Abstract
Information about the position of an object that is held in both hands, such as a golf club or a tennis racquet, is transmitted to the human central nervous system from peripheral sensors in both left and right arms. How does the brain combine these two sources of information? Using a robot to move participant's passive limbs, we performed psychophysical estimates of proprioceptive function for each limb independently and again when subjects grasped the robot handle with both arms. We compared empirical estimates of bimanual proprioception to several models from the sensory integration literature: some that propose a combination of signals from the left and right arms (such as a Bayesian maximum-likelihood estimate), and some that propose using unimanual signals alone. Our results are consistent with the hypothesis that the nervous system both has knowledge of and uses the limb with the best proprioceptive acuity for bimanual proprioception. Surprisingly, a Bayesian model that postulates optimal combination of sensory signals could not predict empirically observed bimanual acuity. These findings suggest that while the central nervous system seems to have information about the relative sensory acuity of each limb, it uses this information in a rather rudimentary fashion, essentially ignoring information from the less reliable limb.
Keywords: bimanual, human, maximum likelihood, proprioception, sensory integration
the human sensorimotor system can combine multiple sensory signals to estimate the position of the body. Several studies have shown data consistent with the hypothesis that the sensory system optimally integrates sensory information: both when combining a prior distribution with current signal variability (Kording and Wolpert 2004) and when integrating visual and haptic sensory information (Ernst and Banks 2002). These studies suggest that the central nervous system may implement some form of Bayesian statistics.
Studies of information processing by the nervous system have found Bayesian models to often be consistent with empirical data for a rather broad set of behaviors that includes infant cognition (Gweon et al. 2010), language (Bannard et al. 2009; Frank and Goodman 2012), face perception (Peterson and Eckstein 2012), rhythm perception (Cicchini et al. 2012), haptics (Squeri et al. 2012), and multisignal integration (Ernst and Banks 2002). It is thus an important current theory for sensory-motor neuroscience and motor control. In some of these studies, however, it is not clear that the Bayesian proposal is unique. It has sometimes been the case that Bayesian models have been applied without the capacity to distinguish between subtle differences in the underlying variability distributions (Zhang et al. 2013). Thus some behaviors might be mistakenly classified as Bayesian as a result of mis-approximation of true sensory or motor variability.
Proprioception of the human upper limb has been explored considerably, and some asymmetries between that of the dominant and nondominant limb have been observed (for a review see Goble and Brown 2008a). In particular there is some suggestion that the nondominant arm may have superior position sense. This is an ecologically relevant question because many behaviors involve the simultaneous use of both hands, and thus the central nervous system may implement some form of sensory integration. Here we directly test the nervous system's integration of proprioceptive signals from the left and right arms.
In this experiment we perform psychophysical estimates of proprioceptive function. By measuring unimanual proprioception of each limb and comparing these measures with bimanual proprioception we can test if and how the human sensorimotor system combines proprioceptive signals from the two limbs. We compare empirically observed bimanual proprioceptive bias and acuity to predictions from several models of sensory integration, including some that propose combining signals from the left and right arms and some that propose using unimanual signals alone. Our results are consistent with the hypothesis that the nervous system both has knowledge of and uses the limb with the best proprioceptive acuity for bimanual proprioception. Our data are not consistent with the hypothesis that the sensorimotor system optimally combines unimanual proprioceptive signals from the two limbs in the bimanual case.
METHODS
Subjects
Thirty-seven (20 female) healthy individuals participated in this study (aged 18–45 yr). All subjects were right-handed as assessed by the Dutch handedness questionnaire (van Strien 1992). Subjects reported no history of neurological or musculoskeletal disorder and had normal or corrected-to-normal vision. All subjects provided written informed consent before participation in the study, which was approved by the University of Western Ontario Research Ethics Board.
Apparatus
Subjects were seated in the dark at a table adjusted to chest height. Subjects grasped the handle of an InMotion robotic linkage (In Motion Technologies, Cambridge, MA) as shown in Fig. 1A. An air sled was used to support the arm and allowed smooth, near frictionless movement along the surface of the table (not shown). The robot was programmed to move the arm from one position to another in a two-dimensional horizontal plane located just below shoulder height. A six-axis force transducer (ATI Industrial Automation, Apex, NC) inside the handle measured forces at the hand. Shoulder straps attached to the chair kept the trunk in a static position, while allowing rotation of the shoulder and elbow joints. A horizontal semisilvered mirror was suspended 31.5 cm above the surface of the table. Vision of the arm and the robotic manipulandum was obscured by opaque curtains in addition to the semisilvered mirror.
Fig. 1.
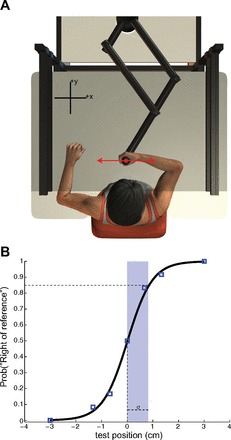
A: subjects sat at a table and grasped the robotic manipulandum during proprioceptive tests of left, right, and bimanual judgments. B: example psychometric function. Squares denote the probability with which a subject reported a given test position (the difference between the judgment and reference positions) to be right of the reference location, as a function of the actual hand location. Subjects' responses were fit to a cumulative normal distribution function. The vertical dashed line indicates the bias; here the estimated bias was 0. The shaded region represents the estimated proprioceptive acuity, σ, of 8.4 mm.
Perceptual Tests
Tests were performed at a single spatial location along the sagittal plane 18 cm away from the body. Three tests were performed in series by each subject: one in which they grasped the handle of the robot using their left hand only, a second in which they grasped the handle using the right hand only, and a third in which they grasped the handle using both hands simultaneously. When testing bimanual proprioception, the subject's fingers were interleaved such that neither hand gripped the handle more directly than the other. The order in which the tests were performed was counterbalanced across subjects.
The test procedure has been described elsewhere (Wilson et al. 2010; Mattar et al. 2013; Wong et al. 2012). Briefly, we employed a two-alternative forced-choice paradigm to estimate the psychophysical relationship between actual and perceived position of the limb(s). On each trial, subjects were instructed to keep their arm muscles relaxed, and their head in a neutral, forwards direction. Vision of the arm was completely blocked by opaque curtains. Each proprioceptive test consisted of 74 trials in which the robot moved the passive limb(s) along a left-right axis.
Subjects were instructed to keep their eyes closed at all times. On each trial, the subject's arm was moved to the reference position by the robotic manipulandum and held there for 2 s. Next, the hand was moved away from the reference position through a distractor movement, before being brought to a judgment position where the hand was held until the subject made a two-alternative forced-choice judgment about which side along the axis of movement (left or right) the judgment position fell with respect to the reference position. The distractor movement displaced the hand 14 cm plus or minus a random distance (chosen from a Gaussian with mean = 14 cm and SD = 2 cm) from the reference position along the test axis to a peripheral position before bringing the hand to a judgment position. Seven judgment positions were tested, at [−30, −13.3, −6.7, 0, +6.7, +13.3, +30] mm. Each judgment position was tested between 6 and 14 times [6, 12, 12, 14, 12, 12, 6]. The positions furthest from the reference position were tested fewer times because subjects were expected to make essentially no errors at these distant positions.
To familiarize the subject with the procedure, blocks of 20 practice trials were performed at the start of the experiment, until subjects demonstrated a clear understanding of the task. The majority of subjects only required a single practice block.
A logistic function was fit to the set of binary response data across test locations (Fig. 1B). Proprioceptive bias was quantified as the 50th percentile, i.e., the point at which subjects were equally likely to report their hand as left or right of the reference position. Proprioceptive acuity was quantified as σ, the distance spanning the 50th to the 84th percentiles of the logistic function. Statistical analysis of changes in proprioception were assessed using ANOVA and Tukey's post hoc tests.
Predictions
We tested several models, some proposed previously, that predict how the central nervous system might use the two unimanual signals to perform a bimanual estimate of hand position. These hypotheses can be divided into those that predict signal combination and those that propose the use of a single signal for perceptual judgments.
Signal combination models.
In this study the variance of a proprioceptive signal σ2 was estimated using the square of the distance between the 50th and 84th percentile of the psychometric function. The reliability of a given signal (r) is the inverse of the variance:
Equal-weight.
The parsimonious signal-combination hypothesis predicts that the position of the bimanual estimate (bias) is the average of the two unimanual positions xL and xR,
where . The reliability of the bimanual estimate is (Oruc et al. 2003):
We refer to this prediction as HBiEqual.
Maximum-likelihood estimation.
A second hypothesis predicts that the central nervous system optimally combines the unimanual signals to generate a maximum likelihood estimate (MLE) of bimanual position (Ernst and Banks 2002). The MLE model predicts that the bimanual estimate is a weighted combination of the two unimanual estimates xL and xR for the left and right arms as above for the equal weight model. The weights are defined as a function of the unimanual reliabilities:
Thus less reliable unimanual estimates contribute to a bimanual estimate with lower weight. The resulting variance of the bimanual estimate is (Ernst and Banks 2002):
For each subject in the experiment, we computed the predicted bias and predicted acuity σLR (the distance between the 50th and 84th percentile of the psychophysical function) under the MLE model and compared the predictions to the observed values when subjects grasped the robot handle with both left and right hands. This prediction was referred to as HBiMLE.
Maximum-likelihood for correlated variables.
Finally, note that maximum likelihood estimation as described above assumes uncorrelated signals. It might rather be the case that unimanual signals are correlated. This might for example arise from noise due to torso movement or within shared pathways in the central nervous system.
To take into account the possibility of correlated signals, we did the following. We assume a constant unknown degree of correlation ρ between left and right unimanual signals across all subjects. We then determined the correlation coefficient ρ that resulted in best fits of the empirically observed bimanual acuity to that predicted by the maximum-likelihood model with correlated signals (Oruç et al. 2003):
Using this estimate of ρ, we computed a new maximum likelihood estimate. The effect of including a correlation between signals is to discount the predicted optimal bimanual prediction, while having no effect on the combined bias. This prediction was referred to as HBiMLEcorr. The position estimate for correlated variables, is identical to the MLE estimate.
Single signal models.
Alternatively, the central nervous system might select a single limb for all perceptual responses. Instead, the set of Bimanual responses might be generated by 1) use of the limb with the best proprioceptive acuity, HUniMin; 2) use of a single unimanual cue chosen at random for each trial, HSwitchRand; and 3) use of a single unimanual cue chosen with probability proportional to the signal reliability, HSwitchWeight.
To generate these last two predictions, we performed simulations of the psychophysical experiments. Using the empirically estimated left and right unimanual psychophysical curves of each subject, we generated random-draw or weighted-draw responses that were used to simulate bimanual responses. To generate predictions for HSwitchRand, we generated simulated responses where on each trial, a binomial response was generated using either the left or right empirically observed psychometric curve, chosen at random (with equal probability) for each trial. To generate predictions for HSwitchWeight, the same procedure was used, except that instead of basing the simulated responses on the left or right psychometric functions chosen at random with equal probability, the probability of using left vs. right was proportional to signal reliability (the inverse of acuity). After generating simulated responses for each subject under both hypotheses, we reestimated psychometric functions and recomputed estimates of bias and acuity.
RESULTS
Bias
Figure 2A (“empirical data”) shows means ± SE of the psychophysical estimates of perceptual bias for the right (blue), left (red), and bimanual (black) data averaged across subjects. Average ± SE of BiasRight was −1.18 ± 0.41; BiasLeft was 3.19 ± .36 mm; and BiasBimanual was 0.60 ± 0.34 mm. A split-plot repeated-measures ANOVA (one within-subjects variable, grasp [R, L, B] and one between-subjects variable, testing order [6 different permutations ]) showed no main effect of order (P > 0.05), a significant main effect of grasp (P < 0.001), and no interaction effect (P > 0.05); post hoc tests showed significant differences between the bias of all three conditions (P < 0.001 in all pairwise comparisons). Thus left, right, and bimanual biases were reliably different from each other. Interestingly, BiasBimanual was between BiasRight and BiasLeft.
Fig. 2.
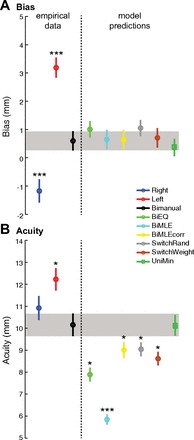
A: bias measures from empirical data (right, left, and bimanual) and predictions (Bi50, BiMLE, BiMLEcorr, SwitchRand, SwitchWeight, and UniMin). ***Reliably different means (all of which were reliable with P < 0.001). B: acuity measures from empirical data (right, left, and bimanual) and predictions (Bi50, BiMLE, BiMLEcorr, SwitchRand, SwitchWeight, and UniMin). *Reliable difference (P < 0.05).
Acuity
Figure 2B (“empirical data”) shows means ± SE of the estimated acuity. Means ± SE acuity measures for right, left, and bimanual were as follows: 10.92 ± 0.55 mm, 12.23 ± 0.51 mm, and 10.15 ± 0.52 mm. A split-plot ANOVA found a marginal main effect of grasp (P = 0.066), no significant main effect of order, and no interaction (P > 0.05); post hoc tests showed that AcuityBimanual was reliably different from AcuityLeft.
Model Predictions
We next tested models of Bimanual proprioception for their ability to predict observed bimanual proprioceptive bias and acuity. These data are summarized in Table 1 and displayed graphically in Fig. 2, A and B (“model predictions”). Bimanual bias predicted from all three signal combination models (HBiMLE, HBiMLEcorr, and HBiEqual) was consistent with the observed data (P > 0.05 in all cases). Predicted acuity from HBiMLE and HBiEqual was observed to be reliably better than empirically observed bimanual proprioceptive acuity (P < 0.0001 in both cases). The hypothesis HBiMLEcorr adjusting MLE for correlation between left and right limbs (using an estimate of groupwise correlation ρ between left and right ρ = 0.33) predicted poorer proprioceptive acuity compared with HMLE (9.00 ± 0.37 mm) but still predicted better acuity than the observed empirical data (P = 0.031). We also investigated the hypothesis that correlation between unimanual signals may be subject specific and in this case fit ρ on a per-subject basis (rather than using the groupwise ρ = 0.33 above). In this case predicted proprioceptive acuity was still better than empirically observed bimanual acuity (P = 0.034).
Table 1.
Summary of empirically estimated and predicted bimanual bias and acuity
| Bias, mm |
Acuity, mm |
|||
|---|---|---|---|---|
| Estimated | P | Estimated | P | |
| Empirical | 0.60 ± 0.34 | 10.15 ± 0.52 | ||
| Bimanual models | ||||
| BiEQ | 1.01 ± 0.29 | >0.05 | 7.89 ± 0.32 | <0.05 |
| BiMLE | 0.65 ± 0.34 | >0.05 | 5.85 ± 0.23 | <0.001 |
| BiMLECorr | 0.65 ± 0.34 | >0.05 | 9.00 ± 0.37 | <0.001 |
| Unimanual | ||||
| SwitchRand | 1.05 ± 0.29 | >0.05 | 9.04 ± 0.32 | <0.05 |
| SwitchWeight | 0.70 ± 0.34 | >0.05 | 8.62 ± 0.31 | <0.01 |
| UniMin | 0.36 ± 0.30 | >0.05 | 10.14 ± 0.47 | >0.05 |
Values means ± SE. Statistical tests (paired t-tests) were performed to test for reliable differences between empirical bimanual data and predictions.
We next investigated the hypotheses which predict that subjects switch between unimanual proprioceptive signals. Predicted proprioceptive biases from the HSwitchRand and HSwitchWeight models were not reliably different from empirically estimated bimanual data (P > 0.05 in both cases). Proprioceptive acuity predicted by the HSwitchRand model was reliably different (predicting better) from the empirical data (P = 0.031), while acuity predicted from the HSwitchWeight model was also reliably better than that observed empirically (P = 0.0045).
One might also hypothesize that a single limb alone is used for bimanual responses. Based on the above data showing reliable bias differences among left, right, and bimanual responses, it is clear that subjects do not solely use the left or right limb exclusively for bimanual proprioception. It might instead be hypothesized that subjects use the limb having the best proprioceptive acuity (the limb with the smallest σ), a hypothesis we label HUniMin. In fact, bias and acuity predicted by HUniMin were consistent with the observed bimanual bias (P = 0.28) and acuity (P = 0.97). Of our 37 subjects, 22 (59%) had best acuity with the left hand.
Figure 3 plots individual subject data showing predicted bias (Fig. 3A) and acuity (Fig. 3B) for the models tested as a function of empirically estimated values. Notably, HBiMLE clearly overestimates bimanual acuity (i.e., underestimates σ).
Fig. 3.
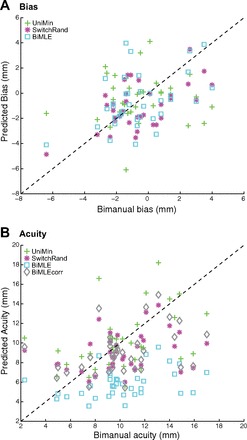
A: predicted bimanual bias as a function of empirically measured bimanual bias, plotted for each subject. B: predicted bimanual acuity as a function of empirically measured bimanual acuity, plotted for each subject.
DISCUSSION
This study examined sensory integration for proprioception of the two limbs. The empirical data are consistent with the hypothesis that the nervous system is aware of and uses the limb with the best proprioceptive acuity for bimanual judgments. Our data are not consistent with the prevailing model that predicts that the nervous system optimally combines sensory signals from the two limbs. In fact the maximum-likelihood model was worst at predicting bimanual acuity, and adjustments made for signal correlation only slightly improved the predictions of the model, which were still reliably different (better) than empirically observed bimanual acuity.
Why did participants not optimally combine proprioceptive signals from the left and right limbs? It may be that the particular task tested here is one for which the sensorimotor system does not have extensive experience. For example, it has been shown that in the absence of practice the human sensorimotor system is not able to optimally combine multiple sources of visual information for behaviors such as navigation (Souman et al. 2009).
In principle it is possible that some additional process exists that adds position judgment noise following the initial estimation stage. For example, it could be that a neural process responsible for combining both signals together to make a decision is variable, and so the resulting bimanual responses are suboptimal. This account is entirely speculative and less parsimonious than the winner take all model proposed here but cannot be ruled out given the current set of experimental data.
The current results are similar to the results of Squeri et al. (2012) in many respects. That study investigated the perception of surface curvature and found that while the nondominant left hand typically was superior at curvature perception, the right hand was a better predictor of bimanual curvature. In our experiment, however, the best predictor of bimanual proprioception was the limb with the best acuity, which was distributed nearly evenly across left and right arms (59% left hand; 22 of 37 subjects). Neither the right nor the left arm was a good predictor of bimanual proprioception in our experiment given the observed differences in proprioceptive bias.
In the current study proprioception was assessed at only one workspace. Some studies have found perceptual differences that vary according to workspace location, such as the bias of tilt angle perception (Henriques and Soechting 2005). One recent study found no systematic differences in perceptual bias to Cartesian displacements across workspace location (Wilson et al. 2010), while a somewhat similar study investigating proprioception of joint angle displacements found biases to proprioception at the extremes of the joint angle range (Fuentes and Bastian 2010). It might be interesting to assess bimanual proprioception in a similar manner.
In our study the model with the most support was one that assumes subjects know in advance which limb has the best proprioceptive acuity. There is some support for the idea that the human sensorimotor system maintains a representation of motor variability for left and right limbs and uses this information both for online correction and for planning of subsequent movement. The motor system makes trial-by-trial adjustments to left and right limb trajectories during bimanual reaching movements and such adjustments are preferentially made to movements of the nondominant hand (White and Diedrichsen 2010). The nondominant hand is in general less accurate during reaching movements and it has thus been proposed that this acuity difference causes the motor system to selectively adjust the control signals for the less-accurate limb. Since our subjects were right handed and left and right limb biases were both different from bimanual bias, these data are not consistent with either a dominant or nondominant hand hypothesis for bimanual proprioception. It is certainly true that our task does not involve active movement, and in fact several studies have shown that during static proprioception (followed by an active matching movement) the majority of subjects are more accurate at static limb proprioception with the nondominant hand (Sainburg 2002; Goble et al. 2006; Goble and Brown 2008a,b).
In the present study we found systematic differences in proprioceptive bias of the left and right arms. This finding mirrors the results of Wilson et al. (2010) in which across several workspace locations subjects tended to perceive that their left arm was to the left of its actual position, and the right arm was judged to be to the right. Similar findings have been reported in the right arm by Vindras et al. (1998) and Desmurget et al. (2000). This perceptual finding of outward bias may result in the observed “overlap effect” during reaches to an unseen hand (van Beers et al. 1998), since these authors found that subjects regularly reached too far rightward for an unseen right hand and too far leftward for an unseen left hand. However, the specific origin of these differences remains unknown.
A criticism of the current study might be related to a potential cognitive component inherent in the psychophysical testing procedure. It may be argued that proprioception could be similar to the visual system with respect to its two-streams hypothesis for perception and action (Volpe et al. 1979; Paillard et al. 1983; Rossetti et al. 1995; Dijkerman and de Haan 2007). Visual information for active movement has been shown to be distinct from visual information for perception (Schneider 1969; Goodale et al. 1991). If such a dissociation exists in the somatosensory system, it may be that signals from the left and right arms are integrated or combined differently for a task that is less “perceptual” (such as the task we used) and more “dorsal” in nature. Recently, however, the double-dissociation hypothesis for the sense of somatosensation, haptic touch specifically, was tested directly in a vibrotactile experiment (Harris et al. 2004). Experimenters fit responses of normal subjects to different signal detection models to determine whether psychophysical responses could be explained by independent parallel processes, or serial processes. Only the serial model successfully described subject responses, leading the authors to conclude that somatosensation for action and perception are not mutually independent processes but rather localization is subsequent to detection. This study illustrates that the two-streams hypothesis may not apply to somatosensory function.
It should be noted that the derived equations for signal combination using maximum likelihood assume that the prior over the variables of interest is uniform. In this experiment the measured perceptual bias of unimanual limbs was nonzero and was in different directions for left and right arms. Previous work involving signal combination has assumed that the prior distribution of individual signals is uniform (Ernst and Banks 2002; Oruc et al. 2003). In Jacobs (1999), the author developed a model that incorporates nonuniform priors into the prediction of the combined signal but assumes that the prior for the combined signal is equal to the prior of the individual signals. In the present study, the biases of each arm are not equal. Moreover, in the absence of independent estimates of the priors, it is not clear how one would incorporate a nonuniform prior in the present analyses.
Bayesian predictions of sensory integration within the nervous system face the scientific challenge that such predictions are consistent with performance in any task for which optimal performance is observed. That is, Bayesian models are a sufficient way of arriving at optimal performance, but it is unclear if they are necessary. Several recent studies have shown that the sensorimotor system's behavior is not always consistent with Bayesian predictions. Two recent studies suggest that the motor system does not always have an accurate estimate of its own motor variability (Mamassian 2008; Zhang et al. 2013), a prerequisite for Bayesian integration of information and the cause of larger-than-optimal pointing errors.
In the present study the results suggest a model of bimanual proprioception in which the nervous system only uses the signals from the limb that has the best acuity. To test this idea in future studies, one might think of ways of independently manipulating the acuity of individual limbs, or alternatively the bias of individual limbs. For example, tendon vibration could be used to manipulate the reliability of proprioceptive signals from one arm (Goodwin et al. 1972). The strong prediction made given the results of the current study is that vibration of the arm that has the poorer acuity would not affect the bimanual estimate. Such an experimental manipulation would be challenging, however, because it would be difficult to implement without unintentionally altering the system in other ways. Moreover there is good reason to consider that such a manipulation would selectively affect bimanual proprioception. Vibration of one limb might result in subjects dedicating more attention to that limb in the bimanual condition. It alternatively might simply act as a distractor that affects performance during the bimanual task. A bimanual robot might similarly be used to manipulate the sensory inputs to the two limbs independently but would involve similar methodological concerns. One virtue of the approach in this article is that we assess bimanual proprioception in a relatively normal task where proprioceptive signals are generated from normal arm motion; thus this task may be considered a reasonable model of bimanual proprioception in vivo.
Future studies may wish to attempt to incorporate some measure of signal correlation in their experimental design. This would alleviate the necessity of analytically fitting correlation coefficients as in the current paper. On the other hand, this would involve some experimental challenges. It is not clear if subjects are capable of accurately reporting the position of both hands simultaneously on each psychophysical test or if such simultaneous responses would accurately reflect the trial-to-trial correlation of unimanual proprioception.
GRANTS
This research was funded by Canadian Institutes of Health Research (CIHR) Grant (to P. L. Gribble), the Netherlands Organization for Scientific Research (to D. A. Kistemaker), a National Sciences and Engineering Council of Canada graduate scholarship (to E. T. Wilson), and a Canada Graduate scholarship from CIHR (to J. D. Wong).
DISCLOSURES
No conflicts of interest, financial or otherwise, are declared by the author(s).
AUTHOR CONTRIBUTIONS
Author contributions: J.D.W. and P.L.G. conception and design of research; J.D.W., E.T.W., and P.L.G. analyzed data; J.D.W., E.T.W., D.A.K., and P.L.G. interpreted results of experiments; J.D.W. prepared figures; J.D.W. and P.L.G. drafted manuscript; J.D.W., D.A.K., and P.L.G. edited and revised manuscript; J.D.W., E.T.W., D.A.K., and P.L.G. approved final version of manuscript; E.T.W. performed experiments.
REFERENCES
- Bannard C, Lieven E, Tomasello M. Modeling children's early grammatical knowledge. Proc Natl Acad Sci USA 106: 17284–17289, 2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini GM, Arrighi R, Cecchetti L, Giusti M, Burr DC. Optimal encoding of interval timing in expert percussionists. J Neurosci 32: 1056–1060, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desmurget M, Vindras P, Gréa H, Viviani P, Grafton ST. Proprioception does not quickly drift during visual occlusion. Exp Brain Res 134: 77, 2000. [DOI] [PubMed] [Google Scholar]
- Dijkerman HC, de Haan EH. Somatosensory processes subserving perception and action. Behav Brain Sci 30: 189–201; discussion 201–39, 2007. [DOI] [PubMed] [Google Scholar]
- Ernst MO, Banks MS. Humans integrate visual and haptic information in a statistically optimal fashion. Nature 415: 429–433, 2002. [DOI] [PubMed] [Google Scholar]
- Frank MC, Goodman ND. Predicting pragmatic reasoning in language games. Science 336: 998, 2012. [DOI] [PubMed] [Google Scholar]
- Fuentes CT, Bastian AJ. Where is your arm? Variations in proprioception across space and tasks. J Neurophysiol 103: 71, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goble D, Brown S. The biological and behavioral basis of upper limb asymmetries in sensorimotor performance. Neurosci Biobehav Rev 32: 598–610, 2008a. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Brown SH. Upper limb asymmetries in the matching of proprioceptive versus visual targets. J Neurophysiol 99: 3063–3074, 2008b. [DOI] [PubMed] [Google Scholar]
- Goble DJ, Lewis CA, Brown SH. Upper limb asymmetries in the utilization of proprioceptive feedback. Exp Brain Res 168: 307–311, 2006. [DOI] [PubMed] [Google Scholar]
- Goodale MA, Milner AD, Jakobson LS, Carey DP. A neurological dissociation between perceiving objects and grasping them. Nature 349: 154–156, 1991. [DOI] [PubMed] [Google Scholar]
- Goodwin GM, McCloskey DI, Matthews PB. The contribution of muscle afferents to kinaesthesia shown by vibration induced illusions of movement and by the effects of paralysing joint afferents. Brain 95, 705–748, 1972. [DOI] [PubMed] [Google Scholar]
- Gweon H, Tenenbaum JB, Schulz LE. Infants consider both the sample and the sampling process in inductive generalization. Proc Natl Acad Sci USA 107: 9066–9071, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris JA, Thein T, Clifford CW. Dissociating detection from localization of tactile stimuli. J Neurosci 24: 3683–3693, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henriques DY, Soechting JF. Approaches to the study of haptic sensing. J Neurophysiol 93: 3036–3043, 2005. [DOI] [PubMed] [Google Scholar]
- Jacobs RA. Optimal integration of texture and motion cues to depth. Vision Res 39: 36213629, 1999. [DOI] [PubMed] [Google Scholar]
- Kording KP, Wolpert DM. The loss function of sensorimotor learning. Proc Natl Acad Sci USA 101: 9839–9842, 2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mamassian P. Overconfidence in an objective anticipatory motor task. Psychol Sci 19: 601–606, 2008. [DOI] [PubMed] [Google Scholar]
- Mattar AA, Darainy M, Ostry DJ. Motor learning and its sensory effects: the time course of perceptual change, and its presence with gradual introduction of load. J Neurophysiol 109: 782–791, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Oruç I, Maloney LT, Landy MS. Weighted linear cue combination with possibly correlated error. Vision Res 43: 2451–2468, 2003. [DOI] [PubMed] [Google Scholar]
- Paillard J, Michel F, Stelmach G. Localization without content. A tactile analogue of “blind sight”. Arch Neurol 40: 548–551, 1983. [DOI] [PubMed] [Google Scholar]
- Peterson MF, Eckstein MP. Looking just below the eyes is optimal across face recognition tasks. Proc Natl Acad Sci USA 109: E3314–23, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rossetti Y, Rode G, Boisson D. Implicit processing of somaesthetic information: a dissociation between where and how? Neuroreport 6: 506–510, 1995. [DOI] [PubMed] [Google Scholar]
- Sainburg RL. Evidence for a dynamic-dominance hypothesis of handedness. Exp Brain Res 142: 241–258, 2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schneider GE. Two visual systems. Science 163: 895–902, 1969. [DOI] [PubMed] [Google Scholar]
- Souman JL, Frissen I, Sreenivasa MN, Ernst MO. Walking straight into circles. Curr Biol 19: 1538–1542, 2009. [DOI] [PubMed] [Google Scholar]
- Squeri V, Sciutti A, Gori M, Masia L, Sandini G, Konczak J. Two hands, one perception: how bimanual haptic information is combined by the brain. J Neurophysiol 107: 544–550, 2012. [DOI] [PubMed] [Google Scholar]
- van Beers RJ, Sittig AC, Denier van der Gon JJ. The precision of proprioceptive position sense. Exp Brain Res 122: 77, 1998. [DOI] [PubMed] [Google Scholar]
- van Strien JW. Classification of left- and right-handed research participants (in Dutch). Ned Tijdschr Psychol 47: 88–92, 1992. [Google Scholar]
- Vindras P, Desmurget M, Prablanc C, Viviani P. Pointing errors reflect biases in the perception of the initial hand position. J Neurophysiol 79: 4, 1998. [DOI] [PubMed] [Google Scholar]
- Volpe BT, LeDoux JE, Gazzaniga MS. Spatially oriented movements in the absence of proprioception. Neurology 29: 1309–1313, 1979. [DOI] [PubMed] [Google Scholar]
- White O, Diedrichsen J. Responsibility assignment in redundant systems. Curr Biol 20: 1290–1295, 2010. [DOI] [PubMed] [Google Scholar]
- Wilson ET, Wong J, Gribble PL. Mapping proprioception across a 2D horizontal workspace. PLoS One 5: e11851, 2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wong JD, Kistemaker DA, Chin A, Gribble PL. Can proprioceptive training improve motor learning? J Neurophysiol 108: 3313–3321, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang H, Daw ND, Maloney LT. Testing whether humans have an accurate model of their own motor uncertainty in a speeded reaching task. PLoS Comput Biol 9: e1003080, 2013. [DOI] [PMC free article] [PubMed] [Google Scholar]


