Abstract
This study assessed clinical scenarios of continuing monoclonal antibody (mAb) treatment for (+)-methamphetamine (METH) addiction, and the implications of missing or discontinuing this therapy. We hypothesized that chronic anti-METH mAb7F9 (METH KD = 9 nM) treatment of rats could significantly decrease METH-induced behaviors; even with repeated METH challenges, use of METH doses in excess of mAb binding sites, and after discontinuing mAb treatment which results in a 10-fold reduction in mAb7F9 serum concentrations. Male Sprague Dawley rats (n=6/group) were treated with i.v. saline or a loading dose of mAb7F9 to achieve instant steady-state conditions followed by two weekly (141 mg/kg) doses ending on day 14. METH (0.56 mg/kg) was administered four hours and three days after each saline or mAb7F9 treatment, and on day 21. This produced locomotion and rearing behavior that lasted about 120 min in control rats. In mAb7F9 treated rats, METH-induced distance traveled was significantly reduced from 60–120 min (P<0.05) on days 0–21 and rearing was significantly reduced from 60–120 min on days 0–17. METH serum concentrations determined 5 hrs after METH dosing was significantly increased in mAb7F9-treated rats after all METH challenges. On days 24 and 28 (the final day), the rats were administered a 3-fold higher METH dose (1.68 mg/kg). MAb7F9 treated rats showed a substantially earlier termination of the METH-induced locomotion on both days, even though the METH dose exceeded mAb7F9’s binding capacity. METH brain concentrations determined 5 hrs after METH on day 28 were also significantly decreased in mAb7F9-treated rats. In conclusion, over one month, mAb7F9 significantly and continuously bound METH and reduced METH-induced locomotor effects even after discontinuation of mAb treatment and challenge with higher METH doses.
Keywords: methamphetamine, drug abuse, monoclonal antibody, preclinical studies, rats, chronic administration
Introduction
METH addiction produces a chronic relapsing cycle of habitual, compulsive drug use which includes binge use, intoxication, withdrawal, and craving [1]. The positive reinforcement of METH is the most common cause for both continued use and relapse [2]. Unfortunately, small molecule treatments for relapse prevention have been unsuccessful [3].
High affinity, anti-METH monoclonal antibodies (mAb) could potentially decrease the rewarding effects of METH, and thereby lessen the impact of a relapse to METH use [4]. The anti-METH mAb rapidly binds METH in the bloodstream. This decreases both the rate of entry and the amount of METH in the brain [5]. Clearance of METH (e.g., metabolism) is also a significant mechanism for regenerating mAb binding capacity for METH [6].
Considering their high specificity for METH-like drugs [7] and non-hepatic metabolism [8], anti-METH mAbs could be safely co-administered with small molecule therapies for existing medical conditions. MAb medication should also lack abuse liability and significant adverse effects [4,6]. The main disadvantages are cost and the potential for eventual anti-mAb immune responses.
Anti-METH mAbs can acutely block METH-induced effects in rats [7,9,10], but there is limited data on anti-METH antibody therapy blocking METH effects over an extended period of time, and at METH doses in significant excess of antibody binding capacity [11,12]. These therapeutic attributes are important because METH-addicted patients are unlikely to remain abstinent from METH use even during treatment and are likely to miss scheduled treatments. For example, in a 16 week clinical study, only 30% of the weekly METH urine screens were negative and only one-third of the patients completed the study [13].
Treatment of METH relapse will require a course of medication lasting for months. To sustain long-term steady-state concentrations within a therapeutic window, a good practice is to administer medications at a rate of once every drug half-life (t1/2). Typically, mAb therapies have a 3–4 week t1/2 in humans, therefore a patient would only need mAb therapy every 3–4 weeks [8]. This is in stark contrast to small molecule medications with a 12 hr t1/2 (for example) that would require daily or twice daily administration. [14–17]. The need for less frequent mAb doses could have significant benefits for patient convenience and compliance.
The current study was performed to assess clinical scenarios of chronic mAb treatment for METH addiction, and the implications of missing or discontinuing mAb treatments. We hypothesized that chronic anti-METH mAb7F9 treatment of rats could significantly decrease METH-induced behaviors; even with repeated METH challenges, use of METH doses in excess of mAb binding sites, and after stopping or missing mAb treatments. Over the one-month study, MAb7F9 significantly and continuously reduced METH-induced locomotor effects, produced substantial METH serum binding, and still had the capacity to lower brain METH concentrations on day 28.
Methods
Drugs and chemicals
(+)-METH hydrochloride and (+)-amphetamine (AMP) sulfate were obtained from NIDA (Rockville, MD). Analytical internal standards and all other chemicals were purchased from Sigma-Aldrich (St. Louis, MO). Doses and standards were calculated as the free base.
Anti-METH mAb
Discovery and large-scale production of mAb7F9 (mouse IgG1 isotype, κ light chain, KD=9 nM for METH) are previously described [7,18,19]. A chimeric version of mAb7F9 is in the process of human clinical trials [6]. The t1/2 of mAb7F9 in rats was estimated to be 7 days based on t1/2 values of three other IgG anti-METH mAbs from our laboratory [20].
Animals
Male Sprague-Dawley rats (n=16) with dual indwelling jugular vein catheters were obtained from Charles River Laboratories (Wilmington, MA). Rats were housed separately, provided water ad libitum, and fed sufficient food pellets to maintain a 300 g body weight. All experiments were conducted in accordance with the NIH Guide for Care and Use of Laboratory Animals, and the University of Arkansas for Medical Sciences Animal Care and Use Committee.
Experimental design
Rats were acclimated to home cages and handling for one-week. They were then habituated 6 hrs a day for 4 days (and 1 day before experiments) in open-top polyethylene behavioral chambers (60×45×40 cm). Rats were administered 0.56 mg/kg METH every three days to obtain stable METH-induced locomotion. Data from the third dose was used to match-pair rats into vehicle and mAb7F9 treatment groups (n=8/group).
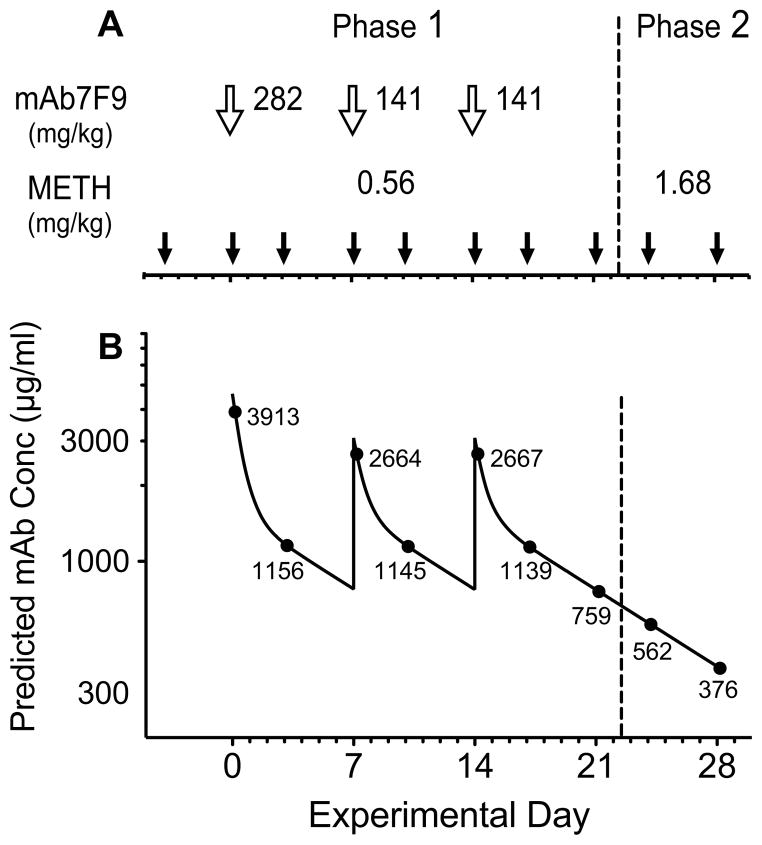
Phase 1 of the studies began 4 days after the last METH conditioning dose. The vehicle and mAb7F9 treatments were administered 4 hrs before i.v. METH challenge doses (see Figure 1A). For mAb7F9, an i.v. loading dose (over 1.5 min) on day 0 was used to achieve immediate steady-state concentrations, followed by two weekly maintenance doses (on days 7 and 14, over 0.75 min) [21]. The maintenance dose of mAb7F9 was calculated to be 0.5 molar in binding sites to the total body burden of a single 0.56 mg/kg METH dose.
Figure 1.
(A) Experimental protocol for dosing with vehicle or mAb7F9 treatment (open arrows) and METH challenges (closed arrows). Doses of mAb7F9 and METH are shown for each study. The dashed line is included to indicate transition from Phase 1 to Phase 2. (B) Predicted serum mAb7F9 concentrations over time (solid line) and predicted mAb concentrations at the times of each METH challenge (black dots, actual values included). After each METH challenge (closed arrows), locomotion and rearing were measured for 4 hrs. Blood samples were collected 5 hrs after all METH doses. Brains and trunk blood were collected after the final METH challenge on day 28.
Behavioral studies
On METH challenge days (Figure 1A), rats were acclimated in the chamber for 1.5 hrs before METH administration. Movement was recorded by overhead video cameras for 5.5 hrs. Ethovision 8 software (Noldus Information Technology, Inc., Sterling, VA) measured locomotion as horizontal distance traveled in 4 min bins. We also validated a method to quantitate rearing events, based on Noldus instructions [22]. We defined a rearing event as a reduction in body surface area of ≥20% of the running average of the previous three seconds.
We analyzed locomotion and rearing data from each 4 hr experiment in both 1 hr intervals and as 4 hrs of total response. We also calculated duration of activity [7] and time of peak activity.
In Phase 2, METH-induced effects in vehicle-treated rats were much longer than expected based on previous studies [23], thus not all animals returned to baseline activity during the data collection period. To determine the end of the METH-induced effects, a best-fit linear regression line was fit to the terminal phase of each group’s distance traveled vs. time data. The x-intercept was considered the end time point.
Of the starting 16 rats (n=8/group), three were excluded due to catheter failure before the final mAb dose. This was anticipated due to our prior experience with long-term catheter maintenance. Another rat was excluded due to repeated jumping out of the behavioral chamber (n=6/group for days 0–24). Before day 28, a rat died due a catheter related issue (n=6 vehicle, n=5 mAb7F9).
In two vehicle- and one mAb7F9-treated rat(s) the METH challenge doses were administered s.c. when catheters lost patency after the final mAb7F9 dose. We determined that neither the pattern nor magnitude of the behavioral data for s.c. doses were outside the range of values produced by i.v. doses. The pharmacological similarities of these routes of administration were also supported by METH pharmacokinetic and behavioral studies after i.v. and s.c. dosing [24].
Determination of METH and AMP metabolite concentrations
Five hours after each METH challenge, blood (0.25 ml) was collected from the tail vein. Five hrs post-METH on day 28, rats were euthanized under isoflurane anesthesia by decapitation for collection of brains and trunk blood. We assessed adequate depth of anesthesia by paw pinch, and respiration frequency and depth. Serum and brain tissues were stored at −80°C.
Samples (0.05 ml) and extraction solvent (0.45 ml of 95% acetonitrile containing 0.1 μg/ml AMP-d11 [1,1,2,3,3,3-hexadeutero-1-pentadeuterophenyl-2-aminopropane] and METH-d5 [(±)-1-phenyl-1,2-dideutero-2-[trideuteromethyl]aminopropane] internal standards in 20 mM ammonium acetate, pH 3.7) were added to a Sirocco extraction plate (Waters Corp, Milford, MA). The solvent containing analytes was collected under vacuum.
Each sample (7.2 μl) was injected into a 50 mm × 2.1 mm, 1.7 μm Kinetex HILIC column (Phenomenex, Torrance, CA) at 50°C. This column was connected to an Acquity Ultra Performance Liquid Chromatography system coupled to a Quattro Premier XE mass spectrometer (Waters Corp) operated in the positive ion mode via an electrospray ionization probe. The mobile phases were 20 mM ammonium acetate pH 3.7 in A: 96% acetonitrile or B: water. The initial flow rate of 0.6 ml/min was increased to 0.7 ml/min at 3.25 min. Mobile phase B was increased over 3 min from 4–15%, over the next 0.25 min from 15–35%, and held at 35% for 0.75 min, and finally decreased over 0.25 min from 65–4%.
Positive ions for AMP, AMP-d11, METH, and METH-d5 were generated using argon collision induced disassociation, and detection was achieved using the known conversion of precursor to product ions. The range of quantitation was 0.3–4000 ng/ml for METH and 1–300 ng/ml for AMP, with standards showing ±20% of predicted values.
Pharmacokinetic simulations and statistical analysis
We used WinNonlin 6.3 (Certara, St. Louis, MO) and pharmacokinetic parameters from a similar anti-METH mAb [20] to simulate anti-METH mAb7F9 serum concentrations over the one month experiment (Figure 1B). For all measures of serum METH (and AMP) concentration and behavior data, a two factor repeated measures analysis of variance was used with the repeated measure being time. Contrasts were predefined to be the difference between mAb7F9 and vehicle treatments on METH challenge days during Phase 1 and 2 studies. A Bonferroni adjustment of p-values was performed with the Stepdown Bonferroni method in proc multtest using SAS 9.3 (SAS Institute, Cary, NC). While the statistical analysis was conducted by this method, the data for each measure was presented as a mean ± standard deviation. Brain drug concentrations (day 28) were compared with a student’s t-test.
Results
Phase 1. Pharmacological effects of mAb7F9 treatment following repeated 0.56 mg/kg METH challenges (days 0–21)
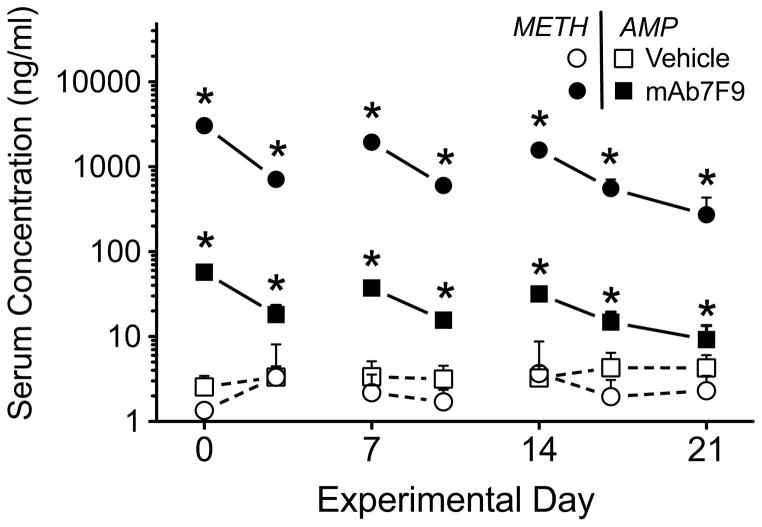
Figure 2 shows representative plots of METH-induced locomotion over time. The summary of METH-induced locomotion and rearing measurements are shown in Figures 3 and 4, and in Tables 1 and 2. Figure 5 shows MAb7F9 effects on METH and AMP serum concentration. Since mAb7F9 does not significantly cross react with AMP (Ki = 370 nM) [6], the elevated AMP serum concentrations (compared to controls) suggested increased METH in the serum led to greater metabolic conversion to AMP.
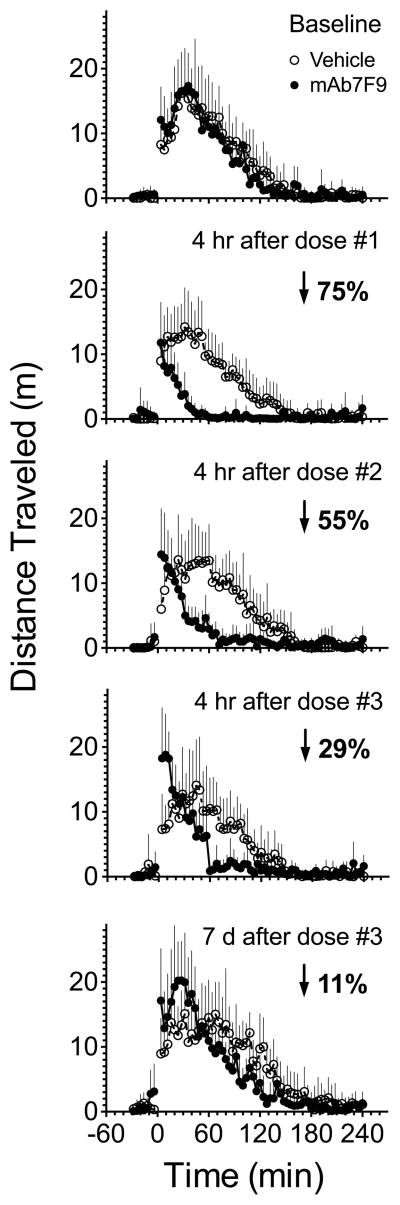
Figure 2.
Changes in the METH-induced (0.56 mg/kg) distance traveled behavioral profile in control and mAb7F9 treated rats. METH was administered at time 0. METH-induced distance traveled or rearing (+SD) is shown in vehicle- (open circles) and mAb7F9-treated (closed circles) rats (n=6 per group). Each point displays the total distance traveled in a 4 min time period. The baseline behavior profile (top panel) shows METH-induced locomotion before vehicle or mAb7F9 administration. The next four plots show the progressive change in the behavioral profile in mAb7F9- compared to vehicle-treated rats at four hrs after three weekly mAb7F9 doses, and finally at seven days after the third mAb7F9 dose. The average percentage decrease in total distance traveled in mAb7F9- compared to vehicle-treated rats is indicated by the arrow and number.
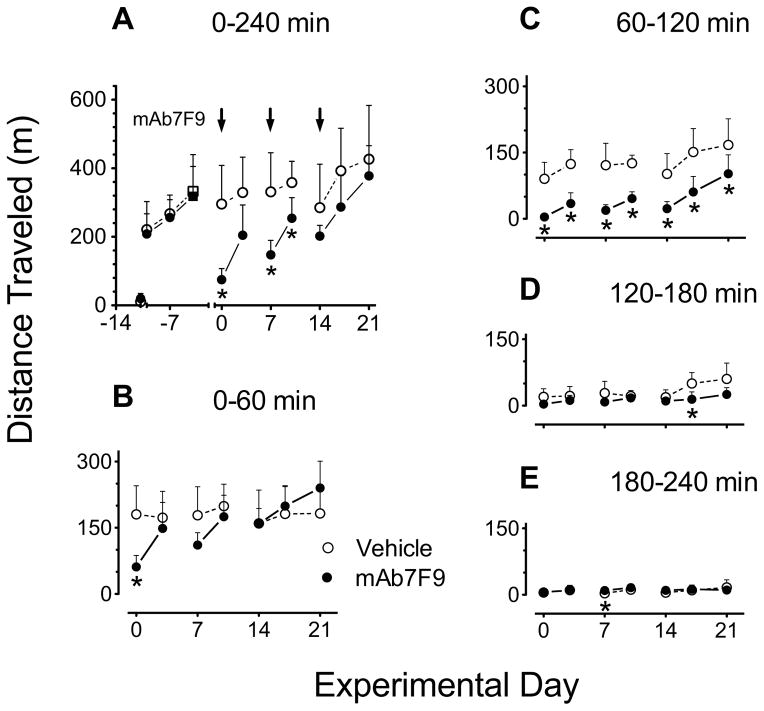
Figure 3.
Comparison of distance traveled after 0.56 mg/kg METH challenge doses in vehicle- (open circles) and mAb7F9-treated (closed circles) rats (n=6 per group) over the 21 days of the Phase 1 studies. Total values measured over 4 hrs are shown in panel A. This plot includes the values for the three METH administrations used to stabilize the effects of METH administration prior to vehicle or mAb7F9 treatment as well as the pre-METH saline dose (data points prior to day 0). Panels B–E show a more detailed analysis of the data from Panel A in 1 hr intervals. Pre-vehicle or pre-mAb baseline measurements used for match pairing into treatment groups are denoted by the square symbols on day −4. The * denotes statistical significance compared to time-matched vehicle controls (P<0.05).
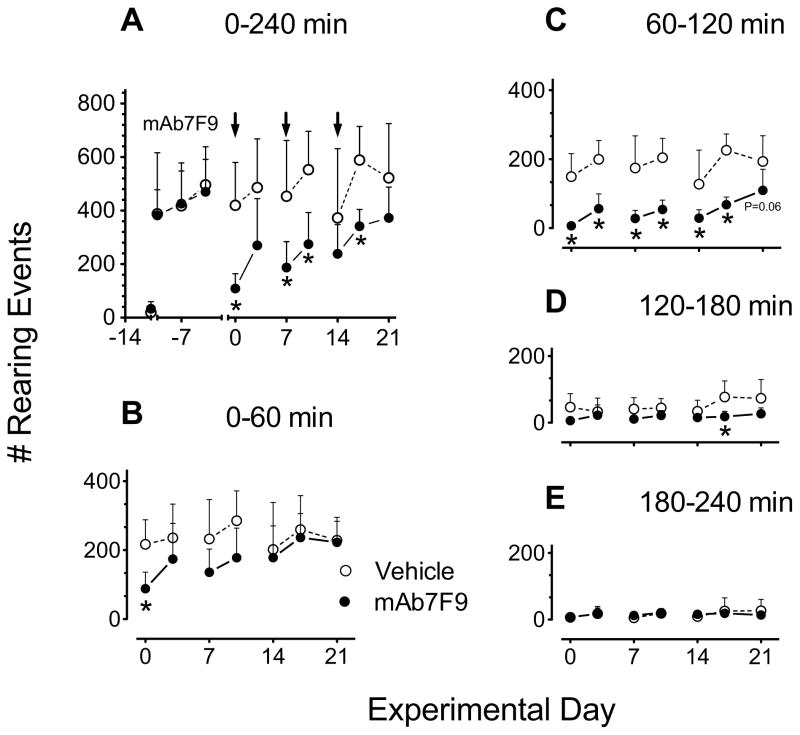
Figure 4.
Comparison of the number of rearing events after 0.56 mg/kg METH challenge doses in vehicle- (open circles) and mAb7F9-treated (closed circles) rats (n=6 per group) over the 21 days of the Phase 1 studies. Total values measured over 4 hrs are shown in panel A. This plot includes the values for the three METH administrations used to stabilize the effects of METH administration prior to vehicle or mAb7F9 treatment as well as the pre-METH saline dose (data points prior to day 0). Panels B-E show a more detailed analysis of the data from Panel A in 1 hr intervals. Pre-vehicle or pre-mAb baseline measurements are denoted by the square symbols on day −4. The * denotes statistical significance compared to time-matched vehicle controls (P<0.05).
Table 1.
Analysis of distance traveled data to determine time of peak effect and duration of action after 0.56 mg/kg METH challenge doses in vehicle- and mAb7F9-treated rats in Phase 1 studies. See Figure 1A for overview of experimental design.
| Day | Vehicle or mAb7F9 Dose # | Time of METH Challenge after Vehicle or mAb7F9 | Time of Peak Effect (min) | Duration (min) | ||
|---|---|---|---|---|---|---|
| Vehicle | mAb | Vehicle | mAb | |||
| −4 | Pre-treatment Baseline | 34 ± 6 | 36 ± 8 | 136 ± 20 | 134 ± 32 | |
| 0 | 1 | 4 hr post | 37 ± 9 | 9 ± 11a | 125 ± 23 | 35 ± 14a |
| 3 | 3 day post | 35 ± 7 | 21 ± 12a | 133 ± 17 | 89 ± 36a | |
| 7 | 2 | 4 hr post | 41 ± 30 | 9 ± 9b | 138 ± 29 | 65 ± 28a |
| 10 | 3 day post | 55 ± 33 | 23 ± 17b | 143 ± 26 | 105 ± 35 | |
| 14 | 3 | 4 hr post | 35 ± 16 | 11 ± 7a | 135 ± 17 | 71 ± 25a |
| 17 | 3 day post | 75 ± 35 | 20 ± 16a | 158 ± 13 | 106 ± 33a | |
| 21 | 7 day post | 67 ± 30 | 32 ± 13a | 157 ± 22 | 135 ± 39 | |
Statistical significance compared to time-matched vehicle controls (P<0.05)
P=0.06
Table 2.
Analysis of rearing data to determine time of peak effect and duration of action after 0.56 mg/kg METH challenge doses in vehicle- and mAb7F9-treated rats in Phase 1 studies. See Figure 1A for overview of experimental design.
| Day | Vehicle or mAb7F9 Dose # | Time of METH Challenge after Vehicle or mAb7F9 | Time of Peak Effect (min) | Duration (min) | ||
|---|---|---|---|---|---|---|
| Vehicle | mAb | Vehicle | mAb | |||
| −4 | Pre-treatment Baseline | 35 ± 15 | 25 ± 10 | 134 ± 21 | 127 ± 29 | |
| 0 | 1 | 4 hr post | 35 ± 18 | 13 ± 11b | 122 ± 27 | 35 ± 17a |
| 3 | 3 day post | 65 ± 23 | 23 ± 11a | 133 ± 17 | 95 ± 46 | |
| 7 | 2 | 4 hr post | 35 ± 18 | 12 ± 7a | 132 ± 31 | 55 ± 29a |
| 10 | 3 day post | 39 ± 24 | 18 ± 13 | 141 ± 32 | 96 ± 45 | |
| 14 | 3 | 4 hr post | 56 ± 46 | 25 ± 18 | 120 ± 47 | 61 ± 24 |
| 17 | 3 day post | 54 ± 26 | 23 ± 16 | 154 ± 14 | 92 ± 19a | |
| 21 | 7 day post | 45 ± 26 | 24 ± 21 | 152 ± 28 | 112 ± 44 | |
Statistical significance compared to time-matched vehicle controls (P<0.05)
P=0.06
Figure 5.
Serum METH and metabolite AMP concentrations 5 hrs after the 0.56 mg/kg METH dose in vehicle- and mAb7F9-treated rats (see figures 3 and 4 for behavioral data from these Phase 1 studies). Open symbols denote the vehicle treatment group, and solid symbols denote the mAb7F9 treatment group. METH concentrations are represented by circles and AMP concentrations are represented by squares. The * denotes statistical significance compared to time-matched vehicle controls (P<0.05).
Phase 2. Pharmacological effects of mAb7F9 treatment after 1.68 mg/kg METH challenges (days 24 and 28)
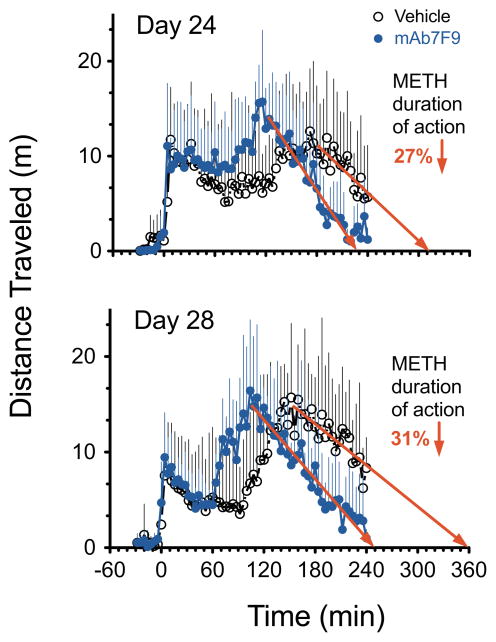
Figure 6 shows averaged distance traveled over time. Table 3 shows comparisons of 4-hr total and 1-hr interval METH-induced distance traveled and rearing data. In the mAb7F9 treatment group, the individual locomotor activity in four of six rats on day 24 and three of five rats on day 28 decreased to saline-treated baseline before the end of the 4-hr measurement period. With vehicle treatment, the return to baseline values was only achieved in two of six rats on day 24 and one of six rats on day 28. For rearing behavior, all six mAb7F9-treated animals, but only one of six vehicle-treated animals returned to baseline activity on day 24. Two of five mAb-treated rats and one of six vehicle-treated animals returned to baseline rearing on day 28. These reductions were corroborated by the significant decrease in rearing on both days, and near significant reductions in locomotion in mAb-treated rats from 180–240 min (Table 3).
Figure 6.
Distance traveled over time after a 1.68 mg/kg METH dose in vehicle- (black, open circle, n=6) and mAb7F9-treated (blue, closed circle, n=5) rats on day 24 and day 28 in Phase 2 studies. This METH dose was 3-fold greater than the METH dose used in the Phase 1 experiments. The best-fit linear regression line (in red) was fit to the terminal portion of the distance traveled-time curve. This line was used to estimate the time of the end of activity and the relative difference in the duration of METH effects between vehicle- and mAb7F9-treatment groups.
Table 3.
Analysis of distance traveled (Figure 6) and rearing data (plot not shown) 1.68 mg/kg METH challenge doses in the Phase 2 studies in vehicle- and mAb7F9-treated rats. This table shows the analysis of the total data from 0–240 min as well as the analysis of 1 hr interval data over the 4 hrs of measurement.
| Distance traveled (in meters) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| 0–240 min (Total) | 0–60 min | 60–120 min | 120–180 min | 180–240 min | ||||||
| Day | Vehicle | mAb7F9 | Vehicle | mAb7F9 | Vehicle | mAb7F9 | Vehicle | mAb7F9 | Vehicle | mAb7F9 |
| 24 | 511 ± 210 | 509 ± 94 | 131 ± 77 | 144 ± 57 | 100 ± 94 | 166 ± 45 | 152 ± 84 | 157 ± 60 | 128 ± 73 | 42 ± 33b |
| 28 | 530 ± 173 | 480 ± 98 | 84 ± 81 | 91 ± 35 | 85 ± 102 | 182 ± 61 | 200 ± 56 | 150 ± 56 | 160 ± 84 | 56 ± 56c |
| Number of rearing events | ||||||||||
| 24 | 684 ± 326 | 732 ± 191 | 252 ± 170 | 287 ± 162 | 140 ± 115 | 221 ± 76 | 160 ± 87 | 175 ± 66 | 132 ± 65 | 49 ± 35a |
| 28 | 637 ± 373 | 639 ± 178 | 178 ± 199 | 243 ± 138 | 124 ± 143 | 176 ± 106 | 170 ± 72 | 155 ± 55 | 166 ± 58 | 65 ± 62a |
Statistical significance compared to time-matched vehicle controls (P<0.05)
P=0.06
P=0.07
In vehicle- and mAb7F9-treated rats, the best-fit line to the terminal phase of the distance traveled versus time curve showed r2 = 0.7 and 0.95 (respectively) on day 24, and 0.68 and 0.94 (respectively) on day 28. In vehicle- and mAb7F9-treated rats, the best-fit line equations were y=−0.085x+26 and y=−0.135x+31 (respectively) on day 24, and y=−0.072x+26 and y=−0.104x+26 (respectively) on day 28. The terminal phase of the rearing vs. time data (not shown) was not sufficiently linear to produce a predictive best-fit line.
Serum METH concentrations 5 hrs after the 1.68 mg/kg METH administration were significantly elevated in the mAb7F9-treated rats relative to the vehicle-treated rats on day 24 (102±63 vs. 8±2 ng/ml) and day 28 (53±39 vs. 6±2 ng/ml; P<0.05). Whole brain METH concentrations were significantly decreased 5 hrs post-METH dosing compared to vehicle-treated animals (47±9 vs 75±20 ng/g; P<0.05). There were no significant changes in serum AMP concentrations on day 24 (13±4 vs 15±6 ng/ml) or 28 (9±2 vs 12±5 ng/ml), or brain AMP concentrations on day 28 (65±15 vs 109±41 ng/g; P=0.05).
Discussion
These studies were designed to test important clinical scenarios related to anti-METH mAb7F9 therapy and relapse to METH use. We were especially interested in the potential changes in METH binding function of mAb7F9 during chronic treatment, and the safety and efficacy of the antibody-METH interactions if mAb treatment was prematurely stopped. Compared to vehicle, mAb7F9 significantly decreased total METH-induced distance traveled in rats on days 0, 7, and 10 (Figure 3A) and rearing events on days 0, 7, 10, and 17 (Figure 4A). However, changes in the apparent behavioral profile in the mAb-treated rats (Figure 2) after each METH dose suggested a progressive decrease in efficacy. At first, this was surprising since METH concentrations (Figure 5) in mAb7F9-treated animals and predicted mAb7F9 concentrations (Figure 1B) suggested mAb7F9 significantly bound and redistributed METH from peripheral compartments.
We realized that measures of total METH-induced behavior (0–240 min) were misleading since they failed to detect important time-dependent changes in the behavioral profile (Figure 2, Tables 1 and 2). Thus, we re-analyzed the data in one-hr time segments. During the period from 0–60 min (Figures 3B and 4B), METH-induced behaviors appeared to progressively increase over the first 21 days in the mAb-treated rats compared to controls. During the 60–120 min interval, however, mAb7F9 treatment significantly reduced METH-induced locomotion after each METH challenge from days 0–21 (Figure 3C). In the period from 120–240 min, all behaviors were back to baseline levels (Figures 3C–D and 4C–D).
For distance traveled, mAb7F9 treatment produced a significantly earlier time of peak METH-induced effects and shortened the duration of action (Table 1) in 5 out of 7 METH challenges from day 0–21; and significantly reduced the total distance traveled from 60–120 min (Figure 3C) through day 21. While mAb7F9 treatment significantly reduced the number of rearing events from 60–120 min through day 17 (Figure 4C), compared to distance traveled, it produced less profound changes (Table 2). Since the mechanisms producing METH-induced horizontal motion and rearing are somewhat different [25], it was not surprising that the results differed.
Interestingly, mAb7F9 treatment appeared to progressively increase distance traveled and rearing (relative to controls) during the 0–60 min interval from day 0–21 (Figure 3B and 4B). These early mAb7F9-induced increases in distance traveled after treatment with a medication is not unprecedented. Pretreatment with haloperidol (a dopamine-2 receptor antagonist) prior to a 4 mg/kg s.c. AMP (a METH-like drug) dose produces immediate high levels of locomotor activity, instead of the substantial early suppression of movement normally caused by a 4 mg/kg AMP dose. However, unlike mAb7F9 treatment of METH, haloperidol does not shorten the duration of action of AMP [26]. Thus we conclude mAb7F9 treatment both slows entry and decreases the amount of METH in the brain, which reduces the overall effects and duration of action.
The Phase 2 experiments were designed to mimic a patient in therapy who misses two anti-METH mAb treatments. Assuming a t1/2 of 3 weeks for a mAb in humans [8] and a 1 week t1/2 of mAb7F9 in rats, a 10 and 14 day lapse of mAb7F9 treatment in rats would be an approximately 30 and 42 day lapse in humans. We calculated that the mAb7F9 concentrations on experimental days 24 and 28 were 79% and 86% lower, respectively, than those on day 14 (Figure 1). We also increased the METH dose three-fold to 1.68 mg/kg, resulting in an 8- and 12-fold excess of METH compared to the predicted number of mAb7F9 binding sites. This simulated a patient’s potentially unsafe attempt to surmount the mAb METH binding capacity. The results showed mAb7F9 still substantially shortened the METH duration of action (Figure 6).
From a safety viewpoint, a METH dose in significant excess of mAb binding capacity did not lead to additive or synergistic effects in behaviors (Table 3 and Figure 6). Instead, mAb7F9 treatment effectively increased the percentage of rats that returned to pre-METH baseline behavioral values on days 24 and 28, resulting in a 27% and 31% (respectively) decrease in the duration of METH-induced locomotion (Figure 6). Also, the 37% reduction in brain concentrations at the end of the study correlated well with the 31% shortening of the duration of action on day 28. However, additional studies will be needed to determine if anti-METH mAb7F9 in the presence of high concentrations of METH is safe for other critical organ systems like the cardiovascular system.
The high doses of mAb7F9 used in these rat studies are not currently practical for human use, mainly due to the high cost. We used high doses because we were interested in studying the mAb7F9 effects over a one-month period, and knew that not treating for two weeks would produce substantially lower mAb7F9 serum concentrations (3913 μg/ml on day 0 and 376 μg/ml on day 28; Figure 1B). This 10-fold mAb7F9 concentration range allowed us to gain insight into the range of effective mAb7F9 doses. We calculated that a weekly 53 mg/kg mAb7F9 dose could produce steady-state concentrations of 409 μg/ml with minimum concentrations ≥376 μg/ml.
In the clinical trial of an anti-cocaine active vaccine (not an anti-cocaine mAb), there was decreased cocaine use in subjects who attained anti-cocaine antibody concentrations ≥43 μg/ml [27]. By our calculations using pharmacokinetic parameters from a mAb clinical trial [28], a 3.5 mg/kg dose of mAb administered once per t1/2 (18.1 days) in humans would lead to a steady-state concentration of 62 μg/ml with a minimum concentration of >43 μg/ml. Unlike active immunization, this could be accomplished in all patients, including immune compromised HIV patients. Nevertheless, only human testing will reveal the dose, frequency of dosing, and serum mAb concentrations needed to treat human METH abuse.
Conclusions
These studies support our hypothesis and showed that mAb7F9 can be chronically administered to provide continuous protection from METH pharmacological effects. This protection was demonstrated even when the METH dose significantly exceeded mAb binding capacity and after missing or discontinuing mAb treatments. We think a treatment that could reduce the impact of a lapse or relapse to METH use by significantly reducing METH effects in conjunction with cognitive behavioral therapy could help to better manage the quality of life of an addicted individual and thereby reduce the cost of METH addiction for society [29].
Highlights.
Anti-METH mAb7F9 treatment for METH abuse was studied in rats over one month
Investigated clinical scenarios of therapy and consequences of missing treatments
MAb7F9 significantly and continuously bound METH in serum and reduced brain levels
MAb7F9 treatment shortened METH locomotor effects, even with repeated METH dosing
MAb7F9 safely provided continuous and long-lasting reductions in METH effects
Acknowledgments
The authors thank Melinda Gunnell, Sherri Wood, Yingni Che, and C. Michael West for technical assistance.
Research funding was provided by the NIH National Institute on Drug Abuse (Grants U01DA23900, DA11560, and T32DA022981); and the National Center for Advancing Translational Science (Grant ULITR000039).
Abbreviations
- AMP
(+)-amphetamine
- i.v
intravenous
- AMP-d11
1,1,2,3,3,3-hexadeutero-1-pentadeuterophenyl-2-aminopropane
- KD
equilibrium dissociation rate constant
- LC-MS/MS
liquid chromatography coupled to tandem mass spectrometry
- mAb
monoclonal antibody
- METH
(+)-methamphetamine
- METH-d5
(±)-1-phenyl-1,2-dideutero-2-[trideuteromethyl]aminopropane
- s.c
subcutaneous
- t1/2
terminal elimination half-life
Footnotes
Conflict of interest statement: SMO is Chief Scientific Officer of and has financial interests in InterveXion Therapeutics, LLC, a pharmaceutical biotech company, whose main interest is the development of antibody medications for the treatment of human diseases, including drug abuse.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Koob GF, Volkow ND. Neurocircuitry of addiction. Neuropsychopharmacology. 2010;35:217–38. doi: 10.1038/npp.2009.110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Newton TF, De La Garza R, 2nd, Kalechstein AD, Tziortzis D, Jacobsen CA. Theories of addiction: methamphetamine users’ explanations for continuing drug use and relapse. Am J Addict. 2009;18:294–300. doi: 10.1080/10550490902925920. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brensilver M, Heinzerling KG, Shoptaw S. Pharmacotherapy of amphetamine-type stimulant dependence: An update. Drug Alcohol Rev. 2013;32:449–60. doi: 10.1111/dar.12048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gentry WB, Rüedi-Bettschen D, Owens SM. Anti-(+)-methamphetamine monoclonal antibody antagonists designed to prevent the progression of human diseases of addiction. Clin Pharmacol Ther. 2010;88:390–3. doi: 10.1038/clpt.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Owens SM, Atchley WT, Hambuchen MD, Peterson EC, Gentry WB. Monoclonal antibodies as pharmacokinetic antagonists for the treatment of (+)-methamphetamine addiction. CNS Neurol Disord-Dr. 2011;10:892–8. doi: 10.2174/187152711799219370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Stevens MW, Tawney RL, West CM, Kight AD, Henry RL, Owens SM, et al. Preclinical characterization of an anti-methamphetamine monoclonal antibody for human use. Mabs. 2014;6:547–55. doi: 10.4161/mabs.27620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Byrnes-Blake KA, Laurenzana EM, Carroll FI, Abraham P, Gentry WB, Landes RD, et al. Pharmacodynamic mechanisms of monoclonal antibody-based antagonism of (+)-methamphetamine in rats. Eur J Pharmacol. 2003;461:119–28. doi: 10.1016/s0014-2999(03)01313-x. [DOI] [PubMed] [Google Scholar]
- 8.Wang W, Wang EQ, Balthasar JP. Monoclonal antibody pharmacokinetics and pharmacodynamics. Clin Pharmacol Ther. 2008;84:548–58. doi: 10.1038/clpt.2008.170. [DOI] [PubMed] [Google Scholar]
- 9.Byrnes-Blake KA, Laurenzana EM, Landes RD, Gentry WB, Owens SM. Monoclonal IgG affinity and treatment time alters antagonism of (+)-methamphetamine effects in rats. Eur J Pharmacol. 2005;521:86–94. doi: 10.1016/j.ejphar.2005.08.016. [DOI] [PubMed] [Google Scholar]
- 10.Gentry WB, Laurenzana EM, Williams DK, West JR, Berg RJ, Terlea T, et al. Safety and efficiency of an anti-(+)-methamphetamine monoclonal antibody in the protection against cardiovascular and central nervous system effects of (+)-methamphetamine in rats. Int Immunopharmacol. 2006;6:968–77. doi: 10.1016/j.intimp.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 11.Rüedi-Bettschen D, Wood SL, Gunnell MG, West CM, Pidaparthi RR, Carroll FI, et al. Vaccination protects rats from methamphetamine-induced impairment of behavioral responding for food. Vaccine. 2013;31:4596–602. doi: 10.1016/j.vaccine.2013.07.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller ML, Moreno AY, Aarde SM, Creehan KM, Vandewater SA, Vaillancourt BD, et al. A methamphetamine vaccine attenuates methamphetamine-induced disruptions in thermoregulation and activity in rats. Biol Psychiat. 2013;73:721–8. doi: 10.1016/j.biopsych.2012.09.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hillhouse MP, Marinelli-Casey P, Gonzales R, Ang A, Rawson RA. Predicting in-treatment performance and post-treatment outcomes in methamphetamine users. Addiction (Abingdon, England) 2007;102 (Suppl 1):84–95. doi: 10.1111/j.1360-0443.2007.01768.x. [DOI] [PubMed] [Google Scholar]
- 14.Shoptaw S, Heinzerling KG, Rotheram-Fuller E, Steward T, Wang J, Swanson A, et al. Randomized, placebo-controlled trial of bupropion for the treatment of methamphetamine dependence. Drug Alcohol Depend. 2008;96:222–32. doi: 10.1016/j.drugalcdep.2008.03.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Jayaram-Lindström N, Hammarberg A, Beck O, Franck J. Naltrexone for the treatment of amphetamine dependence: a randomized, placebo-controlled trial. Am J Psychiatry. 2008;165:1442–8. doi: 10.1176/appi.ajp.2008.08020304. [DOI] [PubMed] [Google Scholar]
- 16.Shearer J, Darke S, Rodgers C, Slade T, van Beek I, Lewis J, et al. A double-blind, placebo-controlled trial of modafinil (200 mg/day) for methamphetamine dependence. Addiction. 2009;104:224–33. doi: 10.1111/j.1360-0443.2008.02437.x. [DOI] [PubMed] [Google Scholar]
- 17.Colfax GN, Santos G, Moupali das, Santos DM, Matheson T, Gasper J, et al. Mirtazapine to reduce methamphetamine use: a randomized controlled trial. Arch Gen Psychiat. 2011;68:1168–75. doi: 10.1001/archgenpsychiatry.2011.124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Peterson EC, Gunnell MG, Che Y, Goforth RL, Carroll FI, Henry R, et al. Using hapten design to discover therapeutic monoclonal antibodies for treating methamphetamine abuse. J Pharmacol Exp Ther. 2007;322:30–9. doi: 10.1124/jpet.106.117150. [DOI] [PubMed] [Google Scholar]
- 19.Carroll FI, Abraham P, Gong PK, Pidaparthi RR, Blough BE, Che Y, et al. The synthesis of haptens and their use for the development of monoclonal antibodies for treating methamphetamine abuse. J Med Chem. 2009;52:7301–9. doi: 10.1021/jm901134w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Laurenzana EM, Hendrickson HP, Carpenter D, Peterson EC, Gentry WB, West M, et al. Functional and biological determinants affecting the duration of action and efficacy of anti(+)-methamphetamine monoclonal antibodies in rats. Vaccine. 2009;27:7011–20. doi: 10.1016/j.vaccine.2009.09.072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rowland M, Tozer TN. Clinical Pharmacokinetics. 2. Malvern, Pennsylvania: Lea and Febiger; 1989. [Google Scholar]
- 22.Cadée N, Grieco F, Mayton T, Piersma T, Spink A, Trienes R. Ethovision reference manual. 3. Wageningen: Noldus Information Technology; 2005. [Google Scholar]
- 23.Milesi-Hallé A, Hendrickson HP, Laurenzana EM, Gentry WB, Owens SM. Sex- and dose-dependency in the pharmacokinetics and pharmacodynamics of (+)-methamphetamine and its metabolite (+)-amphetamine in rats. Toxicol Appl Pharmacol. 2005;209:203–13. doi: 10.1016/j.taap.2005.04.007. [DOI] [PubMed] [Google Scholar]
- 24.Gentry WB, Ghafoor AU, Wessinger WD, Laurenzana EM, Hendrickson HP, Owens SM. (+)-Methamphetamine-induced spontaneous behavior in rats depends on route of (+)METH administration. Pharmacol Biochem Behav. 2004;79:751–60. doi: 10.1016/j.pbb.2004.10.006. [DOI] [PubMed] [Google Scholar]
- 25.Balcells-Olivero M, Vezina P. Effects of naltrexone on amphetamine-induced locomotion and rearing: acute and repeated injections. Psychopharmacology. 1997;131:230–8. doi: 10.1007/s002130050288. [DOI] [PubMed] [Google Scholar]
- 26.Kuczenski R, Segal DS. Sensitization of amphetamine-induced stereotyped behaviors during the acute response. J Pharmacol Exp Ther. 1999;288:699–709. [PubMed] [Google Scholar]
- 27.Martell BA, Orson FM, Poling J, Mitchell E, Rossen RD, Gardner T, et al. Cocaine vaccine for the treatment of cocaine dependence in methadone-maintained patients: a randomized, double-blind, placebo-controlled efficacy trial. Arch Gen Psychiat. 2009;66:1116–23. doi: 10.1001/archgenpsychiatry.2009.128. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Meininger V, Pradat P, Corse A, Al-Sarraj S, Brooks BR, Caress JB, et al. Safety, Pharmacokinetic, and Functional Effects of the Nogo-A Monoclonal Antibody in Amyotrophic Lateral Sclerosis: A Randomized, First-In-Human Clinical Trial. Plos One. 2014;9:e97803. doi: 10.1371/journal.pone.0097803. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Nicosia N, Pacula RL, Kilmer B, Lundberg R, Chiesa J. The Economic Cost of Methamphetamine Use in the United States, 2005. RAND Corporation; 2009. [Google Scholar]