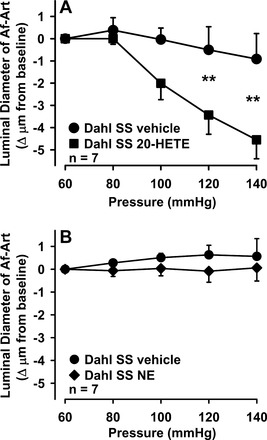
A: effect of a subconstrictor concentration of exogenous 20-HETE on the myogenic response in Dahl SS rats. Addition of 20-HETE enhanced pressure-induced constriction (**P < 0.01 control vs. 20-HETE). B: effect of a subconstrictor concentration of exogenous norepinephrine (NE) on the myogenic response in Dahl SS rats. Unlike 20-HETE, addition of NE has no effect on pressure-induced constriction.
Effect of Exogenous ATP on Af-Art Constriction
Because Af-Art autoregulation has been shown to be mediated by ATP and 20-HETE, with 20-HETE being downstream from ATP (34, 35), we tested whether the response to exogenous ATP was different in Dahl SS and Dahl SR, using the phosphodiesterase-resistant, P2X-selective ATP analog α,β-methylene-ATP. As summarized in Fig. 4, α,β-methylene-ATP 10−6 M decreased Af-Art diameter from 14.6 ± 0.9 to 7.2 ± 0.8 μm in Dahl SR (n = 6), but only from 14.5 ± 0.8 to 13.0 ± 0.6 μm in Dahl SS (n = 8, P < 0.001 Dahl SS vs. Dahl SR), suggesting an impairment in ATP-induced constriction in Dahl SS.
Fig. 4.
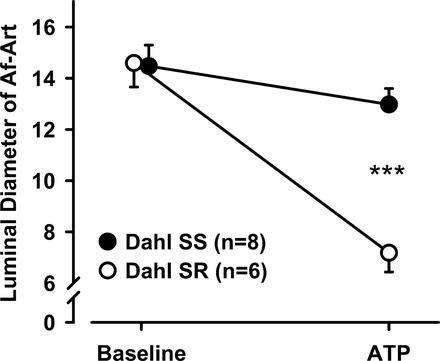
Effect of exogenous ATP on Af-Art constriction. Addition of 10−6 M ATP constricted Af-Arts more in Dahl SR than in Dahl SS rats (***P < 0.001 Dahl SS vs. Dahl SR).
Effect of 20-HEDE on ATP-Induced Af-Art Constriction in Dahl SR and Dahl SS Rats
We then studied whether 20-HETE also participated in ATP-induced constriction. We induced Af-Art constriction two consecutive times, the second in the presence of the 20-HETE antagonist 20-HEDE. As summarized in Fig. 5A, α,β-methylene-ATP decreased Af-Art diameter in Dahl SR from 14.6 ± 0.9 to 7.2 ± 0.8 μm during the control period, whereas after the addition of 20-HEDE (10−6 M), Af-Art diameter decreased only from 13.7 ± 0.9 to 12.1 ± 1.0 μm (n = 6, P < 0.01). As a control, we showed that in the presence of vehicle, ATP-induced constriction was stable and reproducible (first period 16.3 ± 0.6 to 6.8 ± 0.5 μm, second period 16.6 ± 0.7 to 6.2 ± 0.4 μm, Fig. 5B). Next, we repeated these experiments in Dahl SS. α,β-methylene-ATP decreased Af-Art diameter only from 15.1 ± 0.9 to 13.3 ± 0.8 μm in Dahl SS during the control period. This small decrease in Af-Art induced by ATP in Dahl SS was not affected by addition of 20-HEDE (from 14.8 ± 1 to 13.2 ± 0.8 μm, Fig. 5C). In a control experiment, ATP-induced constriction was also reproducible in Dahl SS (first period 14.2 ± 0.8 to 12.3 ± 1.0 μm, second period 14.3 ± 0.9 to 12.3 ± 1.0 μm, Fig. 5D). Similarly to our findings with the myogenic response, these data suggest that under normal conditions ATP-induced constriction also requires 20-HETE, but in Dahl SS rats there is impairment in ATP-induced constriction due to a lack of 20-HETE.
Fig. 5.
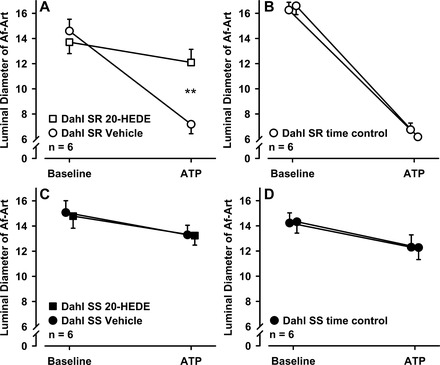
A: effect of 20-HEDE on ATP-induced Af-Art constriction in Dahl SR rats. 20-HEDE significantly blocked ATP-induced Af-Art constriction (**P < 0.01 control vs. 20-HEDE). B: time control of ATP-induced Af-Art constriction in Dahl SR rats. C: effect of 20-HEDE on ATP-induced Af-Art constriction in Dahl SS rats. 20-HEDE has no effect on ATP-induced Af-Art constriction. D: time control of ATP-induced Af-Art constriction in Dahl SS rats.
DISCUSSION
Current knowledge of the mechanisms underlying the myogenic response comes mainly from two separate sets of studies. First, a link between pressure-dependent autoregulation and purinergic receptor activation had been found based on studies performed in the in vitro blood-perfused juxtamedullary nephron technique (14), and we confirmed this is the case in our isolated afferent arteriole preparation. Second, based on other vascular bed studies, it had been suggested that 20-HETE may be a downstream mediator of ATP in Af-Arts (13), and Zhao et al. showed that 20-HETE mediates the calcium response (34) and constriction (35) of renal microvascular cells to P2X receptor activation. Thus, it is reasonable to propose that increases in transmural pressure cause myogenic constriction of Af-Arts by acting through P2X receptors and 20-HETE. Here, we report that indeed this is the case, as our present studies connect the pathway from transmural pressure, to ATP, to 20-HETE. This is consistent with the recent demonstration by Ge et al. (11) that the myogenic response can be blocked by inhibiting 20-HETE. Our studies are also in keeping with previous reports in which 20-HETE appears to be a common pathway for other stimuli that constrict the Af-Art, including endothelin-1 (12), and ANG II (1). Our data indicate that 20-HETE participates in the myogenic response and that a decrease in 20-HETE underlies the impairment seen in myogenic response and ATP-induced constriction in Dahl SS rats. Of note, since a single subconstrictor concentration of 20-HETE was able to fully restore the myogenic response in Dahl SS rats, our studies do not prove a mediator function for 20-HETE, but rather suggest a permissive function to enable Af-Art constriction. Furthermore, a permissive role of 20-HETE in Af-Art constriction is more consistent with the literature showing a participation of 20-HETE in a wide variety of constrictor stimuli [pressure in our experiments, ANG II and endothelin-1 in previous studies (1, 12)]. It is also consistent with Yousif et al. (33), who considered the possibility that 20-HETE acted as a permissive factor in the constrictor response of the mesenteric arteries to NE, endothelin-1, and ANG II.
Although the P2X receptor is in itself a cation channel, it is also possible that 20-HETE contributes to additional calcium entry in response to extracellular ATP. In this regard, Zhao et al. (35) showed that the intracellular increase in calcium concentration seen in vascular smooth muscle cells in response to ATP could be partially attenuated by the 20-HETE antagonist 20-HEDE.
Early experiments by Azar et al. (3) showed that Dahl SS rats have a decreased Af-Art resistance, leading to an increased glomerular capillary pressure. Takenaka et al. (29) expanded on those findings by showing that the myogenic response was impaired in Dahl SS compared with Dahl SR, and this finding held true irrespective of whether the rats were fed a normal- or high-sodium diet. Our studies confirmed those findings, using a different technique (isolated perfused Af-Arts in our experiments vs. isolated perfused hydronephrotic rat kidneys in the experiments of Takenaka et al.) and further delved into the mechanisms underlying this finding. As mentioned above, 20-HETE participates in the myogenic response. Our data indicate that a decrease in 20-HETE underlies the impairment seen in myogenic response and ATP-induced Af-Art constriction in Dahl SS rats. This is in keeping with the recent demonstration by Williams et al. (32) that Dahl SS have decreased renal cortical CYP4A expression and 20-HETE generation compared with a consomic control in which chromosome 5 from the Brown Norway rat has been introgressed.
Our results differ somewhat from those of Frisbee et al. (10). Unlike our results with the Af-Art, they did not find an impairment of the myogenic response in skeletal muscle arteries. Since the myogenic response mechanisms are tissue-specific (9), this is likely due to differences between the vascular beds.
It has been described that even when fed normal-salt diets, older Dahl SS rats have a higher than normal BP, which could potentially affect our results. However, because of technical limitations on dissection of older animals, we limited our study to rats in the 80- to 100-g range. Using these animals, we found no difference between Dahl SS and Dahl SR in BP, as measured by tail cuff (range 90–120 mmHg).
In addition to the Dahl SS rat, other rat strains with impaired myogenic response, such as the Fawn-Hooded rat, present severe renal damage (30). Churchill et al. (8) showed that kidneys from the Brown Norway (BN) rat, a normotensive strain with impaired myogenic response, are very sensitive to renal damage when exposed to increased BP levels. On the other hand, as we previously showed, SHR have enhanced myogenic response (25), which may act to preserve renal function in the face of increased systolic BP (22). In fact, SHR can be rendered susceptible to renal damage by either unilateral nephrectomy (24), or congenic transfer of chromosome 1 from the BN rat (SHR.BN1-D1Mit3/Igf2) (28), i.e., two approaches expected to impair the myogenic response (4, 8). Thus, an impaired myogenic response appears to be a permissive factor for hypertensive glomerulosclerosis to develop.
Studies of whole kidney autoregulation demonstrate a link between reduced autoregulatory capacity and hypertensive injury (22). However, the specific contributions of impairments in myogenic vs. nephron mechanisms [such as TGF and CTGF] are not fully known, due to inherent difficulties of whole kidney models to assess the individual contributions of these interacting systems. Our experiments in isolated Af-Arts directly point to an impairment in Dahl SS myogenic response. Nevertheless, our studies do not exclude a role of nonmyogenic (nephron) mechanisms contributing to an impaired Af-Art autoregulation in the Dahl SS rats. Indeed, we recently showed that Dahl SS have lower TGF and greater CTGF than Dahl SR and that CTGF antagonizes TGF (31). Taken together, these findings may explain the increase in vasodilatation, glomerular capillary pressure, and glomerular damage in SS hypertension.
Put in perspective, our studies suggest that strategies that increase 20-HETE could be used to restore the myogenic response. For example, since peroxisome proliferator-activated receptor α (PPARα) is known to be expressed in the Af-Art (15), and its activation induces CYP4A expression and 20-HETE production, it is possible that PPARα agonists such as the fibric acid derivatives clofibrate and fenofibrate, commonly used in humans as hypolipidemic drugs, could be used in the future to restore the myogenic response in patients with salt-sensitive hypertension.
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: Y.R. performed experiments; Y.R., M.A.D., J.L.G., and O.A.C. interpreted results of experiments; Y.R. and M.A.D. drafted manuscript; Y.R., M.A.D., J.L.G., E.L.P., and O.A.C. approved final version of manuscript; M.A.D., J.L.G., and O.A.C. conception and design of research; M.A.D. and E.L.P. analyzed data; M.A.D. prepared figures; M.A.D., J.L.G., and O.A.C. edited and revised manuscript.
REFERENCES
- 1.Alonso-Galicia M, Maier KG, Greene AS, Cowley AW, Jr, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in the renal and vasoconstrictor actions of angiotensin II. Am J Physiol Regul Integr Comp Physiol 283: R60–R68, 2002. [DOI] [PubMed] [Google Scholar]
- 2.Arima S, Ito S. Role of renal eicosanoids in the control of intraglomerular and systemic blood pressure during development of hypertension. Contrib Nephrol 143: 65–76, 2004. [DOI] [PubMed] [Google Scholar]
- 3.Azar S, Limas C, Iwai J, Weller D. Single nephron dynamics during high sodium intake and early hypertension in Dahl rats. Jpn Heart J 20: 138–140, 1979. [Google Scholar]
- 4.Bidani AK, Hacioglu R, bu-Amarah I, Williamson GA, Loutzenhiser R, Griffin KA. “Step” vs. “dynamic” autoregulation: implications for susceptibility to hypertensive injury. Am J Physiol Renal Physiol 285: F113–F120, 2003. [DOI] [PubMed] [Google Scholar]
- 5.Briggs JP, Schnermann J. The tubuloglomerular feedback mechanism: functional and biochemical aspects. Annu Rev Physiol 49: 251–273, 1987. [DOI] [PubMed] [Google Scholar]
- 6.Campese VM. Salt sensitivity in hypertension. Renal and cardiovascular implications. Hypertension 23: 531–550, 1994. [DOI] [PubMed] [Google Scholar]
- 7.Chobanian AV, Hill M. National Heart, Lung, and Blood Institute Workshop on Sodium and Blood Pressure: a critical review of current scientific evidence. Hypertension 35: 858–863, 2000. [DOI] [PubMed] [Google Scholar]
- 8.Churchill PC, Churchill MC, Bidani AK, Griffin KA, Picken M, Pravenec M, Kren V, St. Lezin E, Wang JM, Wang N, Kurtz TW. Genetic susceptibility to hypertension-induced renal damage in the rat. Evidence based on kidney-specific genome transfer. J Clin Invest 100: 1373–1382, 1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Davis MJ, Hill MA. Signaling mechanisms underlying the vascular myogenic response. Physiol Rev 79: 387–423, 1999. [DOI] [PubMed] [Google Scholar]
- 10.Frisbee JC, Roman RJ, Krishna UM, Falck JR, Lombard JH. 20-HETE modulates myogenic response of skeletal muscle resistance arteries from hypertensive Dahl-SS rats. Am J Physiol Heart Circ Physiol 280: H1066–H1074, 2001. [DOI] [PubMed] [Google Scholar]
- 11.Ge Y, Murphy SR, Lu Y, Falck J, Liu R, Roman RJ. Endogenously produced 20-HETE modulates myogenic and TGF response in microperfused afferent arterioles. Prostaglandins Other Lipid Mediat 102–103: 42–48, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Imig JD, Pham BT, LeBlanc EA, Reddy KM, Falck JR, Inscho EW. Cytochrome P450 and cyclooxygenase metabolites contribute to the endothelin-1 afferent arteriolar vasoconstrictor and calcium responses. Hypertension 35: 307–312, 2000. [DOI] [PubMed] [Google Scholar]
- 13.Inscho EW. Lewis K. Dahl Memorial Lecture. Mysteries of renal autoregulation. Hypertension 53: 299–306, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Inscho EW, Cook AK, Imig JD, Vial C, Evans RJ. Physiological role for P2X1 receptors in renal microvascular autoregulatory behavior. J Clin Invest 112: 1895–1905, 2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ishizuka T, Ito O, Tan L, Ogawa S, Kohzuki M, Omata K, Takeuchi K, Ito S. Regulation of cytochrome P-450 4A activity by peroxisome proliferator-activated receptors in the rat kidney. Hypertens Res 26: 929–936, 2003. [DOI] [PubMed] [Google Scholar]
- 16.Ito S, Arima S, Juncos LA, Carretero OA. Endothelium-derived relaxing factor/nitric oxide modulates angiotensin II action in the isolated microperfused rabbit afferent but not efferent arteriole. J Clin Invest 91: 2012–2019, 1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Just A. Mechanisms of renal blood flow autoregulation: dynamics and contributions. Am J Physiol Regul Integr Comp Physiol 292: R1–R17, 2007. [DOI] [PubMed] [Google Scholar]
- 18.Kauser K, Clark JE, Masters BS, Ortiz de Montellano PR, Ma YH, Harder DR, Roman RJ. Inhibitors of cytochrome P-450 attenuate the myogenic response of dog renal arcuate arteries. Circ Res 68: 1154–1163, 1991. [DOI] [PubMed] [Google Scholar]
- 19.Kimura G, Brenner BM. A method for distinguishing salt-sensitive from non-salt-sensitive forms of human and experimental hypertension. Curr Opin Nephrol Hypertens 2: 341–349, 1993. [PubMed] [Google Scholar]
- 20.Landis EM. The capillary pressure in frog mesentery as determined by micro-injection methods. Am J Physiol 75: 548–570, 1926. [Google Scholar]
- 21.Loutzenhiser R, Bidani AK, Wang X. Systolic pressure and the myogenic response of the renal afferent arteriole. Acta Physiol Scand 181: 407–413, 2004. [DOI] [PubMed] [Google Scholar]
- 22.Loutzenhiser R, Griffin K, Williamson G, Bidani A. Renal autoregulation: new perspectives regarding the protective and regulatory roles of the underlying mechanisms. Am J Physiol Regul Integr Comp Physiol 290: R1153–R1167, 2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Raij L. End-organ susceptibility as a determinant of renal disease in hypertension. Kidney Int 64: 1923–1932, 2003. [DOI] [PubMed] [Google Scholar]
- 24.Raij L, Azar S, Keane WF. Role of hypertension in progressive glomerular immune injury. Hypertension 7: 398–404, 1985. [PubMed] [Google Scholar]
- 25.Ren Y, D'Ambrosio MA, Liu R, Pagano PJ, Garvin JL, Carretero OA. Enhanced myogenic response in the afferent arteriole of spontaneously hypertensive rats. Am J Physiol Heart Circ Physiol 298: H1769–H1775, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ren Y, Garvin JL, Carretero OA. Vasodilator action of angiotensin-(1–7) on isolated rabbit afferent arterioles. Hypertension 39: 799–802, 2002. [DOI] [PubMed] [Google Scholar]
- 27.Ren Y, Garvin JL, Liu R, Carretero OA. Crosstalk between the connecting tubule and the afferent arteriole regulates renal microcirculation. Kidney Int 71: 1116–1121, 2007. [DOI] [PubMed] [Google Scholar]
- 28.St Lezin E, Griffin KA, Picken M, Churchill MC, Churchill PC, Kurtz TW, Liu W, Wang N, Kren V, Zidek V, Pravenec M, Bidani AK. Genetic isolation of a chromosome 1 region affecting susceptibility to hypertension-induced renal damage in the spontaneously hypertensive rat. Hypertension 34: 187–191, 1999. [DOI] [PubMed] [Google Scholar]
- 29.Takenaka T, Forster H, De Micheli A, Epstein M. Impaired myogenic responsiveness of renal microvessels in Dahl salt-sensitive rats. Circ Res 71: 471–480, 1992. [DOI] [PubMed] [Google Scholar]
- 30.van Dokkum RP, Sun CW, Provoost AP, Jacob HJ, Roman RJ. Altered renal hemodynamics and impaired myogenic responses in the fawn-hooded rat. Am J Physiol Regul Integr Comp Physiol 276: R855–R863, 1999. [DOI] [PubMed] [Google Scholar]
- 31.Wang H, D'Ambrosio MA, Garvin JL, Ren Y, Carretero OA. Connecting tubule glomerular feedback in hypertension. Hypertension 62: 738–745, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Williams JM, Fan F, Murphy S, Schreck C, Lazar J, Jacob HJ, Roman RJ. Role of 20-HETE in the antihypertensive effect of transfer of chromosome 5 from Brown Norway to Dahl salt-sensitive rats. Am J Physiol Regul Integr Comp Physiol 302: R1209–R1218, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yousif MH, Benter IF, Dunn KM, hly-Vernon AJ, Akhtar S, Roman RJ. Role of 20-hydroxyeicosatetraenoic acid in altering vascular reactivity in diabetes. Auton Autacoid Pharmacol 29: 1–12, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Zhao X, Falck JR, Gopal VR, Inscho EW, Imig JD. P2X receptor-stimulated calcium responses in preglomerular vascular smooth muscle cells involves 20-hydroxyeicosatetraenoic acid. J Pharmacol Exp Ther 311: 1211–1217, 2004. [DOI] [PubMed] [Google Scholar]
- 35.Zhao X, Inscho EW, Bondlela M, Falck JR, Imig JD. The CYP450 hydroxylase pathway contributes to P2X receptor-mediated afferent arteriolar vasoconstriction. Am J Physiol Heart Circ Physiol 281: H2089–H2096, 2001. [DOI] [PubMed] [Google Scholar]
- 36.Zou AP, Imig JD, Kaldunski M, Ortiz de Montellano PR, Sui Z, Roman RJ. Inhibition of renal vascular 20-HETE production impairs autoregulation of renal blood flow. Am J Physiol Renal Fluid Electrolyte Physiol 266: F275–F282, 1994. [DOI] [PubMed] [Google Scholar]


