Abstract
The basolateral complex of the amygdala (BLA) plays a role in the modulation of emotional memory consolidation through its interactions with other brain regions. In rats, memory enhancing infusions of the β-adrenergic receptor agonist clenbuterol into the BLA immediately after training enhances expression of the protein product of the immediate early gene Arc in the dorsal hippocampus and memory-impairing intra-BLA treatments reduce hippocampal Arc expression. We have proposed that the BLA may modulate memory consolidation through an influence on the local translation of synaptic plasticity proteins, like Arc, in recently active synapses in efferent brain regions. To date, all work related to this hypothesis is based on aversive memory tasks such as inhibitory avoidance (IA). To determine whether BLA modulation of hippocampal Arc protein expression is specific to plasticity associated with inhibitory avoidance memory, or a common mechanism for multiple types of memory, we tested the effect of intra-BLA infusions of clenbuterol on memory and hippocampal synaptic Arc expression following IA or object recognition training. Results indicate that intra-BLA infusions of clenbuterol enhance memory for both tasks; however, Arc expression in hippocampal synaptoneurosomes was significantly elevated only in rats trained on the aversive IA task. These findings suggest that regulation of Arc expression in hippocampal synapses may depend on co-activation of arousal systems. To test this hypothesis, a “high arousal” version of the OR task was used where rats were not habituated to the testing conditions. Posttraining intra-BLA infusions of clenbuterol enhanced consolidation of the high-arousing version of the task and significantly increased Arc protein levels in dorsal hippocampus synaptic fractions. These findings suggest that the BLA modulates multiple forms of memory and affects the synaptic plasticity-associated protein Arc in synapses of the dorsal hippocampus when emotional arousal is elevated.
Keywords: BLA, norepinephrine, aversive learning, dorsal hippocampus, object recognition, emotional arousal
1.1 INTRODUCTION
It is frequently observed that emotionally arousing events are better remembered than non-emotionally arousing events (Cahill, Haier, Fallon, Alkire, Tang, Keator, Wu, and McGaugh, 1996; Hamann, Ely, Grafton, and Kilts, 1999; McGaugh, 2000). Extensive evidence indicates that the basolateral complex of the amygdala (BLA) modulates memory through an influence on efferent brain regions such as the dorsal hippocampus (McGaugh, 2004; McIntyre, Miyashita, Setlow, Marjon, Steward, Guzowski, and McGaugh, 2005; Packard, Cahill, and McGaugh, 1994; Roozendaal, Nguyen, Power, and McGaugh, 1999). One way that the BLA likely influences efferent brain regions is by modulating plasticity or expression of plasticity-related proteins in candidate brain regions (Huff, Frank, Wright-Hardesty, Sprunger, Matus-Amat, Higgins, and Rudy, 2006; Ikegaya, Nakanishi, Saito, and Abe, 1997; McIntyre et al., 2005). The protein product of the immediate early gene, activity-regulated cytoskeletal-associated protein (Arc/Arg 3.1), commonly used as a marker of neuronal plasticity, is of particular interest because it has been shown to undergo local translation (Waung, Pfeiffer, Nosyreva, Ronesi, and Huber, 2008; Yin, Edelman, and Vanderklish, 2002) and its mRNA is localized to discrete regions in hippocampal dendrites that have received direct synaptic stimulation (Steward, Wallace, Lyford, and Worley, 1998). Arc protein expression signifies more than just synaptic activity; blockade of Arc protein expression in the dorsal hippocampus impairs maintenance, but not induction, of hippocampal late-phase long-term potentiation and long-term, but not short-term hippocampus-dependent memory, indicating that Arc expression plays a functional role in long-term plasticity and memory (Guzowski, Lyford, Stevenson, Houston, McGaugh, Worley, and Barnes, 2000; McIntyre et al., 2005; Messaoudi, Kanhema, Soule, Tiron, Dagyte, da Silva, and Bramham, 2007). Noradrenergic activation of the BLA increases Arc protein expression in the dorsal hippocampus following training on the inhibitory avoidance task in a post-transcriptional manner (McIntyre et al., 2005) and noradrenergic manipulation of the BLA can influence corticosterone-induced Arc protein expression in dorsal hippocampal synaptic-enriched tissue following training on the inhibitory avoidance task (McReynolds, Donowho, Abdi, McGaugh, Roozendaal, and McIntyre; 2010) suggesting a role for BLA modulation of synaptic Arc protein expression. Taken together with evidence that Arc and other plasticity-related mRNAs can be translated in isolated synaptoneurosomes (Dong, Caruncho, Liu, Smalheiser, Grayson, Costa, and Guidotti, 2003; Dziembowska, Milek, Janusz, Rejmak, Romanowska, Gorkiewicz, Tiron, Bramham, and Kaczmarek; Richter and Lorenz, 2002; Shin, Kundel, and Wells, 2004; Yin et al., 2002) and Arc is found specifically in stimulated regions of dendrites (Farris, Lewandowski, Cox, and Steward, 2014; Huang, Chotiner, and Steward, 2007; Steward et al., 1998; Steward and Worley, 2001), we have proposed the hypothesis that actions in the BLA may modulate the local translation of plasticity-related mRNAs in downstream synapses that are engaged by the training experience (McIntyre et al., 2005; McReynolds and McIntyre, 2012). This hypothesis is supported by evidence that memory enhancing or impairing drug infusions into the BLA influence levels of Arc and another locally translated protein, calcium-calmodulin-dependent kinase IIα (CaMKIIα), but not the somatically localized immediate early gene c-Fos, in the rostral anterior cingulate cortex (Holloway-Erickson, McReynolds, and McIntyre, 2012). However, it is unclear whether BLA modulation of synaptic mRNAs could be considered a general rule of memory consolidation. Here, we examine whether the described effects are isolated observations associated with inhibitory avoidance memory.
The BLA is involved in the memory modulation of aversive tasks such as inhibitory avoidance (Da Cunha, Roozendaal, Vazdarjanova, and McGaugh, 1999; Ferry, Roozendaal, and McGaugh, 1999; LaLumiere, Buen, and McGaugh, 2003; Roozendaal et al., 1999), conditioned taste aversion (Miranda, Quirarte, Rodriguez-Garcia, McGaugh, and Roozendaal, 2008), auditory fear conditioning (Roozendaal, Hui, Hui, Berlau, McGaugh, and Weinberger, 2006), and spatial and cued water maze (Packard et al., 1994). The BLA also plays a role in memory for appetitive behaviors, such as conditioned place preference (McIntyre, Ragozzino, and Gold, 1998) and conditioned cue preference (Ferbinteanu and McDonald, 2001). Although less is known about the role of the BLA in the formation of long-term memory for tasks with little to no emotional arousal, Roozendaal and colleagues demonstrated that intra-BLA infusions of norepinephrine enhanced memory for a relatively non-arousing object recognition task (Roozendaal, Castello, Vedana, Barsegyan, and McGaugh, 2008). This task does not require any external motivation but exploits a rat’s innate exploratory behavior. To avoid novelty-related anxiety, rats can be extensively habituated to the experimental apparatus prior to training. In fact, posttraining corticosterone injections only enhance long-term memory formation for this task when rats have no prior habituation (Okuda, Roozendaal, and McGaugh, 2004) and this effect is dependent upon arousal-induced norepinephrine (Roozendaal, Okuda, Van der Zee, and McGaugh, 2006). Though there is some discrepancy in the literature about the extent of the involvement of the hippocampus in the object recognition task, there is very strong evidence that the hippocampal system is engaged during training and testing and is critical for long-term memory for this task (Broadbent, Squire, and Clark, 2004; Clarke, Rossato, Monteiro, Bevilaqua, Izquierdo, and Cammarota, 2008; Cohen, Munchow, Rios, Zhang, Asgeirsdottir, and Stackman, 2013; da Silveira, Furini, Benetti, Monteiro Sda, and Izquierdo, 2013; Hammond, Tull, and Stackman, 2004; Jobim, Pedroso, Werenicz, Christoff, Maurmann, Reolon, Schroder, and Roesler, 2012). To examine whether the effect of noradrenergic actions in the BLA on hippocampus-dependent memory and Arc protein expression is generalized across multiple classes of memory, and whether these effects are dependent upon training-induced emotional arousal, we examined the effect of immediate posttraining intra-BLA administration of the β-adrenoceptor agonist, clenbuterol, on memory for the aversive inhibitory avoidance task and the relatively non-arousing novel object recognition task, and quantified synaptic Arc protein levels in the dorsal hippocampus following training.
1.2 MATERIALS AND METHODS
1.2.1 Subjects
Eighty seven male Sprague-Dawley rats (250–275g at the time of arrival), obtained from Charles River Breeding Laboratories (Wilmington, MA), were housed individually in a temperature-controlled (22° C) colony room, with food and water available ad libitum. Rats were maintained on a 12h light- 12h dark cycle (7:00–19:00 h, lights on) and kept in the animal colony room for one week before surgeries or behavioral procedures. All experimental procedures were in compliance with the National Institutes of Health guidelines and were approved by the Institutional Animal Care and Use Committee (University of Texas at Dallas).
1.2.2 Surgery
Rats were anesthetized with isoflurane (1% in O2) (Western Medical Supply) and the skull was positioned into a stereotaxic frame (Stoelting, Wood Dale, Il). For animals used for the intra-BLA cannula experiment, two 15-mm-long stainless steel guide cannulas (23 gauge; Small Parts, Miramar, Fl) were implanted bilaterally 2 mm above the BLA [coordinates: anteroposterior (AP), −2.7 mm from Bregma; mediolateral (ML), ±5.2 mm from midline; dorsoventral (DV), −6.4 mm below skull surface; incisor bar, −3.3 mm from interaural line (Paxinos and Watson, 2005)]. The guide cannulas were fixed in place with acrylic dental cement and two small anchoring screws. Stylets (15-mm long insect dissection pins) were inserted into each cannula to maintain patency. After surgery, rats were given 2.0 mL of saline to prevent dehydration. Rats were allowed to recover a minimum of 7 days before the commencement of behavioral training and testing.
1.2.3 Inhibitory Avoidance
Following recovery from surgery, rats were handled for 2 min per day for five consecutive days before training in order to habituate rats to the experimental procedures. Rats were then trained on an inhibitory avoidance task. The inhibitory avoidance apparatus consisted of a trough-shaped alley (91 cm long, 15 cm deep, 20 cm wide at the top and 6.4 cm wide at the floor) that was divided into two compartments, separated by a manually controlled sliding door that opened by retracting into the floor. The starting “light” compartment (31 cm long) was white and illuminated, whereas the shock “dark” compartment (60 cm long) was made of two dark electrifiable metal plates and was not illuminated. The rats were placed in the light compartment and allowed to cross to the dark shock compartment. After a rat stepped completely into the dark compartment, the sliding door was closed and a single inescapable footshock (0.38 mA, 1 s) was delivered. The rat was removed from the dark compartment 15 s later and, after drug treatment, returned to the home cage. Some rats received a retention test 48 h after training. During the retention test, rats were returned to the light compartment of the inhibitory avoidance apparatus and the latency to reenter the dark compartment with all four paws (maximum latency 600 s) was measured. Memory of the training experience was inferred from longer crossing latencies on the retention test. No shock or drug was delivered during retention testing. Other animals were sacrificed 45 min after training and drug treatment, and brains were used for analysis of Arc protein expression. This time point was chosen as this is the peak of Arc protein expression following exposure to a novel context and significant increases in Arc expression are observed in the dorsal hippocampus (McIntyre et al., 2005; Ramirez-Amaya, Vazdarjanova, Mikhael, Rosi, Worley, and Barnes, 2005).
1.2.4 Novel Object Recognition
The experimental apparatus for the object recognition task was a plastic square box (90 cm × 90 cm), which was placed in a dimly illuminated room. The objects to be discriminated were small green plastic cubes and small orange wooden triangles. Animals were handled for 7 days before training. On each day, animals were handled 5 min each, twice a day. Rats were separated into 2 groups. In one group, rats were excessively habituated to the experimental apparatus (With-Habituation) in order to limit novelty-induced emotional arousal (Okuda et al., 2004). In this condition, rats were habituated twice a day to the apparatus by being placed in the box, without any objects, and allowed to freely explore for 5 min. Another group of rats were handled as described, but were not habituated to the experimental apparatus (Without-Habituation). On the day of training, the two identical objects were placed in two opposite corners of the box. The object presented during training was counterbalanced across animals. For the training session, the rats were placed in the box with the objects and allowed to freely explore for 3 min. After 3 min the rats were removed from the box and given a post-training intra-BLA infusion of clenbuterol (4 ng/0.2 µL) or vehicle. The rat was then placed back in the home cage. The rats were given a 24 hr retention test for the behavioral component of the experiment. During the testing session, one of the objects presented during the training trial was placed in one corner and the other corner had a novel object the rat had not seen (either the cube or the triangle). The location of the novel object was counterbalanced. The rat was placed in the apparatus and allowed to freely explore for 3 min. The testing session was videorecorded. Two people, blind to the training conditions, analyzed the videotapes, measuring the time spent exploring each objects. The box was divided into 4 separate grids and locomotor activity was measured by counting the amount of grid crossings during the testing session. Behavioral data was presented as mean discrimination index for each group. The discrimination index was calculated by: [(Time spent exploring the novel object-time spent exploring the training object)/Total exploration time] × 100. For the experiment examining Arc protein expression, rats were trained on the task and received the intra-BLA infusions as described above but were sacrificed 45 minutes after training and drug treatment. This time point was chosen as this is the peak of Arc protein expression following exposure to a context (Ramirez-Amaya et al., 2005). The primary reason for selecting the novel object recognition task over object-in-context or object location tasks is that Roozendaal and colleagues have established two different versions of the novel object recognition task where arousal level is elevated in one version (no habituation) relative to the other (habituated), but all other performance variables remain constant. This allows examination of the effect of emotional arousal and/or habituation on memory and Arc protein expression in the hippocampus while controlling other task-specific factors. Furthermore, this is a task with a demonstrated involvement of BLA noradrenergic activity (Roozendaal et al., 2006; Roozendaal et al., 2008).
1.2.5 Drug treatment
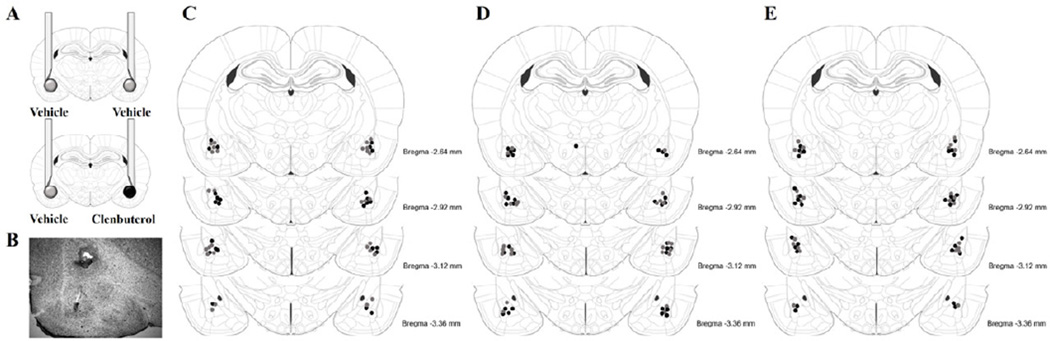
The β-adrenoceptor agonist clenbuterol (4 ng per 0.2 µL; Sigma-Aldrich) or vehicle was infused into the BLA immediately following training. This dose of clenbuterol was chosen as it has been shown to significantly enhance long-term memory for the inhibitory avoidance task and increases Arc protein expression in homogenated dorsal hippocampal tissue (McIntyre et al., 2005). Clenbuterol was dissolved in a vehicle of 0.9% saline. The infusion needles were 25 gauge microinfusion needles that were attached to 10-µL Hamilton microsyringes by polyethylene tubing (PE-20). The drug solution was backfilled into the needle and the infusion was driven by a minipump (Harvard Instruments). The injection needle protruded 2.0 mm beyond the cannula tip and a 0.2-µl injection volume was infused over the course of 32 s. The volume was chosen based on evidence that a 0.2 µL infusion volume of a neurotoxin is small enough to specifically lesion the basolateral, medial, or central nucleus of the amygdala without affecting adjacent nuclei (Roozendaal and McGaugh, 1996). Furthermore, other studies have shown, using a dye, that a larger infusion volume of 0.5 µL is also contained within the BLA (Jobim et al., 2012). For rats used to test memory effects, clenbuterol or vehicle was infused bilaterally into the BLA. For rats that were used for Arc protein expression analysis, each hemisphere was treated differently. Clenbuterol was infused unilaterally into either the left or right BLA and vehicle in the other hemisphere (Fig. 1a) or, in some controls, vehicle was infused bilaterally. In experimental animals, drug and vehicle infusions were counterbalanced across hemispheres in a randomized fashion.
Figure 1.
Posttraining intra-BLA infusions of clenbuterol or vehicle. A.) For rats used for analysis of Arc protein expression, vehicle was infused into the BLA of one hemisphere and clenbuterol was infused into the BLA of the opposite hemisphere. The location of the drug infusion was counter-balanced. For the control group, vehicle was infused bilaterally into the BLA. B.) Representative image of cannula track and needle placement within the BLA. C.) Injection needle tips rats included in the inhibitory avoidance experiment. Black circles represent rats that were used for the behavioral experiment and gray circles represent rats that were used for analysis of Arc protein expression. D.) Injection needle tips rats included in the With-Habituation object recognition experiment. Black circles represent rats that were used for the behavioral experiment and gray circles represent rats that were used for analysis of Arc protein expression. E.) Injection needle tips rats included in the Without-Habituation object recognition experiment. Black circles represent rats that were used for the behavioral experiment and gray circles represent rats that were used for analysis of Arc protein expression. Adapted from Paxinos and Watson (2005).
1.2.6 Tissue Preparation
Rats were deeply anesthetized with isoflurane (Western Medical Supply) 45 min after training and drug treatment and brains were rapidly removed and flash frozen by submersion for 2 min in a beaker filled with 2-methylbutane sitting in a dry-ice ethanol bath. Brains of untrained cage control animals were processed identically. The brains from rats given intra-BLA infusions were cut coronally, just before the fornix, and cut horizontally, just above the rhinal fissure, and several 40-µm thick sections were taken with a Cryostat, mounted on glass slides and stained with thionin. Brain sections were analyzed under a light microscope to identify the location of infusion. Only brains with needle tracks terminating in the BLA were used for analysis (Fig. 1). For Arc comparisons, a cryostat was used to make three 500-µm-thick coronal sections from frozen brain tissue at the level of the dorsal hippocampus (−2.3 to −4.0 mm from Bregma) and tissue punches were taken from the Ammon’s horn and dentate gyrus regions of the dorsal hippocampus using a tissue punch kit (1.22 mm in diameter), and pooled into one sample with each hemisphere collected separately. The tissue punches were stored at −80° C for later Western blot analysis.
1.2.7 Synaptoneurosome Preparation
The hippocampal tissue from each hemisphere was homogenized with a nylon pestle, 16 strokes, in 100 µl homogenization buffer solution [in mM: NaCl, 124; KCl, 5; CaCl2·2 H2O, 0.1; MgCl2·6 H2O, 3.2; NaHCO3, 26; glucose, 10; pH 7.4, containing 20% protease inhibitor cocktail (Sigma), and 10% protease inhibitor cocktail II (Sigma)]. After homogenization, the total volume was brought to 500 µl with the homogenization buffer. The homogenate was backfilled into a 1 ml syringe then filtered through 3 layers of 100 micron nylon mesh (Small Parts) inside a 13 mm syringe filter holder (Pall Life Sciences). The filtered solution was backfilled again into a 1 ml syringe and filtered through a 0.9-µm pore nitrocellulose filter (Sigma-Aldrich) inside a 13 mm syringe filter holder. The final filtered solution was centrifuged at 10,000 × g for 10 min at 4° C. The supernatant was removed and the pellet was resuspended in 100 µl cold homogenization buffer [in mM: Tris, 65.2; NaCl, 150; EDTA, 2; NaH2PO4, 50; Na4P2O7, 10; pH 7.4 containing 0.1% SDS, 0.5% deoxycholate, 20% protease inhibitor cocktail (Sigma) and 10% protease inhibitor cocktail II (Sigma)]. This preparation results in enriched synaptic tissue, as demonstrated by an increase in PSD-95 expression, while does not contain nuclear proteins such as acetyl-H3 (McReynolds et al., 2010).
1.2.8 Protein assay and Immunoblotting
Total protein concentrations from synaptoneurosome tissue were determined using a Qubit fluorometer and Qubit protein assay kit (Invitrogen). Synaptoneurosome tissue containing approximately 15 µg total protein were heated in a sample buffer with a reducing agent (Invitrogen), loaded and then run on 8% Bis-Tris MIDI gels (Invitrogen). Tissue from each condition was loaded onto each gel. The gels were then electroblotted to a nitrocellulose membrane using an iBlot dry-blotting system (Invitrogen). Membranes were then washed in Tris-buffered saline (TBS: 150 mM NaCl/100 mM Tris base, pH 7.5) and incubated with primary antibodies diluted in blocking solution (5% Carnation nonfat dry milk in TBS-Tween) overnight at 4°C. The primary antibodies were anti-Arc (rabbit polyclonal; 1:6000, Synaptic Systems) and anti-actin (rabbit; 1:3000, Sigma). On the next day, the membranes were incubated with a secondary HRP-linked antibody (goat anti-rabbit; 1:6000, Millipore) for 1 h. Immunoreactivity was detected using chemiluminescence (ECL Western Blot Kit; Pierce). Invitrogen markers were run on all gels to determine the relative mobility of the immunoreactive bands. For densitometric quantification of these results, the films were scanned and converted into TIF files for analysis with NIH Image J software.
1.2.9 Statistical Analysis
Inhibitory avoidance retention latencies were analyzed with two-sample t-tests to make pair-wise comparisons between the vehicle- and clenbuterol-infused groups. Mean discrimination indices were analyzed with two-sample t-tests to make pair-wise comparisons between the vehicle-infused and clenbuterol-infused groups. The remaining control behavioral measures were also analyzed using two-sample t-tests to make pair-wise comparisons between the two groups. Western blot densitometry results were expressed as a ratio of Arc to actin and were then expressed as a ratio of the drug-infused hemisphere to the vehicle-infused hemisphere (or a ratio of one hemisphere to the other hemisphere, with conditions being randomly assigned, if the rat received a bilateral vehicle infusion). Finally, those ratios were expressed as a percentage of the bilateral vehicle control group. The percentages were then compared using a Student’s t-test to make pair-wise comparisons between drug groups. A probability level of p < .05 was considered significant. Data are presented as means + SEM.
1.3 RESULTS
1.3.1 Posttraining intra-BLA infusions of clenbuterol enhance memory for the inhibitory avoidance task and increase dorsal hippocampal synaptic Arc protein expression
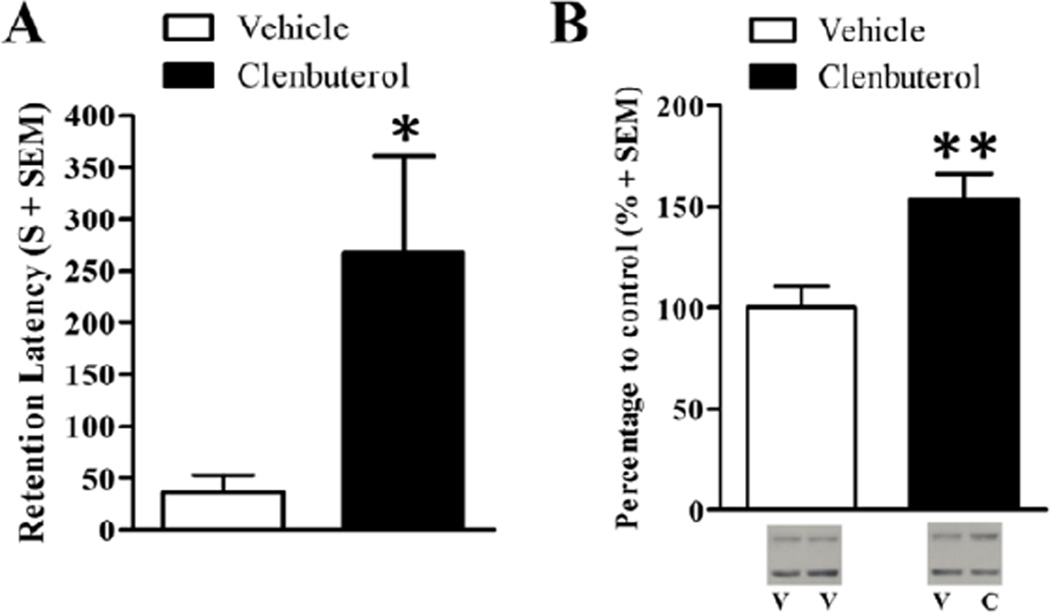
In order to examine whether β-adrenoceptor activation of the BLA influences dorsal hippocampal synaptic Arc protein levels, rats were trained on the inhibitory avoidance task and given immediate posttraining intra-BLA infusions of clenbuterol or vehicle. Memory retention was tested 48 hr later or Arc protein expression was examined in synaptoneurosomes taken from the dorsal hippocampus 45 min after training. Training latencies did not significantly differ between clenbuterol-infused rats (mean training latency ± SEM: 9.83 s ± 2.82) and vehicle-infused rats (mean training latency ± SEM: 16.86 s ± 5.51; t(11)= −1.08; p=.31). However, a two-sample t-test revealed that clenbuterol-treated rats had significantly higher 48-hr retention latencies as compared to the rats given intra-BLA infusions of vehicle (Fig. 2A; t(11)= −2.25; p<.05). Mean retention latencies for the clenbuterol-infused rats (n=7) were 267.43 s ± 93.36 and mean retention latencies for the vehicle-infused rats (n=6) were 36.00 s ± 16.97.
Figure 2.
Posttraining intra-BLA infusions of clenbuterol enhance memory for the inhibitory avoidance task and increase dorsal hippocampal synaptic Arc protein expression. A.) Rats that received immediate posttraining intra-BLA infusions of clenbuterol (n=7; 4 ng/0.2 µL) had a significantly higher retention latencies than those that received intra-BLA infusions of vehicle (n=6; p<.05). B.) Western blot analysis of Arc protein expression 45 min after training on the inhibitory avoidance task and drug treatment. Memory-enhancing clenbuterol (n=9; 4 ng/0.2 µL) significantly increases Arc protein expression in dorsal hippocampal enriched synaptic tissue as compared to intra-BLA vehicle (n=7, p<.01). Representative blot images are shown underneath the graph. V=vehicle, C=clenbuterol. Data are presented as mean + SEM.
Arc protein expression was examined in synaptic fractions from the dorsal hippocampus ipsilateral to the intra-BLA infusion of clenbuterol and compared to the dorsal hippocampal tissue ipsilateral to the intra-BLA infusion of vehicle (or randomly assigned left-right or right-left for the bilateral infusion of vehicle). This design was used because it allows each animal to serve as its own control and limits the variability when comparing between animals (McIntyre et al, 2005). Arc was normalized to actin, then the drug-infused hemisphere was normalized to the vehicle-infused hemisphere (or randomly counterbalanced left-to-right or right-to-left for the bilateral vehicle-infused group), and were finally expressed as a percentage of the corresponding bilateral vehicle-infused group. A two-sample t-test revealed a significant increase in Arc protein expression in dorsal hippocampal synaptic tissue from the clenbuterol-infused rats (n=9) as compared to the vehicle-infused rats (Fig. 2B; vehicle, n=7; t(14) = −3.00, p<.01). Memory-enhancing intra-BLA administration of clenbuterol significantly increased dorsal hippocampal synaptic Arc protein.
1.3.2 Posttraining intra-BLA infusions of clenbuterol enhance memory for the novel object recognition task but do not influence dorsal hippocampal synaptic Arc protein expression
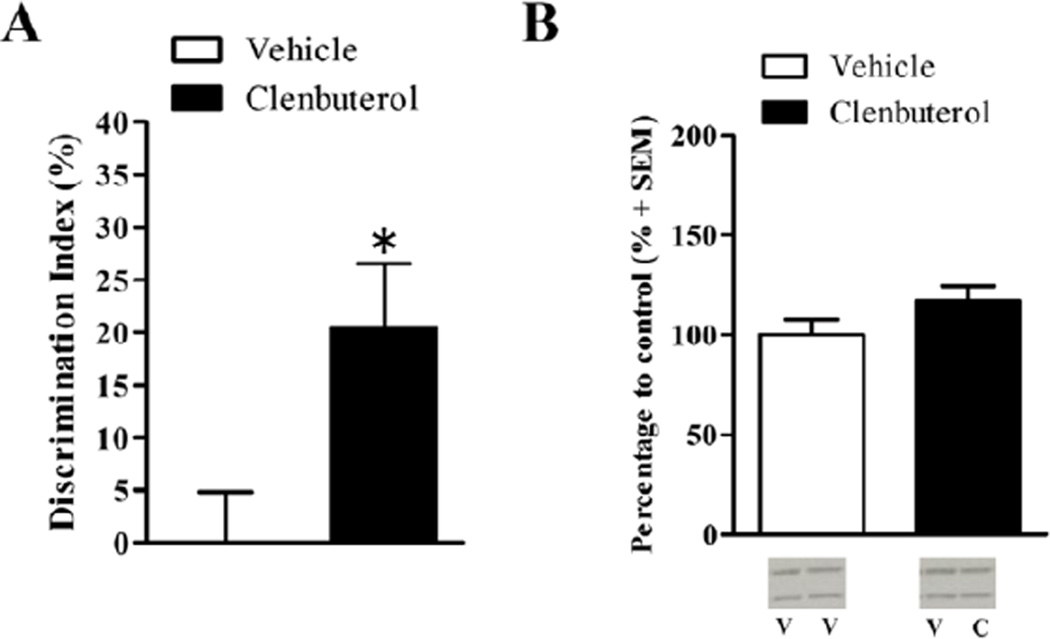
To determine whether mimicking the effects of endogenous arousal on noradrenergic receptors in the BLA enhances memory for otherwise non-arousing information, rats were trained on the relatively non-arousing novel object recognition task wherein the rats are extensively habituated to the experimental apparatus prior to training (With-Habituation). There was no significant difference in time spent exploring the object in either the left or right side for either the vehicle-treated (left: 21.02 s ± 2.98; right: 21.54s ± 2.56; t(7)=−0.15, p=.88) or clenbuterol-treated rats (left: 19.73 s ± 2.14; right: 17.63s ± 2.35; t(7)=0.73, p=.49). Furthermore, there was no significant difference in total exploration time during the training session between vehicle-(mean total exploration time: 42.56s ± 4.48) and clenbuterol-treated rats (mean total exploration time: 37.35s ± 3.47; t(14)=0.92, p=.37). Memory retention was tested 24 hr after training and drug treatment. The discrimination index (DI) was significantly greater in clenbuterol-treated rats (Fig. 3A; n=8; DI: 20.43% ± 6.09) than vehicle-treated rats (n=8; DI: 0.1%+4.79; t(14)=−2.63, p<.05). There was no significant difference in locomotor activity during the retention test, as assessed by the number of grid crossings, between the 2 different drug groups (t(14)= −.07, p=.94). Finally, there was no significant difference in total exploration time between the vehicle- (mean total exploration time: 30.25s ± 3.30) and clenbuterol- infused rats (mean total exploration time: 33.22s ± 5.21; t(14)= −0.48, p=0.64). Rats given intra-BLA infusions of clenbuterol showed significantly enhanced memory for the novel object recognition task.
Figure 3.
Posttraining intra-BLA infusions of clenbuterol enhance memory for the non-arousing object recognition task (With-Habituation) but not affect dorsal hippocampal synaptic Arc protein expression. A.) Posttraining intra-BLA infusions of clenbuterol enhance memory for the novel object recognition task (With-Habituation). Rats that received immediate posttraining intra-BLA infusions of clenbuterol (n=8; 4 ng/0.2 µL) had significantly higher discrimination indices during the retention test than those that received intra-BLA infusions of vehicle (n=8; p<.02). B.) Western blot analysis of Arc protein expression 45 min after training on the non-arousing object recognition task (With-Habituation) and drug treatment. Memory-enhancing clenbuterol (n=8; 4 ng/0.2 µL) did not significantly increase Arc protein expression in dorsal hippocampal enriched synaptic tissue as compared to intra-BLA vehicle (n=7, p=.15). Representative blot images are shown underneath the graph. V=vehicle, C=clenbuterol. Data are presented as mean + SEM.
Arc protein expression was quantified as described above. Intra-BLA infusions of clenbuterol did not influence Arc protein expression in dorsal hippocampal synaptic fractions following training on the non-arousing object recognition task (With-Habituation). In hippocampal synaptic fractions, a two-sample t-test did not reveal a significant difference in Arc protein expression between the clenbuterol-infused (n=8) and vehicle-infused rats (Fig. 3B; n=7; t(11)= −1.53, p=.15). A two-sample t-test also did not reveal a significant difference in Arc protein expression between trained and cage control rats (t(27)= −1.55, p=0.13, data not shown).
1.3.2 Posttraining intra-BLA infusions of clenbuterol enhance memory for the more arousing novel object recognition task (Without-Habituation) and increase dorsal hippocampal synaptic Arc protein expression
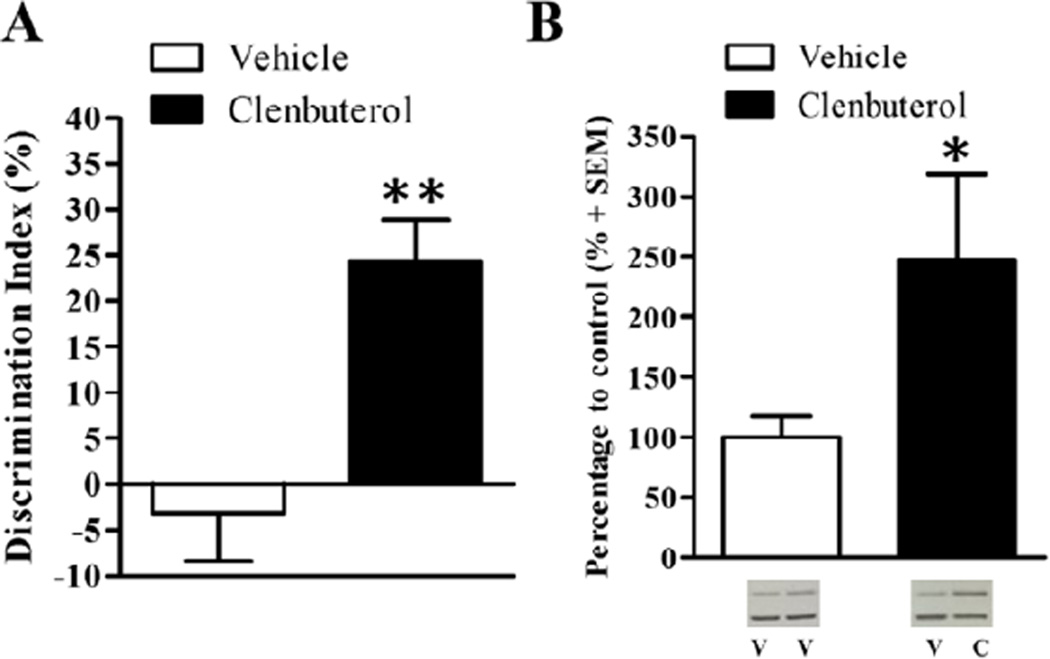
In order to assess whether the effect of intra-BLA clenbuterol on dorsal hippocampal Arc protein expression requires an emotional arousal component, rats were trained on a version of the novel object recognition task that is slightly more stressful (Without-Habituation). This version of the task is designed to drive a stress response in rats, who typically show some neophobia, by exposing them to the objects in the apparatus without prior habituation. Exposure to a novel context results in an increase in norepinephrine levels in the hippocampus (Ihalainen, Riekkinen, and Feenstra, 1999), suggesting that exposure to a novel context elicits a measure of emotional arousal. To examine whether noradrenergic activation of the BLA enhances memory for this version of the task, rats were trained on the object recognition task for 3 min, received intra-BLA infusions of clenbuterol, and were given a retention test 24 hr later. There was no significant difference in time spent exploring the object in either the left or right side for either the vehicle-treated (left: 18.13 s ± 2.32; right: 16.79s ± 2.91; t(7)=0.46, p=.66) or clenbuterol-treated rats (left: 16.87 s ± 2.52; right: 19.12s ± 2.37; t(6)=−0.77, p=.47). Furthermore, there was no significant difference in total exploration time during the training session between vehicle-(mean total exploration time: 34.92s ± 4.40) and clenbuterol-treated rats (mean total exploration time: 35.99s ± 3.92; t(13)=−0.18, p=.86), indicating that the discrimination difference was not due to differences in exploration of the objects during the training or test session. A two-sample t-test revealed that rats treated with intra-BLA clenbuterol (n=8) show a significantly greater discrimination index than rats given intra-BLA infusions of vehicle (Fig. 4A; n=8; t(13)= −3.91, p<.01). Vehicle-treated control rats had a −3.129% ± 5.24 discrimination index and rats given intra-BLA infusions of clenbuterol had a 24.31% ± 4.49 discrimination index. There was no significant difference in locomotor activity during the retention test, as assessed by number of grid crossings during the test, between the 2 different drug groups (t(13)= −.20, p=.84). Finally, there was no significant difference in total exploration time between the vehicle- (mean total exploration time: 34.79s ± 2.60) and clenbuterol-infused rats (mean total exploration time: 38.62s ± 2.39; t(13)= −1.07, p=0.30) during the retention test. Once again, intra-BLA infusions of clenbuterol significantly enhanced memory for the object recognition task.
Figure 4.
Posttraining intra-BLA infusions of clenbuterol enhance memory for the higher-arousing object recognition task (Without-Habituation) and dorsal hippocampal synaptic Arc protein expression. A.) Posttraining intra-BLA infusions of clenbuterol enhance memory for the novel object recognition task (Without-Habituation). Rats that received immediate posttraining intra-BLA infusions of clenbuterol (n=7; 4 ng/0.2 µL) had significantly higher discrimination indices during the retention test than those that received intra-BLA infusions of vehicle (n=8; p<.01). B.) Western blot analysis of Arc protein expression 45 min after training on the low-arousing object recognition task (Without-Habituation) and drug treatment. Memory-enhancing clenbuterol (n=5; 4 ng/0.2 µL) significantly increases Arc protein expression in dorsal hippocampal enriched synaptic tissue as compared to intra-BLA vehicle (n=7, p=.15). Representative blot images are shown underneath the graph. V=vehicle, C=clenbuterol. Data are presented as mean + SEM.
A two-sample t-test revealed a significant increase in Arc protein expression between the clenbuterol-infused (n=5) and vehicle-infused rats (Fig. 4B; n=7; t(10)= −2.36, p<.05). A two-sample t-test also revealed a significant increase in dorsal hippocampal synaptic Arc protein expression in trained rats compared to cage control rats (t(13)= −2.39, p<0.05, data not shown). Training on the more arousing (Without-Habituation) object recognition task increased synaptic Arc protein expression in the dorsal hippocampus and intra-BLA infusions of clenbuterol increased it further.
1.4 DISCUSSION
The main finding of this study is that memory-enhancing intra-BLA administration of the β-adrenoceptor agonist, clenbuterol, increases dorsal hippocampal synaptic Arc protein levels following training on tasks that have a component of emotional arousal. Intra-BLA clenbuterol administration enhanced memory for both the inhibitory avoidance and novel object recognition tasks. Consistent with prior findings examining Arc expression in whole dorsal hippocampal homogenate, the same memory-enhancing intra-BLA clenbuterol treatment significantly increased Arc protein expression following training on the inhibitory avoidance task in synaptic-enriched tissue from the dorsal hippocampus, supporting the hypothesis that BLA actions have consequences on locally translated proteins in downstream brain regions. However, intra-BLA clenbuterol did not increase dorsal hippocampal synaptic Arc expression following training on a low-arousing version of the novel object recognition task wherein rats are extensively habituated to the experimental apparatus. This finding suggests that the influence of the BLA on the plasticity-associated protein Arc in hippocampal synapses is not a general rule of synaptic or memory consolidation of single trial learning, as proposed. Interestingly, in a version of the novel object recognition task wherein rats have no prior habituation to the experimental apparatus, intra-BLA clenbuterol significantly increased dorsal hippocampal synaptic Arc protein expression. These findings suggest that noradrenergic activation of the BLA only influences synaptic Arc protein expression in the hippocampus when the learning and memory task evokes some emotional arousal.
Modulation of hippocampal Arc expression by noradrenergic activation of the BLA appears to occur at a post-transcriptional level (McIntyre et al., 2005). Local translation of synaptic proteins may be necessary for long-term plasticity and memory consolidation (Bramham, 2008; Steward and Worley, 2002; Sutton and Schuman, 2006) as synaptic plasticity in the hippocampus is dependent on local translation of Arc and CaMKII (Waung et al., 2008) and disruption of dendritic targeting of CaMKII impairs memory consolidation (Miller, Yasuda, Coats, Jones, Martone, and Mayford, 2002). We have previously shown that corticosterone-induced increases in hippocampal synaptic Arc protein expression following inhibitory avoidance training is dependent on BLA noradrenergic activity (McReynolds et al., 2010). Here, we extend those findings to show that BLA noradrenergic activation immediately after inhibitory avoidance training modulates synaptic Arc protein expression in the dorsal hippocampus.
Emotional arousal influences memory consolidation but whether it modulates universal memory consolidation mechanisms or depends upon distinct mechanisms is not well understood. Exposure to a stressful stimulus increases norepinephrine levels in the amygdala (Galvez, Mesches, and McGaugh, 1996; Quirarte, Galvez, Roozendaal, and McGaugh, 1998) and is correlated with memory of the aversive inhibitory avoidance task (McIntyre, Hatfield, and McGaugh, 2002) and these processes likely influence memory through modulation of synaptic plasticity in the hippocampus (Ikegaya et al., 1997; McIntyre et al., 2005). Expression of the plasticity-related protein Arc in the dorsal hippocampus is implicated in the consolidation of such memory tasks such as inhibitory avoidance, spatial water maze, and fear conditioning (Czerniawski, Ree, Chia, Ramamoorthi, Kumata, and Otto, 2011; Guzowski et al., 2000; Huff et al., 2006; McIntyre et al., 2005). These tasks require the formation of associations between places or contexts with punishment (footshock) or reward (escape from water). They are, therefore, hippocampus-dependent. However, it remains to be seen whether the emotional arousal associated with footshock or escape is critical for the BLA modulation of learning-related hippocampal Arc expression. Here, we demonstrate that noradrenergic activation of the BLA also increases hippocampal Arc protein expression following training on the novel object recognition task. Arc expression can be regulated by mTOR signaling (Dong et al., 2003), which is consistent with findings that mTOR signaling within the dorsal hippocampus is necessary for the consolidation of memory for the novel object recognition task (Jobim et al., 2012). Here, increases in Arc expression were only seen with object recognition training wherein rats have no prior habituation to the experimental apparatus. Exposure to a novel context on the day of training appears to be a critical factor in the Arc response to BLA manipulations. One potential explanation for the novelty effect is that it elicits a measure of emotional arousal (Ihalainen et al., 1999; Okuda et al., 2004). The object recognition task varies from inhibitory avoidance because object recognition does not employ an external motivator for rats to complete the task, and versions of the task have been suggested to be most similar to what would be considered episodic memory in humans (Crystal, 2009). Therefore, the present findings suggest that the BLA modulates expression of the plasticity-related protein, Arc, in the hippocampus for multiple memory tasks, but only those that elicit some measure of emotional arousal.
Noradrenergic activation of the BLA alone may not be sufficient to influence learning associated plasticity in the hippocampus. According to the “emotional tagging” hypothesis (Richter-Levin and Akirav, 2003), otherwise irrelevant information can be stored as long-term memory when associated with emotional arousal (Bergado, Lucas, and Richter-Levin, 2011). Our own findings support this as training on the novel object recognition task only produces long-term memory when coupled with noradrenergic activation of the BLA (Roozendaal et al., 2008). This hypothesis was extended from Frey and Morris’s “synaptic tagging” hypothesis (Frey and Morris, 1998), which proposed that the conversion of short-term to long-term plasticity occurs through first the setting of a local tag at relevant synapses followed by synthesis of plasticity-related proteins at the “tagged” synapse to produce long-lasting changes in synaptic strength (Redondo and Morris, 2011). The present findings suggest that mimicking endogenous arousal through selective activation of the BLA noradrenergic system is not sufficient to produce measurable changes in synaptic Arc protein. Administration of the β-adrenoceptor agonist into the BLA immediately after training only increased hippocampal Arc protein levels following training on tasks that have an emotional arousal component. This suggests that there is a requirement for arousal-induced activation of not only the BLA but also its efferent brain regions for modification of synaptic Arc protein expression. The initial “synaptic tag” may be set by noradrenergic activation in the hippocampus, allowing further synthesis of plasticity-related proteins at those tagged synapses to support long-lasting changes in plasticity and memory. The endogenous stress response involves locus coeruleus activation and increased norepinephrine in the amygdala and other brain regions at the same time, including the hippocampus (Abercrombie and Jacobs, 1987; Miyashita and Williams, 2004; Tanaka, 1999; Tanaka, Kohno, Nakagawa, Ida, Takeda, Nagasaki, and Noda, 1983; Zigmond, Schon, and Iversen, 1974). In addition, norepinephrine in the hippocampus can lower the threshold for induction of LTP suggesting a required coordination of norepinephrine response in both the BLA and hippocampus for measured synaptic changes in the hippocampus, (Hu, Real, Takamiya, Kang, Ledoux, Huganir, and Malinow, 2007; Thomas, Moody, Makhinson, and O'Dell, 1996).
Posttraining intra-BLA clenbuterol administration enhanced memory for the novel object recognition task wherein rats are extensively habituated to the experimental apparatus even though it did not significantly increase hippocampal synaptic Arc protein levels. However it is possible that relevant changes in Arc protein were too subtle for detection by western blotting of synaptoneurosome fractions under those conditions. It is also possible that there were selective changes in discrete subregions of the dorsal hippocampus that would not have been detected as we dissected the whole dorsal hippocampus. The extensive habituation to the experimental apparatus could have limited the requirement for long-lasting changes in the hippocampus following the novel object exposure trial. In fact, we found that training alone only significantly increased synaptic Arc protein expression when rats had no prior habituation to the experimental apparatus. This is consistent with findings that overtraining rats on an operant conditioning paradigm resulted in decreased Arc mRNA expression compared to newly trained rats (Kelly and Deadwyler, 2002). Furthermore, place fields in the CA1 region are altered when changing the location of an object within a context but not when changing the object itself (Lenck-Santini, Rivard, Muller, and Poucet, 2005), suggesting that there may not be a need for synapse modifications and related Arc expression in the hippocampus when contextual information is already well-consolidated. The findings of the present study suggest that under conditions of low emotional arousal, such as those seen with extensive habituation to the experimental apparatus, increases in hippocampal Arc protein expression are not needed for memory formation and BLA noradrenergic activation may enhance memory through modulation of other plasticity mechanisms. Therefore, enhancement of memory consolidation through BLA noradrenergic activation may involve distinct mechanisms that depend on the level of emotional arousal that the learning task elicits.
1.5 CONCLUSIONS
The present findings present new evidence that the influence of the BLA is generalized across different classes of memory. Noradrenergic activation of the BLA enhances memory for a variety of behaviors including memory for relatively non-arousing information. In addition, the influence of the BLA on dorsal hippocampal synaptic Arc expression is extended beyond fear conditioning or avoidance learning tasks and is generalized to other forms of memory. This supports a role for the BLA in modulating hippocampal synaptic plasticity to enhance long-term memory formation, however, Arc expression in hippocampal synapses was unchanged following intra-BLA treatment that enhanced memory for novel objects after extensive habituation. In conclusion, training on learning and memory tasks that incorporate some measure of novelty or novelty-related emotional arousal produce changes in expression of the synaptic plasticity protein, Arc, in the dorsal hippocampus, and this Arc expression can be modulated by noradrenergic activation of the BLA.
Highlights.
Intra-BLA clenbuterol infusions enhance consolidation of inhibitory avoidance.
Intra-BLA clenbuterol infusions increase Arc protein expression in hippocampal synapses.
Intra-BLA clenbuterol infusions enhance consolidation of object recognition memory.
The BLA influence on hippocampal Arc expression is associated with emotional memory.
Acknowledgements
This research was funded by the Department of Behavioral and Brain Sciences at The University of Texas at Dallas. The authors declare no competing financial interest.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
REFERENCES
- Abercrombie ED, Jacobs BL. Single-unit response of noradrenergic neurons in the locus coeruleus of freely moving cats. I. Acutely presented stressful and nonstressful stimuli. J Neurosci. 1987;7:2837–2843. doi: 10.1523/JNEUROSCI.07-09-02837.1987. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bergado JA, Lucas M, Richter-Levin G. Emotional tagging--a simple hypothesis in a complex reality. Prog Neurobiol. 2011;94:64–76. doi: 10.1016/j.pneurobio.2011.03.004. [DOI] [PubMed] [Google Scholar]
- Bramham CR. Local protein synthesis, actin dynamics, and LTP consolidation. Curr Opin Neurobiol. 2008;18:524–531. doi: 10.1016/j.conb.2008.09.013. [DOI] [PubMed] [Google Scholar]
- Broadbent NJ, Squire LR, Clark RE. Spatial memory, recognition memory, and the hippocampus. Proc Natl Acad Sci U S A. 2004;101:14515–14520. doi: 10.1073/pnas.0406344101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L, Haier RJ, Fallon J, Alkire MT, Tang C, Keator D, Wu J, McGaugh JL. Amygdala activity at encoding correlated with long-term, free recall of emotional information. Proc Natl Acad Sci U S A. 1996;93:8016–8021. doi: 10.1073/pnas.93.15.8016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clarke JR, Rossato JI, Monteiro S, Bevilaqua LR, Izquierdo I, Cammarota M. Posttraining activation of CB1 cannabinoid receptors in the CA1 region of the dorsal hippocampus impairs object recognition long-term memory. Neurobiol Learn Mem. 2008;90:374–381. doi: 10.1016/j.nlm.2008.04.009. [DOI] [PubMed] [Google Scholar]
- Cohen SJ, Munchow AH, Rios LM, Zhang G, Asgeirsdottir HN, Stackman RW., Jr The rodent hippocampus is essential for nonspatial object memory. Curr Biol. 2013;23:1685–1690. doi: 10.1016/j.cub.2013.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crystal JD. Elements of episodic-like memory in animal models. Behav Processes. 2009;80:269–277. doi: 10.1016/j.beproc.2008.09.009. [DOI] [PubMed] [Google Scholar]
- Czerniawski J, Ree F, Chia C, Ramamoorthi K, Kumata Y, Otto TA. The importance of having Arc: expression of the immediate-early gene Arc is required for hippocampus-dependent fear conditioning and blocked by NMDA receptor antagonism. J Neurosci. 2011;31:11200–11207. doi: 10.1523/JNEUROSCI.2211-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Da Cunha C, Roozendaal B, Vazdarjanova A, McGaugh JL. Microinfusions of flumazenil into the basolateral but not the central nucleus of the amygdala enhance memory consolidation in rats. Neurobiol Learn Mem. 1999;72:1–7. doi: 10.1006/nlme.1999.3912. [DOI] [PubMed] [Google Scholar]
- da Silveira CK, Furini CR, Benetti F, Monteiro Sda C, Izquierdo I. The role of histamine receptors in the consolidation of object recognition memory. Neurobiol Learn Mem. 2013;103:64–71. doi: 10.1016/j.nlm.2013.04.001. [DOI] [PubMed] [Google Scholar]
- Dong E, Caruncho H, Liu WS, Smalheiser NR, Grayson DR, Costa E, Guidotti A. A reelin-integrin receptor interaction regulates Arc mRNA translation in synaptoneurosomes. Proc Natl Acad Sci U S A. 2003;100:5479–5484. doi: 10.1073/pnas.1031602100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dziembowska M, Milek J, Janusz A, Rejmak E, Romanowska E, Gorkiewicz T, Tiron A, Bramham CR, Kaczmarek L. Activity-dependent local translation of matrix metalloproteinase-9. J Neurosci. 32:14538–14547. doi: 10.1523/JNEUROSCI.6028-11.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Farris S, Lewandowski G, Cox CD, Steward O. Selective localization of arc mRNA in dendrites involves activity- and translation-dependent mRNA degradation. J Neurosci. 2014;34:4481–4493. doi: 10.1523/JNEUROSCI.4944-13.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferbinteanu J, McDonald RJ. Dorsal/ventral hippocampus, fornix, and conditioned place preference. Hippocampus. 2001;11:187–200. doi: 10.1002/hipo.1036. [DOI] [PubMed] [Google Scholar]
- Ferry B, Roozendaal B, McGaugh JL. Basolateral amygdala noradrenergic influences on memory storage are mediated by an interaction between beta- and alpha1-adrenoceptors. J Neurosci. 1999;19:5119–5123. doi: 10.1523/JNEUROSCI.19-12-05119.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frey U, Morris RG. Synaptic tagging: implications for late maintenance of hippocampal long-term potentiation. Trends Neurosci. 1998;21:181–188. doi: 10.1016/s0166-2236(97)01189-2. [DOI] [PubMed] [Google Scholar]
- Galvez R, Mesches MH, McGaugh JL. Norepinephrine release in the amygdala in response to footshock stimulation. Neurobiol Learn Mem. 1996;66:253–257. doi: 10.1006/nlme.1996.0067. [DOI] [PubMed] [Google Scholar]
- Guzowski JF, Lyford GL, Stevenson GD, Houston FP, McGaugh JL, Worley PF, Barnes CA. Inhibition of activity-dependent arc protein expression in the rat hippocampus impairs the maintenance of long-term potentiation and the consolidation of long-term memory. J Neurosci. 2000;20:3993–4001. doi: 10.1523/JNEUROSCI.20-11-03993.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamann SB, Ely TD, Grafton ST, Kilts CD. Amygdala activity related to enhanced memory for pleasant and aversive stimuli. Nat Neurosci. 1999;2:289–293. doi: 10.1038/6404. [DOI] [PubMed] [Google Scholar]
- Hammond RS, Tull LE, Stackman RW. On the delay-dependent involvement of the hippocampus in object recognition memory. Neurobiol Learn Mem. 2004;82:26–34. doi: 10.1016/j.nlm.2004.03.005. [DOI] [PubMed] [Google Scholar]
- Holloway-Erickson CM, McReynolds JR, McIntyre CK. Memory-enhancing intra-basolateral amygdala infusions of clenbuterol increase Arc and CaMKIIalpha protein expression in the rostral anterior cingulate cortex. Front Behav Neurosci. 2012;6:17. doi: 10.3389/fnbeh.2012.00017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hu H, Real E, Takamiya K, Kang MG, Ledoux J, Huganir RL, Malinow R. Emotion enhances learning via norepinephrine regulation of AMPA-receptor trafficking. Cell. 2007;131:160–173. doi: 10.1016/j.cell.2007.09.017. [DOI] [PubMed] [Google Scholar]
- Huang F, Chotiner JK, Steward O. Actin polymerization and ERK phosphorylation are required for Arc/Arg3.1 mRNA targeting to activated synaptic sites on dendrites. J Neurosci. 2007;27:9054–9067. doi: 10.1523/JNEUROSCI.2410-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huff NC, Frank M, Wright-Hardesty K, Sprunger D, Matus-Amat P, Higgins E, Rudy JW. Amygdala regulation of immediate-early gene expression in the hippocampus induced by contextual fear conditioning. J Neurosci. 2006;26:1616–1623. doi: 10.1523/JNEUROSCI.4964-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ihalainen JA, Riekkinen P, Jr, Feenstra MG. Comparison of dopamine and noradrenaline release in mouse prefrontal cortex, striatum and hippocampus using microdialysis. Neurosci Lett. 1999;277:71–74. doi: 10.1016/s0304-3940(99)00840-x. [DOI] [PubMed] [Google Scholar]
- Ikegaya Y, Nakanishi K, Saito H, Abe K. Amygdala beta-noradrenergic influence on hippocampal long-term potentiation in vivo. Neuroreport. 1997;8:3143–3146. doi: 10.1097/00001756-199709290-00027. [DOI] [PubMed] [Google Scholar]
- Jobim PF, Pedroso TR, Werenicz A, Christoff RR, Maurmann N, Reolon GK, Schroder N, Roesler R. Impairment of object recognition memory by rapamycin inhibition of mTOR in the amygdala or hippocampus around the time of learning or reactivation. Behav Brain Res. 2012;228:151–158. doi: 10.1016/j.bbr.2011.12.004. [DOI] [PubMed] [Google Scholar]
- Kelly MP, Deadwyler SA. Acquisition of a novel behavior induces higher levels of Arc mRNA than does overtrained performance. Neuroscience. 2002;110:617–626. doi: 10.1016/s0306-4522(01)00605-4. [DOI] [PubMed] [Google Scholar]
- LaLumiere RT, Buen TV, McGaugh JL. Post-training intra-basolateral amygdala infusions of norepinephrine enhance consolidation of memory for contextual fear conditioning. J Neurosci. 2003;23:6754–6758. doi: 10.1523/JNEUROSCI.23-17-06754.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lenck-Santini PP, Rivard B, Muller RU, Poucet B. Study of CA1 place cell activity and exploratory behavior following spatial and nonspatial changes in the environment. Hippocampus. 2005;15:356–369. doi: 10.1002/hipo.20060. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. Memory--a century of consolidation. Science. 2000;287:248–251. doi: 10.1126/science.287.5451.248. [DOI] [PubMed] [Google Scholar]
- McGaugh JL. The amygdala modulates the consolidation of memories of emotionally arousing experiences. Annu Rev Neurosci. 2004;27:1–28. doi: 10.1146/annurev.neuro.27.070203.144157. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Hatfield T, McGaugh JL. Amygdala norepinephrine levels after training predict inhibitory avoidance retention performance in rats. Eur J Neurosci. 2002;16:1223–1226. doi: 10.1046/j.1460-9568.2002.02188.x. [DOI] [PubMed] [Google Scholar]
- McIntyre CK, Miyashita T, Setlow B, Marjon KD, Steward O, Guzowski JF, McGaugh JL. Memory-influencing intra-basolateral amygdala drug infusions modulate expression of Arc protein in the hippocampus. Proc Natl Acad Sci U S A. 2005;102:10718–10723. doi: 10.1073/pnas.0504436102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McIntyre CK, Ragozzino ME, Gold PE. Intra-amygdala infusions of scopolamine impair performance on a conditioned place preference task but not a spatial radial maze task. Behav Brain Res. 1998;95:219–226. doi: 10.1016/s0166-4328(97)00161-7. [DOI] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem. 93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, Donowho K, Abdi A, McGaugh JL, Roozendaal B, McIntyre CK. Memory-enhancing corticosterone treatment increases amygdala norepinephrine and Arc protein expression in hippocampal synaptic fractions. Neurobiol Learn Mem. 2010;93:312–321. doi: 10.1016/j.nlm.2009.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McReynolds JR, McIntyre CK. Emotional modulation of the synapse. Rev Neurosci. 2012;23:449–461. doi: 10.1515/revneuro-2012-0047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Messaoudi E, Kanhema T, Soule J, Tiron A, Dagyte G, da Silva B, Bramham CR. Sustained Arc/Arg3.1 synthesis controls long-term potentiation consolidation through regulation of local actin polymerization in the dentate gyrus in vivo. J Neurosci. 2007;27:10445–10455. doi: 10.1523/JNEUROSCI.2883-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miller S, Yasuda M, Coats JK, Jones Y, Martone ME, Mayford M. Disruption of dendritic translation of CaMKIIalpha impairs stabilization of synaptic plasticity and memory consolidation. Neuron. 2002;36:507–519. doi: 10.1016/s0896-6273(02)00978-9. [DOI] [PubMed] [Google Scholar]
- Miranda MI, Quirarte GL, Rodriguez-Garcia G, McGaugh JL, Roozendaal B. Glucocorticoids enhance taste aversion memory via actions in the insular cortex and basolateral amygdala. Learn Mem. 2008;15:468–476. doi: 10.1101/lm.964708. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyashita T, Williams CL. Peripheral arousal-related hormones modulate norepinephrine release in the hippocampus via influences on brainstem nuclei. Behav Brain Res. 2004;153:87–95. doi: 10.1016/j.bbr.2003.11.005. [DOI] [PubMed] [Google Scholar]
- Okuda S, Roozendaal B, McGaugh JL. Glucocorticoid effects on object recognition memory require training-associated emotional arousal. Proc Natl Acad Sci U S A. 2004;101:853–858. doi: 10.1073/pnas.0307803100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Packard MG, Cahill L, McGaugh JL. Amygdala modulation of hippocampal-dependent and caudate nucleus-dependent memory processes. Proc Natl Acad Sci U S A. 1994;91:8477–8481. doi: 10.1073/pnas.91.18.8477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The rat brain in stereotaxic coordinates. 5th ed. Boston: Elsevier Academic Press; 2005. Amsterdam. [Google Scholar]
- Quirarte GL, Galvez R, Roozendaal B, McGaugh JL. Norepinephrine release in the amygdala in response to footshock and opioid peptidergic drugs. Brain Res. 1998;808:134–140. doi: 10.1016/s0006-8993(98)00795-1. [DOI] [PubMed] [Google Scholar]
- Ramirez-Amaya V, Vazdarjanova A, Mikhael D, Rosi S, Worley PF, Barnes CA. Spatial exploration-induced Arc mRNA and protein expression: evidence for selective, network-specific reactivation. J Neurosci. 2005;25:1761–1768. doi: 10.1523/JNEUROSCI.4342-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Redondo RL, Morris RG. Making memories last: the synaptic tagging and capture hypothesis. Nat Rev Neurosci. 2011;12:17–30. doi: 10.1038/nrn2963. [DOI] [PubMed] [Google Scholar]
- Richter JD, Lorenz LJ. Selective translation of mRNAs at synapses. Curr Opin Neurobiol. 2002;12:300–304. doi: 10.1016/s0959-4388(02)00318-5. [DOI] [PubMed] [Google Scholar]
- Richter-Levin G, Akirav I. Emotional tagging of memory formation--in the search for neural mechanisms. Brain Res Brain Res Rev. 2003;43:247–256. doi: 10.1016/j.brainresrev.2003.08.005. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Castello NA, Vedana G, Barsegyan A, McGaugh JL. Noradrenergic activation of the basolateral amygdala modulates consolidation of object recognition memory. Neurobiol Learn Mem. 2008;90:576–579. doi: 10.1016/j.nlm.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Hui GK, Hui IR, Berlau DJ, McGaugh JL, Weinberger NM. Basolateral amygdala noradrenergic activity mediates corticosterone-induced enhancement of auditory fear conditioning. Neurobiol Learn Mem. 2006;86:249–255. doi: 10.1016/j.nlm.2006.03.003. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, McGaugh JL. Amygdaloid nuclei lesions differentially affect glucocorticoid-induced memory enhancement in an inhibitory avoidance task. Neurobiol Learn Mem. 1996;65:1–8. doi: 10.1006/nlme.1996.0001. [DOI] [PubMed] [Google Scholar]
- Roozendaal B, Nguyen BT, Power AE, McGaugh JL. Basolateral amygdala noradrenergic influence enables enhancement of memory consolidation induced by hippocampal glucocorticoid receptor activation. Proc Natl Acad Sci U S A. 1999;96:11642–11647. doi: 10.1073/pnas.96.20.11642. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, Okuda S, Van der Zee EA, McGaugh JL. Glucocorticoid enhancement of memory requires arousal-induced noradrenergic activation in the basolateral amygdala. Proc Natl Acad Sci U S A. 2006;103:6741–6746. doi: 10.1073/pnas.0601874103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin CY, Kundel M, Wells DG. Rapid, activity-induced increase in tissue plasminogen activator is mediated by metabotropic glutamate receptor-dependent mRNA translation. J Neurosci. 2004;24:9425–9433. doi: 10.1523/JNEUROSCI.2457-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steward O, Wallace CS, Lyford GL, Worley PF. Synaptic activation causes the mRNA for the IEG Arc to localize selectively near activated postsynaptic sites on dendrites. Neuron. 1998;21:741–751. doi: 10.1016/s0896-6273(00)80591-7. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley P. Local synthesis of proteins at synaptic sites on dendrites: role in synaptic plasticity and memory consolidation? Neurobiol Learn Mem. 2002;78:508–527. doi: 10.1006/nlme.2002.4102. [DOI] [PubMed] [Google Scholar]
- Steward O, Worley PF. Selective targeting of newly synthesized Arc mRNA to active synapses requires NMDA receptor activation. Neuron. 2001;30:227–240. doi: 10.1016/s0896-6273(01)00275-6. [DOI] [PubMed] [Google Scholar]
- Sutton MA, Schuman EM. Dendritic protein synthesis, synaptic plasticity, and memory. Cell. 2006;127:49–58. doi: 10.1016/j.cell.2006.09.014. [DOI] [PubMed] [Google Scholar]
- Tanaka M. Emotional stress and characteristics of brain noradrenaline release in the rat. Ind Health. 1999;37:143–156. doi: 10.2486/indhealth.37.143. [DOI] [PubMed] [Google Scholar]
- Tanaka M, Kohno Y, Nakagawa R, Ida Y, Takeda S, Nagasaki N, Noda Y. Regional characteristics of stress-induced increases in brain noradrenaline release in rats. Pharmacol Biochem Behav. 1983;19:543–547. doi: 10.1016/0091-3057(83)90132-6. [DOI] [PubMed] [Google Scholar]
- Thomas MJ, Moody TD, Makhinson M, O'Dell TJ. Activity-dependent beta-adrenergic modulation of low frequency stimulation induced LTP in the hippocampal CA1 region. Neuron. 1996;17:475–482. doi: 10.1016/s0896-6273(00)80179-8. [DOI] [PubMed] [Google Scholar]
- Waung MW, Pfeiffer BE, Nosyreva ED, Ronesi JA, Huber KM. Rapid translation of Arc/Arg3.1 selectively mediates mGluR-dependent LTD through persistent increases in AMPAR endocytosis rate. Neuron. 2008;59:84–97. doi: 10.1016/j.neuron.2008.05.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yin Y, Edelman GM, Vanderklish PW. The brain-derived neurotrophic factor enhances synthesis of Arc in synaptoneurosomes. Proc Natl Acad Sci U S A. 2002;99:2368–2373. doi: 10.1073/pnas.042693699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zigmond RE, Schon F, Iversen LL. Increased tyrosine hydroxylase activity in the locus coeruleus of rat brain stem after reserpine treatment and cold stress. Brain Res. 1974;70:547–552. doi: 10.1016/0006-8993(74)90267-4. [DOI] [PubMed] [Google Scholar]