Abstract
Background
Port-wine stain (PWS) is a congenital, progressive vascular malformation but the pathogenesis remains incompletely understood.
Objective
We sought to investigate the activation status of various kinases, including extracellular signal-regulated kinase, c-Jun N-terminal kinase, AKT, phosphatidylinositol 3-kinase, P70 ribosomal S6 kinase, and phosphoinositide phospholipase C γ subunit, in PWS biopsy tissues.
Methods
Immunohistochemistry was performed on 19 skin biopsy samples from 11 patients with PWS.
Results
c-Jun N-terminal kinase, extracellular signal-regulated kinase, and P70 ribosomal S6 kinase in pediatric and adult PWS blood vessels were consecutively activated. Activation of AKT and phosphatidylinositol 3-kinase was found in many adult hypertrophic PWS blood vessels but not in infants. Phosphoinositide phospholipase C γ subunit showed strong activation in nodular PWS blood vessels.
Limitation
Infantile PWS sample size was small.
Conclusion
Our data suggest a subsequent activation profile of various kinases during different stages of PWS: (1) c-Jun N-terminal and extracellular signal-regulated kinases are firstly and consecutively activated in all PWS tissues, which may contribute to both the pathogenesis and progressive development of PWS; (2) AKT and phosphatidylinositol 3-kinase are subsequently activated, and are involved in the hypertrophic development of PWS blood vessels; and (3) phosphoinositide phospholipase C γ subunit is activated in the most advanced stage of PWS and may participate in nodular formation.
Keywords: AKT, c-Jun N-terminal kinase, extracellular signal-regulated kinase, mitogen-activated protein kinase, port-wine stain, vascular malformation
Port-wine stain (PWS) is a congenital, progressive vascular malformation of human skin involving the superficial vascular plexus that occurs in an estimated 3 to 5 children per 1000 live births.1–3 Recently a low-frequency somatic mutation in the guanine nucleotide-binding protein, G alpha subunit q gene (c.548G→A, p.R183Q) was found in PWS lesions, which resulted in an activation of extracellular signal-regulated kinase (ERK).4 However, the activation status of mitogen-activated protein kinase pathways has not yet been examined in PWS tissues. In this study, we attempted to investigate phosphorylation levels of various kinases, including ERK, c-Jun N-terminal kinase (JNK), AKT, phosphati-dylinositol 3-kinase (PI3K), P70 ribosomal S6 kinase (P70S6K), mammalian target of rapamycin (mTOR), and phosphoinositide phospholipase C γ subunit (PLC-γ), in PWS biopsy tissues.
METHODS
The study was approved by the investigational review board at the University of California—Irvine. Deidentified pathological leftover samples or punch biopsy specimens from a selected PWS site and adjacent normal-appearing skin (0.5–1 cm away) were obtained from 11 patients with PWS. Nine hemangioma, 9 normal-appearing pediatric, and 5 normal-appearing adult skin samples were used as controls. The clinical history of PWS and hemangioma biopsy samples were listed in Table I. Immunohistochemistry was performed using routine procedures. The cellular immunoreactivity score was evaluated using a system reported by Populo et al.5
Table I.
Patient description of port-wine stain and hemangioma biopsy samples
| Patient | Gender | Age | Diagnosis | Treatment history | Other vascular malformations |
|---|---|---|---|---|---|
| 1 | M | 22 mo | PWS face | PDL | No |
| 2 | F | 9 mo | PWS arm | None | No |
| 3 | M | 41 y | PWS face | PDL | No |
| 4 | M | 38 y | PWS face | PDL | No |
| 5 | M | 38 y | PWS arm | PDL | No |
| 6 | F | 56 y | PWS face | PDL | No |
| 7 | M | 51 y | PWS face | None | No |
| 8 | F | 13 y | PWS face | PDL | No |
| 9 | F | 16 y | PWS face | None | No |
| 10 | M | 27 y | PWS face | None | No |
| 11 | M | 55 y | PWS face | None | No |
| 12 | M | 6 mo | Proliferative hemangioma | Steroid, PDL | No |
| 13 | F | 6 mo | Proliferative hemangioma | Topical antibiotics for ulceration | Yes |
| 14 | F | 4 y | Involuting hemangioma | None | No |
| 15 | F | 2 y | Involuting hemangioma | None | Yes |
| 16 | F | 2 y | Involuting hemangioma | Topical beta-blockers | No |
| 17 | M | 8 mo | Proliferative hemangioma | Topical antibiotics for ulceration | No |
| 18 | F | 12 mo | Involuting hemangioma | None | Yes |
| 19 | F | 1 y | Involuting hemangioma | None | Yes |
| 20 | F | 3 y | Involuting hemangioma | None | No |
F, Female; M, male; PDL, pulsed dye laser; PWS, port-wine stain.
RESULTS AND DISCUSSION
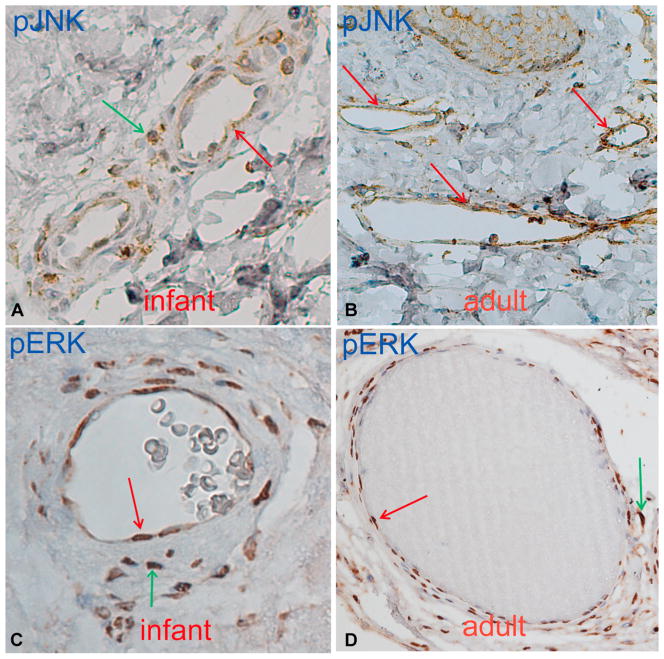
Phosphorylated JNK (pJNK) was observed in the blood vessels of all 19 PWS biopsy samples from 9 adults and 2 infants (Fig 1, A and B, and Table II). In more advanced stages of PWS and 1 nodular sample, pJNK showed the highest immunoreactive score of 6 (Fig 2, A, and Table II). Thus, JNK activation levels appeared correlated to the progressive development of PWS. In the samples containing the edges of PWS lesion sites, scattered moderate JNK activation was found in the dermal superficial vascular plexus in the normal-appearing skin (n = 5 of 5) (Table II). We also found scattered, weak pJNK immunoreactive signals in the dermal superficial vascular plexus/capillary loops in some biopsy samples (n = 3 of 6) taken from the normal-appearing skin adjacent to PWS sites. These results indicated that pathological changes in blood vessels, eg, activation of JNK, happened before morphological abnormalities, eg, blood vessel dilation and skin color changes, in the adjacent areas of PWS lesion sites. JNK was also activated in hemangiomas; we found 8 of 9 hemangioma samples showed pJNK in the blood vessels.
Fig 1.
Activation of c-Jun N-terminal kinase (JNK) (A and B) and extracellular signal-regulated kinase (ERK) (C and D) in infant and adult port-wine stain blood vessels. Positive immunoreactive endothelial cells or blood vessels (red arrows). Positive immunoreactive pericytes (green arrows).
Table II.
The immunoreactive scores of phosphorylated various kinases in abnormal blood vessels from 11 patients with port-wine stain
| Patient | Age | Sample | pJNK* (G7, 6254) | pERK* (E4, 7383) | pP70S6K* (A6, 8416) | pAKT* (7985-R) | pPI3K† (4228) | pmTOR† (49F9, 2976) | pPLC-γ† (2821) |
|---|---|---|---|---|---|---|---|---|---|
| 1 | Infant | Scalp | 2 | 4 | 4 | 0 | 0 | 0 | 0 |
| Normal | 1 | 0 | 4 | 0 | 0 | 0 | 0 | ||
| 2 | Infant | Extremity | 2 | 0 | 6 | 0 | 0 | 0 | 0 |
| Normal | 0 | 0 | 6 | 0 | 0 | 0 | 0 | ||
| 3 | Adult | Extremity | 2 | 1 | 4 | 0 | 0 | 0 | 0 |
| Normal | 0 | 0 | 4 | 0 | 0 | 0 | 0 | ||
| 4 | Adult | Scalp | 6 | 2 | 6 | 1 | 0 | 0 | 0 |
| Normal | 1 | 0 | 6 | 0 | 0 | 0 | 0 | ||
| 5 | Adult | Scalp | 4 | 4 | 4 | 0 | 0 | 0 | 0 |
| Normal | 1 | 0 | 4 | 0 | 0 | 0 | 0 | ||
| 6 | Adult | Facial | 4 | 6 | 6 | 4 | 2 | 0 | 0 |
| Normal | 0 | 0 | 6 | 0 | 0 | 0 | 0 | ||
| Neck | 4 | 6 | 6 | 4 | 1 | 0 | 0 | ||
| 7 | Adult | Facial | 4 | 6 | 6 | 6 | 2 | 0 | 0 |
| Extremity | 4 | 6 | 6 | 4 | 2 | 0 | 0 | ||
| 8 | Adult | Facial | 4 | 4 | 6 | 0 | 0 | 0 | 0 |
| Edge | 4 | 2 | 6 | 2 | 0 | 0 | 0 | ||
| 9 | Adult | Facial | 6 | 6 | 6 | 1 | 0 | 0 | 0 |
| Edge | 6 | 6 | 6 | 0 | 0 | 0 | 0 | ||
| 10 | Adult | Facial | 6 | 6 | 6 | 1 | 0 | 0 | 0 |
| Edge | 6 | 4 | 6 | 0 | 1 | 0 | 0 | ||
| 11 | Adult | Facial | 6 | 6 | 6 | 2 | 0 | 0 | 0 |
| Edge | 6 | 4 | 6 | 2 | 2 | 0 | 0 | ||
| Nodular-1 | 6 | 6 | 6 | 4 | 4 | 0 | 6 | ||
| Nodular-2 | 4 | 4 | 2 | 2 | 2 | 0 | 2 |
Edge, Edge of port-wine stain lesion sites; nodular, nodular port-wine stain; normal, the adjacent normal-appearing skin (0.5–1 cm away from port-wine stain lesion sites).
Antibodies from Santa Cruz Biotechnology, Inc, Santa Cruz, CA.
Antibodies from Cell Signaling, Inc, Danvers, MA.
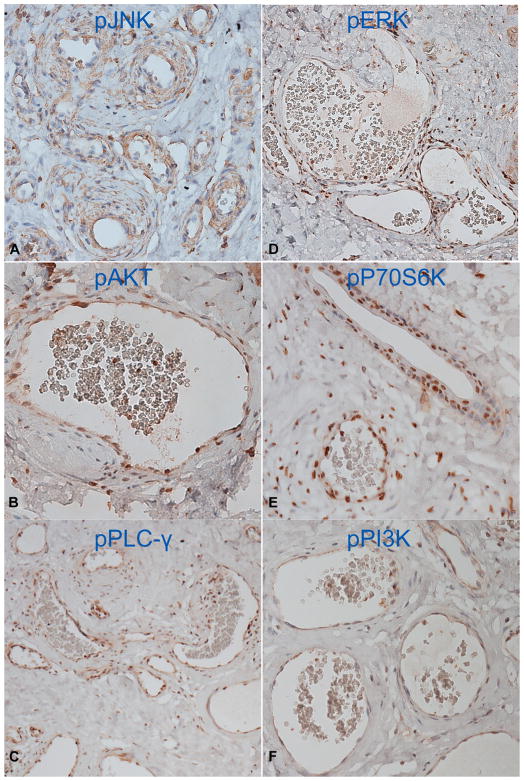
Fig 2.
Activation of c-Jun N-terminal kinase (JNK) (A), AKT (B), phosphoinositide phospholipase C γ subunit (PLC-γ) (C), extracellular signal-regulated kinase (ERK) (D), P70 ribosomal S6 kinase (P70S6K) (E), and phosphatidylinositol 3-kinase (PI3K) (F) in all hypertrophic and dilated blood vessels from a nodular port-wine stain.
Phosphorylated ERK (pERK) was found in 18 of 19 PWS biopsy samples from 10 patients; the exception being 1 infant (Fig 1, C and D, and Table II). The activated ERK was found in all hemangioma samples (n = 9), consistent with other reports.6,7 There was no significant activation of JNK or ERK in the control pediatric (n = 9) and adult (n = 5) skin samples. The trigger that activates JNK and ERK in PWS remains unknown. It may result from the guanine nucleotide-binding protein, G alpha subunit q (R183G) mutation, which has been postulated as the cause of PWS.4 In this study, both JNK and ERK appear to be the predominant activated kinases in PWS and hemangiomas, which is in agreement with the hypothesis that activation of ERK contributes to the pathogenesis of PWS.4 The guanine nucleotide-binding protein, G alpha subunit q (R183G) status in these 11 patients is unknown and will be characterized in a future study.
Phosphorylated P70S6K (pP70S6K) was observed in all PWS tissues with immunoreactive scores ranging from 4 to 6 (Table II). Phosphorylated AKT (pAKT) and PI3K (pPI3K) were found from 7 and 4, respectively, of 11 patients (Table II), but neither was activated in infant tissues. Phosphorylated PLC-γ (pPLC-γ) was found only in 2 nodular PWS but not in any other samples (Fig 2 and Table II). In both nodular PWS, all of the kinases we examined, except mTOR, showed medium to strong activation (Fig 2 and Table II). For the first time, our data have shown the kinase activation profiles in different stages of PWS: JNK and ERK are among the kinases that are first and consecutively activated, then AKT and PI3K, and finally PLC-γ. The subsequent activation of various kinases imply their specific roles in the different stages of PWS: (1) JNK and ERK contribute to both the pathogenesis and progressive development of PWS; (2) AKT and PI3K are involved in hypertrophy of PWS blood vessels; and (3) PLC-γ appears to play a role in nodular formation.
CAPSULE SUMMARY.
The activation status of various mitogen-activated protein kinase pathways and their roles in port-wine stain pathogenesis are unknown.
Our data suggest a subsequent activation profile of various mitogen-activated protein kinases during different stages of port-wine stain.
Our results suggest that mitogen-activated protein kinase inhibitors may be potential agents for port-wine stain treatment.
Acknowledgments
Supported by National Institutes of Health AR063766 (Dr Tan), AR47551 (Dr Nelson), and AR59244 (Dr Nelson), and the American Society for Laser Medicine and Surgery research grant F03.12 and F01.13 (Dr Tan).
We greatly appreciate the assistance of Dr Robert Edwards of the Pathology Department and Amanda Dickson of the Sue and Bill Gross Stem Cell Research Center at the University of California—Irvine during the histology image acquisition process experiments.
Abbreviations used
- ERK
extracellular signal-regulated kinase
- JNK
c-Jun N-terminal kinase
- PI3K
phosphatidylinositol 3-kinase
- PLC-γ
phosphoinositide phospholipase C γ subunit
- PWS
port-wine stain
Footnotes
Conflicts of interest: None declared.
Reprints not available from the authors.
References
- 1.Mulliken JB, Young AR. Vascular birthmarks—hemangiomas and malformations. Philadelphia (PA): WB Saunders Co; 1988. [Google Scholar]
- 2.Jacobs AH, Walton RG. The incidence of birthmarks in the neonate. Pediatrics. 1976;58:218–22. [PubMed] [Google Scholar]
- 3.Pratt AG. Birthmarks in infants. Arch Dermatol Syphilol. 1953;67:302–5. doi: 10.1001/archderm.1953.01540030065006. [DOI] [PubMed] [Google Scholar]
- 4.Shirley MD, Tang H, Gallione CJ, Baugher JD, Frelin LP, Cohen B, et al. Sturge-Weber syndrome and port-wine stains caused by somatic mutation in GNAQ. N Engl J Med. 2013;368:1971–9. doi: 10.1056/NEJMoa1213507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Populo H, Vinagre J, Lopes JM, Soares P. Analysis of GNAQ mutations, proliferation and MAPK pathway activation in uveal melanomas. Br J Ophthalmol. 2011;95:715–9. doi: 10.1136/bjo.2009.174417. [DOI] [PubMed] [Google Scholar]
- 6.Arbiser JL, Bonner MY, Berrios RL. Hemangiomas, angio-sarcomas, and vascular malformations represent the signaling abnormalities of pathogenic angiogenesis. Curr Mol Med. 2009;9:929–34. doi: 10.2174/156652409789712828. [DOI] [PubMed] [Google Scholar]
- 7.Arbiser JL, Weiss SW, Arbiser ZK, Bravo F, Govindajaran B, Caceres-Rios H, et al. Differential expression of active mitogen-activated protein kinase in cutaneous endothelial neoplasms: implications for biologic behavior and response to therapy. J Am Acad Dermatol. 2001;44:193–7. doi: 10.1067/mjd.2000.111632. [DOI] [PubMed] [Google Scholar]