Abstract
This review describes the role of cytokines and their downstream signaling cascades on the modulation of learning and memory. Immune proteins are required for many key neural processes and dysregulation of these functions by systemic inflammation can result in impairments of memory that persist long after the resolution of inflammation. Recent research has demonstrated that manipulations of individual cytokines can modulate learning, memory, and synaptic plasticity. The many conflicting findings, however, have prevented a clear understanding of the precise role of cytokines in memory. Given the complexity of inflammatory signaling, understanding its modulatory role requires a shift in focus from single cytokines to a network of cytokine interactions and elucidation of the cytokine-dependent intracellular signaling cascades. Finally, we propose that whereas signal transduction and transcription may mediate short-term modulation of memory, long-lasting cellular and molecular mechanisms such as epigenetic modifications and altered neurogenesis may be required for the long lasting impact of inflammation on memory and cognition.
Keywords: Cytokine, Memory Modulation, Learning, signal transduction, epigenetic modification, neurogenesis
1. Introduction
Immune proteins and signaling play many key roles in the brain (Shatz, 2009). The central nervous system’s own immune cells, microglia and astrocytes are required for normal synaptic functions including synaptic pruning, synapse formation and synaptic transmission (Benarroch, 2013; Papa, De Luca, Petta, Alberghina, & Cirillo, 2014; Stephan, Barres, & Stevens, 2012). A wealth of literature in animal models of inflammation supports the causal role of inflammatory signaling in memory and cognitive deficits. Systemic injection with lipopolysaccharide (LPS) impairs memory consolidation (Pugh et al., 1998), acquisition of operant conditioning (Aubert, Vega, Dantzer, & Goodall, 1995) and learning in Morris Water Maze tasks (see C. Cunningham & Sanderson, 2008).
In humans, systemic triggers of inflammation, including illness, injury or major surgery (Hudetz et al., 2009; Selnes et al., 2003; Shapira-Lichter et al., 2008) are associated with deficits in a variety of cognitive and memory tasks. Patients with cancer, after myocardial infarction, or major surgery commonly develop post-traumatic stress disorder (Ginzburg & Ein-Dor, 2011; Meister et al., 2013) or cognitive deficits (Fredericks, 2012) long after the illness, suggesting a persistent role for immune function in alterations of memory. Inflammatory signaling is thus considered to be a critical contributor to the short- and long term modulation of mood and cognition. However, the precise role and mechanisms by which cytokines modulate memory remain unknown.
The intricacy of inflammatory signaling presents several complications in understanding the roles and mechanisms of cytokines in neural and cognitive functions. Inflammatory events are not specific to a single cytokine increasing at a single timepoint, instead inflammation produces dynamic regulation of many cytokines (Conti et al., 2008; Gayle, Ilyin, Miele, & Plata-Salamán, 1998; Schindler et al., 1990). Cytokines are also extremely pleiotropic (e.g., Guzmán & Hallal-Calleros, 2010) and exhibit extensive redundancy, with many distinct proteins exerting overlapping effects (X. Liu, Fang, Guo, Mei, & Zhang, 2013). In contrast, the downstream effects of cytokines differ depending on the presence of other cytokines and specific cell types (S. Lund et al., 2006; Norden, Fenn, Dugan, & Godbout, 2014). Despite well-delineated interactions between cytokines within the immune system, the dynamic regulation of cytokines in the central nervous system remains unclear. Similarly, the precise roles of inflammatory signaling in the physiology of neurons, circuits, and cognitive function is not known.
Recent work has made significant progress in establishing the effects of specific cytokines in the brain on learning, memory and plasticity. However, these studies have also uncovered contradictory roles of cytokines in modulation of memory. Given the complexity of inflammatory signaling in the brain, we propose that shifting the focus from individual cytokines to networked activation of cytokines will be a constructive way to understand the impact of inflammatory signaling on memory and cognitive function.
Here we will review the current work on individual cytokines and their effects on learning and plasticity, and begin to unpack potential mechanisms by which cytokine-dependent signaling may intersect with molecular mechanisms of memory. We will discuss both short-lasting effects via intracellular signaling cascades, as well as long lasting effects due to persistent changes in neurogenesis and epigenetic modifications.
2. Modulation of memory by cytokines
Interleukin 1β (IL-1β), Interleukin 6 (IL-6) and tumor necrosis factor α (TNFα) are among the most commonly studied cytokines in the brain (Capuron & Miller, 2011; Goehler, 2008). These proteins are strongly upregulated in the bloodstream after systemic inflammatory events such as LPS injection (Skelly, Hennessy, Dansereau, & Cunningham, 2013), sepsis model (Mina et al., 2013), surgery (Terrando et al., 2011), and other peripheral injuries (O. T. Bağdatoğlu, Polat, Bağdatoğlu, & Atik, 2008). In addition, IL-1β, IL-6 and TNFα are strongly expressed in the hippocampus after manipulations in the periphery (Burton, Sparkman, & Johnson, 2011; Cibelli et al., 2010; Datta & Opp, 2008; Ren et al., 2011) or brain (Belarbi et al., 2012) and are therefore well placed to modulate memory.
There is some evidence for involvement of IL-1β, TNFα, and IL-6 in specific memory processes including acquisition, consolidation, or retrieval. For example, peripheral IL-6 levels correlate with memory retrieval (Elderkin-Thompson, Irwin, Hellemann, & Kumar, 2012), and post-training injection of LPS disrupts consolidation of context fear conditioning via IL-1 (Pugh et al., 1998). Most studies, however, have used transgenic models, chronic injection, or acute injection of cytokine or inflammatory stimulus prior to training, demonstrating roles in modulation of learning and memory, but obscuring their role in specific memory processes.
In this section, we will describe current findings and conflicting results on the role of specific cytokines in learning and memory. Furthermore, we will describe how an interactive framework of cytokine signaling may begin to resolve difficulties in understanding the role of inflammatory signaling in the modulation of learning and memory.
2.1 Interleukin 1β
Several studies demonstrate a critical role for IL-1β in the formation of hippocampal dependent memory. IL-1β is upregulated by context fear conditioning (Goshen et al., 2007) and LTP (Balschun et al., 2003; del Rey, Balschun, Wetzel, Randolf, & Besedovsky, 2013; Schneider et al., 1998), suggesting a role for this cytokine in normal memory processing. Consistent with this, small increases (1ng) of IL-1β injected centrally enhance context fear conditioning (Goshen et al., 2007), passive avoidance and spatial memory (C. Song, Phillips, & Leonard, 2003; Yirmiya, 2002). Adding to the evidence for the requirement of IL-1β are studies of the endogenous IL-1 receptor antagonist, IL-1ra. Overexpression of IL-1ra blocks context fear conditioning (Goshen et al., 2007), passive avoidance (Depino et al., 2004), and spatial memory (Spulber, Mateos, Oprica, Cedazo-Minguez, et al., 2009b; Yirmiya, 2002), as well as LTP (Goshen et al., 2007; Ross, Allan, Rothwell, & Verkhratsky, 2003). Together these findings strongly suggest that IL-1β is required for hippocampal-dependent learning and memory.
In contrast, acute intrahippocampal injection of IL-1β leads to impairments of both context fear conditioning (Gonzalez, Schiöth, Lasaga, & Scimonelli, 2009) and reconsolidation (Machado, González, Schiöth, Lasaga, & Scimonelli, 2010). Chronic overexpression of IL-1β in the hippocampus also leads to impairments of spatial memory (Moore, Wu, Shaftel, Graham, & O’Banion, 2009) and context fear conditioning (Hein et al., 2010). Similarly, application of IL-1β impairs induction and maintenance of LTP (Loscher, 2003; Ross et al., 2003; Schneider et al., 1998; Vereker, O’Donnell, & Lynch, 2000) demonstrating an IL-1β-induced deficit in hippocampal memory processes.
Adding to the complexity of the role of IL-1β, three different lines of IL-1 receptor (IL-1R) knockout mice suggest three different roles of IL-1R in memory. In IL-1R knockout mice, several studies demonstrated impaired hippocampal LTP, spatial memory, or context fear conditioning, but intact auditory fear conditioning (Avital et al., 2003; Goshen et al., 2009). In direct contradiction of this finding, other groups have shown that IL-1R knockout mice exhibit enhanced context and auditory fear conditioning (Koo & Duman, 2009). A third IL-1R knockout line failed to show any alterations in spatial or non-spatial learning tasks, or context fear conditioning (C. L. Murray, Obiang, Bannerman, & Cunningham, 2013).
These findings suggest that although IL-1β does play a role in modulating memory, the precise function strongly depends on the site of injection, timing, and dose (Goshen et al., 2007; Yirmiya, 2002). The effects are consistent with the tight negative regulation of cytokine activity by endogenous receptor antagonists (IL-1ra) (Spulber, Bartfai, & Schultzberg, 2009a) and decoy receptors (IL-1R2) (Garlanda, Dinarello, & Mantovani, 2013). Further supporting the synergistic role of interactions between IL-1β and IL-1ra is the finding that despite the impairing effects of either on their own, application of IL-1β and IL-1ra together normalizes the maintenance of LTP (A. J. Cunningham, Murray, O’Neill, Lynch, & O’Connor, 1996; Loscher, 2003; Ross et al., 2003).
Interactions between IL-1β and other IL-1 family members (Garlanda et al., 2013) likely contribute the effects on memory. For example, IL-1α is increased after passive avoidance (Depino et al., 2004). Another IL-1 family cytokine, IL-18, also regulates memory. IL-18 knockout mice (Yaguchi, Nagata, Yang, & Nishizaki, 2010), or application of IL-18 (Cumiskey, Curran, Herron, & O’Connor, 2007; Curran & O’Connor, 2001) impair memory and LTP, respectively. The ambiguity of the effects of IL-1β and IL-1R on memory, therefore is likely due to co-regulation and compensatory mechanisms of IL-1 family cytokines and their receptors (Garlanda et al., 2013).
2.2 Tumor necrosis factor α
In contrast to the bidirectional effects of IL-1β, inhibition of TNFα alone does not impair memory (Belarbi et al., 2012) and TNFα has been consistently implicated in deficits of memory and plasticity. Specifically, overexpression of TNFα in neurons or glial cells impairs passive avoidance memory (Fiore et al., 2000), synaptic plasticity (Butler, O’Connor, & Moynagh, 2004; A. J. Cunningham et al., 1996; Tancredi et al., 1992) and cerebellar learning (Paredes, Acosta, Gemma, & Bickford, 2010). Consistent with a memory impairing effect of this cytokine, TNFα mediates memory deficits after chronic LPS administration (Belarbi et al., 2012). Whereas these results suggest TNFα is not required for normal learning or memory consolidation, both TNFα and its family member TNFβ are increased after learning (Cartford, Gemma, & Bickford, 2002), and genetic deletion of both TNFα and β results in deficits across a variety of learning paradigms (Baune et al., 2008; Camara et al., 2013). Together, these studies suggestthat TNFβ, but not TNFα, is required for normal memory processes.
Supporting a role for TNFβ in learning and memory, genetic manipulations of TNF receptors alter both memory and plasticity. Deletion of all TNFR results in aberrant LTD (Albensi & Mattson, 2000) and either TNFR1 or TNFR2 impairs spatial memory in the Barnes maze (Baune et al., 2008; Camara et al., 2013). In comparison to TNFR1−/−, TNFR2 −/− mice have additional impairments in Y-maze (Camara et al., 2013) and in some cases, novel object recognition (Baune et al., 2008; Naude et al., 2014). The available evidence suggests that TNFα impairs, but TNFβ is required for learning and memory. The precise role for TNFβ in learning, and its interactions with TNFα and other cytokines in the brain are yet to be explored.
2.3 Interleukin 6
Studies of IL-6 in learning and plasticity show a similar pattern as seen for TNFα, where genetic deletion of IL-6 fails to disrupt learning and memory (Braida et al., 2004) whereas overexpression (Heyser, Masliah, Samimi, Campbell, & Gold, 1997; Wei et al., 2012) or application of IL-6 (A.-J. Li, Katafuchi, Oda, Hori, & Oomura, 1997; Tancredi et al., 2000) cause broad memory impairments and diminished LTP, respectively. Together, these findings suggest that IL-6 is not required for learning and memory, but contributes to impairments in cognitive function after an inflammatory event.
Additional evidence, however, suggests a more subtle modulatory role of IL-6 in learning and memory. Hippocampal IL-6 levels are increased after learning (del Rey et al., 2013) and as a consequence of LTP induction (Balschun et al., 2004; Jankowsky, Derrick, & Patterson, 2000). In addition, IL-6 application after tetanic stimulation results in a decrease of LTP maintenance, suggesting that expression of IL-6 after learning may be an endogenous mechanism for limiting plasticity (Balschun et al., 2004). Consistent with this interpretation, IL-6−/− mice show enhanced learning of the radial arm maze compared with wild type mice (Braida et al., 2004). Therefore, IL-6 plays a limiting role in plasticity during memory formation in the absence of inflammation and further impairs learning and memory during inflammatory events.
2.4 Networked inflammatory signaling
The evidence described above clearly shows a role for IL-1β, TNFα, and IL-6 in the modulation of memory, however there are many instances in which the findings are clearly contradictory despite similar methods and manipulations across studies. For example, IL-1β has been shown to both enhance (Goshen et al., 2007) and impair (Gonzalez et al., 2009; 2013) context fear conditioning. Such discrepancies may arise because rather than direct effects on memory, cytokines exert their effects indirectly via network properties of inflammatory signaling. IL-1β, for example, is not increased in isolation, and also leads to increases in TNFα, IL-6, IL-1 family proteins, and cytokine receptors (Anisman, Gibb, & Hayley, 2008; Moore et al., 2009; Shaftel et al., 2007; Skelly et al., 2013) across multiple brain regions. Similarly, targeting either TNFα or IL-6 leads to changes in expression of other inflammatory cytokines (Balschun et al., 2004; del Rey et al., 2013; Schindler et al., 1990; Skelly et al., 2013). The feed forward nature of cytokine expression means that many of the effects on learning and memory attributed to any individual cytokine are more likely due to the cumulative effects of all active cytokines.
Network interactions of cytokines are not limited to regulation of, and between IL-1β, TNFα, and IL-6. Rather, activation of any of these cytokines results in altered expression of a variety of additional cytokines including IL-10 (C. Platzer, Meisel, Vogt, Platzer, & Volk, 1995; Steensberg, Fischer, Keller, Møller, & Pedersen, 2003) and IL-4 (Nolan et al., 2005), chemokines such as macrophage inflammatory protein (MIP-2, CXCL2), the monocyte chemotactic protein (MCP-1, CCL2), and karatinocyte derived cytokine (KC; CXCL1) (Moore et al., 2009), as well as growth factors including NGF and BDNF (Y.-L. Lin & Wang, 2014; C. Song, Zhang, & Dong, 2013). The modulation of learning memory by commonly studied inflammatory markers is therefore likely due to indirect effects via a network of inflammation-related signals. Of particular interest in understanding the influence of networked cytokines on learning and memory are regulators of the inflammatory response, including IL-4 and IL-10 (A. M. Lynch et al., 2004; Nolan et al., 2005; Steensberg et al., 2003), and CCL2 (Cazareth, Guyon, Heurteaux, Chabry, & Petit-Paitel, 2014).
Consistent with a regulatory role, IL-4 and IL-10 alleviate the deleterious impact of inflammatory processes on memory and plasticity (A. M. Lynch et al., 2004; Nolan et al., 2005; Richwine, Sparkman, Dilger, Buchanan, & Johnson, 2009). IL-10 alleviates the impairing effects of LPS (A. M. Lynch et al., 2004) or IL-1β (Kelly et al., 2001; Nolan et al., 2005) on LTP. Both IL-4 and IL-10 can abrogate learning and memory deficits observed in inflammatory models of Alzheimer’s disease (Kawahara et al., 2012; Kiyota et al., 2012; 2010). Finally, decreased IL-4 correlates with upregulation of IL-1β (Maher, Martin, & Lynch, 2004) and contributes to IL-1β-associated memory deficits (Maher, Nolan, & Lynch, 2005; Nolan et al., 2005).
Chemokines also play a key role in the inflammatory network. The monocyte chemoattractant protein 1 (MCP-1/CCL2) is induced by inflammatory signaling (Cazareth et al., 2014) and together with its receptor CCR2, has been implicated in modulation of memory (Naert & Rivest, 2011; 2012). Like IL-4 and IL-10, most effects of CCL2 on learning and memory observed thus far are protective. Overexpression of CCL2 prevents the impairments in fEPSP due to acute ethanol exposure (Bray, Reyes, Roberts, Ransohoff, & Gruol, 2013). Similarly, chronic transgenic overexpression of CCL2 protects against ethanol-induced impairments in context and cued fear conditioning (Bray et al., 2013). Consistent with this protective effect of CCL2, CCR2 deficiency leads to exaggerated deficits in spatial memory and contextual fear conditioning deficits in an Alzheimer’s model (Naert & Rivest, 2011). Together with IL-4 and IL-10, chemokines including CCL2, are therefore central to the modulation of learning and memory by inflammatory signaling, and differential activation of these cytokines may explain inconsistent effects of IL-1β, TNFα and IL-6 in other studies.
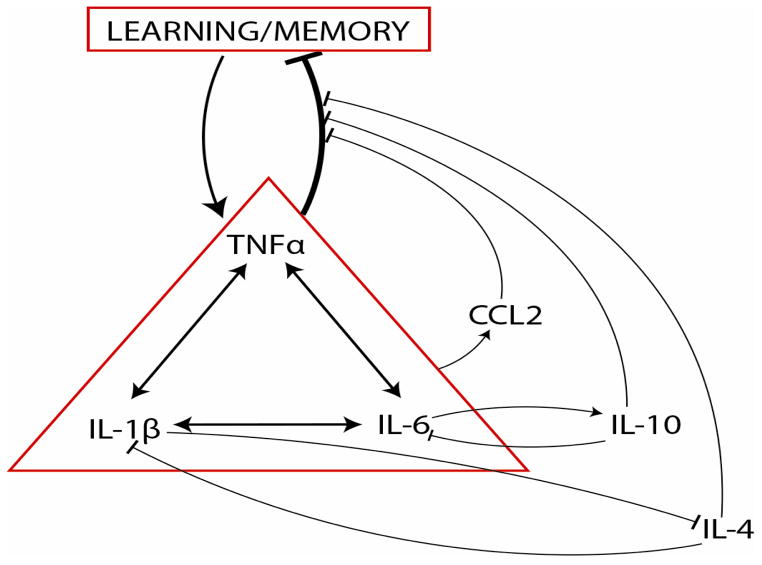
Many of the conflicting results from studies of individual cytokines on the modulation of memory may thus be explained by compensatory and synergistic effects of other cytokines, chemokines, and growth factors (Yogeetha et al., 2013) in the network of inflammatory signaling. For example, IL-4, IL-10 and CCL2 interact with IL-1β, IL-6, and TNFα in the modulation of memory processes (Figure 1). Elucidating the larger network of cytokine interactions will be required to understand the conditions under which inflammatory signaling enhances or impairs learning and memory. This network-level analysis will provide a framework for understanding the contradictory effects of individual cytokines on learning and memory
Figure 1.
Modulation of learning and memory by networked activation of cytokines. IL- 1β, TNFα, and IL-6 indirectly modify memory processes via interactions and regulation of cytokines and chemokines with similar and opposing effects. The state of the cytokine network is therefore more predictive of the effect on memory than the level of any single cytokine. Arrows represent excitatory connections, bars represent inhibitory connections.
IL-1β Interleukin 1β; TNFα Tumor Necrosis Factor α; IL-6 Interleukin 6; IL-4 Interleukin 4; IL-10 interleukin 10; CCL2 C-C-motif ligand 2. Arrows represent positive influence. Blocked head represents negative regulation.
3. Kinase signaling and transcriptional regulation
Given the complexity of inflammatory networks at the level of cytokines and their receptors, an alternative approach to studying the role and mechanisms of inflammatory signaling is to focus on their downstream effectors. Here, the patterns of intracellular signaling provide a “readout” of the summed cytokine activity during an inflammatory event. Therefore, defining the intracellular mechanisms of cytokine signaling may provide a way to move from the study of individual cytokines toward understanding the effect of cytokine networks on learning and memory.
Multiple cytokines converge on the same signal transduction pathways. In particular, many exert their action via Janus Kinase (JAK)-Signal Transduction and Activator of Transcription (STAT) cascade (Heim, 1999; Murray, 2007) (see Figure 2) and mitogen activated protein kinase (MAPK) pathways (Kaminska, 2005). Second messenger and transcriptional pathways are also largely conserved across systems and the same signaling cascades are required for both immune and cognitive functions. For example, the mitogen- and stress-activated protein kinase families, including promiscuous extracellular signal regulated kinase (ERK)-1/2 and p38MAPK (Alonso, Bevilaqua, Izquierdo, Medina, & Cammarota, 2003; Shalin et al., 2004) play critical roles in memory formation as well as immune signaling (Ashwell, 2006; Kaminska, 2005). Points of convergence between cytokine-dependent signal transduction and those of memory may be one way in which these systems interact.
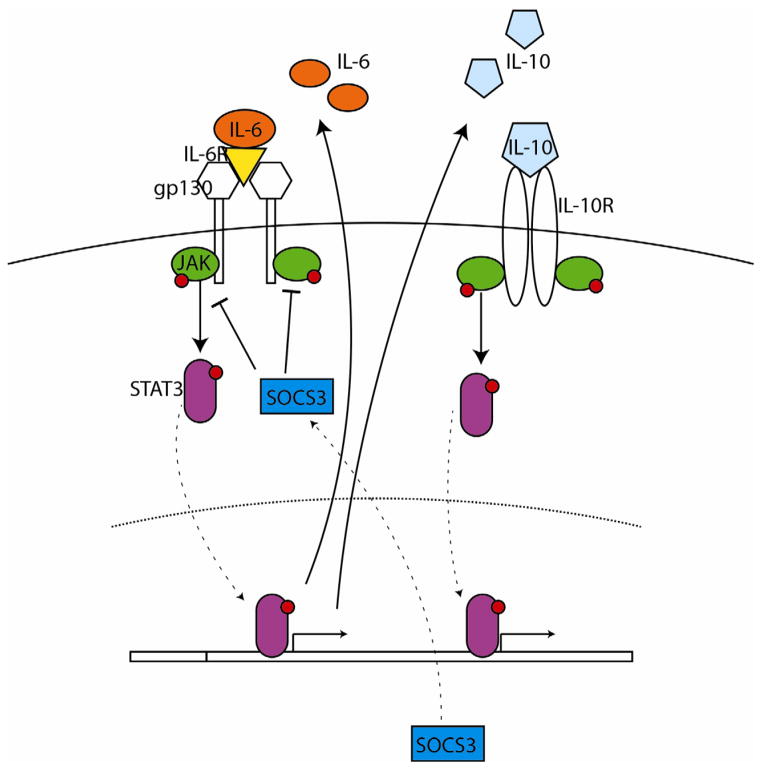
Figure 2.
Signal transduction pathways integrate activity of multiple cytokines. For example, despite opposing effects on the modulation of memory, IL-6 and IL-10 converge on the JAK/STAT3 pathway. STAT3 mediates transcription of SOCS3 which forms an inhibitory feedback pathway, blocking JAK/STAT3 signaling specifically at the IL-6 receptor complex. Thus high STAT3 activity with low SOCS3 suggests preferential IL-6 mediated outcomes, whereas STAT3 and high SOCS3 activity suggests IL-10 dominated signaling. Patterns of intracellular signaling cascades during inflammation therefore provide complementary information about the role of networked cytokine activation on learning and memory.
JAK Janus kinase; STAT3 Signal transduction and transcription 3; IL-6 Interleukin 6; IL-10 interleukin 10; IL-6R Interleukin 6 receptor; IL-10R Interleukin 10 receptor; gp130 Glycoprotein130; SOCS3 Suppressor of Cytokine Signaling 3
3.1 MAPK signaling
Indeed, decreased ERK1/2 activation after IL-6 or IL-1β application correlates with impairments of LTP (Tancredi et al., 2000) and fear conditioning (Gonzalez et al., 2013), respectively. In contrast, p38MAPK and c-Jun-N-Terminal Kinase (JNK) are increased in the hippocampus after systemic administration of LPS (Lonergan, Martin, Horrobin, & Lynch, 2004), and inhibition of p38 blocks the LPS-induced alterations in plasticity (Barry, Nolan, Clarke, Lynch, & Lynch, 2005). Consistent with these findings, p38MAPK and JNK are activated during (Vereker et al., 2000) and play causal roles in the inhibition of LTP by IL-1β (Coogan, O’Neill, & O’Connor, 1999; Kelly, Laroche, & Davis, 2003a; Kelly et al., 2003b; Tong et al., 2012), IL-18 (Curran, Murray, & O’Connor, 2003), or TNFα (Butler et al., 2004). This is also true for memory, where inhibition of p38MAPK prevents the impairing effects of IL-1β (Gonzalez et al., 2009). Therefore, the disruptive effects of inflammatory signaling on memory and plasticity are mediated, at least in part, by p38MAPK and JNK.
More interesting is that interactions between cytokines also seem to be mediated via p38MAPK and JNK. For example, either IL-10 (Kelly et al., 2001) or IL-4 (Nolan et al., 2005) counteract the effects of IL-1β on LTP via inhibition of this pathway. The role of p38MAPK may also be involved in the regulation of inflammatory signaling by other hormones including melanocortin stimulating hormone (Gonzalez et al., 2009) and glucocorticoids. The role here is not straightforward, however, as activity of this kinase is increased by both inflammatory stimulation, and glucocorticoid receptor activation (Munhoz, Sorrells, Caso, Scavone, & Sapolsky, 2010). Multiple mediators of inflammatory signaling, therefore, converge onto p38MAPK and JNK activation, suggesting that this pathway may integrate information from not just individual cytokines, but patterns of cytokine activity.
3.2 JAK-STAT signaling
STAT has been extensively studied for its role in the canonical cytokine and neurotrophin signaling pathway. STAT3 is downstream of many cytokines, several with opposing effects including IL-2, IL-6, IL-10, and TNFα (Lai et al., 1996; P. J. Murray, 2007). Although few studies have examined a role for STAT3 in learning, there is evidence for a role of JAK/STAT signaling in memory and synaptic plasticity. In drosophila, JAK/STAT plays a key role in formation of long term olfactory avoidance memory (Copf, Goguel, Lampin-Saint-Amaux, Scaplehorn, & Preat, 2011). More recently, STAT3 has been demonstrated to play a key role in NMDA receptor dependent LTD (Nicolas et al., 2013; 2012). The presence of STAT in the post-synaptic density (Murata, Usuda, Okano, Kobayashi, & Suzuki, 2000) further suggests that this kinase/transcription factor plays a broader role in synaptic plasticity than is currently known. As a kinase, STAT may play a role in NMDA receptor phosphorylation, and AMPA receptor trafficking (Nicolas et al., 2013; Sacktor, 2012). In its role as a transcription factor, STAT may influence both interactions between cytokines (Yasukawa et al., 2003) and plasticity via expression of Suppressors of Cytokine Signaling (SOCS) (Campbell, 2005) (Figure 2). This pathway also mediates the effects of growth factors including Brain Derived Neurotrophic Factor (BDNF) on memory (I. V. Lund et al., 2008). At this stage, however, the direct role of STAT signaling in the integration of inflammatory effects on memory remains unknown.
3.3 C/EPBβ and NFκB
Two additional transcription factors, CCAAT enhancing binding protein β (C/EPBβ, Nuclear Factor-IL6, NF-IL6) and Nuclear kappa B (NFκB) are of particular interest for the intersection of inflammatory signaling and memory. Whether C/EBPβ plays a role in the integration of inflammatory signaling and memory is unknown, however this transcription factor is critical for both memory consolidation and the effects of IL-6. In memory research, C/EBPβ is well known to play a critical role in consolidation of long-term memory in Alplysia (Alberini, Ghirardl, Metz, & Kandel, 1994), as well as consolidation of hippocampal-dependent memory (Taubenfeld, Milekic, Monti, & Alberini, 2001a; Taubenfeld et al., 2001b) and amygdala-dependent reconsolidation (Milekic, Pollonini, & Alberini, 2007) in rodents. Dysregulation of C/EPBβ as a consequence of inflammation may prevent its normal role in memory consolidation.
NFκB also has well-described roles in both memory (Ahn et al., 2008; Freudenthal & Romano, 2000; Freudenthal et al., 2005; Kaltschmidt et al., 2006; Yeh, Lin, & Gean, 2004) and regulation of inflammatory effects in the brain (Moynagh, 2005; Munhoz et al., 2008), and thus may be a mechanism by which inflammatory signaling may exert effects on memory. Several studies have identified NFκB as playing a key role in the interactions between inflammation and memory. Inhibition of NFκB reverses the impairing effects of inflammation (Choi et al., 2012), IL-1β (Kelly et al., 2003b), or TNFα (Albensi & Mattson, 2000) on memory and synaptic plasticity. These findings strongly implicate NFκB in the modulation of memory by inflammation. It is not clear, however whether NFκB affects memory by upregulation of the inflammatory network (Z.-Q. Li et al., 2013), or via direct influence on memory-related signaling pathways (Chou et al., 2011). The precise role of NFκB in the modulation of memory by inflammation, and the second messenger pathways that mediate these effects, require additional study.
Modulation of memory by an inflammatory event requires integration of signaling mechanisms from both systems. This likely occurs at multiple levels, including kinase signaling and transcriptional regulation. The MAPK family, in particular p38MAPK and JNK, appear to play an important role in mediating inflammatory effects on memory. Less is known about the effects of other kinases and transcription factors, including STAT, C/EPBβ and NFκB, all of which are well placed to act as signal integrators in the modulation of memory by inflammatory signaling.
4. Long lasting effects of inflammatory event
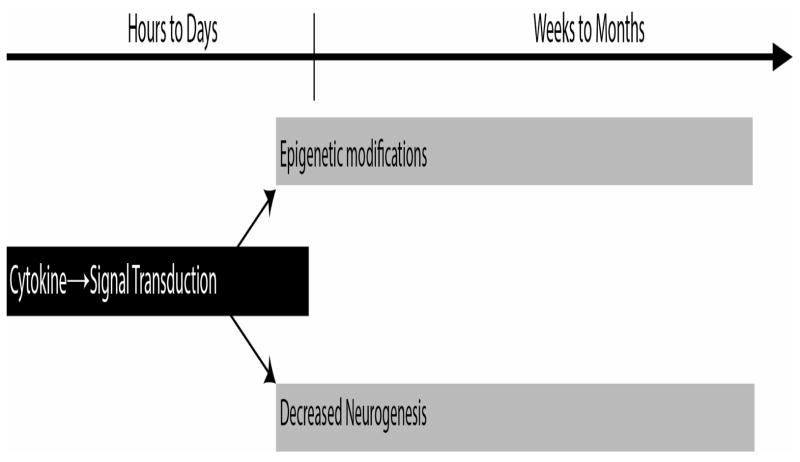
Research on the modulation of memory and synaptic plasticity by inflammatory signaling has focused on the short-term effects of cytokines and immediate activation of signaling cascades and gene transcription. However, a single inflammatory event has long lasting consequences for cognition, memory, and mood. After surgery, many patients exhibit both memory loss (Fredericks, 2012) and cognitive decline (Selnes et al., 2003) in the months after surgery. Similarly, in animal models, a single inflammatory insult impairs later memory (Y. Wang et al., 2013) and results in lasting changes in gene expression (Bilbo et al., 2008). Animals previously exposed to an inflammatory event show reductions of hippocampal BDNF expression after learning, and altered BDNF and IL-1β expression after a second inflammatory stimulus (Bilbo et al., 2008; Yin et al., 2013). Such persistent changes in memory cannot be explained solely by the cytokine-dependent signaling during inflammation. Therefore, the initial inflammatory event must trigger intervening variables that mediate these long lasting changes in memory and gene expression (Figure 3).
Figure 3.
Effects of an inflammatory event on memory and cognition may be mediated by different mechanisms at different times. Cytokine-dependent signaling likely interacts directly with mechanisms of memory processes in the hours to days after inflammation. These initial events also trigger persistent changes in neuronal function via altered neurogenesis and epigenetic modifications which mediate the memory and cognitive deficits observed in the weeks to months after the resolution of inflammatory signaling.
No studies to date have directly examined the mechanisms by which cytokines cause persistent changes in gene expression and cognitive function. Two candidate mechanisms for mediating these long lasting effects are adult neurogenesis and epigenetic modifications. These processes have previously been shown to be directly modulated by immune signaling (J. Liu, Solway, Messing, & Sharp, 1998; Monje, Toda, & Palmer, 2003; Takeshima et al., 2012), and independently, are known to alter behavior long after an initial insult (Rudenko & Tsai, 2014; Shors et al., 2001; Shors, Townsend, Zhao, Kozorovitskiy, & Gould, 2002). Here we discuss the possibility that neurogenesis and epigenetic modifications mediate the sustained effects of inflammatory signaling on memory.
4.1 Neurogenesis
Adult neurogenesis plays a key role in hippocampal-dependent memory (Monje & Dietrich, 2012; Shors et al., 2001) and synaptic plasticity (Ge, Yang, Hsu, Ming, & Song, 2007; Schmidt-Hieber, Jonas, & Bischofberger, 2004), and is modulated by immune signaling. Upregulation of neurogenesis occurs following ischemia (J. Liu et al., 1998) and downregulation occurs as a consequence of LPS-triggered inflammatory signaling (Monje et al., 2003).
As in memory, IL-1β, TNFα, and IL-6 are the main focus of studies on inflammatory modulation of neurogenesis. Chronic exposure to IL-1 consistently impairs neurogenesis (Goshen et al., 2008; Koo & Duman, 2008; Seguin, Brennan, Mangano, & Hayley, 2009; Wu et al., 2012; Zunszain et al., 2012). The effects of IL-1 on neurogenesis are mediated via SAPK/JNK pathway (X. Wang et al., 2007) and NFκB pathway (Koo, Russo, Ferguson, Nestler, & Duman, 2010). Similarly, TNFα (Chen & Palmer, 2013; Iosif et al., 2006) and IL-6 (Monje et al., 2003; Vallières, Campbell, Gage, & Sawchenko, 2002) decrease neurogenesis, in part via NFκB signaling (Chen & Palmer, 2013).
There is also evidence suggesting that the overall pattern of cytokine activation is more important than any individual cytokine in disrupting neurogenesis. For example, overexpression IL-1ra leads to decreased neurogenesis, despite its antagonistic effect on IL-1β (Spulber, Bartfai, & Schultzberg, 2009a). In addition, peripheral but not central administration of TNFα impairs neurogenesis, with the reverse pattern true for IL-6 (Seguin et al., 2009). These findings suggest that the network activation of cytokines is crucial to the regulation of neurogenesis.
The results described here demonstrate that short-term inflammatory signaling results in decreased neurogenesis within a limited time frame. However, there is also evidence that the disruption of neurogenesis after transient inflammatory signaling has long lasting consequences. For example, six weeks after LPS-induced disruption of neurogenesis, there were still 65% fewer synaptic connections in the neurons born in the days after LPS suggesting a persistently decreased capacity for plasticity. More striking, animals injected with LPS six weeks prior to testing exhibited deficits in spatial learning (Valero, Mastrella, Neiva, Sánchez, & Malva, 2014). Similarly, persistent effects of prenatal exposure to LPS are also, in part, mediated by neurogenesis. In these animals, neurogenesis is reduced until at least 90 days of age, spine density is decreased and depression-like behavior is persistently increased (Y.-L. Lin & Wang, 2014), despite no lasting increase in baseline cytokine levels (Bilbo et al., 2008). Disruption of neurogenesis is therefore a viable candidate for the sustained alterations of memory and cognitive functions long after the resolution of cytokine-dependent signaling. Whether alterations in cognitive ability persist after a transient change in neurogenesis remains to be explored.
4.2 Epigenetic modifications
Epigenetic modifications are also of interest for both the immediate effects of inflammatory signaling on learning and memory, and for changes to memory processes and cognition long after resolution of the inflammatory event. Histone modifications are considered to be mechanisms for dysregulation of subsequent gene expression, leading to cognitive decline and memory impairments in a variety of disorders (Rudenko & Tsai, 2014) and aging (Peleg et al., 2010). In particular, histone methylation and demethylation have been linked with cognitive disabilities (Parkel, Lopez-Atalaya, & Barco, 2013). Therefore, lasting epigenetic modification as a consequence of transient cytokine signaling (Y. Wang et al., 2013) are a potential mediator of persistent changes in cognition and memory.
Inflammatory signaling triggers histone acetylation (Ottaviani et al., 2013), histone methylation (Takeshima et al., 2012), and DNA methylation (Hmadcha, Bedoya, Sobrino, & Pintado, 1999), leading to increased cfos gene expression and methylation dependent silencing of the memory-related fragile X mental retardation 1 gene (FMR1). Downstream effectors of cytokines, STAT3 and NF B, also mediate DNA methylation (Thomas, 2012) and histone acetylation (Lubin & Sweatt, 2007). These inflammation-mediated epigenetic modifications can be very long lasting. For example, methylation at histone 3, lysine 27 (H3K27) and lysine 9 (H3K9) in a model of inflammatory colitis persists for at least 16 weeks, resulting in sustained dysregulation of gene expression (Takeshima et al., 2012).
The role of cumulative histone acetylation during aging is an example of the long lasting impact of epigenetic modifications on subsequent cognition. Specifically, age dependent memory impairments are associated with acetylation of histone 4, lysine 12 (H4K12) (Peleg et al., 2010). Here increased acetylation leads to impairments of memory and cognitive function, most likely due to dysregulated gene expression.
Whether similar changes in histone acetylation or methylation, or DNA methylation mediate persistent effects of an inflammatory event on learning and cognition is yet to be examined. In addition, little is known about the specific histone residues that may lead to impairments in memory processes. However, together with previous studies demonstrating altered gene expression after prior inflammatory stimulation (Bilbo et al., 2008), the findings reviewed here suggest that histone modifications are a strong candidate for mediating persistent impairments in learning, memory, and cognition after an inflammatory event.
The long lasting nature of memory alterations and cognitive deficits after inflammatory events including surgery (Fredericks, 2012) suggest that immune signaling results in both acute changes in signaling during inflammation as well as alterations in neuronal function that persist beyond the cessation of cytokine-dependent signaling. Here we propose that alterations in neurogenesis and epigenetic modification are two candidate mechanisms that may mediate such persistent dysregulation of cognitive function and memory processes.
5. Summary and Perspective
Inflammatory signaling triggered during learning plays a critical role in the normal formation and regulation of memory and plasticity. In parallel, illness or injury can enhance or impair memory depending on the patterns of cytokines activated in the brain. One major complication in understanding the role of inflammatory signaling in the modulation of memory is that cytokines do not function in isolation. Rather, manipulation of a single cytokine causes network-level changes in inflammatory signaling by regulating levels of other cytokines and their endogenous suppressors. Furthermore, relatively short-lasting cytokine activity cannot explain the long lasting memory and cognitive changes after an inflammatory event. Moving beyond single cytokines, analyses that focus on patterns of cytokine activity or expression are required to unravel the complexity of network-level activity and function.
We propose that signaling cascades and transcription factors at the intersection of memory and inflammatory signaling, including MAPK, STAT, NFκB and C/EBPβ, mediate the immediate modulation of memory by network activation of cytokines during an inflammatory event. Furthermore, we suggest that epigenetic modifications and neurogenesis may mediate the long lasting effects of inflammation on memory and cognitive function. Understanding both the short- and long-term mechanisms by which cytokine-dependent signaling affect neural processes will be vital for treating and preventing the debilitating memory loss and cognitive impairments after illness, injury, and major surgery.
Highlights.
Inflammatory signaling modulates learning, memory and synaptic plasticity
Networked interactions of cytokines are crucial to understanding effects on memory
Intracellular signaling integrates the effects of opposing cytokines
Epigenetic modifications and neurogenesis may mediate persistent changes in memory
Acknowledgments
This work was funded by the National Institute of Mental Health R00 MH093459 to NCT. We would like to thank Natalie Nevárez, Ashley Keiser and Lacie Turnbull for their comments on the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Contributor Information
Elissa J. Donzis, Email: edonzis@umich.edu.
Natalie C. Tronson, Email: ntronson@umich.edu.
References
- Ahn HJ, Hernandez CM, Levenson JM, Lubin FD, Liou HC, Sweatt JD. c-Rel, an NF-κB family transcription factor, is required for hippocampal long-term synaptic plasticity and memory formation. Learning & Memory. 2008;15(7):539–549. doi: 10.1101/lm.866408. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albensi BC, Mattson MP. Evidence for the involvement of TNF and NF-κB in hippocampal synaptic plasticity. Synapse. 2000;35(2):151–159. doi: 10.1002/(SICI)1098-2396(200002)35:2<151::AID-SYN8>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- Alberini CM, Ghirardl M, Metz R, Kandel ER. C/EBP is an immediate-early gene required for the consolidation of long-term facilitation in Aplysia. Cell. 1994;76(6):1099–1114. doi: 10.1016/0092-8674(94)90386-7. [DOI] [PubMed] [Google Scholar]
- Alonso M, Bevilaqua LRM, Izquierdo I, Medina JH, Cammarota M. Memory formation requires p38MAPK activity in the rat hippocampus. NeuroReport. 2003;14(15):1989–1992. doi: 10.1097/01.wnr.0000091129.97039.f6. [DOI] [PubMed] [Google Scholar]
- Anisman H, Gibb J, Hayley S. Influence of continuous infusion of interleukin-1β on depression-related processes in mice: corticosterone, circulating cytokines, brain monoamines, and cytokine mRNA expression. Psychopharmacology. 2008;199(2):231–244. doi: 10.1007/s00213-008-1166-z. [DOI] [PubMed] [Google Scholar]
- Ashwell JD. The many paths to p38 mitogen-activated protein kinase activation in the immune system. Nature Reviews Immunology. 2006;6(7):532–540. doi: 10.1038/nri1865. [DOI] [PubMed] [Google Scholar]
- Aubert A, Vega C, Dantzer R, Goodall G. Pyrogens specifically disrupt the acquisition of a task involving cognitive processing in the rat. Brain Behavior and Immunity. 1995;9(2):129–148. doi: 10.1006/brbi.1995.1013. [DOI] [PubMed] [Google Scholar]
- Avital A, Goshen I, Kamsler A, Segal M, Iverfeldt K, Richter-Levin G, Yirmiya R. Impaired interleukin-1 signaling is associated with deficits in hippocampal memory processes and neural plasticity. Hippocampus. 2003;13(7):826– 834. doi: 10.1002/hipo.10135. [DOI] [PubMed] [Google Scholar]
- Bağdatoğlu OT, Polat G, Bağdatoğlu C, Atik U. Effects of peripheral nerve ischemia-reperfusion model on serum cytokine levels. Turkish Neurosurgery. 2008;18(2):149–156. [PubMed] [Google Scholar]
- Balschun D, Randolf A, Pitossi F, SCHNEIDER H, REY A, Besedovsky HO. Hippocampal Interleukin-1β Gene Expression during Long-Term Potentiation Decays with Age. Annals of the New York Academy of Sciences. 2003;992(1):1–8. doi: 10.1111/j.1749-6632.2003.tb03132.x. [DOI] [PubMed] [Google Scholar]
- Balschun D, Wetzel W, del Rey A, Pitossi F, Schneider H, Zuschratter W, Besedovsky HO. Interleukin-6: a cytokine to forget. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2004;18(14):1788–1790. doi: 10.1096/fj.04-1625fje. [DOI] [PubMed] [Google Scholar]
- Barry CE, Nolan Y, Clarke RM, Lynch A, Lynch MA. Activation of c- Jun-N-terminal kinase is critical in mediating lipopolysaccharide-induced changes in the rat hippocampus. Journal of Neurochemistry. 2005;93(1):221–231. doi: 10.1111/j.1471-4159.2004.03011.x. [DOI] [PubMed] [Google Scholar]
- Baune BT, Wiede F, Braun A, Golledge J, Arolt V, Koerner H. Cognitive dysfunction in mice deficient for TNF- and its receptors. American Journal of Medical Genetics Part C: Seminars in Medical Genetics. 2008;147B(7):1056– 1064. doi: 10.1002/ajmg.b.30712. [DOI] [PubMed] [Google Scholar]
- Belarbi K, Jopson T, Tweedie D, Arellano C, Luo W, Greig NH, Rosi S. TNF-α protein synthesis inhibitor restores neuronal function and reverses cognitive deficits induced by chronic neuroinflammation. Journal of Neuroinflammation. 2012;9(1):23. doi: 10.1038/nn1708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Benarroch EE. Microglia: Multiple roles in surveillance, circuit shaping, and response to injury. Neurology. 2013;81(12):1079–1088. doi: 10.1212/WNL.0b013e3182a4a577. [DOI] [PubMed] [Google Scholar]
- Bilbo SD, Barrientos RM, Eads AS, Northcutt A, Watkins LR, Rudy JW, Maier SF. Early-life infection leads to altered BDNF and IL-1β mRNA expression in rat hippocampus following learning in adulthood. Brain Behavior and Immunity. 2008;22(4):451–455. doi: 10.1016/j.bbi.2007.10.003. [DOI] [PubMed] [Google Scholar]
- Braida D, Sacerdote P, Panerai AE, Bianchi M, Aloisi AM, Iosuè S, Sala M. Cognitive function in young and adult IL (interleukin)-6 deficient mice. Behavioural Brain Research. 2004;153(2):423–429. doi: 10.1016/j.bbr.2003.12.018. [DOI] [PubMed] [Google Scholar]
- Bray JG, Reyes KC, Roberts AJ, Ransohoff RM, Gruol DL. Synaptic plasticity in the hippocampus shows resistance to acute ethanol exposure in transgenic mice with astrocyte-targeted enhanced CCL2 expression. Neuropharmacology. 2013;67:115–125. doi: 10.1016/j.neuropharm.2012.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burton MD, Sparkman NL, Johnson RW. Inhibition of interleukin-6 trans-signaling in the brain facilitates recovery from lipopolysaccharide-induced sickness behavior. Journal of Neuroinflammation. 2011;8(1):54. doi: 10.1186/1742-2094-8-54. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Butler MP, O’Connor JJ, Moynagh PN. Dissection of tumor-necrosis factor-α inhibition of long-term potentiation (LTP) reveals a p38 mitogen-activated protein kinase-dependent mechanism which maps to early—but not late—phase LTP. Neuroscience. 2004;124(2):319–326. doi: 10.1016/j.neuroscience.2003.11.040. [DOI] [PubMed] [Google Scholar]
- Camara ML, Corrigan F, Jaehne EJ, Jawahar MC, Anscomb H, Koerner H, Baune BT. TNF-α and its receptors modulate complex behaviours and neurotrophins in transgenic mice. Psychoneuroendocrinology. 2013;38(12):3102–3114. doi: 10.1016/j.psyneuen.2013.09.010. [DOI] [PubMed] [Google Scholar]
- Campbell IL. Cytokine-mediated inflammation, tumorigenesis, and diseaseassociated JAK/STAT/SOCS signaling circuits in the CNS. Brain Research Reviews. 2005;48(2):166–177. doi: 10.1016/j.brainresrev.2004.12.006. [DOI] [PubMed] [Google Scholar]
- Capuron L, Miller AH. Immune system to brain signaling: neuropsychopharmacological implications. Pharmacology & Therapeutics. 2011;130(2):226–238. doi: 10.1016/j.pharmthera.2011.01.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cartford MC, Gemma C, Bickford PC. Eighteen-month-old Fischer 344 rats fed a spinach-enriched diet show improved delay classical eyeblink conditioning and reduced expression of tumor necrosis factor alpha (TNFalpha ) and TNFbeta in the cerebellum. Journal of Neuroscience. 2002;22(14):5813–5816. doi: 10.1523/JNEUROSCI.22-14-05813.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cazareth J, Guyon A, Heurteaux C, Chabry J, Petit-Paitel A. Molecular and cellular neuroinflammatory status of mouse brain after systemic lipopolysaccharide challenge: importance of CCR2/CCL2 signaling. Journal of Neuroinflammation. 2014;11(1):132. doi: 10.1186/1742-2094-11-132. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Z, Palmer TD. Differential roles of TNFR1 and TNFR2 signaling in adult hippocampal neurogenesis. Brain Behavior and Immunity. 2013;30:45–53. doi: 10.1016/j.bbi.2013.01.083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Choi DY, Lee JW, Peng J, Lee YJ, Han JY, Lee YH, et al. Obovatol improves cognitive functions in animal models for Alzheimer’s disease. Journal of Neurochemistry. 2012;120(6):1048–1059. doi: 10.1111/j.1471-4159.2011.07642.x. [DOI] [PubMed] [Google Scholar]
- Chou CW, Wong GTC, Lim G, McCabe MF, Wang S, Irwin MG, Mao J. Peripheral nerve injury alters the expression of NF-κB in the rat’s hippocampus. Brain Research. 2011;1378:66–71. doi: 10.1016/j.brainres.2011.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cibelli M, Fidalgo AR, Terrando N, Ma D, Monaco C, Feldmann M, et al. Role of interleukin-1β in postoperative cognitive dysfunction. Annals of Neurology. 2010;68(3):360–368. doi: 10.1002/ana.22082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti B, Tabarean I, Sanchez-Alavez M, Davis C, Brownell S, Behrens M, Bartfai T. Cytokines and the Brain. Vol. 6. Elsevier; 2008. Cytokine Receptors in the Brain; pp. 19–38. [DOI] [Google Scholar]
- Coogan AN, O’Neill LA, O’Connor JJ. The P38 mitogen-activated protein kinase inhibitor SB203580 antagonizes the inhibitory effects of interleukin-1beta on long-term potentiation in the rat dentate gyrus in vitro. Neuroscience. 1999;93(1):57–69. doi: 10.1016/s0306-4522(99)00100-1. [DOI] [PubMed] [Google Scholar]
- Copf T, Goguel V, Lampin-Saint-Amaux A, Scaplehorn N, Preat T. Cytokine signaling through the JAK/STAT pathway is required for long-term memory in Drosophila. Proceedings of the National Academy of Sciences. 2011;108(19):8059–8064. doi: 10.1073/pnas.1012919108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cumiskey D, Curran BP, Herron CE, O’Connor JJ. A role for inflammatory mediators in the IL-18 mediated attenuation of LTP in the rat dentate gyrus. Neuropharmacology. 2007;52(8):1616–1623. doi: 10.1016/j.neuropharm.2007.03.006. [DOI] [PubMed] [Google Scholar]
- Cunningham AJ, Murray CA, O’Neill LA, Lynch MA, O’Connor JJ. Interleukin-1 beta (IL-1 beta) and tumour necrosis factor (TNF) inhibit long-term potentiation in the rat dentate gyrus in vitro. Neuroscience Letters. 1996;203(1):17–20. doi: 10.1016/0304-3940(95)12252-4. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Sanderson DJ. Malaise in the water maze: untangling the effects of LPS and IL-1beta on learning and memory. Brain Behavior and Immunity. 2008;22(8):1117–1127. doi: 10.1016/j.bbi.2008.05.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curran BP, Murray HJ, O’Connor JJ. A role for c-Jun N-terminal kinase in the inhibition of long-term potentiation by interleukin-1beta and long-term depression in the rat dentate gyrus in vitro. Neuroscience. 2003;118(2):347–357. doi: 10.1016/s0306-4522(02)00941-7. [DOI] [PubMed] [Google Scholar]
- Curran B, O’Connor JJ. The pro-inflammatory cytokine interleukin-18 impairs long-term potentiation and NMDA receptor-mediated transmission in the rat hippocampus in vitro. Neuroscience. 2001;108(1):83–90. doi: 10.1016/s0306-4522(01)00405-5. [DOI] [PubMed] [Google Scholar]
- Datta SC, Opp MR. Lipopolysaccharide-induced increases in cytokines in discrete mouse brain regions are detectable using Luminex xMAP® technology. Journal of Neuroscience Methods. 2008;175(1):119–124. doi: 10.1016/j.jneumeth.2008.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rey A, Balschun D, Wetzel W, Randolf A, Besedovsky HO. A cytokine network involving brain-borne IL-1β, IL-1ra, IL-18, IL-6, and TNFα operates during long-term potentiation and learning. Brain Behavior and Immunity. 2013;33:15–23. doi: 10.1016/j.bbi.2013.05.011. [DOI] [PubMed] [Google Scholar]
- Depino AM, Alonso M, Ferrari C, del Rey A, Anthony D, Besedovsky H, et al. Learning modulation by endogenous hippocampal IL-1: Blockade of endogenous IL-1 facilitates memory formation. Hippocampus. 2004;14(4):526–535. doi: 10.1002/hipo.10164. [DOI] [PubMed] [Google Scholar]
- Elderkin-Thompson V, Irwin MR, Hellemann G, Kumar A. Interleukin-6 and memory functions of encoding and recall in healthy and depressed elderly adults. The American Journal of Geriatric Psychiatry : Official Journal of the American Association for Geriatric Psychiatry. 2012;20(9):753–763. doi: 10.1097/JGP.0b013e31825d08d6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fiore M, Angelucci F, Alleva E, Branchi I, Probert L, Aloe L. Learning performances, brain NGF distribution and NPY levels in transgenic mice expressing TNF-alpha. Behavioural Brain Research. 2000;112(1–2):165–175. doi: 10.1016/s0166-4328(00)00180-7. [DOI] [PubMed] [Google Scholar]
- Fredericks S. Memory loss following coronary artery bypass graft surgery: a discussion of the implications for nursing. Canadian Journal of Cardiovascular Nursing= …. 2012;22(2):33–36. [PubMed] [Google Scholar]
- Freudenthal R, Romano A. Participation of Rel/NF-κB transcription factors in long-term memory in the crab Chasmagnathus. Brain Research. 2000;855(2):274–281. doi: 10.1016/S0006-8993(99)02358-6. [DOI] [PubMed] [Google Scholar]
- Freudenthal R, Boccia MM, Acosta GB, Blake MG, Merlo E, Baratti CM, Romano A. NF-κB transcription factor is required for inhibitory avoidance long-term memory in mice. European Journal of Neuroscience. 2005;21(10):2845–2852. doi: 10.1111/j.1460-9568.2005.04126.x. [DOI] [PubMed] [Google Scholar]
- Garlanda C, Dinarello CA, Mantovani A. The Interleukin-1 Family: Back to the Future. Immunity. 2013;39(6):1003–1018. doi: 10.1016/j.immuni.2013.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gayle D, Ilyin SE, Miele ME, Plata-Salamán CR. Modulation of TNF-α mRNA production in rat C6 glioma cells by TNF-α, IL-1β, IL-6, and IFN-α: In vitro analysis of cytokine-cytokine interactions. Brain Research Bulletin. 1998;47(3):231–235. doi: 10.1016/S0361-9230(98)00078-1. [DOI] [PubMed] [Google Scholar]
- Ge S, Yang CH, Hsu KS, Ming GL, Song H. A Critical Period for Enhanced Synaptic Plasticity in Newly Generated Neurons of the Adult Brain. Neuron. 2007;54(4):559–566. doi: 10.1016/j.neuron.2007.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ginzburg K, Ein-Dor T. Posttraumatic stress syndromes and health-related quality of life following myocardial infarction: 8-year follow-up. General Hospital Psychiatry. 2011;33(6):565–571. doi: 10.1016/j.genhosppsych.2011.08.015. [DOI] [PubMed] [Google Scholar]
- Goehler LE. Cytokines and the Brain. Vol. 6. Elsevier; 2008. Cytokines in Neural Signaling to the Brain; pp. 337–352. [DOI] [Google Scholar]
- Gonzalez PV, Schiöth HB, Lasaga M, Scimonelli TN. Memory impairment induced by IL-1beta is reversed by alpha-MSH through central melanocortin-4 receptors. Brain Behavior and Immunity. 2009;23(6):817–822. doi: 10.1016/j.bbi.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Gonzalez P, Machado I, Vilcaes A, Caruso C, Roth GA, Schiöth H, et al. Molecular mechanisms involved in interleukin 1-beta (IL-1β)-induced memory impairment. Modulation by alpha-melanocyte-stimulating hormone (α-MSH) Brain Behavior and Immunity. 2013;34:141–150. doi: 10.1016/j.bbi.2013.08.007. [DOI] [PubMed] [Google Scholar]
- Goshen I, Avital A, Kreisel T, Licht T, Segal M, Yirmiya R. Environmental Enrichment Restores Memory Functioning in Mice with Impaired IL-1 Signaling via Reinstatement of Long-Term Potentiation and Spine Size Enlargement. Journal of Neuroscience. 2009;29(11):3395–3403. doi: 10.1523/JNEUROSCI.5352-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ben-Menachem-Zidon O, Licht T, Weidenfeld J, Ben-Hur T, Yirmiya R. Brain interleukin-1 mediates chronic stress-induced depression in mice via adrenocortical activation and hippocampal neurogenesis suppression. Molecular Psychiatry. 2008;13(7):717–728. doi: 10.1038/sj.mp.4002055. [DOI] [PubMed] [Google Scholar]
- Goshen I, Kreisel T, Ounallah-Saad H, Renbaum P, Zalzstein Y, Ben-Hur T, et al. A dual role for interleukin-1 in hippocampal-dependent memory processes. Psychoneuroendocrinology. 2007;32(8–10):1106–1115. doi: 10.1016/j.psyneuen.2007.09.004. [DOI] [PubMed] [Google Scholar]
- Guzmán C, Hallal-Calleros C. Interleukin-6: a cytokine with a pleiotropic role in the neuroimmunoendocrine network. The Open … 2010 [Google Scholar]
- Heim MH. The Jak-STAT pathway: cytokine signalling from the receptor to the nucleus. Journal of Receptors and Signal Transduction. 1999;19(1–4):75–120. doi: 10.3109/10799899909036638. [DOI] [PubMed] [Google Scholar]
- Hein AM, Stasko MR, Matousek SB, Scott-McKean JJ, Maier SF, Olschowka JA, et al. Sustained hippocampal IL-1β overexpression impairs contextual and spatial memory in transgenic mice. Brain Behavior and Immunity. 2010;24(2):243–253. doi: 10.1016/j.bbi.2009.10.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heyser CJ, Masliah E, Samimi A, Campbell IL, Gold LH. Progressive decline in avoidance learning paralleled by inflammatory neurodegeneration in transgenic mice expressing interleukin 6 in the brain. Proceedings of the National Academy of Sciences. 1997;94(4):1500–1505. doi: 10.1073/pnas.94.4.1500. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hmadcha A, Bedoya FJ, Sobrino F, Pintado E. Methylation-Dependent Gene Silencing Induced by Interleukin 1 via Nitric Oxide Production. Journal of Experimental Medicine. 1999;190(11):1595–1604. doi: 10.1126/science.7690156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hudetz JA, Iqbal Z, Gandhi SD, Patterson KM, Byrne AJ, HUDETZ AG, et al. Ketamine attenuates post-operative cognitive dysfunction after cardiac surgery. Acta Anaesthesiologica Scandinavica. 2009;53(7):864–872. doi: 10.1111/j.1399-6576.2009.01978.x. [DOI] [PubMed] [Google Scholar]
- Iosif RE, Ekdahl CT, Ahlenius H, Pronk CJH, Bonde S, Kokaia Z, et al. Tumor necrosis factor receptor 1 is a negative regulator of progenitor proliferation in adult hippocampal neurogenesis. Journal of Neuroscience. 2006;26(38):9703–9712. doi: 10.1523/JNEUROSCI.2723-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jankowsky JL, Derrick BE, Patterson PH. Cytokine responses to LTP induction in the rat hippocampus: a comparison of in vitro and in vivo techniques. Learning & Memory. 2000;7(6):400–412. doi: 10.1101/lm.32600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaltschmidt B, Ndiaye D, Korte M, Pothion S, Arbibe L, Prullage M, et al. NF-κB Regulates Spatial Memory Formation and Synaptic Plasticity through Protein Kinase A/CREB Signaling. Molecular and Cellular Biology. 2006;26(8):2936– 2946. doi: 10.1128/MCB.26.8.2936-2946.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy--from molecular mechanisms to therapeutic benefits. Biochimica Et Biophysica Acta. 2005;1754(1–2):253–262. doi: 10.1016/j.bbapap.2005.08.017. [DOI] [PubMed] [Google Scholar]
- Kawahara K, Suenobu M, Yoshida A, Koga K, Hyodo A, Ohtsuka H, et al. Intracerebral microinjection of interleukin-4/interleukin-13 reduces β-amyloid accumulation in the ipsilateral side and improves cognitive deficits in young amyloid precursor protein 23 mice. Neuroscience. 2012;207:243–260. doi: 10.1016/j.neuroscience.2012.01.049. [DOI] [PubMed] [Google Scholar]
- Kelly Á, Laroche S, Davis S. Activation of mitogen-activated protein kinase/extracellular signal-regulated kinase in hippocampal circuitry is required for consolidation and reconsolidation of recognition memory. The Journal of Neuroscience. 2003a;23(12):5354–5360. doi: 10.1523/JNEUROSCI.23-12-05354.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kelly Á, Lynch A, Vereker E, Nolan Y, Queenan P, Whittaker E, et al. The anti-inflammatory cytokine, interleukin (IL)-10, blocks the inhibitory effect of IL-1 beta on long term potentiation. A role for JNK. The Journal of Biological Chemistry. 2001;276(49):45564–45572. doi: 10.1074/jbc.M108757200. [DOI] [PubMed] [Google Scholar]
- Kelly Á, Vereker E, Nolan Y, Brady M, Barry C, Loscher CE, et al. Activation of p38 plays a pivotal role in the inhibitory effect of lipopolysaccharide and interleukin-1β on long term potentiation in rat dentate gyrus. Journal of Biological Chemistry. 2003b;278(21):19453–19462. doi: 10.1074/jbc.M301938200. [DOI] [PubMed] [Google Scholar]
- Kiyota T, Ingraham KL, Swan RJ, Jacobsen MT, Andrews SJ, Ikezu T. AAV serotype 2/1-mediated gene delivery of anti-inflammatory interleukin-10 enhances neurogenesis and cognitive function in APP+PS1 mice. Gene Therapy. 2012;19(7):724–733. doi: 10.1038/gt.2011.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiyota T, Okuyama S, Swan RJ, Jacobsen MT, Gendelman HE, Ikezu T. CNS expression of anti-inflammatory cytokine interleukin-4 attenuates Alzheimer’s disease-like pathogenesis in APP+PS1 bigenic mice. FASEB Journal : Official Publication of the Federation of American Societies for Experimental Biology. 2010;24(8):3093–3102. doi: 10.1096/fj.10-155317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. IL-1 is an essential mediator of the antineurogenic and anhedonic effects of stress. Proceedings of the National Academy of Sciences. 2008;105(2):751–756. doi: 10.1073/pnas.0708092105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Duman RS. Interleukin-1 receptor null mutant mice show decreased anxiety-like behavior and enhanced fear memory. Neuroscience Letters. 2009;456(1):39–43. doi: 10.1016/j.neulet.2009.03.068. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koo JW, Russo SJ, Ferguson D, Nestler EJ, Duman RS. Nuclear factor-κB is a critical mediator of stress-impaired neurogenesis and depressive behavior. Proceedings of the National Academy of Sciences. 2010;107(6):2669–2674. doi: 10.1073/pnas.0910658107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai CF, Ripperger J, Morella KK, Jurlander J, Hawley TS, Carson WE, et al. Receptors for interleukin (IL)-10 and IL-6-type cytokines use similar signaling mechanisms for inducing transcription through IL-6 response elements. The Journal of Biological Chemistry. 1996;271(24):13968–13975. doi: 10.1074/jbc.271.24.13968. [DOI] [PubMed] [Google Scholar]
- Li AJ, Katafuchi T, Oda S, Hori T, Oomura Y. Interleukin-6 inhibits long-term potentiation in rat hippocampal slices. Brain Research. 1997;748(1–2):30–38. doi: 10.1016/S0006-8993(96)01283-8. [DOI] [PubMed] [Google Scholar]
- Li ZQ, Rong XY, Liu YJ, Ni C, Tian XS, Mo N, et al. Activation of the canonical nuclear factor-κB pathway is involved in isoflurane-induced hippocampal interleukin-1β elevation and the resultant cognitive deficits in aged rats. Biochemical and Biophysical Research Communications. 2013;438(4):628–634. doi: 10.1016/j.bbrc.2013.08.003. [DOI] [PubMed] [Google Scholar]
- Lin YL, Wang S. Prenatal lipopolysaccharide exposure increases depression-like behaviors and reduces hippocampal neurogenesis in adult rats. Behavioural Brain Research. 2014;259:24–34. doi: 10.1016/j.bbr.2013.10.034. [DOI] [PubMed] [Google Scholar]
- Liu J, Solway K, Messing RO, Sharp FR. Increased neurogenesis in the dentate gyrus after transient global ischemia in gerbils. The Journal of Neuroscience. 1998;18(19):7768–7778. doi: 10.1523/JNEUROSCI.18-19-07768.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu X, Fang L, Guo TB, Mei H, Zhang JZ. Drug targets in the cytokine universe for autoimmune disease. Trends in Immunology. 2013;34(3):120–128. doi: 10.1016/j.it.2012.10.003. [DOI] [PubMed] [Google Scholar]
- Lonergan PE, Martin DSD, Horrobin DF, Lynch MA. Neuroprotective actions of eicosapentaenoic acid on lipopolysaccharide-induced dysfunction in rat hippocampus. Journal of Neurochemistry. 2004;91(1):20–29. doi: 10.1111/j.1471-4159.2004.02689.x. [DOI] [PubMed] [Google Scholar]
- Loscher C. Interleukin-1 receptor antagonist exerts agonist activity in the hippocampus independent of the interleukin-1 type I receptor. Journal of Neuroimmunology. 2003;137(1–2):117–124. doi: 10.1016/S0165-5728(03)00072-9. [DOI] [PubMed] [Google Scholar]
- Lubin FD, Sweatt JD. The IκB Kinase Regulates Chromatin Structure during Reconsolidation of Conditioned Fear Memories. Neuron. 2007;55(6):942–957. doi: 10.1016/j.neuron.2007.07.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund IV, Hu Y, Raol YH, Benham RS, Faris R, Russek SJ, Brooks-Kayal AR. BDNF Selectively Regulates GABAA Receptor Transcription by Activation of the JAK/STAT Pathway. Science Signaling. 2008;1(41):ra9–ra9. doi: 10.1126/scisignal.1162396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund S, Christensen KV, Hedtjärn M, Mortensen AL, Hagberg H, Falsig J, et al. The dynamics of the LPS triggered inflammatory response of murine microglia under different culture and in vivo conditions. Journal of Neuroimmunology. 2006;180(1–2):71–87. doi: 10.1016/j.jneuroim.2006.07.007. [DOI] [PubMed] [Google Scholar]
- Lynch AM, Walsh C, Delaney A, Nolan Y, Campbell VA, Lynch MA. Lipopolysaccharide-induced increase in signalling in hippocampus is abrogated by IL-10 - a role for IL-1β? Journal of Neurochemistry. 2004;88(3):635–646. doi: 10.1046/j.1471-4159.2003.02157.x. [DOI] [PubMed] [Google Scholar]
- Machado I, González P, Schiöth HB, Lasaga M, Scimonelli TN. α-Melanocyte-stimulating hormone (α-MSH) reverses impairment of memory reconsolidation induced by interleukin-1 beta (IL-1 beta) hippocampal infusions. Peptides. 2010;31(11):2141–2144. doi: 10.1016/j.peptides.2010.07.018. [DOI] [PubMed] [Google Scholar]
- Maher FO, Martin DSD, Lynch MA. Increased IL-1beta in cortex of aged rats is accompanied by downregulation of ERK and PI-3 kinase. Neurobiology of Aging. 2004;25(6):795–806. doi: 10.1016/j.neurobiolaging.2003.08.007. [DOI] [PubMed] [Google Scholar]
- Maher FO, Nolan Y, Lynch MA. Downregulation of IL-4-induced signalling in hippocampus contributes to deficits in LTP in the aged rat. Neurobiology of Aging. 2005;26(5):717–728. doi: 10.1016/j.neurobiolaging.2004.07.002. [DOI] [PubMed] [Google Scholar]
- Meister R, Princip M, Schmid JP, Schnyder U, Barth J, Znoj H, et al. Myocardial Infarction - Stress PRevention INTervention (MI-SPRINT) to reduce the incidence of posttraumatic stress after acute myocardial infarction through trauma-focused psychological counseling: study protocol for a randomized controlled trial. Trials. 2013;14(1):329. doi: 10.1097/01.psy.0000221275.75056.d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Milekic MH, Pollonini G, Alberini CM. Temporal requirement of C/EBP in the amygdala following reactivation but not acquisition of inhibitory avoidance. Learning & Memory. 2007;14(7):504–511. doi: 10.1101/lm.598307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mina F, Comim CM, Dominguini D, Cassol OJ, Jr, Dall Igna DM, Ferreira GK, et al. Il1-β Involvement in Cognitive Impairment after Sepsis. Molecular Neurobiology. 2013;49(2):1069–1076. doi: 10.1007/s12035-013-8581-9. [DOI] [PubMed] [Google Scholar]
- Monje ML, Toda H, Palmer TD. Inflammatory Blockade Restores Adult Hippocampal Neurogenesis. Science. 2003;302:1760–1765. doi: 10.1126/science.1088417. [DOI] [PubMed] [Google Scholar]
- Monje M, Dietrich J. Cognitive side effects of cancer therapy demonstrate a functional role for adult neurogenesis. Behavioural Brain Research. 2012;227(2):376– 379. doi: 10.1016/j.bbr.2011.05.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moore AH, Wu M, Shaftel SS, Graham KA, O’Banion MK. Sustained expression of interleukin-1β in mouse hippocampus impairs spatial memory. Neuroscience. 2009;164(4):1484–1495. doi: 10.1016/j.neuroscience.2009.08.073. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moynagh PN. The interleukin-1 signalling pathway in astrocytes: a key contributor to inflammation in the brain. Journal of Anatomy. 2005;207(3):265–269. doi: 10.1111/j.1469-7580.2005.00445.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Munhoz CD, García-Bueno B, Madrigal JLM, Lepsch LB, Scavone C, Leza JC. Stress-induced neuroinflammation: mechanisms and new pharmacological targets. Brazilian Journal of Medical and Biological Research. 2008;41(12):1037–1046. doi: 10.1590/S0100-879X2008001200001. [DOI] [PubMed] [Google Scholar]
- Munhoz CD, Sorrells SF, Caso JR, Scavone C, Sapolsky RM. Glucocorticoids Exacerbate Lipopolysaccharide-Induced Signaling in the Frontal Cortex and Hippocampus in a Dose-Dependent Manner. Journal of Neuroscience. 2010;30(41):13690–13698. doi: 10.1523/JNEUROSCI.0303-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murata S, Usuda N, Okano A, Kobayashi S, Suzuki T. Occurrence of a transcription factor, signal transducer and activators of transcription 3 (Stat3), in the postsynaptic density of the rat brain. Molecular Brain Research. 2000;78(1–2):80–90. doi: 10.1016/s0169-328x(00)00077-2. [DOI] [PubMed] [Google Scholar]
- Murray CL, Obiang P, Bannerman D, Cunningham C. Endogenous IL-1 in cognitive function and anxiety: a study in IL-1RI−/− mice. PloS One. 2013;8(10):e78385. doi: 10.1371/journal.pone.0078385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Murray PJ. The JAK-STAT signaling pathway: input and output integration. The Journal of Immunology. 2007;178(5):2623–2629. doi: 10.4049/jimmunol.178.5.2623. [DOI] [PubMed] [Google Scholar]
- Naert G, Rivest S. CC chemokine receptor 2 deficiency aggravates cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Journal of Neuroscience. 2011;31(16):6208–6220. doi: 10.1523/JNEUROSCI.0299-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naert G, Rivest S. Hematopoietic CC-chemokine receptor 2 (CCR2) competent cells are protective for the cognitive impairments and amyloid pathology in a transgenic mouse model of Alzheimer’s disease. Molecular Medicine (Cambridge, Mass) 2012;18(2):297–313. doi: 10.2119/molmed.2011.00306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naude PJW, Dobos N, van der Meer D, Mulder C, Pawironadi KGD, Boerden JA, et al. Analysis of cognition, motor performance and anxiety in young and aged tumor necrosis factor alpha receptor 1 and 2 deficient mice. Behavioural Brain Research. 2014;258:43–51. doi: 10.1016/j.bbr.2013.10.006. [DOI] [PubMed] [Google Scholar]
- Nicolas CS, Amici M, Bortolotto ZA, Doherty A, Csaba Z, Fafouri A, et al. The role of JAK-STAT signaling within the CNS. Jak-Stat. 2013;2(1) doi: 10.4161/jkst.22925. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nicolas CS, Peineau S, Amici M, Csaba Z, Fafouri A, Javalet C, et al. The JAK/STAT Pathway Is Involved in Synaptic Plasticity. Neuron. 2012;73(2):374–390. doi: 10.1016/j.neuron.2011.11.024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nolan Y, Mahler FO, Martin DS, Clarke RM, Brady MT, Bolton AE, et al. Role of Interleukin-4 in Regulation of Age-related Inflammatory Changes in the Hippocampus. Journal of Biological Chemistry. 2005;280(10):9354–9362. doi: 10.1074/jbc.M412170200. [DOI] [PubMed] [Google Scholar]
- Norden DM, Fenn AM, Dugan A, Godbout JP. TGFβ produced by IL-10 redirected astrocytes attenuates microglial activation. Glia. 2014;62(6):881–895. doi: 10.1002/glia.22647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ottaviani E, Accorsi A, Rigillo G, Malagoli D, Blom JMC, Tascedda F. Epigenetic modification in neurons of the mollusc Pomacea canaliculata after immune challenge. Brain Research. 2013;1537:18–26. doi: 10.1016/j.brainres.2013.09.009. [DOI] [PubMed] [Google Scholar]
- Papa M, De Luca C, Petta F, Alberghina L, Cirillo G. Astrocyte-neuron interplay in maladaptive plasticity. Neuroscience & Biobehavioral Reviews. 2014;42C:35–54. doi: 10.1016/j.neubiorev.2014.01.010. [DOI] [PubMed] [Google Scholar]
- Paredes D, Acosta S, Gemma C, Bickford PC. Role of TNFα Induced Inflammation in Delay Eyeblink Conditioning in Young and Aged Rats. Aging and Disease. 2010;1(3):191–198. [PMC free article] [PubMed] [Google Scholar]
- Parkel S, Lopez-Atalaya JP, Barco A. Histone H3 lysine methylation in cognition and intellectual disability disorders. Learning & Memory (Cold Spring Harbor, NY) 2013;20(10):570–579. doi: 10.1101/lm.029363.112. [DOI] [PubMed] [Google Scholar]
- Peleg S, Sananbenesi F, Zovoilis A, Burkhardt S, Bahari-Javan S, Agis-Balboa RC, et al. Altered histone acetylation is associated with age-dependent memory impairment in mice. Science. 2010;328(5979):753–756. doi: 10.1126/science.1186088. [DOI] [PubMed] [Google Scholar]
- Platzer C, Meisel C, Vogt K, Platzer M, Volk HD. Up-regulation of monocytic IL-10 by tumor necrosis factor-alpha and cAMP elevating drugs. International Immunology. 1995;7(4):517–523. doi: 10.1093/intimm/7.4.517. [DOI] [PubMed] [Google Scholar]
- Pugh CR, Kumagawa K, Fleshner M, Watkins LR, Maier SF, Rudy JW. Selective effects of peripheral lipopolysaccharide administration on contextual and auditory-cue fear conditioning. Brain Behavior and Immunity. 1998;12(3):212–229. doi: 10.1006/brbi.1998.0524. [DOI] [PubMed] [Google Scholar]
- Ren WJ, Liu Y, Zhou LJ, Li W, Zhong Y, Pang RP, et al. Peripheral Nerve Injury Leads to Working Memory Deficits and Dysfunction of the Hippocampus by Upregulation of TNF-α in Rodents. Neuropsychopharmacology. 2011;36(5):979–992. doi: 10.1038/npp.2010.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richwine AF, Sparkman NL, Dilger RN, Buchanan JB, Johnson RW. Cognitive deficits in interleukin-10-deficient mice after peripheral injection of lipopolysaccharide. Brain Behavior and Immunity. 2009;23(6):794–802. doi: 10.1016/j.bbi.2009.02.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ross FM, Allan SM, Rothwell NJ, Verkhratsky A. A dual role for interleukin-1 in LTP in mouse hippocampal slices. Journal of Neuroimmunology. 2003;144(1–2):61–67. doi: 10.1016/j.jneuroim.2003.08.030. [DOI] [PubMed] [Google Scholar]
- Rudenko A, Tsai L-H. Epigenetic modifications in the nervous system and their impact upon cognitive impairments. Neuropharmacology. 2014 doi: 10.1016/j.neuropharm.2014.01.043. [DOI] [PubMed] [Google Scholar]
- Sacktor TC. JAK/STAT: The Enigma within the Mystery of NMDAR-LTD. Neuron. 2012;73(2):211–213. doi: 10.1016/j.neuron.2012.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schindler R, Mancilla J, Endres S, Ghorbani R, Clark SC, Dinarello CA. Correlations and interactions in the production of interleukin-6 (IL-6), IL-1, and tumor necrosis factor (TNF) in human blood mononuclear cells: IL-6 suppresses IL-1 and TNF. Blood. 1990;75(1):40–47. [PubMed] [Google Scholar]
- Schmidt-Hieber C, Jonas P, Bischofberger J. Enhanced synaptic plasticity in newly generated granule cells of the adult hippocampus. Nature. 2004;429(6988):184–187. doi: 10.1038/nature02553. [DOI] [PubMed] [Google Scholar]
- Schneider H, Pitossi F, Balschun D, Wagner A, del Rey A, Besedovsky HO. A neuromodulatory role of interleukin-1beta in the hippocampus. Proceedings of the National Academy of Sciences of the United States of America. 1998;95(13):7778–7783. doi: 10.1073/pnas.95.13.7778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seguin JA, Brennan J, Mangano E, Hayley S. Proinflammatory cytokines differentially influence adult hippocampal cell proliferation depending upon the route and chronicity of administration. Neuropsychiatric Disease and Treatment. 2009;5:5–14. [PMC free article] [PubMed] [Google Scholar]
- Selnes OA, Grega MA, Borowicz LM, Royall RM, McKhann GM, Baumgartner WA. Cognitive changes with coronary artery disease: a prospective study of coronary artery bypass graft patients and nonsurgical controls. The Annals of Thoracic Surgery. 2003;75(5):1377–84. doi: 10.1016/s0003-4975(03)00021-3. discussion 1384–6. [DOI] [PubMed] [Google Scholar]
- Shaftel SS, Kyrkanides S, Olschowka JA, Miller JNH, Johnson RE, O’Banion MK. Sustained hippocampal IL-1 beta overexpression mediates chronic neuroinflammation and ameliorates Alzheimer plaque pathology. The Journal of Clinical Investigation. 2007;117(6):1595–1604. doi: 10.1172/JCI31450. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shalin SC, Zirrgiebel U, Honsa KJ, Julien JP, Miller FD, Kaplan DR, Sweatt JD. Neuronal MEK is important for normal fear conditioning in mice. Journal of Neuroscience Research. 2004;75(6):760–770. doi: 10.1002/jnr.20052. [DOI] [PubMed] [Google Scholar]
- Shapira-Lichter I, Beilin B, Ofek K, Bessler H, Gruberger M, Shavit Y, et al. Cytokines and cholinergic signals co-modulate surgical stress-induced changes in mood and memory. Brain Behavior and Immunity. 2008;22(3):388–398. doi: 10.1016/j.bbi.2007.09.006. [DOI] [PubMed] [Google Scholar]
- Shatz CJ. MHC Class I: An Unexpected Role in Neuronal Plasticity. Neuron. 2009;64(1):40–45. doi: 10.1016/j.neuron.2009.09.044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shors TJ, Miesegaes G, Beylin A, Zhao M, Rydel T, Gould E. Neurogenesis in the adult is involved in the formation of trace memories. Nature. 2001;410(6826):372–376. doi: 10.1038/35066584. [DOI] [PubMed] [Google Scholar]
- Shors TJ, Townsend DA, Zhao M, Kozorovitskiy Y, Gould E. Neurogenesis may relate to some but not all types of hippocampal-dependent learning. Hippocampus. 2002;12(5):578–584. doi: 10.1002/hipo.10103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skelly DT, Hennessy E, Dansereau MA, Cunningham C. A systematic analysis of the peripheral and CNS effects of systemic LPS, IL-1B, TNF-α and IL-6 challenges in C57BL/6 mice. PloS One. 2013;8(7):e69123. doi: 10.1371/journal.pone.0069123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song C, Phillips AG, Leonard B. Interleukin 1 beta enhances conditioned fear memory in rats: possible involvement of glucocorticoids. European Journal of Neuroscience. 2003;18(7):1739–1743. doi: 10.1046/j.1460-9568.2003.02886.x. [DOI] [PubMed] [Google Scholar]
- Song C, Zhang Y, Dong Y. Acute and subacute IL-1β administrations differentially modulate neuroimmune and neurotrophic systems: possible implications for neuroprotection and neurodegeneration. Journal of Neuroinflammation. 2013;10:59. doi: 10.1186/1742-2094-10-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spulber S, Bartfai T, Schultzberg M. IL-1/IL-1ra balance in the brain revisited - evidence from transgenic mouse models. Brain Behavior and Immunity. 2009a;23(5):573–579. doi: 10.1016/j.bbi.2009.02.015. [DOI] [PubMed] [Google Scholar]
- Spulber S, Mateos L, Oprica M, Cedazo-Minguez A, Bartfai T, Winblad B, Schultzberg M. Impaired long term memory consolidation in transgenic mice overexpressing the human soluble form of IL-1ra in the brain. Journal of Neuroimmunology. 2009b;208(1–2):46–53. doi: 10.1016/j.jneuroim.2009.01.010. [DOI] [PubMed] [Google Scholar]
- Steensberg A, Fischer CP, Keller C, Møller K, Pedersen BK. IL-6 enhances plasma IL-1ra, IL-10, and cortisol in humans. American Journal of Physiology Endocrinology and Metabolism. 2003;285(2):E433–7. doi: 10.1152/ajpendo.00074.2003. [DOI] [PubMed] [Google Scholar]
- Stephan AH, Barres BA, Stevens B. The Complement System: An Unexpected Role in Synaptic Pruning During Development and Disease. Annual Review of Neuroscience. 2012;35:369–389. doi: 10.1146/annurev-neuro-061010-113810. [DOI] [PubMed] [Google Scholar]
- Takeshima H, Ikegami D, Wakabayashi M, Niwa T, Kim YJ, Ushijima T. Induction of aberrant trimethylation of histone H3 lysine 27 by inflammation in mouse colonic epithelial cells. Carcinogenesis. 2012;33(12):2384–2390. doi: 10.1093/carcin/bgs294. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Antuono M, Cafè C, Giovedì S, Buè MC, D’Arcangelo G, et al. The Inhibitory Effects of Interleukin-6 on Synaptic Plasticity in the Rat Hippocampus Are Associated with an Inhibition of Mitogen-Activated Protein Kinase ERK. Journal of Neurochemistry. 2000;75(2):634–643. doi: 10.1046/j.1471-4159.2000.0750634.x. [DOI] [PubMed] [Google Scholar]
- Tancredi V, D’Arcangelo G, Grassi F, Tarroni P, Palmieri G, Santoni A, Eusebi F. Tumor necrosis factor alters synaptic transmission in rat hippocampal slices. Neuroscience Letters. 1992;146(2):176–178. doi: 10.1016/0304-3940(92)90071-e. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Milekic MH, Monti B, Alberini CM. The consolidation of new but not reactivated memory requires hippocampal C/EBP|[beta]|. Nature Neuroscience. 2001a;4(8):813–818. doi: 10.1038/90520. [DOI] [PubMed] [Google Scholar]
- Taubenfeld SM, Wiig KA, Monti B, Dolan B, Pollonini G, Alberini CM. Fornix-dependent induction of hippocampal CCAAT enhancer-binding protein [beta] and [delta] Co-localizes with phosphorylated cAMP response element-binding protein and accompanies long-term memory consolidation. Journal of Neuroscience. 2001b;21(1):84–91. doi: 10.1523/JNEUROSCI.21-01-00084.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terrando N, Eriksson LI, Kyu Ryu J, Yang T, Monaco C, Feldmann M, et al. Resolving postoperative neuroinflammation and cognitive decline. Annals of Neurology. 2011;70(6):986–995. doi: 10.1002/ana.22664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thomas NSB. The STAT3-DNMT1 connection. Jak-Stat. 2012;1(4):257–260. doi: 10.4161/jkst.22436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tong L, Prieto GA, Kramár EA, Smith ED, Cribbs DH, Lynch G, Cotman CW. Brain-Derived Neurotrophic Factor-Dependent Synaptic Plasticity Is Suppressed by Interleukin-1 via p38 Mitogen-Activated Protein Kinase. Journal of Neuroscience. 2012;32(49):17714–17724. doi: 10.1523/JNEUROSCI.1253-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Valero J, Mastrella G, Neiva I, Sánchez S, Malva JO. Long-term effects of an acute and systemic administration of LPS on adult neurogenesis and spatial memory. Neurogenesis. 2014;8 doi: 10.3389/fnins.2014.00083. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vallières L, Campbell IL, Gage FH, Sawchenko PE. Reduced hippocampal neurogenesis in adult transgenic mice with chronic astrocytic production of interleukin-6. Journal of Neuroscience. 2002;22(2):486–492. doi: 10.1523/JNEUROSCI.22-02-00486.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vereker E, O’Donnell E, Lynch MA. The inhibitory effect of interleukin-1beta on long-term potentiation is coupled with increased activity of stress-activated protein kinases. The Journal of Neuroscience. 2000;20(18):6811–6819. doi: 10.1523/JNEUROSCI.20-18-06811.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang X, Fu S, Wang Y, Yu P, Hu J, Gu W, et al. Interleukin-1β mediates proliferation and differentiation of multipotent neural precursor cells through the activation of SAPK/JNK pathway. Molecular and Cellular Neuroscience. 2007;36(3):343–354. doi: 10.1016/j.mcn.2007.07.005. [DOI] [PubMed] [Google Scholar]
- Wang Y, Chen Z, Zhao Y, Shi R, Wang Y, Xu J, et al. Epigenetics as a new therapeutic target for postoperative cognitive dysfunction. Medical Hypotheses. 2013;80(3):249–251. doi: 10.1016/j.mehy.2012.11.041. [DOI] [PubMed] [Google Scholar]
- Wei H, Chadman KK, McCloskey DP, Sheikh AM, Malik M, Brown WT, Li X. Brain IL-6 elevation causes neuronal circuitry imbalances and mediates autism-like behaviors. Biochimica Et Biophysica Acta. 2012;1822(6):831–842. doi: 10.1016/j.bbadis.2012.01.011. [DOI] [PubMed] [Google Scholar]
- Wu MD, Hein AM, Moravan MJ, Shaftel SS, Olschowka JA, O’Banion MK. Adult murine hippocampal neurogenesis is inhibited by sustained IL-1β and not rescued by voluntary running. Brain Behavior and Immunity. 2012;26(2):292– 300. doi: 10.1016/j.bbi.2011.09.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yaguchi T, Nagata T, Yang D, Nishizaki T. Interleukin-18 regulates motor activity, anxiety and spatial learning without affecting synaptic plasticity. Behavioural Brain Research. 2010;206(1):47–51. doi: 10.1016/j.bbr.2009.08.033. [DOI] [PubMed] [Google Scholar]
- Yasukawa H, Ohishi M, Mori H, Murakami M, Chinen T, Aki D, et al. IL-6 induces an anti-inflammatory response in the absence of SOCS3 in macrophages. Nature Immunology. 2003;4(6):551–556. doi: 10.1038/ni938. [DOI] [PubMed] [Google Scholar]
- Yeh SH, Lin CH, Gean PW. Acetylation of nuclear factor-kappaB in rat amygdala improves long-term but not short-term retention of fear memory. Molecular Pharmacology. 2004;65(5):1286–1292. doi: 10.1124/mol.65.5.1286. [DOI] [PubMed] [Google Scholar]
- Yin P, Li Z, Wang YY, Qiao NN, Huang SY, Sun RP, Wang JW. Neonatal immune challenge exacerbates seizure-induced hippocampus-dependent memory impairment in adult rats. Epilepsy & Behavior. 2013;27(1):9–17. doi: 10.1016/j.yebeh.2012.12.015. [DOI] [PubMed] [Google Scholar]
- Yirmiya R. Brain Interleukin-1 Is Involved in Spatial Memory and Passive Avoidance Conditioning. Neurobiology of Learning and Memory. 2002;78(2):379–389. doi: 10.1006/nlme.2002.4072. [DOI] [PubMed] [Google Scholar]
- Yogeetha BS, Haupt LM, McKenzie K, Sutherland HG, Okolicsyani RK, Lea RA, et al. BDNF and TNF-α polymorphisms in memory. Molecular Biology Reports. 2013;40(9):5483–5490. doi: 10.1007/s11033-013-2648-6. [DOI] [PubMed] [Google Scholar]
- Zunszain PA, Anacker C, Cattaneo A, Choudhury S, Musaelyan K, Myint AM, et al. Interleukin-1β: a new regulator of the kynurenine pathway affecting human hippocampal neurogenesis. Neuropsychopharmacology. 2012;37(4):939–949. doi: 10.1038/npp.2011.277. [DOI] [PMC free article] [PubMed] [Google Scholar]