Abstract
Innate immune responses are vital for pathogen defense but can result in septic shock when excessive. A key mediator of septic shock is tumor necrosis factor–α (TNFα), which is shed from the plasma membrane after cleavage by the TNFα convertase (TACE). We report that the rhomboid family member iRhom2 interacted with TACE and regulated TNFα shedding. iRhom2 was critical for TACE maturation and trafficking to the cell surface in hematopoietic cells. Gene-targeted iRhom2-deficient mice showed reduced serum TNFα in response to lipopolysaccharide (LPS) and could survive a lethal LPS dose. Furthermore, iRhom2-deficient mice failed to control the replication of Listeria monocytogenes. Our study has identified iRhom2 as a regulator of innate immunity that may be an important target for modulating sepsis and pathogen defense.
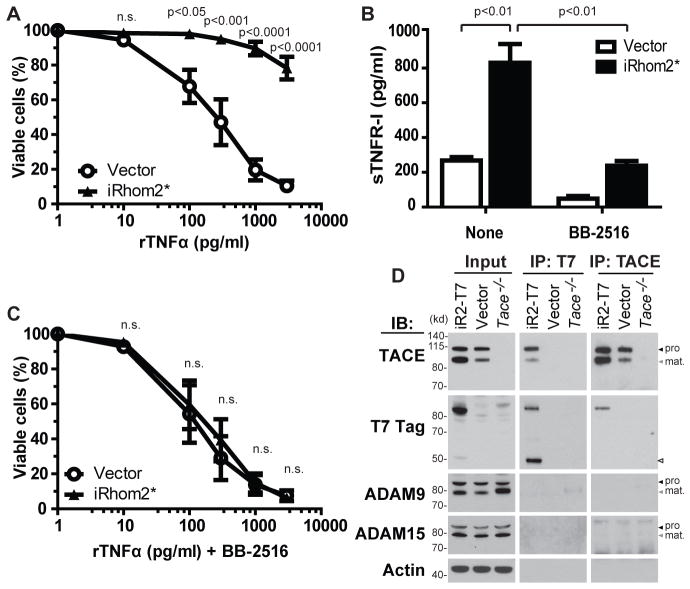
Tumor necrosis factor–α (TNFα) is both crucial for effective innate immunity and a pathologic contributor to inflammatory diseases, including sepsis and rheumatoid arthritis (1–4). How TNFα signaling is regulated, however, is still not fully understood. To identify new molecules involved in regulating TNFα signaling, we performed an unbiased cyclic packaging rescue screen (5) to isolate cDNAs conferring TNFα resistance. One such candidate was a short cDNA (iRhom2*) derived from the gene encoding iRhom2 (Rhbdf2) (fig. S1), which is a largely uncharacterized member of the rhomboid protein family (6–8). Stable overexpression of iRhom2* in L929 cells revealed its localization in the endoplasmic reticulum (ER), consistent with previous studies of iRhoms (8–10), and partial localization in the Golgi apparatus (fig. S2). iRhom2* overexpression protected L929 cells from TNFα-induced apoptosis (Fig. 1A). Experiments using the metalloproteinase inhibitor BB-2516 suggested that this TNFα resistance was the result of metalloprotease (MP)-dependent release of TNFα receptors (TNFRs) from the cell surface (Fig. 1, B and C). Because TNFR shedding is mediated by TNFα convertase (TACE) (also known as a disintegrin and MP (ADAM) 17) (11), we investigated whether iRhom2 interacts with TACE. Mature TACE is generated after processing of its prodomain in the Golgi (12, 13). By preparing cell lysates in the presence of MP inhibitors to prevent autocatalytic degradation of mature TACE (12) and by performing Concanavalin A (ConA) lectin purification, we were able to use immunoblotting to clearly distinguish immature (or pro-TACE) from the active mature form of TACE (fig. S3). Immunoprecipitation of lysates of iRhom2-overexpressing fibroblasts followed by immunoblotting revealed a physical association between tagged-iRhom2 and both pro- and mature forms of TACE, which suggested that iRhom2 and TACE are associated through multiple stages of the secretory pathway (Fig. 1D). This interaction appears specific, as no such association with other ADAM family members, including ADAM9 and ADAM15, could be detected (Fig. 1D). These data suggested that association with iRhom2 might be important for regulating TACE activity.
Fig. 1.
iRhom2 confers resistance to TNFα in a MP-dependent manner and interacts with TACE. (A) L929 cells stably overexpressing iRhom2* or control vector were treated with recombinant TNFα (rTNFα) at the indicated concentrations, and percent viability was determined by Annexin-7AAD exclusion (means ± SEM; n = 4 experiments). (B) Untreated cells from (A) were cultured with or without metalloproteinase inhibitor BB-2516 (20 μM) for 24 hours. Soluble TNFR1 in the culture supernatant was measured by enzyme-linked immunosorbent assay (ELISA) (means ± SEM; n = 3 experiments). (C) Cells from (A) were treated for 24 hours with TNFα at the indicated concentrations plus 20 μM BB-2516, and viability was assessed as in (A) (means ± SEM; n = 4 experiments). (D) WT immortalized mouse embryonic fibroblasts (MEFs) stably overexpressing T7-tagged iRhom2 (iR2-T7) or control vector and Adam17−/−(TACE−/−) MEFs (negative control) were immunoprecipitated (IP) by using antibodies against T7 or TACE followed by immunoblotting (IB) to detect T7 or TACE. ADAM9 and ADAM15 were specificity controls; actin, loading control. Black arrowheads indicate immature pro-forms, whereas gray arrowheads indicate mature forms, of TACE and other ADAMs (for all figures). Empty arrowhead is likely a processed form of iRhom2. ConA purification was used to enhance detection of TACE in input lanes (unpurified input appears in fig. S3). Results are representative of three trials.
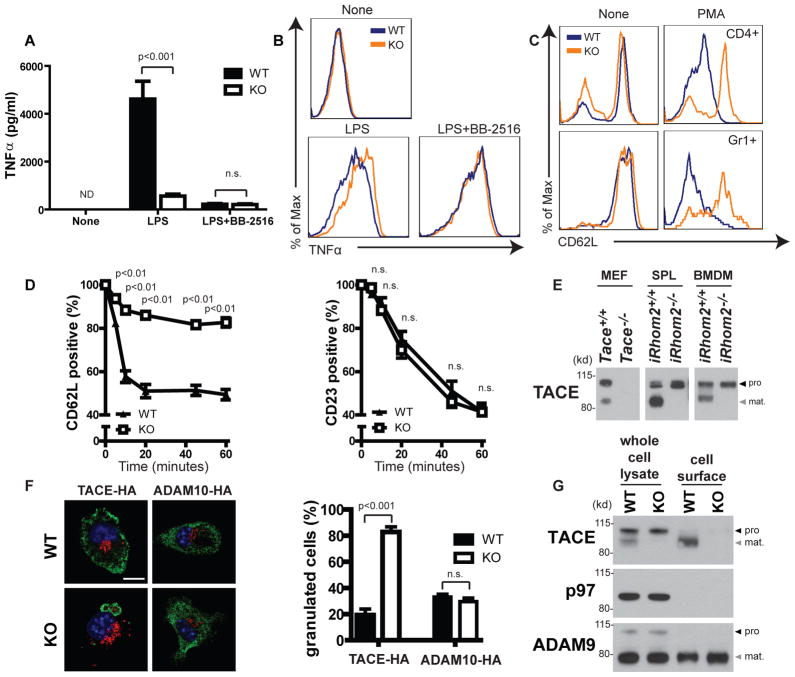
To investigate whether the relation between iRhom2 and TACE was physiologically relevant, we generated mice deficient for the gene that encodes iRhom2 (iRhom2−/−) in which exons 4 to 14 of the iRhom2 gene were deleted, which abolished expression of iRhom2 mRNA (fig. S4). iRhom2−/− mice are viable and fertile, show no obvious defects, have a normal life-span, and exhibit a normal immune cell distribution (table S1). Because TACE is classically known to be the enzyme responsible for production of soluble TNFα through surface shedding (14, 15), we analyzed TNFα production by macrophages. When thioglycollate-elicited peritoneal macrophages (TGEMs) were isolated from control (iRhom2+/+ or iRhom+/−) mice and stimulated in vitro with the Toll-like receptor (TLR) 4 ligand lipopolysaccharide (LPS), the mRNA levels of iRhom2, TACE, and TNFα were all increased (fig. S5, A to C). TACE and TNFα mRNA levels were comparably up-regulated in LPS-stimulated iRhom2−/− TGEMs (fig. S5, B and C), but significantly less TNFα protein was shed into mutant cell culture supernatants than into control supernatants (Fig. 2A). Consistent with a block in membrane-bound TNFα cleavage (16) in the absence of iRhom2, LPS-treated iRhom2−/− TGEMs accumulated higher expression of membrane-bound TNFα than controls (Fig. 2B, left). Treatment with BB-2516 mimicked iRhom2 deficiency, as it increased levels of membrane-bound TNFα on LPS-stimulated wild-type (WT) TGEMs to levels observed on untreated iRhom2−/− TGEMs (Fig. 2B, right). No difference in the secretion of other LPS-induced cytokines, such as interleukin-6 (IL-6) or IL-12, was observed (fig. S5, D and E). Although a mechanism of triggering IL-12 production involving processing of the TNFα intracellular domain has been described (17), proficient IL-12 production in iRhom2−/− macrophages after LPS is consistent with other mouse models incapable of producing soluble TNFα (18).
Fig. 2.
iRhom2 deficiency reduces TACE activity in vitro. (A and B) WT and iRhom2−/− TGEMs were stimulated in vitro with 1 μg/ml LPS, with or without 20 μM BB-2516. (A) TNFα in culture supernatants was determined by ELISA after 24 hours of treatment (means ± SEM of triplicates). (B) Membrane-bound TNFα was assayed by flow cytometry after 3 hours of treatment. Results are representative of three trials. (C) Isolated WT and iRhom2−/− total splenocytes were stimulated in vitro with 25 ng/ml PMA for 3 hours, and CD62L expression on CD4+ T cells and Gr1+ granulocytes was determined by flow cytometry. Results are representative of three trials. (D) WT and iRhom2−/− total splenocytes were stimulated with 2 mM BzATP for the indicated times, and surface levels of CD62L and CD23 (ADAM10 substrate) on B220+CD3− B cells were determined by flow cytometry (means ± SEM; n = 3 mice per group). (E) ConA-purified lysates of control and iRhom2−/− splenocytes (SPL) or BMDMs were immunoblotted to detect pro- (black arrowhead) and mature (gray arrowhead) TACE. Adam17−/−(TACE−/−) MEFs, negative control. Additional controls appear in fig. S6A. Results are representative of three trials. (F) (Top) Control and iRhom2−/− BMDMs were transfected with vectors expressing TACE-HA or ADAM10-HA (green), stimulated with LPS, and visualized by confocal immunofluorescence microscopy. Giantin (red) and 4′,6′-diamidino-2-phenylindole (DAPI) stain (blue). Scale bar, 10 μm. (Bottom) Percentages of control and iRhom2−/− BMDMs that exhibited granulated vesicular appearance of TACE or ADAM10 localization (means ± SEM; n = 4 to 6 experiments). (G) (Top) Immunoblot to detect pro- (black arrowhead) and mature (gray arrowhead) TACE in whole-cell lysates and purified cell surface fractions of control and iRhom2−/− BMDMs. (Middle) P97, intracellular protein (negative control). (Bottom) Pro- (black arrowhead) and mature (gray arrowhead) ADAM9 (positive control). Results are representative of three trials.
TACE is also crucial for the stimulus-dependent cleavage of other substrates from the surfaces of immune cells, including L-selectin (CD62L) (11). Granulocytes and CD4+ T cells that were isolated from iRhom2−/− mice and stimulated in vitro with phorbol-12-myristate-13-acetate (PMA) to activate TACE (11) showed impaired CD62L surface down-regulation compared with controls (Fig. 2C). Similarly, when WT and iRhom2−/− B cells were stimulated with the nucleotide analog 2′(3′)-O-(4-benzoyl)benzoyl adenosine 5′-triphosphate (BzATP) to induce shedding of both CD62L and CD23 (an ADAM10 substrate), only CD62L shedding was inhibited in iRhom2−/− B cells (Fig. 2D). We also detected elevated surface expression of another TACE substrate, intercellular adhesion molecule–1 (19), on iRhom2−/− TGEMs (fig. S5F). Taken together, these results suggest that iRhom2 is specifically required for TACE-mediated shedding of multiple surface molecules, including TNFα, from immune cell surfaces.
To determine potential mechanisms by which iRhom2 might control TACE activity, we examined the status of TACE maturation in the absence of iRhom2. Using immunoblotting, we readily detected both the inactive pro- and active mature forms of TACE in splenocytes and bone marrow–derived macrophages (BMDMs) from control mice. However, iRhom2−/− splenocytes and BMDMs exhibited only pro-TACE expression (Fig. 2E and fig. S6A). When we analyzed the subcellular localization of hemagglutinin (HA)–tagged TACE (TACE-HA) in WT BMDMs by immunofluorescence microscopy, TACE was broadly distributed, which included prominence in the cell periphery. In contrast, TACE appeared mislocalized in iRhom2−/− BMDMs, as it was restricted to granular vesicular compartments. (Fig. 2F and fig. S6, B and C). No discernible differences were observed in ADAM10-HA localization between WT and iRhom2−/− BMDMs (Fig. 2F and fig. S6B). To examine if this phenotype held true for endogenous TACE, we isolated cell surface proteins from BMDMs by biotinylation and probed for endogenous TACE by immunoblotting. Consistent with our microscopy data, mature TACE was correctly localized in cell surface fractions of WT BMDMs, whereas iRhom2−/− BMDMs exclusively expressed only minute quantities of pro-TACE at the cell surface (Fig. 2G). These data suggest that iRhom2 is critical for triggering TACE maturation and trafficking to the cell surface and may explain why iRhom2 is necessary for TACE activity in immune cells.
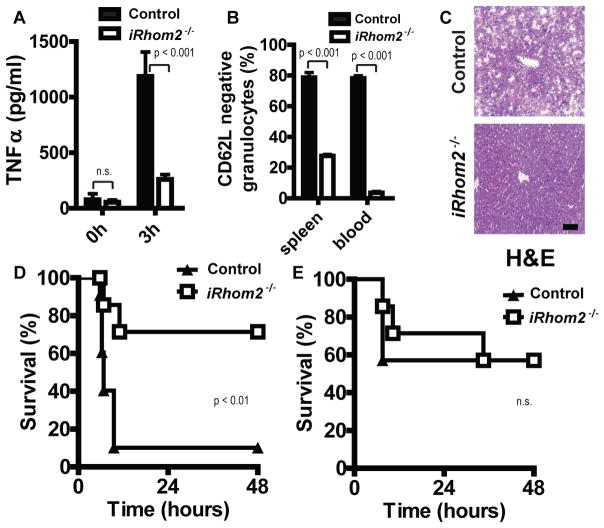
To examine the consequences of iRhom2-mediated regulation of TACE maturation in vivo, we injected control and iRhom2−/− mice with LPS and determined serum TNFα levels. The mutants showed dramatically less serum TNFα than controls (Fig. 3A), and granulocytes isolated from these animals exhibited decreased LPS-stimulated down-regulation of CD62L (Fig. 3B and fig. S7A). A well-known model of TNFα-mediated septic shock and liver damage involves the combined injection of LPS and D-galactosamine (GalN) (4). When we injected control and iRhom2−/− mice with LPS and GalN, serum TNFα was reduced in the mutants, whereas IL-6, IL-12, and interferon-γ (IFNγ) levels were comparable with those in controls (fig. S7B). Examination of liver histology 6 hours after injection showed that 87% of LPS- and GalN–treated control mice showed disrupted liver architecture, compared with only 35% of LPS- and GalN–treated iRhom2−/− mice (Fig. 3C). In terms of lethality, whereas most LPS- and GalN–treated control mice died within 24 hours, most LPS- and GalN–treated iRhom2−/− mice survived beyond the 48 hours of the experiment (Fig. 3D). However, treatment of control and iRhom2−/− mice with recombinant TNFα and GalN led to similar rates of death (Fig. 3E and fig. S7, C and D). Thus, although in vivo responses to exogenous TNFα are normal in iRhom2−/− mice, endogenous production of soluble TNFα is impaired, such that these mutants are resistant to LPS lethality.
Fig. 3.
iRhom2 deficiency prevents LPS-induced liver pathology by inhibiting TNFα shedding. (A and B) Control and iRhom2−/− mice (n = 4 per group) were intravenously injected with 0.14 mg per mouse LPS O111:B4. Serum TNFα (A) and CD62L−Gr1+CD11b+ cells in spleen and blood (B) were measured 3 hours after injection by ELISA or flow cytometry, respectively. (C) Control (n = 7) and iRhom2−/−(n = 8) mice were intraperitoneally injected with 10 mg GalN, followed 20 min later by intravenous injection of 0.05 mg LPS O111:B4. Livers were snap-frozen 6 hours after injection and sections stained with hematoxylin and eosin (H&E). Control panel is representative for six out of seven samples. iRhom2−/− panel is representative for five out of eight samples. Scale bar, 100 μm. (D) Control and iRhom2−/− mice (n = 10 to 11 per group) were injected with GalN and LPS as for (C), and survival was monitored for 48 hours. (E) Control and iRhom2−/− mice (n = 7 per group) were intraperitoneally injected with 10 mg GalN, followed 20 min later by intravenous injection of 0.15 μg rTNFα. Survival was monitored for 48 hours.
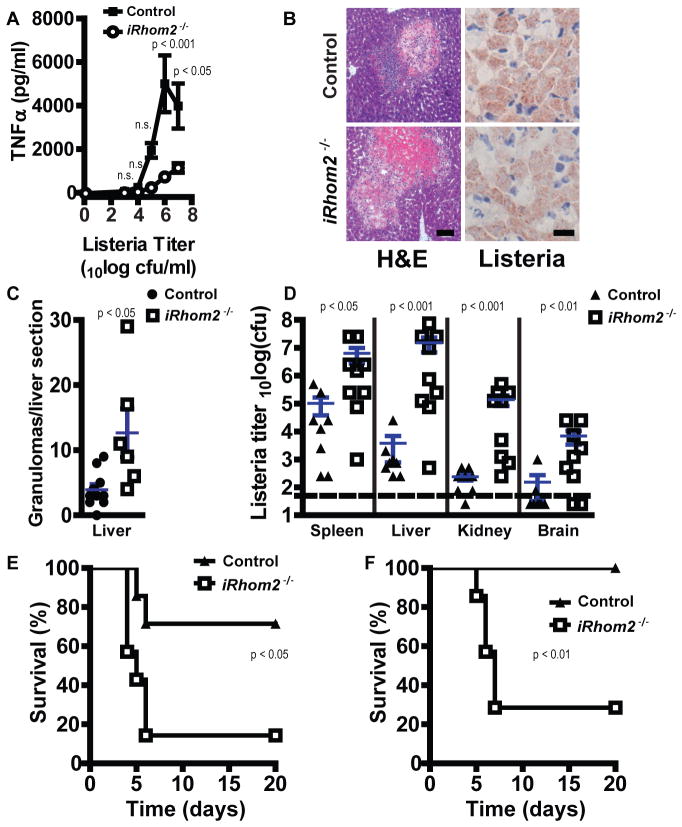
TNFα and TNFRI are crucial for defense against bacterial infections (4, 16, 20, 21). To determine whether iRhom2 is required for TNFα-mediated antibacterial activity, we infected TGEMs from untreated control and iRhom2−/− mice with the intracellular bacterium, L. monocytogenes. Little TNFα was detected in the supernatants of infected iRhom2−/− TGEM cultures (Fig. 4A). When control and iRhom2−/− mice were infected with L. monocytogenes, serum levels of IL-6, IL-12, and IFNγ were comparable (fig. S8A), and no differences in granulocyte infiltration were observed in spleen or liver (fig. S8B). Although granuloma formation and intracellular L. monocytogenes were detected in liver tissues of both control and iRhom2−/− mice (Fig. 4B), more granulomas were present in infected iRhom2−/− liver than in the control (Fig. 4C). In addition, L. monocytogenes titers in spleen, liver, kidney, and brain were all significantly higher in iRhom2−/− mice than in controls 4 days after infection (Fig. 4D). As a result, iRhom2−/− mice rapidly succumbed to the infection (Fig. 4E), a pattern that held true even at bacterial doses that were nonlethal for control mice (Fig. 4F). Thus, iRhom2 is critical for defense against L. monocytogenes.
Fig. 4.
iRhom2 is crucial for control of L. monocytogenes. (A) TGEMs (105) isolated from control or iRhom2−/− mice (n = 5 to 8 per group) were exposed to the indicated titers of L. monocytogenes for 24 hours. TNFα in culture supernatants was determined by ELISA (means ± SEM). (B) Control and iRhom2−/− mice (n = 6 to 10 per group) were infected with 104 colony-forming units (cfu) L. monocytogenes. Livers were isolated on day 4 after infection, sectioned, and stained with H&E (left) or with antibody against Listeria (right). Scale bars: left, 100 μm; right, 20 μm. (C) Granulomas were counted in F4/80-stained liver sections (not shown) from the mice in (B). Data points are granulomas or liver of individual mice. Blue lines, means ± SEM (n = 6 to 10 per group). (D) Control and iRhom2−/− mice (n = 8 to 9 mice per group) were infected with 105 cfu L. monocytogenes, and bacterial titers were determined in spleen, liver, kidney, and brain on day 4 after infection. Data points are titers of individual mice. Dashed line, limit of detection. Blue lines, means ± SEM. (E and F) Control and iRhom2−/− mice (n = 7 per group) were infected with 5 × 104 cfu (E) or 5 × 103 cfu (F) L. monocytogenes, and mouse survival was monitored for 20 days.
Our data support a role of iRhom2 as an essential factor for the activity and trafficking of TACE in hematopoietic cells and are supported by the results presented in the accompanying manuscript by Adrain et al. (22). Mice with a myeloid cell–specific deletion in TACE, are similar to iRhom2−/− mice in that both are resistant to LPS-induced septic shock and defective in generating soluble TNFα (23). Unlike what we observed in the iRhom2−/− mice, TACE-deficient (Adam17−/−) mice often die perinatally (11). These differences may be the result of cell-, or context-specific effects of iRhom2 function. The inhibition of iRhom2 may represent a potential new therapeutic approach for treating TNFα-mediated diseases.
Supplementary Material
Acknowledgments
The authors thank S. Le Gall, S. McCracken, A. Elia, E. Arpaia, and J. Height for experimental assistance and M. Saunders for scientific editing. The data reported in this manuscript are tabulated in the main paper and in the Supporting Online Material. P.A.L. was supported by the Sofja Kovalevskaja Award 2010 of the Alexander von Humboldt Foundation and the Strategic Research Fund of the Heinrich Heine University. K.S.L. was funded by the Sofja Kovalevskaja Award 2008 of the Alexander von Humboldt Foundation; Deutsche Forschungsgemeinschaft grant LA1419/3-1; and the Molecules of Infection Center, Manchot Graduate School (Jürgen Manchot Foundation). This study was supported by the Collaborative Research Center 575 (SFB575: Experimental Hepatology; Coordinator: D.H.). C.P.B. was supported by NIH GM64750, and T.M. by the Emerald Foundation. K.O. was supported in part by the Naito Foundation; the Mochida Memorial Foundation for Medical and Pharmaceutical Research; the Senri Life Science Foundation; and the Ministry of Education, Culture, Sports, Science and Technology of Japan. T.W.M., D.R.M., and K.O. have filed U.S. Patent Application No: 61/426,396 regarding the use of iRhom2 for regulating innate immunity. This work was generously supported by funding from the Canadian Institutes of Health Research and the Terry Fox Foundation.
Footnotes
Link to final version published in Science: www.sciencemag.org/content/335/6065/229
www.sciencemag.org/cgi/content/full/VOL/ISSUE/PAGE/DC1
Materials and Methods
References and Notes
- 1.Scott DL, Kingsley GH. Tumor necrosis factor inhibitors for rheumatoid arthritis. N Engl J Med. 2006;355:704. doi: 10.1056/NEJMct055183. [DOI] [PubMed] [Google Scholar]
- 2.Palladino MA, Bahjat FR, Theodorakis EA, Moldawer LL. Anti-TNF-alpha therapies: The next generation. Nat Rev Drug Discov. 2003;2:736. doi: 10.1038/nrd1175. [DOI] [PubMed] [Google Scholar]
- 3.Feldmann M. Development of anti-TNF therapy for rheumatoid arthritis. Nat Rev Immunol. 2002;2:364. doi: 10.1038/nri802. [DOI] [PubMed] [Google Scholar]
- 4.Pfeffer K, et al. Mice deficient for the 55 kd tumor necrosis factor receptor are resistant to endotoxic shock, yet succumb to L. monocytogenes infection. Cell. 1993;73:457. doi: 10.1016/0092-8674(93)90134-C. [DOI] [PubMed] [Google Scholar]
- 5.Bhattacharya D, Logue EC, Bakkour S, DeGregori J, Sha WC. Identification of gene function by cyclical packaging rescue of retroviral cDNA libraries. Proc Natl Acad Sci USA. 2002;99:8838. doi: 10.1073/pnas.132274799. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Koonin EV, et al. The rhomboids: A nearly ubiquitous family of intramembrane serine proteases that probably evolved by multiple ancient horizontal gene transfers. Genome Biol. 2003;4:R19. doi: 10.1186/gb-2003-4-3-r19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lemberg MK, Freeman M. Functional and evolutionary implications of enhanced genomic analysis of rhomboid intramembrane proteases. Genome Res. 2007;17:1634. doi: 10.1101/gr.6425307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Zettl M, Adrain C, Strisovsky K, Lastun V, Freeman M. Rhomboid family pseudoproteases use the ER quality control machinery to regulate intercellular signaling. Cell. 2011;145:79. doi: 10.1016/j.cell.2011.02.047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Zou H, et al. Human rhomboid family-1 gene RHBDF1 participates in GPCR-mediated transactivation of EGFR growth signals in head and neck squamous cancer cells. FASEB J. 2009;23:425. doi: 10.1096/fj.08-112771. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Nakagawa T, et al. Characterization of a human rhomboid homolog, p100hRho/RHBDF1, which interacts with TGF-alpha family ligands. Dev Dyn. 2005;233:1315. doi: 10.1002/dvdy.20450. [DOI] [PubMed] [Google Scholar]
- 11.Peschon JJ, et al. An essential role for ectodomain shedding in mammalian development. Science. 1998;282:1281. doi: 10.1126/science.282.5392.1281. [DOI] [PubMed] [Google Scholar]
- 12.Schlöndorff J, Becherer JD, Blobel CP. Intracellular maturation and localization of the tumour necrosis factor alpha convertase (TACE) Biochem J. 2000;347:131. doi: 10.1042/0264-6021:3470131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Reiss K, Saftig P. The “a disintegrin and metalloprotease” (ADAM) family of sheddases: Physiological and cellular functions. Semin Cell Dev Biol. 2009;20:126. doi: 10.1016/j.semcdb.2008.11.002. [DOI] [PubMed] [Google Scholar]
- 14.Black RA, et al. A metalloproteinase disintegrin that releases tumour-necrosis factor-alpha from cells. Nature. 1997;385:729. doi: 10.1038/385729a0. [DOI] [PubMed] [Google Scholar]
- 15.Moss ML, et al. Cloning of a disintegrin metalloproteinase that processes precursor tumour-necrosis factor-alpha. Nature. 1997;385:733. doi: 10.1038/385733a0. [DOI] [PubMed] [Google Scholar]
- 16.Alexopoulou L, et al. Transmembrane TNF protects mutant mice against intracellular bacterial infections, chronic inflammation and autoimmunity. Eur J Immunol. 2006;36:2768. doi: 10.1002/eji.200635921. [DOI] [PubMed] [Google Scholar]
- 17.Friedmann E, et al. SPPL2a and SPPL2b promote intramembrane proteolysis of TNFalpha in activated dendritic cells to trigger IL-12 production. Nat Cell Biol. 2006;8:843. doi: 10.1038/ncb1440. [DOI] [PubMed] [Google Scholar]
- 18.Torres D, et al. Membrane tumor necrosis factor confers partial protection to Listeria infection. Am J Pathol. 2005;167:1677. doi: 10.1016/S0002-9440(10)61250-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Tsakadze NL, et al. Tumor necrosis factor-alpha-converting enzyme (TACE/ADAM-17) mediates the ectodomain cleavage of intercellular adhesion molecule-1 (ICAM-1) J Biol Chem. 2006;281:3157. doi: 10.1074/jbc.M510797200. [DOI] [PubMed] [Google Scholar]
- 20.Pasparakis M, Alexopoulou L, Episkopou V, Kollias G. Immune and inflammatory responses in TNF alpha-deficient mice: A critical requirement for TNF alpha in the formation of primary B cell follicles, follicular dendritic cell networks and germinal centers, and in the maturation of the humoral immune response. J Exp Med. 1996;184:1397. doi: 10.1084/jem.184.4.1397. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rothe J, et al. Mice lacking the tumour necrosis factor receptor 1 are resistant to TNF-mediated toxicity but highly susceptible to infection by Listeria monocytogenes. Nature. 1993;364:798. doi: 10.1038/364798a0. [DOI] [PubMed] [Google Scholar]
- 22.Adrain C, Zettl M, Christova Y, Taylor N, Freeman M. Tumor necrosis factor signaling requires iRhom2 to promote trafficking and activation of TACE. Science. 2012;335:6065. doi: 10.1126/science.1214400. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Horiuchi K, et al. Cutting edge: TNF-alpha-converting enzyme (TACE/ADAM17) inactivation in mouse myeloid cells prevents lethality from endotoxin shock. J Immunol. 2007;179:2686. doi: 10.4049/jimmunol.179.5.2686. [DOI] [PubMed] [Google Scholar]
- 24.Horiuchi K, et al. Substrate selectivity of epidermal growth factor-receptor ligand sheddases and their regulation by phorbol esters and calcium influx. Mol Biol Cell. 2007;18:176. doi: 10.1091/mbc.E06-01-0014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.McIlwain DR, et al. Smg1 is required for embryogenesis and regulates diverse genes via alternative splicing coupled to nonsense-mediated mRNA decay. Proc Natl Acad Sci USA. 2010;107:12186. doi: 10.1073/pnas.1007336107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Weskamp G, Krätzschmar J, Reid MS, Blobel CP. MDC9, a widely expressed cellular disintegrin containing cytoplasmic SH3 ligand domains. J Cell Biol. 1996;132:717. doi: 10.1083/jcb.132.4.717. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lum L, Reid MS, Blobel CP. Intracellular maturation of the mouse metalloprotease disintegrin MDC15. J Biol Chem. 1998;273:26236. doi: 10.1074/jbc.273.40.26236. [DOI] [PubMed] [Google Scholar]
- 28.Lang PA, et al. Tissue macrophages suppress viral replication and prevent severe immunopathology in an interferon-I-dependent manner in mice. Hepatology. 2010;52:25. doi: 10.1002/hep.23640. [DOI] [PubMed] [Google Scholar]
- 29.Lang PA, et al. Aggravation of viral hepatitis by platelet-derived serotonin. Nat Med. 2008;14:756. doi: 10.1038/nm1780. [DOI] [PubMed] [Google Scholar]
- 30.Lang PA, et al. Hematopoietic cell-derived interferon controls viral replication and virus-induced disease. Blood. 2009;113:1045. doi: 10.1182/blood-2007-10-117861. [DOI] [PubMed] [Google Scholar]
- 31.Navarini AA, et al. Increased susceptibility to bacterial superinfection as a consequence of innate antiviral responses. Proc Natl Acad Sci USA. 2006;103:15535. doi: 10.1073/pnas.0607325103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Sauer B, Henderson N. Site-specific DNA recombination in mammalian cells by the Cre recombinase of bacteriophage P1. Proc Natl Acad Sci USA. 1988;85:5166. doi: 10.1073/pnas.85.14.5166. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Bernsel A, Viklund H, Hennerdal A, Elofsson A. TOPCONS: Consensus prediction of membrane protein topology. Nucleic Acids Res. 2009;37:W465. doi: 10.1093/nar/gkp363. Web Server issue. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.