Abstract
Different alcohol drinking patterns, involving either small and frequent drinking bouts or large and long-lasting bouts, are found to differentially affect the risk for developing alcohol-related diseases, suggesting that they have different underlying mechanisms. Such mechanisms may involve orexigenic peptides known to stimulate alcohol intake through their actions in the hypothalamic paraventricular nucleus (PVN). These include orexin (OX), which is expressed in the perifornical lateral hypothalamus, and galanin (GAL) and enkephalin (ENK), which are expressed within as well as outside the PVN. To investigate the possibility that these peptides affect different aspects of consumption, a microstructural analysis of ethanol drinking behavior was performed in male, Sprague-Dawley rats trained to drink 7% ethanol and implanted with guide shafts aimed at the PVN. While housed in specialized cages containing computerized intake monitors (BioDAQ Laboratory Intake Monitoring System, Research Diets Inc., New Brunswick, NJ) that measure bouts of ethanol drinking, these rats were given PVN injections of OX (0.9 nmol), GAL (1.0 nmol), or the ENK analog D-Ala2-met-enkephalinamide (DALA) (14.2 nmol), as compared to saline vehicle. Results revealed clear differences between the effects of these peptides. While all 3 stimulated ethanol intake, they had distinct effects on patterns of drinking, with OX increasing the number of drinking bouts, GAL increasing the size of the drinking bouts, and DALA increasing both the size and duration of the bouts. In contrast, these peptides had little impact on water or food intake. These results support the idea that different peptides can increase ethanol consumption by promoting distinct aspects of the ethanol drinking response. The stimulatory effect of OX on drinking frequency may be related to its neuronally stimulatory properties, while the stimulatory effect of GAL and ENK on bout size and duration may reflect a suppressive effect of these neuronally inhibitory peptides on the satiety-controlling PVN.
Keywords: enkephalin, ethanol, galanin, orexin, pattern, rat
Introduction
Alcohol drinking can lead to various medical diseases, including cirrhosis of the liver, hepatitis, and heart disease (Koob & Le Moal, 2006; Zakhari & Li, 2007). Accumulating evidence suggests that these diseases are associated not only with the total amount of alcohol consumed but also with specific patterns of drinking that may involve either a high frequency of drinking bouts or a large size and duration of drinking bouts (Zakhari & Li, 2007). These different patterns of alcohol consumption are found to have differential effects on liver function (Kamper-Jørgensen, Grønbaek, Tolstrup, & Becker, 2004; Stranges et al., 2004) and coronary heart disease (Dorn et al., 2003), underscoring the importance of understanding the specific brain mechanisms that mediate them. Patterns of alcohol drinking are also found to be differentially responsive to therapeutic drugs, with some medications more effective in preventing the relapse to consuming alcohol, which corresponds with the initiation or frequency of intake, and others more effective in suppressing chronic excessive drinking, which relates to the size and duration of drinking bouts (Mann, Lehert, & Morgan, 2004; O’Malley, Krishnan-Sarin, Farren, Sinha, & Kreek, 2002; Ooteman, Koeter, Verheul, Schippers, & van den Brink, 2007). While there is indirect evidence suggesting that the specific drugs which prevent relapse or reduce ongoing drinking act through different neurochemical systems in the brain (Mann, Lehert, & Morgan, 2004; Ooteman et al., 2007), there are few studies to date that have directly examined particular neurochemicals in the brain in terms of their role in mediating specific characteristics of alcohol drinking patterns.
The available evidence that may help in understanding the mechanisms mediating specific aspects of alcohol consumption comes from studies of orexigenic peptides and their effects on patterns of eating behavior (Baird et al., 2009; Clifton, 2000; Glass, Grace, Cleary, Billington, & Levine, 2001). Analyses of meal frequency and meal size have implicated specific hypothalamic peptides in the appetitive (frequency) or consummatory (size) aspects of feeding (Berthoud & Münzberg, 2011), suggesting that these peptides promote eating through different actions. In particular, the peptide orexin/hypocretin (OX), which is closely associated with appetitive behavior (Harris & Aston-Jones, 2006), has with cerebroventricular administration been shown to enhance sucrose consumption by increasing the frequency of its intake (Baird et al., 2009). This is in contrast to the peptides galanin (GAL) and enkephalin (ENK), which are known to be important promoters of consummatory behavior (Leibowitz & Wortley, 2004; Naleid, Grace, Chimukangara, Billington, & Levine, 2007) and have been shown with agonist injections to suppress satiety signals (Kehr et al., 2002; Ogren et al., 1998; Rada, Mark, & Hoebel, 1998) and with antagonist injections to specifically decrease meal size and duration (Glass et al., 2001; Kirkham & Blundell, 1987).
These 3 peptides have also been found to stimulate ethanol consumption when administered in the medial hypothalamus (Barson et al., 2010; Schneider, Rada, Darby, Leibowitz, & Hoebel, 2007). These studies of eating patterns lead us to ask whether they can similarly affect different patterns of ethanol drinking, with OX primarily stimulating the frequency of drinking bouts, and GAL and ENK stimulating the size and/or duration of bouts. This possibility receives indirect support from studies examining the paraventricular nucleus of the hypothalamus (PVN), an important satiety center (Leibowitz & Alexander, 1998; Leibowitz & Wortley, 2004). With OX from the perifornical lateral hypothalamus known to increase arousal (Kiwaki, Kotz, Wang, Lanningham-Foster, & Levine, 2004) and to be neuronally stimulatory (de Lecea et al., 1998; Sakurai et al., 1998), this peptide in the PVN may act to initiate drinking bouts. In contrast, with GAL and ENK known to be neuronally inhibitory (Barson, Morganstern, & Leibowitz, 2013; Nicoll, Siggins, Ling, Bloom, & Guillemin, 1977), these peptides may disinhibit activity within the PVN and consequently increase the size or duration of drinking bouts. Thus, while all 3 hypothalamic peptides act to promote consummatory behavior, they likely do so by affecting different aspects of this behavior.
The present study used the BioDAQ system, an automatic episodic intake monitor, to investigate the role of these hypothalamic peptides in controlling specific patterns of ethanol drinking. Sprague-Dawley rats were first trained to voluntarily consume 7% ethanol. After hypothalamic injection of OX, GAL, or ENK compared to saline vehicle, they were then examined over a 3-h period for changes in the frequency, average size, and average duration of their ethanol drinking bouts, as well as their intake of food and water, which were simultaneously available. All 3 peptides were tested after injection in the PVN, where they have been shown to be endogenously released (Barson, Chang, Poon, Morganstern, & Leibowitz, 2011; Beck & Max, 2007; Chang et al., 2007; Peyron et al., 1998) and to have a stimulatory effect on overall ethanol intake (Barson et al., 2010; Schneider et al., 2007). The results obtained with this microstructural analysis revealed clear differences between these peptides in their effects on patterns of ethanol consumption.
Materials and methods
Subjects
Adult male Sprague-Dawley rats (N = 24), weighing 225–250 g at the start of the experiments, were obtained from Taconic Farms (Germantown, NY). They were individually housed in hanging wire cages or plastic shoebox cages with a wired floor insert, and maintained on a reversed 12:12-h light/dark cycle, with lights off at 6:00 AM. Subjects had ad libitum access to water, either through an automatic piping system or through graduated cylinders with non-drip sippers (Integrated Laboratory Equipment, Fort Smith, AR) after ethanol had been introduced. They had ad libitum access to chow (LabDiet 5001 Rodent Chow, St. Louis, MO) prior to ethanol training. Once ethanol training began, access to chow was restricted to 12 h per day (along with ethanol) in order to increase ethanol intake. Animals were allowed 1 week to acclimate to the facility before experiments were initiated. All procedures were approved in advance by the Princeton University Institutional Animal Care and Use Committee, and conformed to the National Institutes of Health guidelines on the ethical use of animals.
Ethanol training
As in our previous publications (Chen, Barson, Chen, Hoebel, & Leibowitz, 2013; Chen, Morganstern, Barson, Hoebel, & Leibowitz, 2013), subjects were trained to drink ethanol by gradually acclimating them to unsweetened ethanol, increasing the concentration every 4 days, from 1, 2, 4, to 7% (v/v). Animals were allowed access to ethanol solution for 12 h per day, along with ad libitum water and cycled chow, starting 3 h into the dark period. Using this procedure, animals can consume an average of 1.09 ± 0.13 g/kg in 3 h and 2.50 ± 0.51 g/kg over a 12-h period. Injection tests were begun after the subjects had at least 11 days of access to 7% ethanol.
Surgery
Subjects were anesthetized using ketamine (80 mg/kg, intra-peritoneally [i.p.]) and xylazine (10 mg/kg, i.p.), supplemented with ketamine when necessary. Guide shafts (10 mm in length) made of 21-gauge stainless steel were implanted perpendicularly and unilaterally in the midline, aimed at the PVN (A: −1.8; L: ± 0.4; V: 3.8 mm) with reference to bregma, the midsagittal sinus, and the level skull surface. Injectors protruded 4.5 mm beyond the guide shafts to reach the PVN (V: 8.3 mm). Subjects received cannulation surgeries after having at least 4 days of access to 7% ethanol, and they were given at least 1 week of post-surgery recovery before microinjections. Other than the time of injections, stainless-steel stylets were left in the guide shafts to prevent occlusion.
Microinjection procedures
All solutions were delivered through concentric microinjectors made of 26-gauge stainless steel, with fused-silica tubing inside (74 μm ID, 154 μm OD; Polymicro Technologies, Phoenix, AZ) that protruded 2.5 mm beyond the stainless steel part of injectors to reach the PVN (V: 8.3 mm). Doses were chosen based on the ethanol literature (Barson et al., 2010; Schneider et al., 2007), and on pilot tests. The following drugs were used: i) orexin-A (0.9 nmol); ii) galanin (1.0 nmol); iii) D-Ala2-met-enkephalinamide (DALA, 14.2 nmol). Drugs were purchased from Sigma-Aldrich Co. (St. Louis, MO; galanin and orexin-A) or American Peptide Co., Inc. (Sunnyvale, CA; DALA). During pilot testing, a lower dose of each drug was tested and found not to significantly change overall consumption of ethanol; therefore, only the higher dose was tested for microstructural effects. Drugs were dissolved in preservative-free 0.9% NaCl solution (Hospira Inc., Lake Forest, IL) and prepared fresh immediately prior to microinjection. To minimize stress, animals were handled on an almost daily basis throughout their ethanol training prior to the initiation of microinjections. Injection tests were counterbalanced in a within-subject design, so that each animal received vehicle or drug in opposing order on 2 consecutive days. In the 8 animals tested with 2 drugs (e.g., orexin vs. saline and galanin vs. saline), at least 1 week of recovery was given between the tests, and the order of the tests was counterbalanced. While individual intake after vehicle injection was stable between the tests (Pearson product-moment correlation coefficient = 0.85, p < 0.01), there was variability in the intake of the whole groups between the tests as they were composed of different subjects. Injections were given immediately prior to daily ethanol presentation. They were made using a syringe pump, which delivered 0.5 μL during 47 sec at a flow rate of 0.6 μL/min, as previously published (Chen, Barson, et al., 2013; Chen, Morganstern, et al., 2013). The microinjectors were left in place for another 47 sec to allow diffusion of the solution into the injection site. The intake of ethanol, food, and water was measured for 3 h after the injections, with ethanol being measured automatically by an episodic monitoring system (BioDAQ Laboratory Intake Monitoring System, Research Diets Inc., New Brunswick, NJ) and food and water measured manually at 1 h, 2 h, and 3 h.
Episodic ethanol intake measurement
To examine the effects of the peptides on the microstructure of ethanol drinking, the BioDAQ Laboratory Intake Monitoring System was modified to measure episodic ethanol intake behavior. In this system, plastic bottles containing 7% ethanol were placed on the hoppers, which in turn were mounted on electronic strain-gauge load cells to measure ethanol intake. The hopper assembly, with bottle and ethanol contents, was weighed 50 times per second (accurate to 0.01 g), and a mean and standard deviation (S.D.) over approximately 1 sec was calculated by computer. The computer was programmed such that drinking behavior was signaled by a fluctuation in the hopper weight (defined as a S.D. > 2000 mg) caused by the animal’s drinking. The end of a drinking response was signaled when the hopper was left undisturbed for 1 min, and the duration of the drinking response and amount consumed (initial minus final hopper weight) was calculated. Each ethanol drinking bout was defined as having at least a 600 sec inter-meal interval and an overall consumption of at least 0.1 g. These criteria are similar to those used in microstructural analysis of liquid intake (Baird et al., 2008; Davis & Smith, 1992). Information about each drinking bout, with measures of drinking duration and amount consumed, was exported to a central laptop. During the experiment, the rats were housed individually in specialized cages connected to the computerized BioDAQ monitoring system and were allowed to acclimate for 1 week prior to the start of the injection tests. The ethanol drinking patterns (number of drinking bouts, volume, and duration) during the first 3 h after injection were automatically recorded by the system, and the data obtained were then exported to Microsoft Excel for further analysis.
Histological analysis
To verify the site of microinjection, rats were sacrificed by rapid decapitation. Their brains were sliced using a freezing microtome as 40-μm coronal sections and were examined microscopically. Six animals had probes 0.5 mm or farther from the target region and were removed from the analysis.
Data analysis
Cumulative ethanol, food, and water intake at the end of the 3-h measurement period was measured by paired, 2-tailed t tests. Ethanol intake measures across each hour (intake, number of drinking bouts, duration of bouts, and size of bouts) was measured via 2-way repeated-measures ANOVA, with drug and time as within-subject factors. As the behavioral effects of peptides such as opioids have been shown to vary over time (Barson et al., 2010; Craft, 2008), an a priori decision was made to follow up the ANOVAs with tests of the simple effects at each time point (using paired, 2-tailed t tests) regardless of significance. Values of p < 0.05 were considered significant. Data from animals consuming little or no ethanol on measurement days (less than 0.25 g/kg during 3 h) were excluded. Data in the figures are expressed as mean ± standard error of the mean.
Results
Experiment 1: PVN OX injection increases ethanol intake by increasing the frequency of drinking bouts
Using the BioDAQ system, we examined the microstructure of ethanol drinking behavior during the first 3 h after microinjection of OX in the PVN, a peptide that projects to this nucleus from neurons localized in the perifornical lateral hypothalamus. The specific injection sites for this study are illustrated in Fig. 1. In rats trained to voluntarily drink 7% ethanol (n = 8), analysis of cumulative ethanol intake showed that OX in the PVN (0.9 nmol) tended to increase overall ethanol intake across the 3-h recording time (t = 1.8, df = 7, p = 0.11) (Fig. 2A), while having no effect on intake of water (t = 0.9, df = 7, p = 0.42) or food (t = 1.0, df = 7, p = 0.37) (Table 1). Examination of ethanol intake across each hour revealed a trend for a main effect of drug [F(l,7) = 3.26, p = 0.11] as well as for an interaction effect between drug and time [F(2,14) =2.81, p = 0.09]. Specifically, ethanol drinking significantly increased during the first hour post-injection (t = 4.4, df = 7, p < 0.01) (Fig. 2A) but not during the second (t = 0.6, df = 7, p = 0.58) and third (t = 1.1, df = 7, p = 0.32) hours. Further analysis of the data across each hour also revealed a small trend for an interaction effect between drug and time in the number of the drinking bouts [F(2,14) = 1.87, p = 0.19], with a significant increase occurring in the first hour after OX injection (t = 3.4, df = 7, p = 0.05) (Fig. 2B), although there was not a significant main effect on this measure [F(l,7) = 0.18, p = 0.69]. Importantly, there were no significant main or interaction effects observed for average size of the ethanol drinking bouts (main effect: [F(l,7) = 0.46, p = 0.52]; interaction effect: [F(2,14) = 1.81, p = 0.20]). In addition, there were no significant main or interaction effects observed for duration of the ethanol drinking bouts (main effect: [F(l,7) = 1.67, p = 0.23]; interaction effect: [F(2,14) = 0.58, p = 0.57]). These results demonstrate that OX in the PVN enhances ethanol consumption by initiating more ethanol drinking responses.
Figure 1.
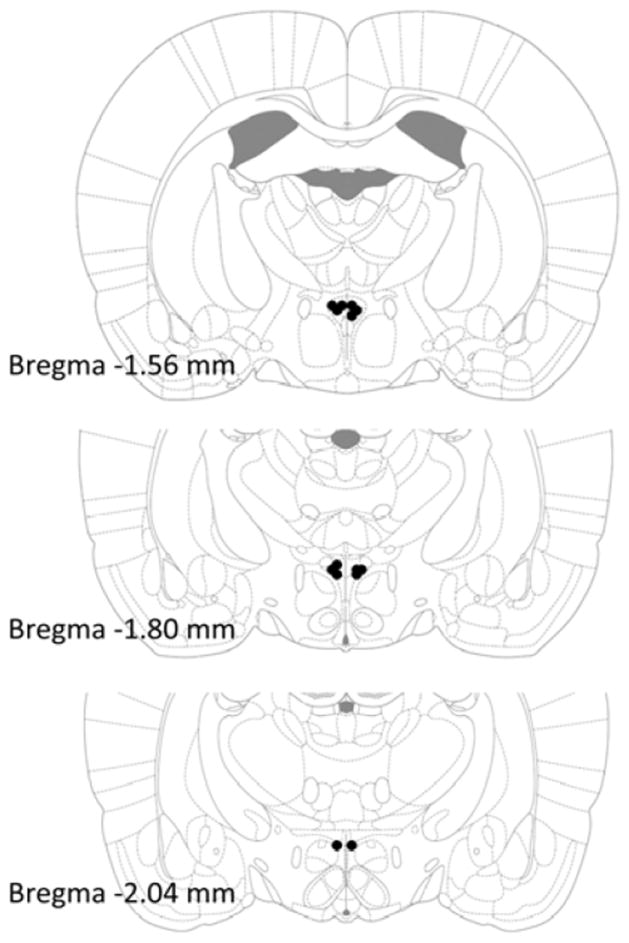
Injection sites from animals included in the analysis, as indicated by black dots. Adapted from The Rat Brain, compact 6th edition, G. Paxinos and C. Watson, Copyright 2007, with permission from Elsevier.
Figure 2.
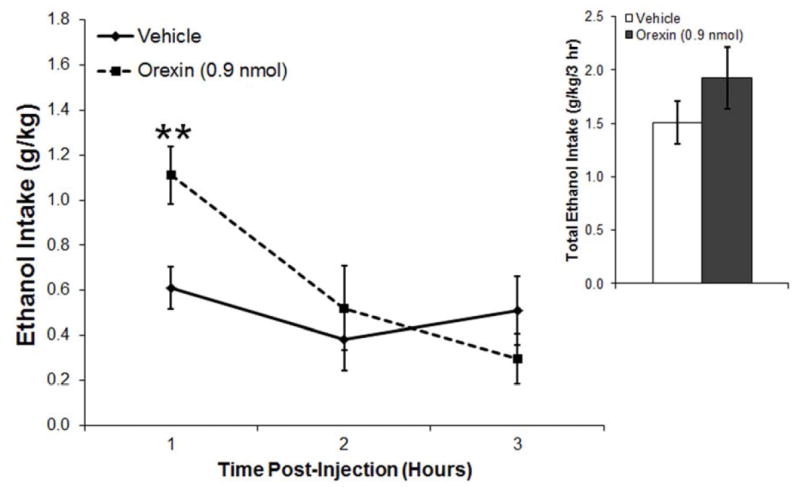
A. Injection of orexin (0.9 nmol) into the paraventricular nucleus of the hypothalamus of ethanol-drinking rats (n = 8) increased ethanol consumption during the first hour post-injection. B. Injection of orexin significantly increased the number of ethanol drinking bouts, while leaving the size and duration of bouts relatively unaffected. Values are mean ± S.E.M.; **p < 0.01, *p < 0.05.
Table 1.
Food and water intake was not significantly affected by injections of the peptides into the paraventricular nucleus of the hypothalamus.
| Nutrient | Hour 1 | Hour 2 | Hour 3 | Total |
|---|---|---|---|---|
| Food (g) | ||||
| Vehicle | 3.2 ± 0.5 | 1.1 ± 0.1 | 1.7 ± 0.2 | 6.0 ± 0.5 |
| Galanin (1.0 nmol) | 4.4 ± 0.9 | 1.3 ± 0.2 | 1.7 ± 0.2 | 7.4 ± 0.9 |
| Water (ml) | ||||
| Vehicle | 2.5 ± 0.4 | 1.4 ± 0.3 | 1.8 ± 0.2 | 5.7 ± 0.7 |
| Galanin (1.0 nmol) | 3.2 ± 0.5 | 1.1 ± 0.2 | 1.4 ± 0.2 | 5.7 ± 0.6 |
| Food (g) | ||||
| Vehicle | 6.3 ± 0.8 | 1.4 ± 0.5 | 1.6 ± 0.5 | 9.4 ± 0.7 |
| DALA (14.2 nmol) | 6.9 ± 0.8 | 0.6 ± 0.5 | 1.8 ± 0.5 | 9.4 ± 0.7 |
| Water (ml) | ||||
| Vehicle | 9.4 ± 1.1 | 4.0 ± 1.0 | 3.3 ± 0.8 | 16.7 ± 1.7 |
| DALA (14.2 nmol) | 7.5 ± 2.0 | 3.5 ± 0.7 | 3.5 ± 1.1 | 14.5 ± 3.2 |
| Food (g) | ||||
| Vehicle | 5.3 ± 0.8 | 1.4 ± 0.8 | 1.6 ± 0.4 | 8.3 ± 0.7 |
| Orexin (0.9 nmol) | 7.8 ± 1.3 | 1.8 ± 0.7 | 0.6 ± 0.3 | 10.2 ± 1.3 |
| Water (ml) | ||||
| Vehicle | 6.9 ± 1.5 | 4.6 ± 1.4 | 4.5 ± 1.1 | 16.0 ± 3.2 |
| Orexin (0.9 nmol) | 7.5 ± 1.9 | 5.6 ± 1.4 | 4.5 ± 0.9 | 17.8 ± 3.6 |
Experiment 2: PVN GAL injection increases ethanol intake by increasing the size of drinking bouts
This experiment examined the effect of another hypothalamic peptide, GAL, which is both transcribed and released in the PVN. Injection of GAL (1.0 nmol) in the PVN of ethanol-drinking rats (n = 8) significantly increased cumulative ethanol intake by the end of 3 h (t = 2.6, df = 7, p < 0.05) (Fig. 3A), while having little impact on water intake (t = 0.3, df = 7, p = 0.98) and showing a tendency toward an increase in food consumption (t = 2.0, df = 7, p = 0.08) (Table 1). In examining hourly ethanol intake, there was a significant main effect of drug [F(l,7) = 9.67, p < 0.05] as well as a small trend for an interaction effect between drug and time [F(2,14) = 2.25, p = 0.14], with an increase in ethanol drinking after GAL injection apparent at both 1 h (t = 3.9, df = 7, p < 0.01) and 3 h (t = 2.5, df = 7, p < 0.05) post-injection (Fig. 3A). While GAL compared to vehicle had no main or interaction effect during this time on the number of drinking bouts (main effect: [F(1,7) = 0.78, p = 0.41]; interaction effect: [F(2,14) = 0.11, p = 0.90]) or duration of the bouts (main effect: [F(l,7) = 2.04, p = 0.20]; interaction effect: [F(2,14) = 1.48, p = 0.26]), it was found to significantly increase the size of the ethanol drinking bouts (main effect: [F(1,7) = 7.45, p < 0.05]; interaction effect: [F(2,14) = 0.20, p = 0.82]). This effect occurred predominantly in the first hour (t = 3.5, df = 7, p < 0.01), although there was also a small trend in the second hour post-injection (t = 2.0, df = 7, p = 0.09) (Fig. 3B). These results demonstrate that, in contrast to OX, PVN GAL has a stimulatory effect specifically on the size of ethanol drinking bouts, without changing the frequency or duration of bouts or the ingestion of water or food.
Figure 3.
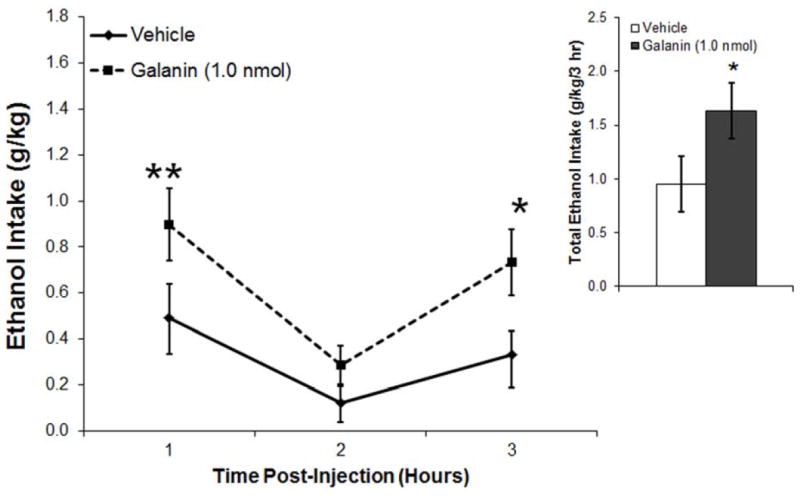
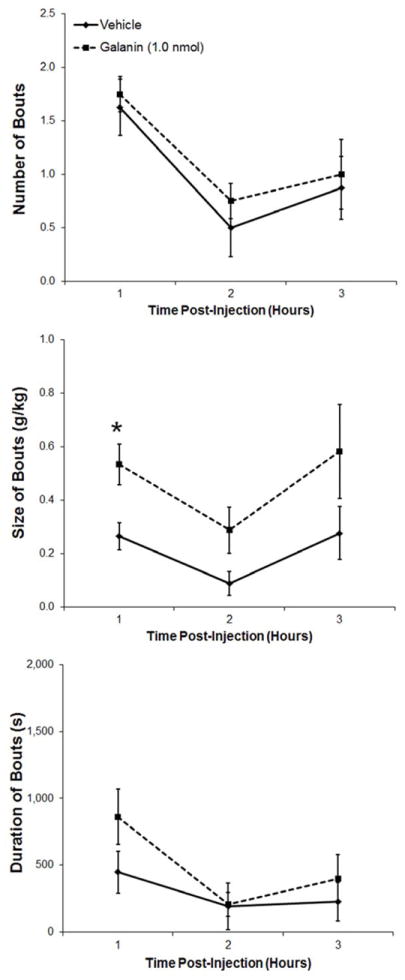
A. Injection of galanin (1.0 nmol) into the paraventricular nucleus of the hypothalamus of ethanol-drinking rats (n = 8) significantly increased ethanol consumption over the 3 h post-injection. B. Injection of galanin significantly increased the size of ethanol drinking bouts, leaving the number and duration of the bouts relatively unaffected. Values are mean ± S.E.M.; **p < 0.01, *p < 0.05.
Experiment 3: PVN ENK injection increases ethanol intake by increasing the size and duration of drinking bouts
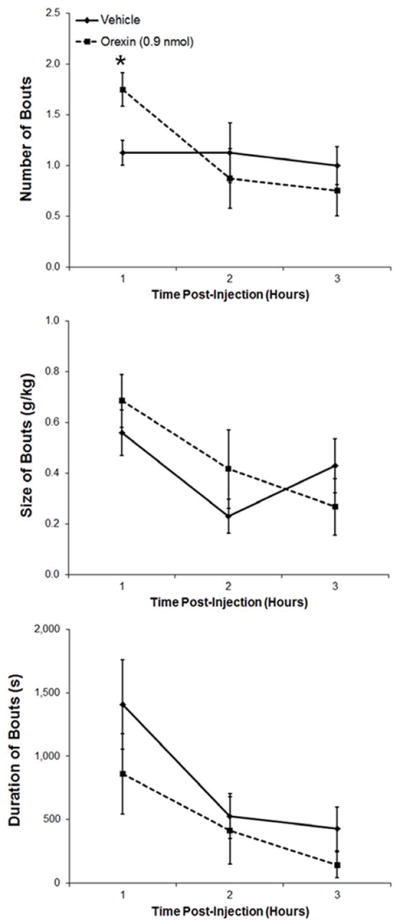
This experiment used the ENK analog, DALA, to examine the effect of ENK in the PVN where, like GAL, this peptide is both transcribed and released. In ethanol-drinking rats (n = 8), PVN injection of DALA (14.2 nmol) significantly increased cumulative ethanol intake by the end of the 3 h (t = 4.6, df = 7, p < 0.05) (Fig. 4A), while leaving unaffected both water (t = 1.0, df = 7, p = 0.33) and food intake (t = 0.1, df = 7, p = 0.96) (Table 1). Examining hourly intake, for which there was a significant main effect of drug [F(1,7) = 20.33, p < 0.01] as well as a significant interaction effect between drug and time [F(2,14) = 7.96, p < 0.01], this increased ethanol drinking occurred during the first hour post-injection (t = 4.3, df = 7, p < 0.01) (Fig. 4A). As with GAL, injection of DALA compared to vehicle had no significant effect on the number of ethanol drinking bouts (main effect: [F(1,7] = 3.32, p = 0.11]; interaction effect: [F(2,14) = 2.26, p = 0.14]). Whereas DALA also had no main effect across the 3 h on the size [F(l,7) = 1.32, p = 0.29] or duration [F(l,7) = 0.21, p = 0.66] of the bouts, it did produce significant interaction effects with time for the measures of both size [F(2,14) = 7.30, p < 0.01] and duration [F(2,14) = 7.19, p < 0.01]. Specifically, during the first hour post-injection, DALA increased the size of the ethanol drinking bouts (t = 3.0, df = 7, p< 0.05) and the duration of the bouts (t = 2.8, df = 7, p < 0.05) (Fig. 4B). Taken together, these results show that ENK is similar to GAL in the PVN in enhancing ethanol consumption, but not water or food intake, primarily by increasing the size of ethanol drinking bouts. Unlike GAL, however, it additionally causes an increase in the duration of these bouts.
Figure 4.
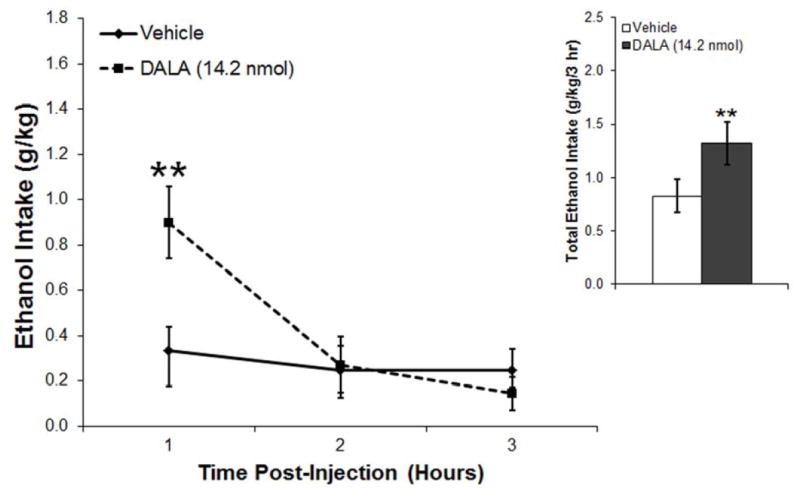
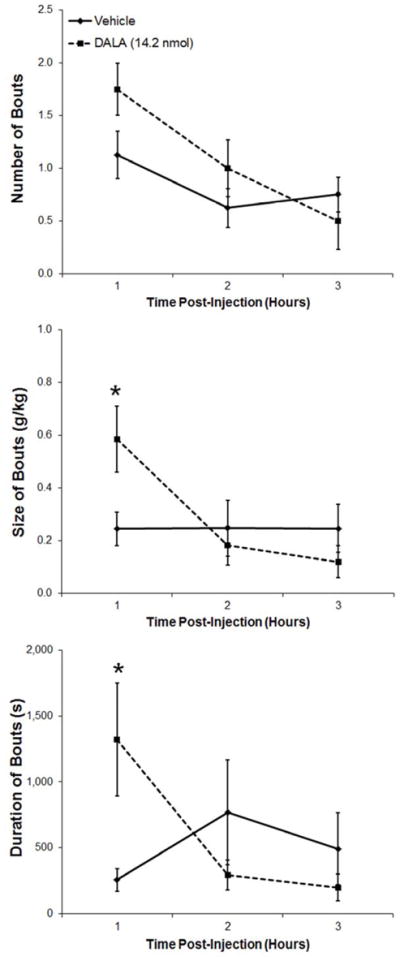
A. Injection of the enkephalin analogue D-Ala2-met-enkephalinamide (DALA, 14.2 nmol) into the paraventricular nucleus of the hypothalamus of ethanol-drinking rats (n = 8) significantly increased ethanol consumption. B. Injection of DALA significantly increased the size and duration of ethanol drinking bouts, leaving the number of bouts relatively unaffected. Values are mean ± S.E.M.; **p < 0.01, *p < 0.05.
Discussion
This microstructural analysis of specific components of voluntary ethanol drinking shows that, in controlling ethanol intake through their actions in the PVN, the peptides OX, GAL, and ENK each have unique effects on the patterns of drinking. These findings provide the first evidence supporting the idea that specific aspects of ethanol drinking are mediated by different neurochemical mechanisms, with some of them more involved in the initiation of drinking and others more involved in the prolongation of drinking.
Orexin in the PVN stimulates frequency of ethanol drinking
The peptide OX is known to have a stimulatory effect on the intake of drugs of abuse, including the drinking of ethanol (Aston-Jones et al., 2010), and this effect has been demonstrated with injection of OX directly into the PVN (Schneider et al., 2007). The results of the present study confirm this finding and, with microstructural analysis, additionally show that this OX-induced ethanol drinking response is achieved through an increase in the frequency of drinking bouts evident during the first hour after injection. This suggests that OX, which is expressed in the perifornical lateral hypothalamic area known to be responsible for meal initiation (Rada, Mendialdua, Hernandez, & Hoebel, 2003), is also important in the initiation of ethanol drinking behavior. This is consistent with published studies showing central injection of this peptide to stimulate the frequency of licking for a sucrose solution (Baird et al., 2009) and showing that the integrity of the OX system is important for the reinstatement of ethanol seeking (Aston-Jones et al., 2010; Lawrence, Cowen, Yang, Chen, & Oldfield, 2006). Together with evidence that OX-ablated mice fail to exhibit anticipatory behaviors that normally precede the initiation of food consumption (Akiyama et al., 2004; Mieda et al., 2004; Mistlberger, Antle, Kilduff, & Jones, 2003; Mizushige et al., 2006), our results demonstrate a distinct role for OX in initiating bouts of ethanol drinking.
Galanin and enkephalin in the PVN increase the size of ethanol drinking bouts
The peptides GAL and ENK have been shown to stimulate ethanol drinking when injected into the cerebral ventricles or directly into the PVN (Barson et al., 2010; Lewis, Johnson, Waldman, Leibowitz, & Hoebel, 2004; Rada, Avena, Leibowitz, & Hoebel, 2004). Our results confirm these findings with PVN injections, showing that both peptides increase total ethanol consumption within the first 3 h after injection. With the microstructural analysis, they additionally demonstrate that this greater intake after both GAL and ENK is due to a significant increase in the size of the ethanol drinking bouts, with no change in the number of drinking bouts, and that after ENK, there is also an increase in the duration of the drinking bouts. This finding with GAL, suggesting that it enhances drinking specifically by increasing bout size, is consistent with evidence that a peripherally administered GAL-3 receptor antagonist decreases ethanol consumption only during initial ethanol access, by reducing the number of earned ethanol rewards specifically during the first 5 min of a 20-min session (Ash, Zanatta, Williams, Lawrence, & Djouma, 2011). The role of ENK in increasing both the size and duration of ethanol drinking bouts is consistent with evidence showing that the feeding-suppressive effect of ENK antagonists is due to a reduction in meal size and duration with no change in meal frequency (Glass et al., 2001; Kirkham & Blundell, 1987), and also that μ-opioid receptor stimulation in the accumbens shell increases the duration of licking for a lipid emulsion (Katsuura, Heckmann, & Taha, 2011). The present result, showing that GAL increases bout size without significantly affecting duration, suggests that the effect of GAL might have occurred through an increase in the rate of drinking. While no studies to date have examined this phenomenon, one finding that indirectly supports this possibility is that transgenic mice overexpressing GAL show higher levels of spontaneous locomotor activity (Kuteeva, Hokfelt, & Ogren, 2005) that might lead them to a higher rate of consumption. Thus, the evidence suggests that, in controlling the drinking of ethanol, both GAL and ENK in the PVN have a more important role in increasing ongoing ethanol drinking rather than in initiating this behavior.
Possible implications for clinical medications and the control of long-term drinking
The differences observed here in the peptide systems’ control of ethanol drinking patterns may help to elucidate the mechanisms mediating the differential efficacy of various drugs in treating the different aspects of alcoholism, specifically relapse (initiation) or ongoing excessive drinking (prolongation). For example, our results showing that OX in the PVN acts to initiate ethanol drinking may explain, in part, the specific efficacy of OX antagonists in preventing the reinstatement of ethanol-seeking behavior (Lawrence et al., 2006). Notably, studies have shown that repeated injections of OX, while initially stimulating food intake, subsequently cause a compensatory inhibition that ultimately leaves overall intake unaffected (Novak & Levine, 2009; Yamanaka, Sakurai, Katsumoto, Yanagisawa, & Goto, 1999). In addition, the expression of OX, while stimulated by short bursts of ethanol intake or acute ethanol administration, is suppressed by chronic consumption of ethanol (Morganstern et al., 2010). This pattern with OX contrasts markedly with that seen with GAL and ENK, which when repeatedly injected can continue to enhance food intake (Jalowiec, Panksepp, Zolovick, Najam, & Herman, 1981; Yun et al., 2005), and, with chronic exposure to ethanol, still show increased endogenous expression (Chang et al., 2007). Together with the present findings, this evidence suggests that the OX system may play a more important role in initiating alcohol drinking responses, while GAL and ENK act to sustain an already established drinking response. Given the extensive literature linking them with eating behavior, it is notable that these peptides in the present experiments affected ethanol rather than food intake. We have also found this to be the case in a number of our previous studies involving animals trained to drink ethanol (see, for example, Barson et al., 2010; Schneider et al., 2007). Therefore, we propose that the availability of ethanol may mask or supplant any effects on feeding behavior induced by PVN peptide injections.
Conclusions
The results of this microstructural analysis reveal some similarities between GAL and ENK, as well as differences between these peptides themselves and between them and OX, in terms of their role in mediating distinct patterns of ethanol drinking. They underscore the importance of performing such analyses, to elucidate the mechanisms which underlie specific drinking patterns that differentially affect health conditions and which contribute to initial drinking compared with chronic overconsumption. Further efforts to identify such mechanisms may help to determine the appropriate medications for treating patients with specific patterns of alcohol drinking or at different stages of alcohol use disorders.
Acknowledgments
This research was supported by the National Institute on Alcohol Abuse and Alcoholism of the National Institutes of Health under Award Numbers R01AA12882 (SFL and BGH) and K99AA021782 (JRB), and by the E. H. Lane Foundation (BGH). The content is solely the responsibility of the authors and does not necessarily represent the official views of the NIH.
Footnotes
There are no commercial affiliations or consultant roles of any authors that could be construed as a conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Akiyama M, Yuasa T, Hayasaka N, Horikawa K, Sakurai T, Shibata S. Reduced food anticipatory activity in genetically orexin (hypocretin) neuron-ablated mice. The European Journal of Neuroscience. 2004;20:3054–3062. doi: 10.1111/j.1460-9568.2004.03749.x. [DOI] [PubMed] [Google Scholar]
- Ash BL, Zanatta SD, Williams SJ, Lawrence AJ, Djouma E. The galanin-3 receptor antagonist, SNAP 37889, reduces operant responding for ethanol in alcohol-preferring rats. Regulatory Peptides. 2011;166:59–67. doi: 10.1016/j.regpep.2010.08.009. [DOI] [PubMed] [Google Scholar]
- Aston-Jones G, Smith RJ, Sartor GC, Moorman DE, Massi L, Tahsili-Fahadan P, et al. Lateral hypothalamic orexin/hypocretin neurons: A role in reward-seeking and addiction. Brain Research. 2010;1314:74–90. doi: 10.1016/j.brainres.2009.09.106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Choe A, Loveland JL, Beck J, Mahoney CE, Lord JS, et al. Orexin-A hyperphagia: hindbrain participation in consummatory feeding responses. Endocrinology. 2009;150:1202–1216. doi: 10.1210/en.2008-0293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baird JP, Rios C, Loveland JL, Beck J, Tran A, Mahoney CE. Effects of hindbrain melanin-concentrating hormone and neuropeptide Y administration on licking for water, saccharin, and sucrose solutions. American Journal of Physiology Regulative, Integrative and Comparative Physiology. 2008;294:R329–343. doi: 10.1152/ajpregu.00611.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Carr AJ, Soun JE, Sobhani NC, Rada P, Leibowitz SF, et al. Opioids in the hypothalamic paraventricular nucleus stimulate ethanol intake. Alcoholism: Clinical and Experimental Research. 2010;34:214–222. doi: 10.1111/j.1530-0277.2009.01084.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Chang GQ, Poon K, Morganstern I, Leibowitz SF. Galanin and the orexin 2 receptor as possible regulators of enkephalin in the paraventricular nucleus of the hypothalamus: relation to dietary fat. Neuroscience. 2011;193:10–20. doi: 10.1016/j.neuroscience.2011.07.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barson JR, Morganstern I, Leibowitz SF. Galanin. In: Kastin AJ, editor. Handbook of Biologically Active Peptides. New York, NY: Elsevier Inc; 2013. [Google Scholar]
- Beck B, Max JP. Hypothalamic galanin and plasma leptin and ghrelin in the maintenance of energy intake in the Brattleboro rat. Biochemical and Biophysical Research Communications. 2007;364:60–65. doi: 10.1016/j.bbrc.2007.09.092. [DOI] [PubMed] [Google Scholar]
- Berthoud HR, Münzberg H. The lateral hypothalamus as integrator of metabolic and environmental needs: from electrical self-stimulation to opto-genetics. Physiology & Behavior. 2011;104:29–39. doi: 10.1016/j.physbeh.2011.04.051. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chang GQ, Karatayev O, Ahsan R, Avena NM, Lee C, Lewis MJ, et al. Effect of ethanol on hypothalamic opioid peptides, enkephalin, and dynorphin: relationship with circulating triglycerides. Alcoholism: Clinical and Experimental Research. 2007;31:249–259. doi: 10.1111/j.1530-0277.2006.00312.x. [DOI] [PubMed] [Google Scholar]
- Chen YW, Barson JR, Chen A, Hoebel BG, Leibowitz SF. Opioids in the perifornical lateral hypothalamus suppress ethanol drinking. Alcohol. 2013;47:31–38. doi: 10.1016/j.alcohol.2012.11.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen YW, Morganstern I, Barson JR, Hoebel BG, Leibowitz SF. Differential role of D1 and D2 receptors in the perifornical lateral hypothalamus in controlling ethanol drinking and food intake: possible interaction with local orexin neurons. Alcoholism: Clinical and Experimental Research. 2013;38:777–786. doi: 10.1111/acer.12313. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clifton PG. Meal patterning in rodents: psychopharmacological and neuroanatomical studies. Neuroscience and Biobehavioral Reviews. 2000;24:213–222. doi: 10.1016/s0149-7634(99)00074-3. [DOI] [PubMed] [Google Scholar]
- Craft RM. Sex differences in analgesic, reinforcing, discriminative, and motoric effects of opioids. Experimental and Clinical Psychopharmacology. 2008;16:376–385. doi: 10.1037/a0012931. [DOI] [PubMed] [Google Scholar]
- Davis JD, Smith GP. Analysis of the microstructure of the rhythmic tongue movements of rats ingesting maltose and sucrose solutions. Behavioral Neuroscience. 1992;106:217–228. [PubMed] [Google Scholar]
- de Lecea L, Kilduff TS, Peyron C, Gao X, Foye PE, Damelson PE, et al. The hypocretins: hypothalamus-specific peptides with neuroexcitatory activity. Proceedings of the National Academy of Sciences of the United States of America. 1998;95:322–327. doi: 10.1073/pnas.95.1.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dorn JM, Hovey K, Muti P, Freudenheim JL, Russell M, Nochajski TH, et al. Alcohol drinking patterns differentially affect central adiposity as measured by abdominal height in women and men. The Journal of Nutrition. 2003;133:2655–2662. doi: 10.1093/jn/133.8.2655. [DOI] [PubMed] [Google Scholar]
- Glass MJ, Grace MK, Cleary JP, Billington CJ, Levine AS. Naloxone’s effect on meal microstructure of sucrose and cornstarch diets. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2001;281:R1605–1612. doi: 10.1152/ajpregu.2001.281.5.R1605. [DOI] [PubMed] [Google Scholar]
- Harris GC, Aston-Jones G. Arousal and reward: a dichotomy in orexin function. Trends in Neuroscience. 2006;29:571–577. doi: 10.1016/j.tins.2006.08.002. [DOI] [PubMed] [Google Scholar]
- Jalowiec JE, Panksepp J, Zolovick AJ, Najam N, Herman BH. Opioid modulation of ingestive behavior. Pharmacology, Biochemistry, and Behavior. 1981;15:477–484. doi: 10.1016/0091-3057(81)90280-x. [DOI] [PubMed] [Google Scholar]
- Kamper-Jørgensen M, Grønbaek M, Tolstrup J, Becker U. Alcohol and cirrhosis: dose--response or threshold effect? Journal of Hepatology. 2004;41:25–30. doi: 10.1016/j.jhep.2004.03.002. [DOI] [PubMed] [Google Scholar]
- Katsuura Y, Heckmann JA, Taha SA. mu-Opioid receptor stimulation in the nucleus accumbens elevates fatty tastant intake by increasing palatability and suppressing satiety signals. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2011;301:R244–254. doi: 10.1152/ajpregu.00406.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kehr J, Yoshitake T, Wang FH, Razani H, Gimenez-Llort L, Jansson A, et al. Galanin is a potent in vivo modulator of mesencephalic serotonergic neurotransmission. Neuropsychopharmacology. 2002;27:341–356. doi: 10.1016/S0893-133X(02)00309-3. [DOI] [PubMed] [Google Scholar]
- Kirkham TC, Blundell JE. Effects of naloxone and naltrexone on meal patterns of freely-feeding rats. Pharmacology, Biochemistry, and Behavior. 1987;26:515–520. doi: 10.1016/0091-3057(87)90158-4. [DOI] [PubMed] [Google Scholar]
- Kiwaki K, Kotz CM, Wang C, Lanningham-Foster L, Levine JA. Orexin A (hypocretin 1) injected into hypothalamic paraventricular nucleus and spontaneous physical activity in rats. American Journal of Physiology Endocrinology and Metabolism. 2004;286:E551–559. doi: 10.1152/ajpendo.00126.2003. [DOI] [PubMed] [Google Scholar]
- Koob GF, Le Moal M. Neurobiology of Addiction. New York, NY: Academic Press; 2006. [Google Scholar]
- Kuteeva E, Hökfelt T, Ogren SO. Behavioural characterisation of transgenic mice overexpressing galanin under the PDGF-B promoter. Neuropeptides. 2005;39:299–304. doi: 10.1016/j.npep.2005.01.004. [DOI] [PubMed] [Google Scholar]
- Lawrence AJ, Cowen MS, Yang HJ, Chen F, Oldfield B. The orexin system regulates alcohol-seeking in rats. British Journal of Pharmacology. 2006;148:752–759. doi: 10.1038/sj.bjp.0706789. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leibowitz SF, Alexander JT. Hypothalamic serotonin in control of eating behavior, meal size, and body weight. Biological Psychiatry. 1998;44:851–864. doi: 10.1016/s0006-3223(98)00186-3. [DOI] [PubMed] [Google Scholar]
- Leibowitz SF, Wortley KE. Hypothalamic control of energy balance: different peptides, different functions. Peptides. 2004;25:473–504. doi: 10.1016/j.peptides.2004.02.006. [DOI] [PubMed] [Google Scholar]
- Lewis MJ, Johnson DF, Waldman D, Leibowitz SF, Hoebel BG. Galanin microinjection in the third ventricle increases voluntary ethanol intake. Alcoholism: Clinical and Experimental Research. 2004;28:1822–1828. doi: 10.1097/01.alc.0000148099.12344.c8. [DOI] [PubMed] [Google Scholar]
- Mann K, Lehert P, Morgan MY. The efficacy of acamprosate in the maintenance of abstinence in alcohol-dependent individuals: results of a meta-analysis. Alcoholism: Clinical and Experimental Research. 2004;28:51–63. doi: 10.1097/01.ALC.0000108656.81563.05. [DOI] [PubMed] [Google Scholar]
- Mieda M, Williams SC, Sinton CM, Richardson JA, Sakurai T, Yanagisawa M. Orexin neurons function in an efferent pathway of a food-entrainable circadian oscillator in eliciting food-anticipatory activity and wakefulness. The Journal of Neuroscience. 2004;24:10493–10501. doi: 10.1523/JNEUROSCI.3171-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mistlberger RE, Antle MC, Kilduff TS, Jones M. Food- and light-entrained circadian rhythms in rats with hypocretin-2-saporin ablations of the lateral hypothalamus. Brain Research. 2003;980:161–168. doi: 10.1016/s0006-8993(03)02755-0. [DOI] [PubMed] [Google Scholar]
- Mizushige T, Kawai T, Matsumura S, Yoneda T, Kawada T, Tsuzuki S, et al. POMC and orexin mRNA expressions induced by anticipation of a corn-oil emulsion feeding are maintained at the high levels until oil ingestion. Biomedical Research. 2006;27:227–232. doi: 10.2220/biomedres.27.227. [DOI] [PubMed] [Google Scholar]
- Morganstern I, Chang GQ, Barson JR, Ye Z, Karatayev O, Leibowitz SF. Differential effects of acute and chronic ethanol exposure on orexin expression in the perifornical lateral hypothalamus. Alcoholism: Clinical and Experimental Research. 2010;34:886–896. doi: 10.1111/j.1530-0277.2010.01161.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Naleid AM, Grace MK, Chimukangara M, Billington CJ, Levine AS. Paraventricular opioids alter intake of high-fat but not high-sucrose diet depending on diet preference in a binge model of feeding. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2007;293:R99–105. doi: 10.1152/ajpregu.00675.2006. [DOI] [PubMed] [Google Scholar]
- Nicoll RA, Siggins GR, Ling N, Bloom FE, Guillemin R. Neuronal actions of endorphins and enkephalins among brain regions: a comparative microiontophoretic study. Proceedings of the National Academy of Sciences of the United States of America. 1977;74:2584–2588. doi: 10.1073/pnas.74.6.2584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Novak CM, Levine JA. Daily intraparaventricular orexin-A treatment induces weight loss in rats. Obesity (Silver Spring, Md) 2009;17:1493–1498. doi: 10.1038/oby.2009.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ogren SO, Schött PA, Kehr J, Yoshitake T, Misane I, Mannström P, et al. Modulation of acetylcholine and serotonin transmission by galanin. Relationship to spatial and aversive learning. Annals of the New York Academy of Sciences. 1998;863:342–363. doi: 10.1111/j.1749-6632.1998.tb10706.x. [DOI] [PubMed] [Google Scholar]
- O’Malley SS, Knshnan-Sann S, Farren C, Sinha R, Kreek MJ. Naltrexone decreases craving and alcohol self-administration in alcohol-dependent subjects and activates the hypothalamo-pituitary-adrenocortical axis. Psychopharmacology. 2002;160:19–29. doi: 10.1007/s002130100919. [DOI] [PubMed] [Google Scholar]
- Ooteman W, Koeter MW, Verheul R, Schippers GM, van den Brink W. The effect of naltrexone and acamprosate on cue-induced craving, autonomic nervous system and neuroendocrine reactions to alcohol-related cues in alcoholics. European Neuropsychopharmacology. 2007;17:558–566. doi: 10.1016/j.euroneuro.2007.02.012. [DOI] [PubMed] [Google Scholar]
- Peyron C, Tighe DK, van den Pol AN, de Lecea L, Heller HC, Sutcliffe JG, et al. Neurons containing hypocretin (orexin) project to multiple neuronal systems. The Journal of Neuroscience. 1998;18:9996–10015. doi: 10.1523/JNEUROSCI.18-23-09996.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rada P, Avena NM, Leibowitz SF, Hoebel BG. Ethanol intake is increased by injection of galanin in the paraventricular nucleus and reduced by a galanin antagonist. Alcohol. 2004;33:91–97. doi: 10.1016/j.alcohol.2004.05.002. [DOI] [PubMed] [Google Scholar]
- Rada P, Mark GP, Hoebel BG. Galanin in the hypothalamus raises dopamine and lowers acetylcholine release in the nucleus accumbens: a possible mechanism for hypothalamic initiation of feeding behavior. Brain Research. 1998;798:1–6. doi: 10.1016/s0006-8993(98)00315-1. [DOI] [PubMed] [Google Scholar]
- Rada P, Mendialdua A, Hernandez L, Hoebel BG. Extracellular glutamate increases in the lateral hypothalamus during meal initiation, and GABA peaks during satiation: microdialysis measurements every 30 s. Behavioral Neuroscience. 2003;117:222–227. doi: 10.1037/0735-7044.117.2.222. [DOI] [PubMed] [Google Scholar]
- Sakurai T, Amemiya A, Ishii M, Matsuzaki I, Chemelli RM, Tanaka H, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92:573–585. doi: 10.1016/s0092-8674(00)80949-6. [DOI] [PubMed] [Google Scholar]
- Schneider ER, Rada P, Darby RD, Leibowitz SF, Hoebel BG. Orexigenic peptides and alcohol intake: differential effects of orexin, galanin, and ghrelin. Alcoholism: Clinical and Experimental Research. 2007;31:1858–1865. doi: 10.1111/j.1530-0277.2007.00510.x. [DOI] [PubMed] [Google Scholar]
- Stranges S, Freudenheim JL, Muti P, Farinaro E, Russell M, Nochajski TH, et al. Differential effects of alcohol drinking pattern on liver enzymes in men and women. Alcoholism: Clinical and Experimental Research. 2004;28:949–956. doi: 10.1097/01.alc.0000128229.23396.42. [DOI] [PubMed] [Google Scholar]
- Yamanaka A, Sakurai T, Katsumoto T, Yanagisawa M, Goto K. Chronic intracerebroventricular administration of orexin-A to rats increases food intake in daytime, but has no effect on body weight. Brain Research. 1999;849:248–252. doi: 10.1016/s0006-8993(99)01905-8. [DOI] [PubMed] [Google Scholar]
- Yun R, Dourmashkin JT, Hill J, Gayles EC, Fried SK, Leibowitz SF. PVN galanin increases fat storage and promotes obesity by causing muscle to utilize carbohydrate more than fat. Peptides. 2005;26:2265–2273. doi: 10.1016/j.peptides.2005.04.005. [DOI] [PubMed] [Google Scholar]
- Zakhari S, Li TK. Determinants of alcohol use and abuse: Impact of quantity and frequency patterns on liver disease. Hepatology. 2007;46:2032–2039. doi: 10.1002/hep.22010. [DOI] [PubMed] [Google Scholar]


