Abstract
The innate immune system and inflammatory pathways play key roles in numerous diseases of the central nervous system (CNS). Recent evidence indicates that innate immunity induces both pathogenesis and protection during neuronal injury. To test the possibility that the conflicting roles of innate immunity in the CNS depends on the cellular environment in which innate immunity is stimulated, we analyzed the effect of toll-like receptor 3 (TLR3) activation on neuronal survival in the presence and absence of oxidative injury in a mouse model system. We demonstrated that activation of TLR3 by the double stranded RNA activator, Poly (I:C), during paraquat induced oxidative stress, significantly protected mouse photoreceptors, as measured by increased retinal structure, function, and improved visual acuity. In contrast, TLR3 activation without concurrent oxidative injury was neurotoxic. The neurotoxic and protective effects of Poly (I:C) stimulation were absent in TLR3 knockout animals, which indicates that protection by Poly (I:C) is dependent on the TLR3 signaling pathway. Furthermore, we identified the pro-survival transcription factor Stat3 as a necessary mechanism for protection. Knockdown of Stat3 using lentivirally delivered shRNA abolished the protective effects of TLR3 signaling in the retina during oxidative stress. Therefore, TLR3 activation in the context of oxidative stress triggers protective instead of pathogenic signaling, suggesting that TLR3 is a potential therapeutic target for neurodegeneration where oxidative stress is a significant contributor.
Keywords: innate immunity, Toll-Like Receptor 3 (TLR3), oxidative stress, neuroprotection, photoreceptors, Stat3, retinal degeneration, age-related macular degeneration (AMD)
Introduction
A major challenge for designing new therapeutic targets for neuronal diseases is identifying the critical cellular receptors and pathways that regulate neuronal responses to injury. Recently, abnormal innate immune activity and oxidative damage have become recognized as key contributors to neuronal death and diseases (Moreira et al., 2005; Drouin-Ouellet and Cicchetti, 2012). Toll-like receptors (TLRs) are a branch of innate immune signaling that protects against invading pathogens by inducing pro-inflammatory pathways and inflammatory cell migration (Owens, 2009). There are ten members of the TLR family of innate immune receptors in humans. With the exception of TLR3, all TLRs signal through the MyD88 mediated pathway, resulting in NF-kB activation and cytokine secretion (Anderson, 2000). TLR3 signals through the TRIF adaptor mediated pathway and results in interferon production, in addition to cytokine release (Alexopoulou et al., 2001).
Recent evidence implicates multiple TLRs in the initiation and progression of non-pathogen (sterile) tissue injury, including experimental stroke and ischemia, by acting in non-immune regulatory cell types such as neurons and glia, in addition to immune cells (Shichita et al., 2012; Lu et al., 2014). Levels of various TLRs increase in neurons and glia following damage or injury to the brain and spinal cord (Walter et al., 2007; Letiembre et al., 2009), and genetic knockdown of TLR2 and TLR4 resulted in increased neuronal survival in the brain and retina in mouse models of CNS disease (Walter et al., 2007; Kilic et al., 2008; Yi et al., 2012). Paradoxically, activation of several TLRs has been shown to be necessary for cell survival in some situations, and TLR2 and TLR4 signaling following peripheral nerve injury promoted axonal regeneration (Boivin et al., 2007; Kigerl et al., 2007). Therefore, the complex roles of TLRs during neurodegeneration lead to the important question of what are the conditions that promote TLR signaling to be either pathogenic or protective.
While there are many studies that have examined the MyD88-mediated TLR pathways during neuronal degeneration, the role of TLR3 signaling, a viral sentinel, during neuronal disease remains to be fully explored. There is increasing evidence that TLR3 has essential activities in the absence of viral stimulation. TLR3 activation in the retina led to loss of retinal pigmented epithelium (RPE) and photoreceptors (Shiose et al., 2011; Kleinman et al., 2012), while activation in astrocytes during brain injury led to the secretion of neuroprotective molecules (Bsibsi et al., 2006). Identifying mechanisms through which TLR3 regulates cell survival will reveal new points of cross-talk between innate immunity and survival pathways. We previously demonstrated that TLR3 signaling was protective to cultured RPE during oxidative injury (Patel and Hackam, 2012), but how this translates to neuronal cells in an in vivo model is unclear. In this study, we examined how TLR3 activation regulates oxidative stress induced neurodegeneration. We demonstrated that TLR3 has a novel neuroprotective role in the retina during injury, and that it acts through the Stat3 pathway. Therefore, these results suggest that TLR3 is a novel therapeutic target for neuronal degenerative diseases in which oxidative stress is a major contributor.
Materials and Methods
Animal Studies
All procedures involving mice were performed according to the ARVO Statement for the Use of Animals in Ophthalmic and Vision Research and were approved by the Animal Care and Use Committee at the University of Miami. The wild type mice are strain B6;129SF2/J and TLR3 knockout mice are strain B6;129S1-Tlr3tm1Flv/J, which have a targeted mutation resulting in a non-functional TLR3 (Jackson Labs, Bar Harbor, ME).
Subretinal injection
Adult mice (age 8 weeks, both sexes) were anaesthetized using a ketamine/xylazine cocktail delivered through intraperitoneal injection. Eyes were locally anesthetized using 1 drop of Proparacaine Hydrochloride Ophthalmic Solution (0.5%, Akorn, Lake Forest, IL). A small incision was made in the conjunctiva and sclera of the eye exposing the subretinal space and a 1.5 cm 33-gauge Hamilton needle (Hamilton Company, Reno, NV) was inserted between the RPE and retina. The mice were subretinally injected in one eye with two microliters of PBS, polyinosinic:polycytidylic acid (Poly (I:C), 0.5μg/μl, InvivoGen, San Diego, CA), and/or paraquat (1mM, Sigma-Aldrich, St. Louis, MO). Stat3 was knocked down in the retina using subretinal injections of lentiviral Stat3 shRNA (2×108 IFU) (Haghikia et al., 2011). Successful subretinal injections were indicated by bleb formation and detachment, both of which resolved rapidly, as verified in each animal by OCT imaging. Mice with unresolved retinal detachments or bleeding were excluded from further analysis. Investigators were masked to the identity of the injected compound for all analyses.
Immunohistochemistry and molecular analysis
Immunohistochemistry was performed as in Yi et al. using rabbit anti-TLR3 (1:100 dilution, Abcam, Cambridge, MA), mouse anti-rhodopsin (1:300 dilution, Millipore, Billerica, MA), goat anti-rabbit Alexa 488 (1:600, Santa Cruz, Dallas, TX), and goat anti-mouse Alexa 546 (1:600, Santa Cruz, Dallas, TX) antibodies (Yi et al., 2012). QPCR analysis was performed using primers for IRF3, IL-6, Stat3, and TLR3 (Table 1). Western blotting was performed using the following antibodies: phosphorylated Stat3 (1:200 dilution, Cell Signaling, Danvers, MA), total Stat3 (1:200 dilution, Cell Signaling), and β-actin (1:8000 dilution, Sigma, St. Louis, MO) as in Yi et al (Yi et al., 2012).
Table 1.
List of primer sequences
| Gene | Sequence | |
|---|---|---|
| ARP | Forward | 5′-ATCTGCTGCATCTGCTTG-3′ |
| Reverse | 5′-CGACCTGGAAGTCCAACTAC-3′ | |
|
| ||
| TLR3 | Mutant | 5′-GCCAGAGGCCACTTGTGTAG-3′ |
| Wild-type | 5′-GCAACCCTTTCAAAAACCAG-3′ | |
| Common | 5′-AATTCATCAGTGCCATGAGTTT-3′ | |
|
| ||
| Stat3 | Forward | 5′-AATGGAAATTGCCCGGATCG-3′ |
| Reverse | 5′-TCCTGAAGATGCTGCTCCAA-3′ | |
|
| ||
| IRF3 | Forward | 5′-ACGTGTCAACCTGGAAGAGG-3′ |
| Reverse | 5′-AGGCACCCAGATGTACGAAG-3′ | |
|
| ||
| IL6 | Forward | 5′-CCAATTTCCAATGCTCTCCT-3′ |
| Reverse | 5′-ACCACAGTGAGGAATGTCCA-3′ | |
OCT analysis
In vivo imaging of the mouse retina was conducted using an SD-OCT system (Bioptigen, Research Triangle Park, NC) optimized for small animals. Mice were anesthetized and placed on a stage with the body of the animal wrapped in a heating blanket. Eyes were dilated with topical phenylephrine (Akorn, Lake Forest, IL) and kept moist with the application of artificial tears (Systane, Alcon, TX). Scans were centered on optic disk and consisted of 100 × 100 (horizontal × vertical) depth scans covering a volume of 1.3 × 1.3 × 1.56 mm3 of the mouse retina. Average photoreceptor layer thicknesses across retinas were obtained through segmentation of the OCT images using MATLAB software and programs developed by the Ophthalmic Biophysics Center at the University of Miami (Ruggeri et al., 2007).
ERG analysis
Mice were dark adapted and then anesthetized, and were wrapped in a heating blanket with an attached water bath to maintain a constant body temperature. The eyes were dilated and kept moist, as described above. The ground electrode was placed in the tail of the mouse and the reference electrode was placed under the skin between the eyes. Silver wire electrodes were placed on the corneas of mice and the mice were placed into a Ganzfeld light emitting chamber (Li et al., 2010). Mice were exposed to flashes of white light ranging from 0.01 to 10 cd·s/m2. For photopic recordings, green flashes were used in the presence of a green low background light intensity. Both eyes were recorded simultaneously and an average of ten 250 μs flashes per intensity was recorded with an interstimulus time of 5 sec, which was conducted using the UTAS system controlled by EM for Windows software (LKC Technologies, Gaithersburg, MD).
Optokinetic exam
Mice were placed on a raised platform in the center of a chamber surrounded by four monitors with an optokinetic display of rotating sinusoidal gratings of alternating white and black colored stripes, following the method of Prusky et al. (Prusky et al., 2004). The rotation direction was changed every 30 seconds for a total of 6 changes per stripe thickness. Stripe thickness of the gratings was decreased stepwise by a factor of 2 until the animal could no longer track the direction of movement of the gratings. Vision was scored based on whether the mice tracked the direction of stripe movement with their head and upper body. Two observers monitored the movement of the animals, and the observers were masked to the identity of the treatment. Visual acuity was defined as the highest spatial frequency yielding a response from the mouse, which was derived from the angular frequency of the stripe rotation.
Statistics
Statistical analysis was performed using one-way analysis of variance (ANOVA) with appropriate post-hoc analysis or Student’s t-test using GraphPad Prism software. P values <0.05 were considered significant.
Results
TLR3 activation in the retina protects photoreceptors from oxidative stress-induced death
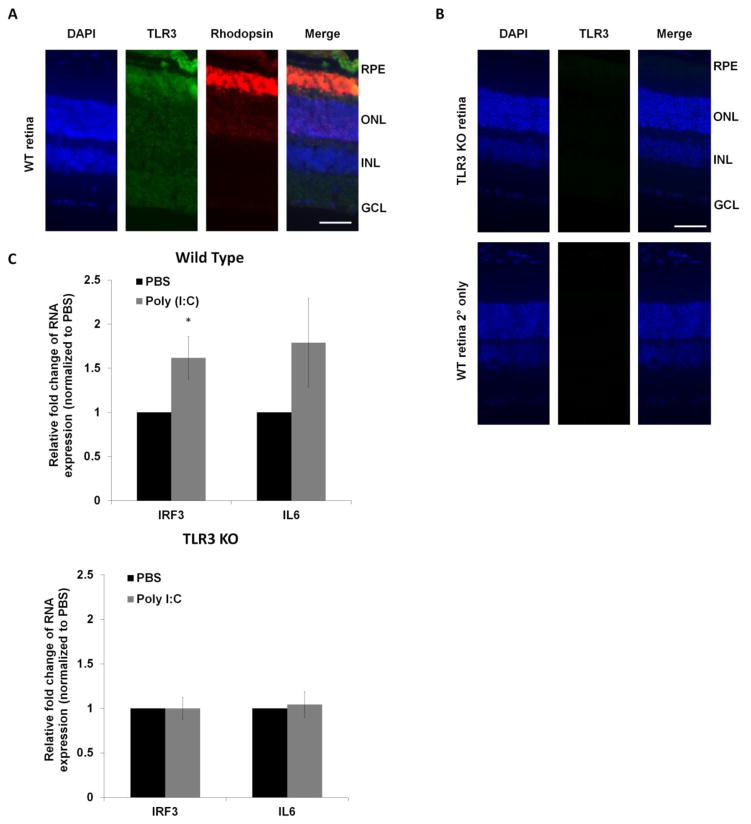
To verify that TLR3 was expressed in photoreceptors in the retina, we identified the cellular localization of TLR3 using immunohistochemical analysis in wild-type mice. As shown in Figure 1A, immunodetection of TLR3 was observed throughout the retinal cell layers, with significant localization in photoreceptors (shown by codetection with rhodopsin). Antibody specificity was indicated by lack of immunostaining in the control sections that omitted the primary antibody or that were obtained from TLR3-deficient (TLR3 KO) mice (Figure 1B).
Figure 1. TLR3 is expressed in the retina.
(A) TLR3 (green) showed prominent localization in photoreceptors and retinal pigmented epithelium (RPE) in wild-type mice retinal sections by immunohistochemistry. TLR3 colocalized with the photoreceptor marker rhodopsin (red) indicating that TLR3 is expressed in photoreceptors. (B) The specificity of the antibody was verified using TLR3 knockout mouse retinal sections (top) and wild-type sections incubated with only 2° antibody (bottom). 4′,6-diamidino-2-phenylindole (DAPI) staining (blue) was used as a marker for cell nuclei. (Scale Bar: 50 μm, RPE: retinal pigmented epithelium, ONL: outer nuclear layer, INL: inner nuclear layer, GCL: ganglion cell layer) (C) Confirmation of TLR3 activation in wild-type retinas following subretinal injection of Poly (I:C) was verified using QPCR analysis of TLR3 induced molecules IRF3 (*p<0.05, n=3) and IL6 (n=3). Poly (I:C) did not alter either IRF3 or IL6 expression in TLR3 KO mice (n=3). Expression of the housekeeping gene ARP was used as a normalization control.
To determine the level of TLR3 signaling induction in the retina, we subretinally injected Poly (I:C), a prototypic double-stranded RNA and measured downstream signaling molecules of TLR3, the IRF3 and IL6 genes (Shiose et al., 2011). Subretinal injections deliver molecules to RPE and photoreceptors that are adjacent to the subretinal space. Poly (I:C) increased IRF3 and IL6 expression compared with control injections, as measured by QPCR (Figure 1C). In contrast, Poly (I:C) did not induce IL6 or IRF3 expression in TLR3 KO mice (Figure 1C), demonstrating specificity of Poly (I:C) to the TLR3 pathway. Therefore, TLR3 is expressed and active in the retina.
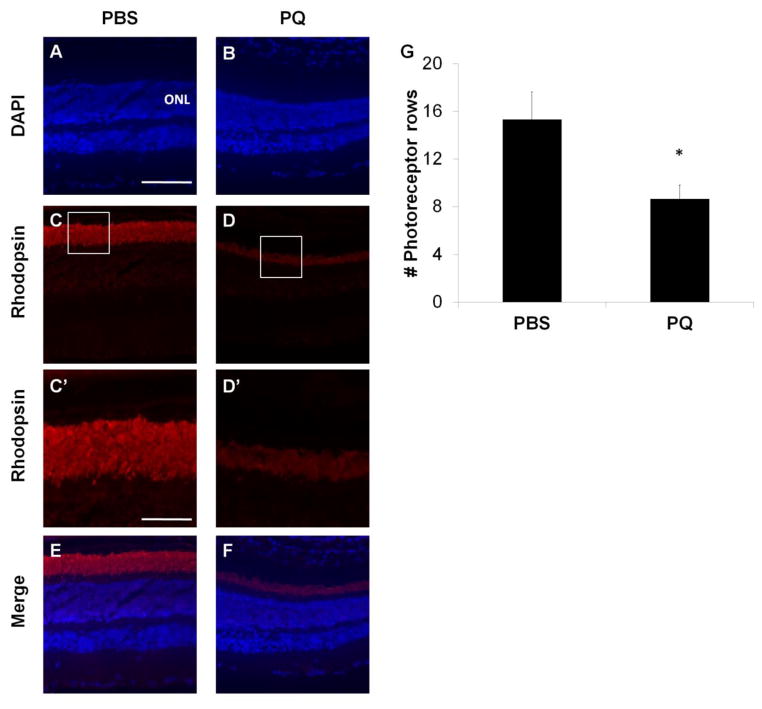
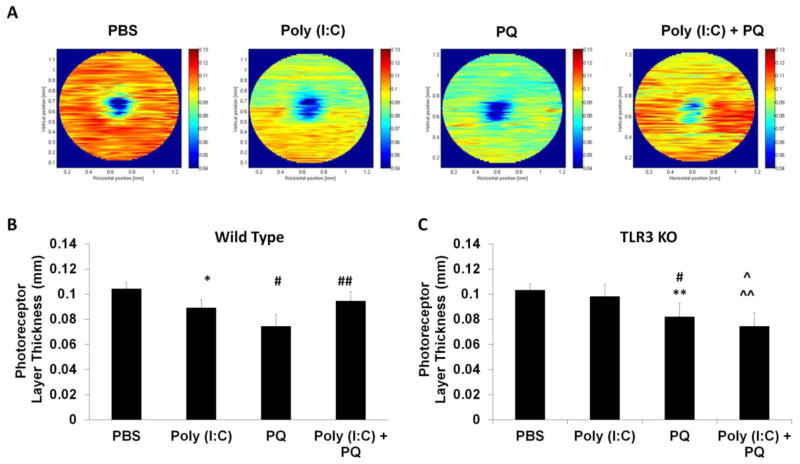
Elevated oxidative stress is a major risk factor for numerous neurodegenerative diseases (Moreira et al., 2005). A commonly used molecule to mimic the effects of oxidative stress in the CNS is paraquat, 1,1′-Dimethyl-4,4′-bipyridinium dichloride, which generates oxygen free radicals through redox cycling and NADPH oxidase regulation (Corasaniti et al., 1998; McCormack et al., 2005; Cingolani et al., 2006; Chen et al., 2013). In this study, we used paraquat (PQ) to induce acute oxidative stress in the retina. Subretinal injection of 1 mM PQ resulted in 45% loss of photoreceptor cell nuclei in the retina compared with eyes injected with PBS (Figure 2), similar to previously reported studies (Cingolani et al., 2006). Co-application of PQ and Poly (I:C) allowed us to identify the role of TLR3 during neuronal oxidative stress injury in the retina. The mice were injected subretinally with either PBS, Poly (I:C), PQ, or a combination of Poly (I:C) and PQ and, two weeks following injection, we quantified changes in photoreceptor structure, survival, retinal function, and visual behavior. As shown in Figure 3, TLR3 activation during oxidative stress injury significantly increased photoreceptor survival, as measured by optical coherence tomography (OCT). Eyes injected with a combination of Poly (I:C) + PQ had 21% thicker outer nuclear/inner and outer segment layers than eyes injected with PQ alone (Figure 3A, B). Oxidative stress alone significantly decreased photoreceptor layer thickness by 28% compared with control eyes (PBS injected) (Figure 3A, B). TLR3 activation in the absence of oxidative stress induced photoreceptor death, resulting in a 14% reduction of photoreceptor layer thickness compared with control eyes (Figure 3A, B), which is consistent with previous studies that showed toxicity of TLR3 in the absence of concurrent injury (Shiose et al., 2011; Kleinman et al., 2012). These results demonstrate the requirement of an oxidative stress stimulus for TLR3 to promote neuronal survival while TLR3 activation alone is harmful to the retina.
Figure 2. Paraquat induces toxicity in photoreceptors.
Retinal sections obtained from mouse eyes subretinally injected with PQ (B) had significant photoreceptor loss compared with eyes injected with PBS (A). (C–D) Retinal sections from mice injected with PQ show significant degeneration of rod photoreceptors, measured by loss of rhodopsin from the outer segments of the photoreceptors (boxed) compared with mice injected with PQ. (C′–D′) Magnified outer segment layer stained with rhodopsin antibodies. (G) Photoreceptor survival was quantified following PQ injection by counting the number of DAPI positive cells in the columns of the outer nuclear layer (ONL). Scale bar = 100 μm (A–F) and 40 μm (C′–D′), n=3, p<0.05.
Figure 3. TLR3 rescued photoreceptors from oxidative stress.
(A) In vivo imaging of retinal cell layers using optical coherence tomography (OCT) showed that co-injection of Poly (I:C) + PQ resulted in significantly larger photoreceptor layer thickness compared with retinas of mice injected with PQ alone. Representative heat maps generated by segmentation analysis software are shown. Colors that approach red indicate thicker areas of the retina while blue indicates thinning of the retina. (B) Quantification of average thickness across the retinas of injected wild-type mice shows that Poly (I:C) co-injected with PQ significantly protected against PQ induced loss of photoreceptors (left, n=7). Poly (I:C) co-injected with PQ did not protect photoreceptors in TLR3 KO retinas, indicating that protection by Poly (I:C) during oxidative stress is TLR3 dependent (right, n=5). Photoreceptor cell layer thickness was quantified by measuring the outer nuclear layer (ONL) and inner/outer segment layer (IS/OS) thickness using MATLAB software. OCT quantification was conducted by outlining 70–80 cross-sectional images approximately 0.5–0.6 mm from the optic disc. A statistical significance of p<0.05 is marked as * PBS vs Poly (I:C), # PBS vs PQ, ^ PBS vs Poly (I:C)+PQ, ** Poly (I:C) vs PQ, and ^^ Poly (I:C) vs Poly (I:C)+PQ and ## PQ vs Poly (I:C)+PQ.
Because there are a number of dsRNA-detecting receptors in mammalian cells in addition to TLR3, we wanted to specifically confirm the role of TLR3 in Poly (I:C)-induced protection. We used TLR3 knockout (KO) mice to verify that the protective effects of Poly (I:C) on photoreceptors are due to TLR3 signaling and not to other dsRNA receptors. In contrast to the significant Poly (I:C)-induced toxicity and protection observed in wild type mice, injection of Poly (I:C) into TLR3 KO mice did not significantly change photoreceptor layer thickness compared with mice injected with PBS, and did not protect photoreceptors from oxidative stress induced death. Injection of PQ resulted in an equivalent amount of photoreceptor loss as the wild type mice, indicating that loss of TLR3 signaling does not alter oxidative stress induced death (Figure 3B). Therefore, the PQ and Poly (I:C) experiment in the wild type and TLR3 KO mice lead to the conclusion that Poly (I:C)-induced protection during oxidative stress in the retina is primarily driven via TLR3.
TLR3 rescues photoreceptor function and vision behavior during oxidative stress
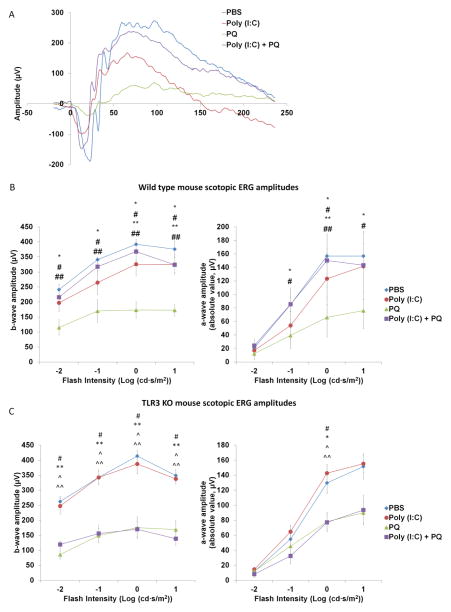
It has been established that neurons lose functions such as electrical signaling well before cell death occurs (Porciatti and Ventura, 2012). Therefore, we next investigated whether TLR3 signaling also protects against loss of photoreceptor function. Electroretinography (ERG) was used to quantify the electrical signaling activity of photoreceptors. Wild-type mice injected with PBS, Poly (I:C), and/or PQ were dark adapted and then stimulated with light-flashes at increasing intensities from 0.01 to 10 cd·s/m2. Mice injected with PQ had a significant loss of photoreceptor function with a maximum reduction of 56% and 58% in a-wave and b-wave amplitude, respectively (Figure 4B), compared with mice injected with PBS. Poly (I:C) significantly protected against oxidative stress-induced loss of scotopic rod photoreceptor function at all intensities of light, with a maximum increase of 230% and 210% in a-wave and b-wave amplitudes, respectively, compared with animals injected with PQ alone (Figure 4B). Furthermore, TLR3 activation on its own lead to a modest loss of photoreceptors compared with PBS injected animals, with a maximum decrease of 36% and 22% in a-wave and b-wave amplitudes respectively (Figure 4B). There was no significant change in photopic cone photoreceptor responses, likely due to the low number of cone photoreceptors in the mouse retina relative to the injection site. These results indicate that TLR3 signaling protects against functional loss of photoreceptor neurons during oxidative injury.
Figure 4. TLR3 activation protected against the loss of scotopic photoreceptor function during oxidative stress in wild type mice.
(A) Representative scotopic ERG waves of wild type mice injected with PBS, Poly (I:C), PQ, and Poly (I:C)+PQ stimulated at 1 cd·s/m2. (B) Average ERG amplitudes of wild type mice at increasing flash intensities. Eyes subretinally injected with Poly (I:C) + PQ had significantly higher a-wave (right) and b-wave (left) scotopic amplitudes compared with eyes injected with PQ alone across different flash intensities (n=7 mice injected with PBS, Poly (I:C), and Poly (I:C) + PQ; n=6, mice injected with PQ). (C) Average ERG amplitudes of TLR3 KO mice at increasing flash intensities are shown. TLR3 KO mice injected with Poly (I:C) + PQ did not have significantly different a-wave or b-wave amplitudes compared with eyes injected with PQ alone (n=5). ERG recordings were conducted two weeks post-injection and flash intensities ranged from 0.01 to 10 cd·s/m2. A statistical significance of p<0.05 is marked as * PBS vs Poly (I:C), # PBS vs PQ, ^ PBS vs Poly (I:C)+PQ, ** Poly (I:C) vs PQ, and ^^ Poly (I:C) vs Poly (I:C)+PQ and ## PQ vs Poly (I:C)+PQ.
To further confirm that Poly (I:C)-mediated functional protection is a TLR3 dependent mechanism, we repeated the ERG measurements on TLR3 KO mice following injections. As expected, we found that Poly (I:C) did not reduce ERG amplitudes on its own, nor did it protect against photoreceptor functional loss during oxidative stress (Figure 4C), consistent with the retina thickness analyses shown in Figure 3. These results indicate that TLR3 signaling is responsible for rescue of both function and morphological survival.
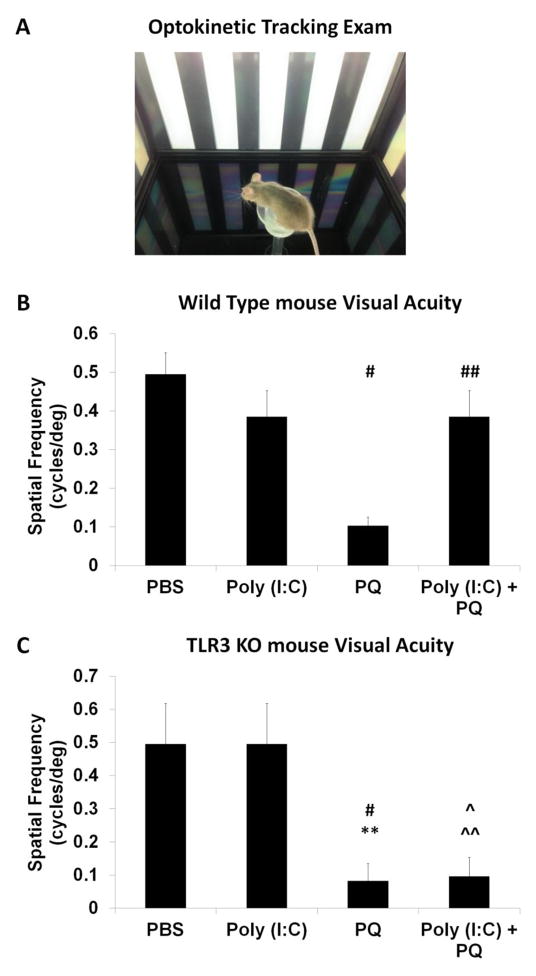
The injected mice were next analyzed for improvements in vision at the behavioral level in order to determine whether the structural and functional protective effects of TLR3 signaling during oxidative stress translated to overall better vision. The visual acuity of the mice was assessed using the optokinetic tracking reflex, in which the ability of the mouse to track a moving stimulus, in this case a series of rotating sinusoidal stripes of decreasing thickness (see Methods), is recorded (Tabata et al., 2010; Pearson et al., 2012). To determine the threshold of visual acuity, the stripe thickness was decreased step-wise until the animal could no longer track the column rotation. As shown in Figure 5, mice injected with PBS maintained a normal visual acuity of greater than 0.49 cycles/deg (Figure 5B), similar to reported previously (Pearson et al., 2012). Mice injected with PQ had reduced visual acuities, averaging at 0.10 cycles/deg (Figure 5B), whereas mice injected with a combination of Poly (I:C) + PQ had significantly higher visual acuities compared with PQ-injected mice, averaging at 0.38 cycles/deg vs 0.1 cycles/deg (Figure 5B). TLR3 KO mice injected with PBS and Poly (I:C) did not have statistically different visual acuities, nor were eyes injected with Poly (I:C) + PQ statistically different from eyes injected with PQ alone (Figure 5C). Therefore, TLR3 activation prevents visual degradation during oxidative insult, in addition to protecting against photoreceptor loss.
Figure 5. TLR3 protected against oxidative stress-induced loss of visual acuity.
(A) Picture of the optokinetic tracking apparatus. Mice were placed on a stage in the center of the optokinetic chamber and surrounded by computer monitors, which displayed a rotating sinusoidal grating of black and white stripes. (B) Wild type mice subretinally injected with Poly (I:C) and PQ had significantly increased visual acuities compared with mice injected with PQ alone (n=5). (C) TLR3 KO mice injected with Poly (I:C)+PQ did not show rescued visual acuity compared with mice with oxidative injury alone, indicating TLR3 signaling is a necessary pathway for Poly (I:C) mediated protection (n=5). Visual acuity is quantified in terms of spatial frequency, a measurement of how well an animal can detect the different grating thicknesses. A statistical significance of p<0.05 is marked as * PBS vs Poly (I:C), # PBS vs PQ, ^ PBS vs Poly (I:C)+PQ, ** Poly (I:C) vs PQ, and ^^ Poly (I:C) vs Poly (I:C)+PQ and ## PQ vs Poly (I:C)+PQ.
Stat3 signaling is required for TLR3-dependent photoreceptor protection
The Stat3 pathway is a key signaling pathway in neuronal survival and regeneration that protects photoreceptors from various neuronal insults, including oxidative stress (Aaronson and Horvath, 2002). While the Stat3 signaling pathway is activated by multiple cytokines and growth factors (Taga and Kishimoto, 1997; Zhang et al., 2008; Patel and Hackam, 2012), the relationship between TLR3 and Stat3 has not been examined in the context of neuronal protection. We have previously demonstrated that TLR3-induced protection of cultured RPE during oxidative stress requires Stat3 signaling (Patel and Hackam, 2012). Therefore, we tested the hypothesis that TLR3 mediates photoreceptor protection during oxidative stress by acting through the Stat3 pathway.
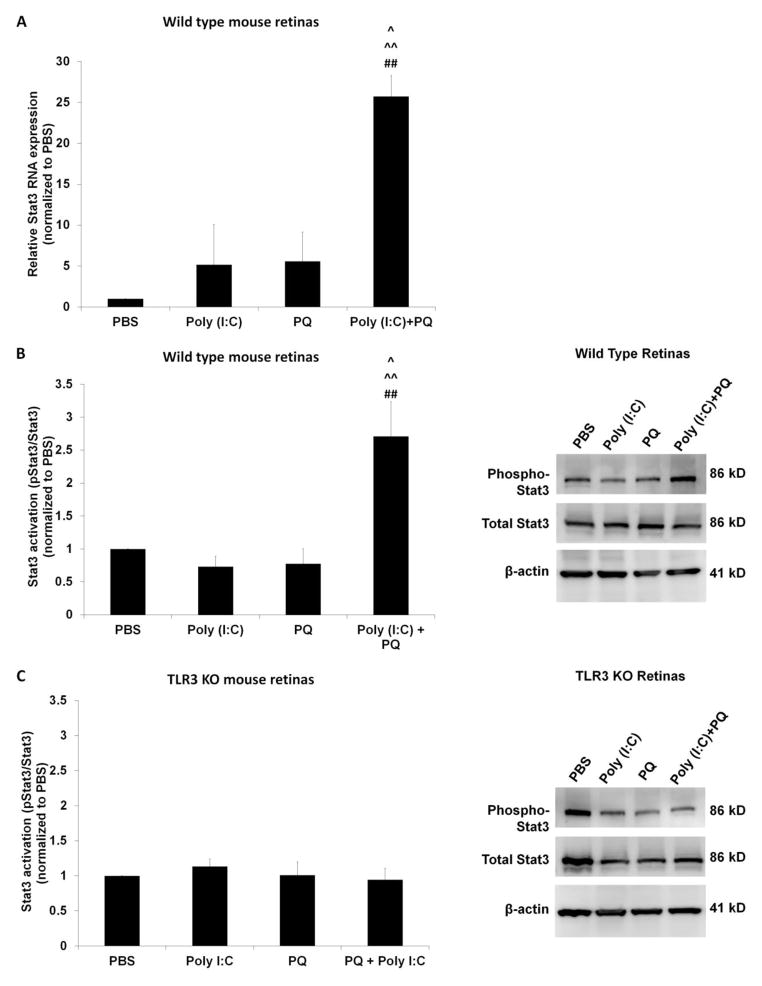
QPCR analysis of cDNA from retinas injected with Poly (I:C) + PQ showed a 23-fold increase in Stat3 mRNA compared with retinas injected with PBS (Figure 6A), whereas Stat3 amounts after injections of Poly (I:C) or PQ alone were not significantly different from retinas injected with PBS injected. Similarly, protein analysis of retinas injected with Poly (I:C) + PQ showed significantly increased Stat3 activation, by 175% compared with PBS, whereas Poly (I:C) alone or PQ alone did not significantly change Stat3 activation levels (Figure 6B). In TLR3 KO mice retinas, mice injected with Poly (I:C), PQ, or a combination of Poly (I:C) and PQ did not have significantly different levels of Stat3 activation compared with mice injected with PBS (Figure 6C). These results demonstrate that increased Stat3 activity is associated with TLR3-dependent neuroprotection.
Figure 6. TLR3 increased Stat3 signaling during oxidative stress.
(A) QPCR of cDNA from mice injected with Poly (I:C) revealed a 23-fold increase in Stat3 mRNA expression compared with mice injected with PBS (n=3). (B) Retinal lysates from wild type mice injected with either PBS, Poly (I:C), or PQ were examined using Western blotting. Phosphorylated Stat3 to total Stat3 ratio was increased in mice injected with Poly (I:C)+PQ injected compared with injections of PBS, Poly (I:C), or PQ (n=4, normalized to PBS injections). (C) There was no increase in Stat3 activation when Poly (I:C)+PQ was injected in TLR3 KO mice compared with injection of PBS (n=4), indicating that the increase of Stat3 activation during concurrent Poly (I:C) and oxidative stress stimulation is TLR3 dependent. Stat3 activation was determined by measuring the ratio of phosphorylated Stat3 protein to total Stat3 protein and both phosphorylated and total Stat3 were normalized with β-actin levels. A statistical significance of p<0.05 is marked as * PBS vs Poly (I:C), # PBS vs PQ, ^ PBS vs Poly (I:C)+PQ, ** Poly (I:C) vs PQ, and ^^ Poly (I:C) vs Poly (I:C)+PQ and ## PQ vs Poly (I:C)+PQ.
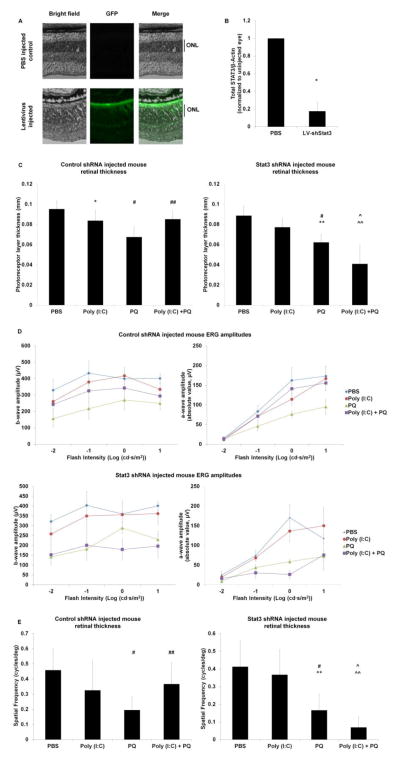
To test whether the Stat3 pathway is a necessary component of TLR3-mediated protection, we knocked down Stat3 in the mouse retina using subretinally injected Stat3-specific shRNA delivered by lentiviral vector. Mice injected with control scrambled shRNA in lentivirus were used as to control for the effects of viral and shRNA delivery. Mice injected with the control shRNA and co-injected with Poly (I:C) + PQ showed a significant increase in photoreceptor layer thickness, visual acuity, and photoreceptor function compared with eyes injected with control shRNA and PQ (Figure 7 C–E), equivalent to our results without lentivirus (Figures 3–6). This finding indicates that potential TLR3 activation from the lentivirus itself does not confound our results. In contrast, knockdown of Stat3 abolished TLR3-induced photoreceptor protection during oxidative stress. Photoreceptor layer thickness, function, and overall visual acuity decreased by up to 64% following TLR3 activation during oxidative stress in the presence of Stat3 shRNA. Efficiency of Stat3 knockdown by Stat3 shRNA was verified by Western blotting and showed that total Stat3 protein was reduced by 90% in eyes injected with LV-Stat3 shRNA compared with control eyes (Figure 7B). Interestingly, the control shRNA increased total Stat3 in the retina by 4 fold compared with retinas injected with PBS. Together, these results demonstrate that Stat3 is a necessary mediator of TLR3 induced protection of photoreceptors during oxidative stress.
Figure 7. Stat3 signaling is required for TLR3 induced protection during oxidative stress.
(A) Wild type mouse eyes were subretinally injected with lentiviral Stat3 shRNA co-expressing GFP. Widespread distribution of GFP expression was detected throughout the retina, with significant localization with the photoreceptors and RPE. A representative image of a retinal section with lentiviral GFP expression is displayed. No GFP expression is seen in the retinas injected with PBS as a vehicle control. Scale bar: 100 μm. (B) Wild type mice subretinally injected with lentiviral Stat3 shRNA had significantly reduced amount of total Stat3 (87% reduction) compared with eyes injected with PBS, measured by Western blotting. β-Actin levels were measured as a loading control (n=4). (C, E) In mice injected with lentiviral scrambled control shRNA, Poly (I:C) + PQ significantly increased photoreceptor layer thickness (C) and visual acuity (E) compared with mice injected with PQ alone (n=6), similar to mice injected in the absence of shRNA (Figure 3–4). (D) Mice injected with Poly (I:C)+PQ and the control shRNA had increased a-wave and b-wave amplitudes compared with injections of PQ alone (n=6, PQ and Poly (I:C) + PQ injections; n=5, PBS and Poly (I:C) injections; p=.071). (C–E) Mice injected with lentiviral Stat3 siRNA (shStat3) and co-injected with Poly (I:C) + PQ did not result in significantly different photoreceptor thickness, visual acuity, or a-wave and b-wave amplitudes compared with shStat3 and PQ injected animals (n=6). A statistical significance of p<0.05 is marked as * PBS vs Poly (I:C), # PBS vs PQ, ^ PBS vs Poly (I:C)+PQ, ** Poly (I:C) vs PQ, and ^^ Poly (I:C) vs Poly (I:C)+PQ and ## PQ vs Poly (I:C)+PQ.
Discussion
Interactions between the various contributors of neuronal degeneration diseases remain largely unknown. In this study, we demonstrated that activation of the TLR3 innate immune receptor leads to neuronal degeneration in the retina, but that activation of TLR3 during oxidative stress is protective to photoreceptor survival and visual function. We identified STAT3 as a necessary component in TLR3 induced protection during injury. To our knowledge, this is the first study to link Stat3 protection to an immune mediated protective response in neurons, and is supported by a vast literature on the protective role of Stat3 signaling in neurodegeneration and retinal health (Zhang et al., 2008; Fragoso et al., 2012; Patel and Hackam, 2012). We observed that neither TLR3 activation nor oxidative stress induced significant increases in Stat3 activation; however, the combination of these two pathways triggers increased Stat3 signaling. Although we have previously shown in culture that TLR3 stimulation increases Stat3 activity, it does not seem to be the case in vivo. One reason for the lack of increase of Stat3 following TLR3 stimulation may be due to the two week timepoint at which the retinas were examined. Poly (I:C) may have increased Stat3 initially, but the increase may have subsided over the time period. It is plausible that only the combination of TLR3 and oxidative stress induced pathways synergistically triggered a specific set of target genes that promote increased long term Stat3 phosphorylation. Future studies will examine the target genes that are activated by TLR3 and oxidative stress and how those targets interact to induce Stat3 signaling and neuroprotection.
The localization of TLR3 within the retina indicates that the cellular mediators of protective TLR3 signaling could be RPE, acting in a paracrine manner, or photoreceptors themselves, via autocrine signaling. Unfortunately, identifying the cellular mediator through the detection of activated Stat3 in the retina, as a marker of TLR3 signaling, is complicated by Stat3 expression throughout the retina during injury (Peterson et al., 2000; Fasler-Kan et al., 2005; Zhang et al., 2008). In addition to TLR3 protecting photoreceptors directly, TLR3 mediated signaling between RPE and photoreceptors may also increase the level of neural protection. We have previously shown that TLR3 protects RPE from oxidative stress induced death (Patel and Hackam, 2012) suggesting that TLR3 activation may maintain RPE homeostasis in vivo during oxidative injury. Ongoing studies are focusing on identifying the roles of TLR3 activation in photoreceptors and RPE.
Elevated oxidative stress and innate immune activity have both been implicated as major risk factors for development of AMD and other retinal degenerations (Cai et al., 2000; Liang and Godley, 2003; Hollyfield et al., 2008; Kinnunen et al., 2012). There is increasing evidence for TLR3 involvement in AMD. Endogenous activators of TLR3, including dsRNA, are released during cell death in AMD (Hanus et al., 2013; Murakami et al., 2013). It has been previously shown that TLR3 activation on its own is detrimental to retinal health, similar to the findings in this study. Subretinal injection of dsRNA induced disruption of the outer retina and RPE, indicating degeneration of photoreceptors (Kleinman et al., 2012). Furthermore, it has been shown that ablation of TLR3 protects against retinal cell loss, including photoreceptors (Shiose et al., 2011). However, in contrast to the previous findings, we found that TLR3 was protective during injury conditions. The difference between our study and others is that we tested concurrent TLR3 activation and injury, which is more significant to a disease condition where both oxidative stress and inflammatory components are present. It is plausible that Poly (I:C) injection alone is toxic to the retina due to its stimulation of pro-apoptotic molecules via NFkB and pro-inflammatory cytokines but that the combination of Poly (I:C) and oxidative injury induced large increases in Stat3 levels that suppressed these apoptotic pathways. In the retina, mRNA or dsRNA released from dying necrotic cells may be a potential source of endogenous activators for TLR3 (Kariko et al., 2004; Cavassani et al., 2008; Bernard et al., 2012). Additionally, dsRNA has been identified as a component in drusen in AMD affected eyes (Murakami et al., 2013). However, it is likely that other agonists for TLR3 are present in injured tissue. Shiose et al. found that TLR3 signaling increased when the endogenous products of dying cells were applied to healthy cells (Shiose et al., 2011). Therefore, identifying endogenous activators of TLR3 will be important for determining the conditions that direct the TLR3 pathway to being pro-survival.
There is precedence for innate immunity having a protective role during disease. A recent study demonstrated that inflammatory cytokines protect against oxidative stress induced degeneration of the RPE (Chen et al., 2013; Juel et al., 2013). TLR3 signaling within astrocytes leads to secretion of neuroprotective mediators and promotes tissue repair responses (Bsibsi et al., 2006). The dual pathogenic/protective role of TLR3 that we found in this study is supported by a study conducted by Jin et al, who showed that TLR3 activation has a dual role during Theiler’s virus-induced demyelinating disease (Jin et al., 2011). Similar to our findings where TLR3 signaling is pathogenic on its own, but protective during injury, Jin et at showed that TLR3 activation prior to viral infection enhances disease development in the brain and spinal cord, while TLR3 activation during disease decreased progression of demyelination.
In this study, we examined the effects of acute TLR3 activation on retinal health. While acute TLR3 activation is protective during injury, chronic TLR3 activation may not be (Galimberti and Scarpini, 2011). It has been shown that acute or low levels of inflammation can have beneficial effects following injury, such as during optic nerve regeneration (Benowitz and Popovich, 2011). However, chronic inflammation has been shown to exacerbate neurodegenerative diseases, through increased secondary degeneration. There is evidence of over-activated innate immunity in several neurodegenerative diseases, including polymorphisms in innate immune regulators, leading to chronic inflammation (Klein et al., 2005; Edwards et al., 2008; Galimberti and Scarpini, 2011; Drouin-Ouellet and Cicchetti, 2012). Therefore, it is likely that precise control of innate immune activity is essential for controlling progression of disease. We have shown that activation of TLR3 during oxidative stress leads to increased retinal cell survival, neuronal function, and overall better vision, which indicates that some level of innate immune activity is important for cellular survival during injury.
TLR3 induced protection of photoreceptors during oxidative stress may be occurring through a preconditioning paradigm. TLRs have the ability to induce tolerance to injury and insults following an initial exposure (Tasaki et al., 1997; Leung et al., 2012; Yi et al., 2012). Because TLR3 is activated rapidly by Poly (I:C) while oxidative stress is induced over time through metabolization of paraquat in the mitochondria into free radicals, it is possible that the kinetics of these two pathways are sufficiently different that TLR3 activation itself is a preconditioning stimulus for the oxidative stress injury (Bus and Gibson, 1984; Field et al., 2010).
Overall, the findings of this study indicate that TLR3 activation in the context of injury is protective via Stat3 signaling, suggesting that combining inflammatory pathways and oxidative stress triggers protective instead of pathogenic signaling. These results identify the TLR3 and Stat3 pathways as novel therapeutic targets for age-related macular degeneration, retinal degenerations, and diseases of the central nervous system.
Highlights.
TLR3 activation protects photoreceptor structure, function and visual behavior during oxidative stress
TLR3 induces neuroprotection during oxidative stress through the Stat3 pathway
Innate immunity acts as a double-edged blade in the context of injury
TLR3 is a potential therapeutic target for neurodegenerations involving oxidative stress
Acknowledgments
This study was supported by a Research to Prevent Blindness Ernest & Elizabeth Althouse Special Scholar Award, the Karl Kirchgessner Foundation, NIH grant RO1 EY017837, and a Fight for Sight Student Fellowship. Institutional support to BPEI was from a Research to Prevent Blindness Unrestricted Grant and an NEI Center Core Grant P30EY014801. The shRNA constructs used in this study were provided by Dr. Denise Hilfiker-Kleiner and Michaela Scherr from Hannover Medical School. Assistance with OCT analysis techniques was provided by Dr. Marco Ruggeri and the Ophthalmic Biophysics Center of University of Miami. We are grateful to William J. Feuer, Bascom Palmer Eye Institute, for guidance with statistical analysis.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aaronson DS, Horvath CM. A road map for those who don’t know JAK-STAT. Science. 2002;296:1653–1655. doi: 10.1126/science.1071545. [DOI] [PubMed] [Google Scholar]
- Alexopoulou L, Holt AC, Medzhitov R, Flavell RA. Recognition of double-stranded RNA and activation of NF-kappaB by Toll-like receptor 3. Nature. 2001;413:732–738. doi: 10.1038/35099560. [DOI] [PubMed] [Google Scholar]
- Anderson KV. Toll signaling pathways in the innate immune response. Current opinion in immunology. 2000;12:13–19. doi: 10.1016/s0952-7915(99)00045-x. [DOI] [PubMed] [Google Scholar]
- Benowitz LI, Popovich PG. Inflammation and axon regeneration. Current opinion in neurology. 2011;24:577–583. doi: 10.1097/WCO.0b013e32834c208d. [DOI] [PubMed] [Google Scholar]
- Bernard JJ, Cowing-Zitron C, Nakatsuji T, Muehleisen B, Muto J, Borkowski AW, Martinez L, Greidinger EL, Yu BD, Gallo RL. Ultraviolet radiation damages self noncoding RNA and is detected by TLR3. Nat Med. 2012 doi: 10.1038/nm.2861. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boivin A, Pineau I, Barrette B, Filali M, Vallieres N, Rivest S, Lacroix S. Toll-like receptor signaling is critical for Wallerian degeneration and functional recovery after peripheral nerve injury. J Neurosci. 2007;27:12565–12576. doi: 10.1523/JNEUROSCI.3027-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bsibsi M, Persoon-Deen C, Verwer RW, Meeuwsen S, Ravid R, Van Noort JM. Toll-like receptor 3 on adult human astrocytes triggers production of neuroprotective mediators. Glia. 2006;53:688–695. doi: 10.1002/glia.20328. [DOI] [PubMed] [Google Scholar]
- Bus JS, Gibson JE. Paraquat: model for oxidant-initiated toxicity. Environ Health Perspect. 1984;55:37–46. doi: 10.1289/ehp.845537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cai J, Nelson KC, Wu M, Sternberg P, Jr, Jones DP. Oxidative damage and protection of the RPE. Progress in retinal and eye research. 2000;19:205–221. doi: 10.1016/s1350-9462(99)00009-9. [DOI] [PubMed] [Google Scholar]
- Cavassani KA, Ishii M, Wen H, Schaller MA, Lincoln PM, Lukacs NW, Hogaboam CM, Kunkel SL. TLR3 is an endogenous sensor of tissue necrosis during acute inflammatory events. J Exp Med. 2008;205:2609–2621. doi: 10.1084/jem.20081370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen M, Luo C, Penalva R, Xu H. Paraquat-induced retinal degeneration is exaggerated in CX3CR1-deficient mice and is associated with increased retinal inflammation. Invest Ophthalmol Vis Sci. 2013;54:682–690. doi: 10.1167/iovs.12-10888. [DOI] [PubMed] [Google Scholar]
- Cingolani C, Rogers B, Lu L, Kachi S, Shen J, Campochiaro PA. Retinal degeneration from oxidative damage. Free Radic Biol Med. 2006;40:660–669. doi: 10.1016/j.freeradbiomed.2005.09.032. [DOI] [PubMed] [Google Scholar]
- Corasaniti MT, Strongoli MC, Rotiroti D, Bagetta G, Nistico G. Paraquat: a useful tool for the in vivo study of mechanisms of neuronal cell death. Pharmacology & toxicology. 1998;83:1–7. doi: 10.1111/j.1600-0773.1998.tb01434.x. [DOI] [PubMed] [Google Scholar]
- Drouin-Ouellet J, Cicchetti F. Inflammation and neurodegeneration: the story ‘retolled’. Trends Pharmacol Sci. 2012;33:542–551. doi: 10.1016/j.tips.2012.07.002. [DOI] [PubMed] [Google Scholar]
- Edwards AO, Chen D, Fridley BL, James KM, Wu Y, Abecasis G, Swaroop A, Othman M, Branham K, Iyengar SK, Sivakumaran TA, Klein R, Klein BE, Tosakulwong N. Toll-like receptor polymorphisms and age-related macular degeneration. Invest Ophthalmol Vis Sci. 2008;49:1652–1659. doi: 10.1167/iovs.07-1378. [DOI] [PubMed] [Google Scholar]
- Fasler-Kan E, Wunderlich K, Hildebrand P, Flammer J, Meyer P. Activated STAT 3 in choroidal neovascular membranes of patients with age-related macular degeneration. Ophthalmologica. 2005;219:214–221. doi: 10.1159/000085730. [DOI] [PubMed] [Google Scholar]
- Field R, Campion S, Warren C, Murray C, Cunningham C. Systemic challenge with the TLR3 agonist poly I:C induces amplified IFNalpha/beta and IL-1beta responses in the diseased brain and exacerbates chronic neurodegeneration. Brain, behavior, and immunity. 2010;24:996–1007. doi: 10.1016/j.bbi.2010.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fragoso MA, Patel AK, Nakamura RE, Yi H, Surapaneni K, Hackam AS. The Wnt/beta-Catenin Pathway Cross-Talks with STAT3 Signaling to Regulate Survival of Retinal Pigment Epithelium Cells. PLoS One. 2012;7:e46892. doi: 10.1371/journal.pone.0046892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Galimberti D, Scarpini E. Inflammation and oxidative damage in Alzheimer’s disease: friend or foe? Front Biosci (Schol Ed) 2011;3:252–266. doi: 10.2741/s149. [DOI] [PubMed] [Google Scholar]
- Haghikia A, Missol-Kolka E, Tsikas D, Venturini L, Brundiers S, Castoldi M, Muckenthaler MU, Eder M, Stapel B, Thum T, Haghikia A, Petrasch-Parwez E, Drexler H, Hilfiker-Kleiner D, Scherr M. Signal transducer and activator of transcription 3-mediated regulation of miR-199a-5p links cardiomyocyte and endothelial cell function in the heart: a key role for ubiquitin-conjugating enzymes. European heart journal. 2011;32:1287–1297. doi: 10.1093/eurheartj/ehq369. [DOI] [PubMed] [Google Scholar]
- Hanus J, Zhang H, Wang Z, Liu Q, Zhou Q, Wang S. Induction of necrotic cell death by oxidative stress in retinal pigment epithelial cells. Cell death & disease. 2013;4:e965. doi: 10.1038/cddis.2013.478. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hollyfield JG, Bonilha VL, Rayborn ME, Yang X, Shadrach KG, Lu L, Ufret RL, Salomon RG, Perez VL. Oxidative damage-induced inflammation initiates age-related macular degeneration. Nat Med. 2008;14:194–198. doi: 10.1038/nm1709. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin YH, Kaneyama T, Kang MH, Kang HS, Koh CS, Kim BS. TLR3 signaling is either protective or pathogenic for the development of Theiler’s virus-induced demyelinating disease depending on the time of viral infection. J Neuroinflammation. 2011;8:178. doi: 10.1186/1742-2094-8-178. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juel HB, Faber C, Svendsen SG, Vallejo AN, Nissen MH. Inflammatory cytokines protect retinal pigment epithelial cells from oxidative stress-induced death. PLoS One. 2013;8:e64619. doi: 10.1371/journal.pone.0064619. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kariko K, Ni H, Capodici J, Lamphier M, Weissman D. mRNA is an endogenous ligand for Toll-like receptor 3. J Biol Chem. 2004;279:12542–12550. doi: 10.1074/jbc.M310175200. [DOI] [PubMed] [Google Scholar]
- Kigerl KA, Lai W, Rivest S, Hart RP, Satoskar AR, Popovich PG. Toll-like receptor (TLR)-2 and TLR-4 regulate inflammation, gliosis, and myelin sparing after spinal cord injury. Journal of neurochemistry. 2007;102:37–50. doi: 10.1111/j.1471-4159.2007.04524.x. [DOI] [PubMed] [Google Scholar]
- Kilic U, Kilic E, Matter CM, Bassetti CL, Hermann DM. TLR-4 deficiency protects against focal cerebral ischemia and axotomy-induced neurodegeneration. Neurobiol Dis. 2008;31:33–40. doi: 10.1016/j.nbd.2008.03.002. [DOI] [PubMed] [Google Scholar]
- Kinnunen K, Petrovski G, Moe MC, Berta A, Kaarniranta K. Molecular mechanisms of retinal pigment epithelium damage and development of age-related macular degeneration. Acta Ophthalmol. 2012;90:299–309. doi: 10.1111/j.1755-3768.2011.02179.x. [DOI] [PubMed] [Google Scholar]
- Klein RJ, Zeiss C, Chew EY, Tsai JY, Sackler RS, Haynes C, Henning AK, SanGiovanni JP, Mane SM, Mayne ST, Bracken MB, Ferris FL, Ott J, Barnstable C, Hoh J. Complement factor H polymorphism in age-related macular degeneration. Science. 2005;308:385–389. doi: 10.1126/science.1109557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleinman ME, Kaneko H, Cho WG, Dridi S, Fowler BJ, Blandford AD, Albuquerque RJ, Hirano Y, Terasaki H, Kondo M, Fujita T, Ambati BK, Tarallo V, Gelfand BD, Bogdanovich S, Baffi JZ, Ambati J. Short-interfering RNAs induce retinal degeneration via TLR3 and IRF3. Mol Ther. 2012;20:101–108. doi: 10.1038/mt.2011.212. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Letiembre M, Liu Y, Walter S, Hao W, Pfander T, Wrede A, Schulz-Schaeffer W, Fassbender K. Screening of innate immune receptors in neurodegenerative diseases: a similar pattern. Neurobiology of aging. 2009;30:759–768. doi: 10.1016/j.neurobiolaging.2007.08.018. [DOI] [PubMed] [Google Scholar]
- Leung PY, Stevens SL, Packard AE, Lessov NS, Yang T, Conrad VK, van den Dungen NN, Simon RP, Stenzel-Poore MP. Toll-like receptor 7 preconditioning induces robust neuroprotection against stroke by a novel type I interferon-mediated mechanism. Stroke. 2012;43:1383–1389. doi: 10.1161/STROKEAHA.111.641522. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li Y, Tao W, Luo L, Huang D, Kauper K, Stabila P, Lavail MM, Laties AM, Wen R. CNTF induces regeneration of cone outer segments in a rat model of retinal degeneration. PLoS One. 2010;5:e9495. doi: 10.1371/journal.pone.0009495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang FQ, Godley BF. Oxidative stress-induced mitochondrial DNA damage in human retinal pigment epithelial cells: a possible mechanism for RPE aging and age-related macular degeneration. Exp Eye Res. 2003;76:397–403. doi: 10.1016/s0014-4835(03)00023-x. [DOI] [PubMed] [Google Scholar]
- Lu C, Ren D, Wang X, Ha T, Liu L, Lee EJ, Hu J, Kalbfleisch J, Gao X, Kao R, Williams D, Li C. Toll-like receptor 3 plays a role in myocardial infarction and ischemia/reperfusion injury. Biochimica et biophysica acta. 2014;1842:22–31. doi: 10.1016/j.bbadis.2013.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCormack AL, Atienza JG, Johnston LC, Andersen JK, Vu S, Di Monte DA. Role of oxidative stress in paraquat-induced dopaminergic cell degeneration. Journal of neurochemistry. 2005;93:1030–1037. doi: 10.1111/j.1471-4159.2005.03088.x. [DOI] [PubMed] [Google Scholar]
- Moreira PI, Smith MA, Zhu X, Nunomura A, Castellani RJ, Perry G. Oxidative stress and neurodegeneration. Ann N Y Acad Sci. 2005;1043:545–552. doi: 10.1196/annals.1333.062. [DOI] [PubMed] [Google Scholar]
- Murakami Y, Matsumoto H, Roh M, Giani A, Kataoka K, Morizane Y, Kayama M, Thanos A, Nakatake S, Notomi S, Hisatomi T, Ikeda Y, Ishibashi T, Connor KM, Miller JW, Vavvas DG. Programmed necrosis, not apoptosis, is a key mediator of cell loss and DAMP-mediated inflammation in dsRNA-induced retinal degeneration. Cell Death Differ. 2013 doi: 10.1038/cdd.2013.109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Owens T. Toll-like receptors in neurodegeneration. Current topics in microbiology and immunology. 2009;336:105–120. doi: 10.1007/978-3-642-00549-7_6. [DOI] [PubMed] [Google Scholar]
- Patel AK, Hackam AS. Toll-like receptor 3 (TLR3) protects retinal pigmented epithelium (RPE) cells from oxidative stress through a STAT3-dependent mechanism. Mol Immunol. 2012;54:122–131. doi: 10.1016/j.molimm.2012.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pearson RA, Barber AC, Rizzi M, Hippert C, Xue T, West EL, Duran Y, Smith AJ, Chuang JZ, Azam SA, Luhmann UF, Benucci A, Sung CH, Bainbridge JW, Carandini M, Yau KW, Sowden JC, Ali RR. Restoration of vision after transplantation of photoreceptors. Nature. 2012;485:99–103. doi: 10.1038/nature10997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peterson WM, Wang Q, Tzekova R, Wiegand SJ. Ciliary neurotrophic factor and stress stimuli activate the Jak-STAT pathway in retinal neurons and glia. J Neurosci. 2000;20:4081–4090. doi: 10.1523/JNEUROSCI.20-11-04081.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Porciatti V, Ventura LM. Retinal ganglion cell functional plasticity and optic neuropathy: a comprehensive model. Journal of neuro-ophthalmology: the official journal of the North American Neuro-Ophthalmology Society. 2012;32:354–358. doi: 10.1097/WNO.0b013e3182745600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prusky GT, Alam NM, Beekman S, Douglas RM. Rapid quantification of adult and developing mouse spatial vision using a virtual optomotor system. Invest Ophthalmol Vis Sci. 2004;45:4611–4616. doi: 10.1167/iovs.04-0541. [DOI] [PubMed] [Google Scholar]
- Ruggeri M, Wehbe H, Jiao S, Gregori G, Jockovich ME, Hackam A, Duan Y, Puliafito CA. In vivo three-dimensional high-resolution imaging of rodent retina with spectral-domain optical coherence tomography. Invest Ophthalmol Vis Sci. 2007;48:1808–1814. doi: 10.1167/iovs.06-0815. [DOI] [PubMed] [Google Scholar]
- Shichita T, Ago T, Kamouchi M, Kitazono T, Yoshimura A, Ooboshi H. Novel therapeutic strategies targeting innate immune responses and early inflammation after stroke. Journal of neurochemistry. 2012;123(Suppl 2):29–38. doi: 10.1111/j.1471-4159.2012.07941.x. [DOI] [PubMed] [Google Scholar]
- Shiose S, Chen Y, Okano K, Roy S, Kohno H, Tang J, Pearlman E, Maeda T, Palczewski K, Maeda A. Toll-like receptor 3 is required for development of retinopathy caused by impaired all-trans-retinal clearance in mice. J Biol Chem. 2011;286:15543–15555. doi: 10.1074/jbc.M111.228551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tabata H, Shimizu N, Wada Y, Miura K, Kawano K. Initiation of the optokinetic response (OKR) in mice. Journal of vision. 2010:10. doi: 10.1167/10.1.13. [DOI] [PubMed] [Google Scholar]
- Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annual review of immunology. 1997;15:797–819. doi: 10.1146/annurev.immunol.15.1.797. [DOI] [PubMed] [Google Scholar]
- Tasaki K, Ruetzler CA, Ohtsuki T, Martin D, Nawashiro H, Hallenbeck JM. Lipopolysaccharide pre-treatment induces resistance against subsequent focal cerebral ischemic damage in spontaneously hypertensive rats. Brain research. 1997;748:267–270. doi: 10.1016/s0006-8993(96)01383-2. [DOI] [PubMed] [Google Scholar]
- Walter S, Letiembre M, Liu Y, Heine H, Penke B, Hao W, Bode B, Manietta N, Walter J, Schulz-Schuffer W, Fassbender K. Role of the toll-like receptor 4 in neuroinflammation in Alzheimer’s disease. Cell Physiol Biochem. 2007;20:947–956. doi: 10.1159/000110455. [DOI] [PubMed] [Google Scholar]
- Yi H, Patel AK, Sodhi CP, Hackam DJ, Hackam AS. Novel role for the innate immune receptor Toll-like receptor 4 (TLR4) in the regulation of the Wnt signaling pathway and photoreceptor apoptosis. PLoS One. 2012;7:e36560. doi: 10.1371/journal.pone.0036560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang C, Li H, Liu MG, Kawasaki A, Fu XY, Barnstable CJ, Shao-Min Zhang S. STAT3 activation protects retinal ganglion cell layer neurons in response to stress. Exp Eye Res. 2008;86:991–997. doi: 10.1016/j.exer.2008.03.020. [DOI] [PubMed] [Google Scholar]