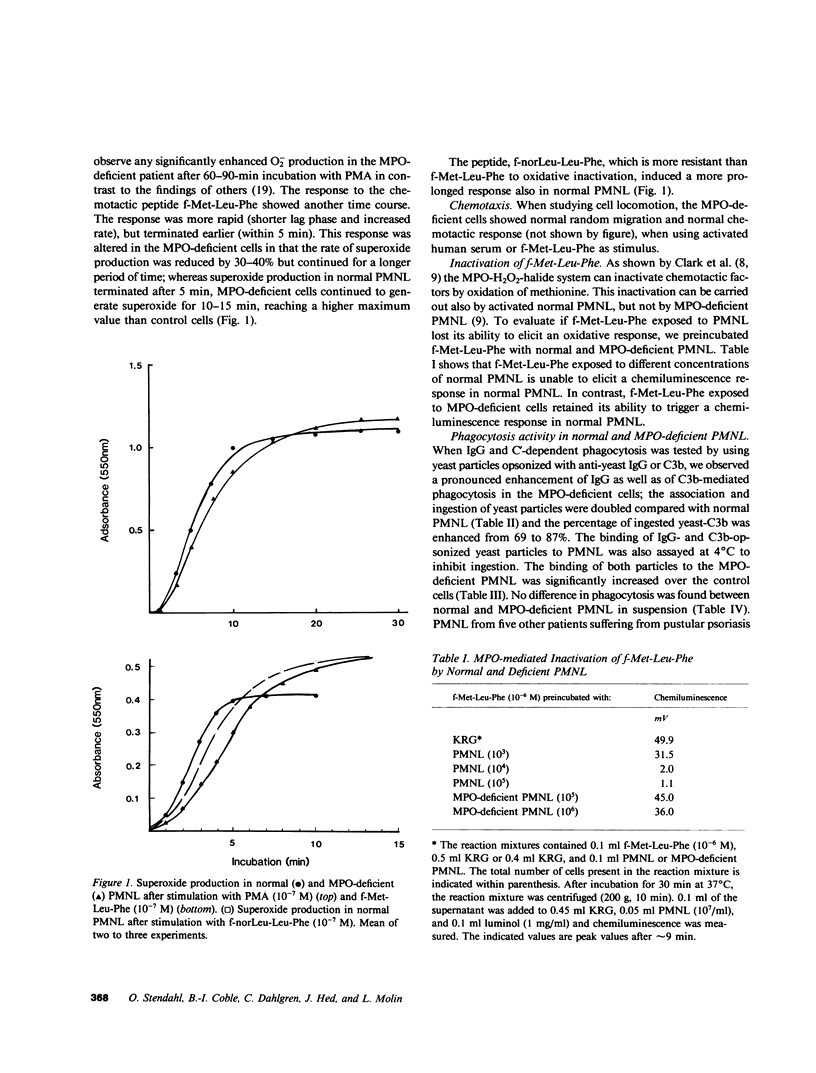
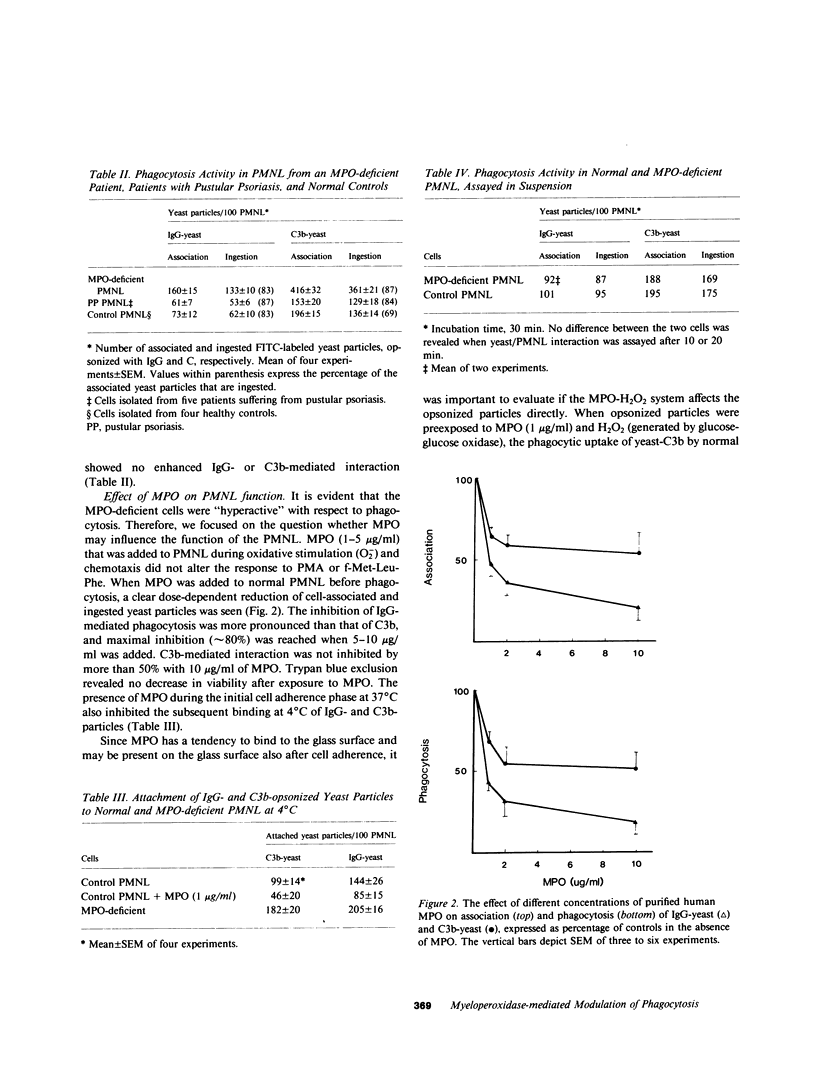
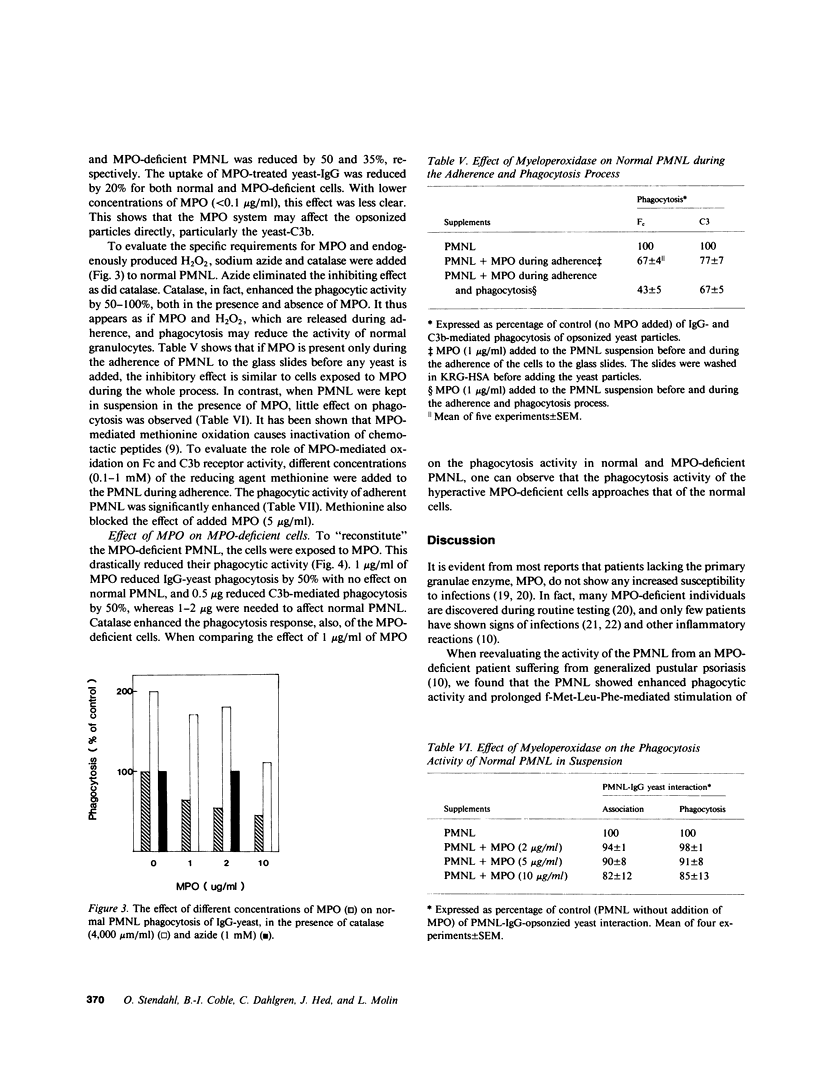
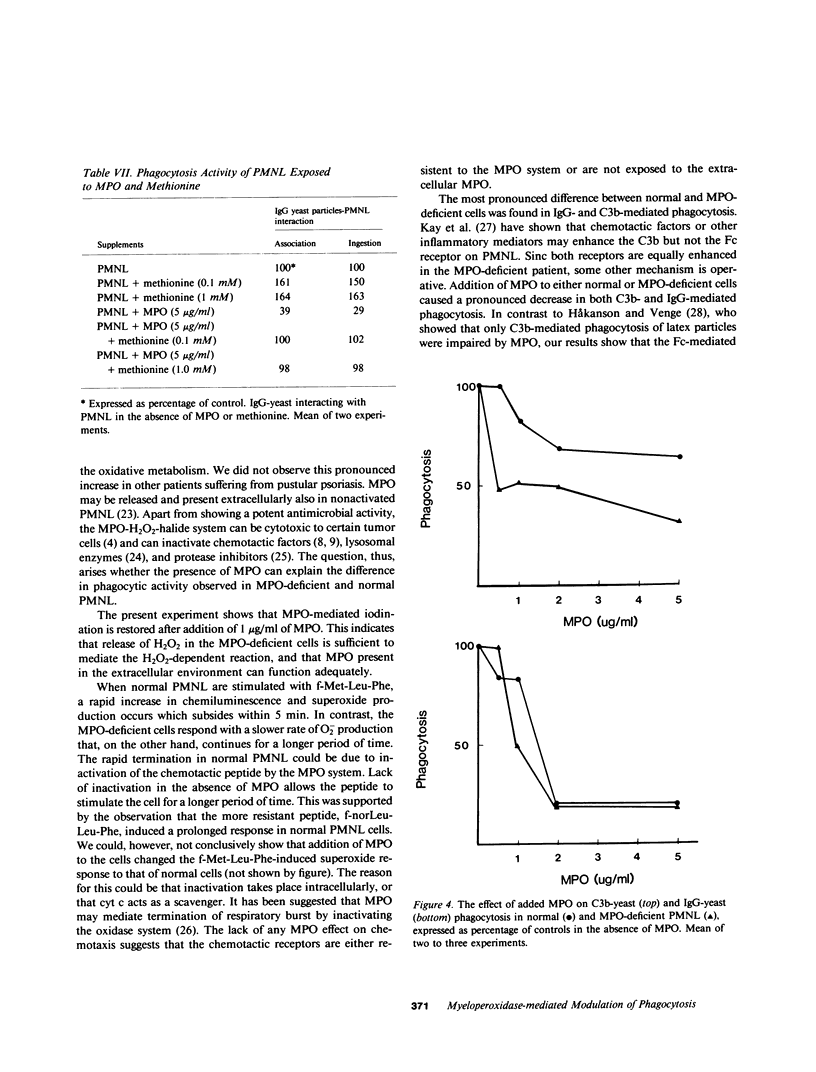
Abstract
Patients lacking the primary granulae enzyme, myeloperoxidase (MPO), do not usually show any increased susceptibility to infection or altered inflammatory response, in contrast to several other biochemical defects in polymorphonuclear neutrophils. We have now evaluated the role of MPO on phagocyte function in a patient with complete MPO deficiency suffering from generalized pustular psoriasis. We found that the MPO-deficient neutrophils showed enhanced phagocytosis (greater than 200% of normal) of IgG- and C3b-opsonized yeast particles and prolonged N-formylmethionyl-leucyl-phenylaline-mediated stimulation of superoxide production. When purified human MPO was added to normal neutrophils during cell adhesion, their Fc- and C3b-mediated phagocytosis was reduced without affecting cell viability. 1 microgram/ml of MPO reduced the Fc and C3b phagocytosis to 47 and 65%, respectively, whereas 10 micrograms/ml reduced the activity to 20 and 54%. Both attachment and ingestion were reduced to a similar extent, indicating that MPO affected the receptor function per se. When MPO was added to the hyperactive MPO-deficient cells, phagocytosis was reduced more rapidly. Catalase, azide, and methionine eliminated the inhibitory effect, and catalase and methionine, in fact, enhanced the phagocytic activity of adherent neutrophils. These data indicate that, apart from being a potent antimicrobial system, the oxidizing activity of the MPO-H2O2-halide system may modulate the inflammatory response by impairing certain receptor-mediated recognition mechanisms of phagocytic cells, which otherwise could elicit inflammatory reactions and tissue injury.
Full text
PDF



Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Babior B. M. Oxygen-dependent microbial killing by phagocytes (second of two parts). N Engl J Med. 1978 Mar 30;298(13):721–725. doi: 10.1056/NEJM197803302981305. [DOI] [PubMed] [Google Scholar]
- Baehner R. L., Boxer L. A., Allen J. M., Davis J. Autooxidation as a basis for altered function by polymorphonuclear leukocytes. Blood. 1977 Aug;50(2):327–335. [PubMed] [Google Scholar]
- Cech P., Stalder H., Widmann J. J., Rohner A., Miescher P. A. Leukocyte myeloperoxidase deficiency and diabetes mellitus associated with Candida albicans liver abscess. Am J Med. 1979 Jan;66(1):149–153. doi: 10.1016/0002-9343(79)90507-2. [DOI] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Chemotactic factor inactivation by the myeloperoxidase-hydrogen peroxide-halide system. J Clin Invest. 1979 Oct;64(4):913–920. doi: 10.1172/JCI109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J., Einstein A. B., Fefer A. Peroxidase-H2O2-halide system: Cytotoxic effect on mammalian tumor cells. Blood. 1975 Feb;45(2):161–170. [PubMed] [Google Scholar]
- Clark R. A., Klebanoff S. J. Myeloperoxidase-mediated platelet release reaction. J Clin Invest. 1979 Feb;63(2):177–183. doi: 10.1172/JCI109287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark R. A., Szot S. Chemotactic factor inactivation by stimulated human neutrophils mediated by myeloperoxidase-catalyzed methionine oxidation. J Immunol. 1982 Apr;128(4):1507–1513. [PubMed] [Google Scholar]
- Cohen H. J., Chovaniec M. E. Superoxide generation by digitonin-stimulated guinea pig granulocytes. A basis for a continuous assay for monitoring superoxide production and for the study of the activation of the generating system. J Clin Invest. 1978 Apr;61(4):1081–1087. doi: 10.1172/JCI109007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dahlgren C., Stendahl O. Effect of in vitro preincubation of polymorphonuclear leukocytes on formylmethionyl-leucyl-phenylalanine-induced chemiluminescence. Infect Immun. 1982 Jul;37(1):34–39. doi: 10.1128/iai.37.1.34-39.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldstein I. M. Polymorphonuclear Leukocyte lysosomes and immune tissue injury. Prog Allergy. 1976;20:301–340. [PubMed] [Google Scholar]
- Hed J., Stendahl O. Differences in the ingestion mechanisms of IgG and C3b particles in phagocytosis by neutrophils. Immunology. 1982 Apr;45(4):727–736. [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Chi E. Y., Klebanoff S. J. Eosinophil peroxidase-induced mast cell secretion. J Exp Med. 1980 Aug 1;152(2):265–279. doi: 10.1084/jem.152.2.265. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henderson W. R., Kaliner M. Mast cell granule peroxidase: location, secretion, and SRS-A inactivation. J Immunol. 1979 Apr;122(4):1322–1328. [PubMed] [Google Scholar]
- Håkansson L., Venge P. Kinetic studies of neutrophil phagocytosis. V. studies on the co-operation between the Fc and C3b receptors. Immunology. 1982 Dec;47(4):687–694. [PMC free article] [PubMed] [Google Scholar]
- Jandl R. C., André-Schwartz J., Borges-DuBois L., Kipnes R. S., McMurrich B. J., Babior B. M. Termination of the respiratory burst in human neutrophils. J Clin Invest. 1978 May;61(5):1176–1185. doi: 10.1172/JCI109033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kay A. B., Glass E. J., Salter D. M. Leucoattractants enhance complement receptors on human phagocytic cells. Clin Exp Immunol. 1979 Nov;38(2):294–299. [PMC free article] [PubMed] [Google Scholar]
- Mogensen C. E. The glomerular permeability determined by dextran clearance using Sephadex gel filtration. Scand J Clin Lab Invest. 1968;21(1):77–82. doi: 10.3109/00365516809076979. [DOI] [PubMed] [Google Scholar]
- Nauseef W. M., Metcalf J. A., Root R. K. Role of myeloperoxidase in the respiratory burst of human neutrophils. Blood. 1983 Mar;61(3):483–492. [PubMed] [Google Scholar]
- Paredes J. M., Weiss S. J. Human neutrophils transform prostaglandins by a myeloperoxidase-dependent mechanism. J Biol Chem. 1982 Mar 25;257(6):2738–2740. [PubMed] [Google Scholar]
- Parry M. F., Root R. K., Metcalf J. A., Delaney K. K., Kaplow L. S., Richar W. J. Myeloperoxidase deficiency: prevalence and clinical significance. Ann Intern Med. 1981 Sep;95(3):293–301. doi: 10.7326/0003-4819-95-3-293. [DOI] [PubMed] [Google Scholar]
- Roos D., Weening R. S., Wyss S. R., Aebi H. E. Protection of human neutrophils by endogenous catalase: studies with cells from catalase-deficient individuals. J Clin Invest. 1980 Jun;65(6):1515–1522. doi: 10.1172/JCI109817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Root R. K., Cohen M. S. The microbicidal mechanisms of human neutrophils and eosinophils. Rev Infect Dis. 1981 May-Jun;3(3):565–598. doi: 10.1093/clinids/3.3.565. [DOI] [PubMed] [Google Scholar]
- Sahlin S., Hed J., Rundquist I. Differentiation between attached and ingested immune complexes by a fluorescence quenching cytofluorometric assay. J Immunol Methods. 1983 May 27;60(1-2):115–124. doi: 10.1016/0022-1759(83)90340-x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Dahlgren C., Hed J. Physicochemical and functional changes in human leukemic cell line HL-60. J Cell Physiol. 1982 Aug;112(2):217–221. doi: 10.1002/jcp.1041120209. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Lindgren S. Function of granulocytes with deficient myeloperoxidase-mediated iodination in a patient with generalized pustular psoriasis. Scand J Haematol. 1976 Feb;16(2):144–153. doi: 10.1111/j.1600-0609.1976.tb01130.x. [DOI] [PubMed] [Google Scholar]
- Stendahl O., Molin L., Dahlgren C. The inhibition of polymorphonuclear leukocyte cytotoxicity by dapsone. A possible mechanism in the treatment of dermatitis herpetiformis. J Clin Invest. 1978 Jul;62(1):214–220. doi: 10.1172/JCI109109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stendahl O., Molin L., Lindroth M. Granulocyte-mediated release of histamine from mast cells. Effect of myeloperoxidase and its inhibition by antiinflammatory sulfone compounds. Int Arch Allergy Appl Immunol. 1983 Mar;70(3):277–284. doi: 10.1159/000233335. [DOI] [PubMed] [Google Scholar]
- Thomas E. L., Grisham M. B., Jefferson M. M. Myeloperoxidase-dependent effect of amines on functions of isolated neutrophils. J Clin Invest. 1983 Aug;72(2):441–454. doi: 10.1172/JCI110992. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsan M. F., Chen J. W. Oxidation of methionine by human polymorphonuclear leukocytes. J Clin Invest. 1980 May;65(5):1041–1050. doi: 10.1172/JCI109756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Voetman A. A., Weening R. S., Hamers M. N., Meerhof L. J., Bot A. A., Roos D. Phagocytosing human neutrophils inactivate their own granular enzymes. J Clin Invest. 1981 May;67(5):1541–1549. doi: 10.1172/JCI110185. [DOI] [PMC free article] [PubMed] [Google Scholar]


