Abstract
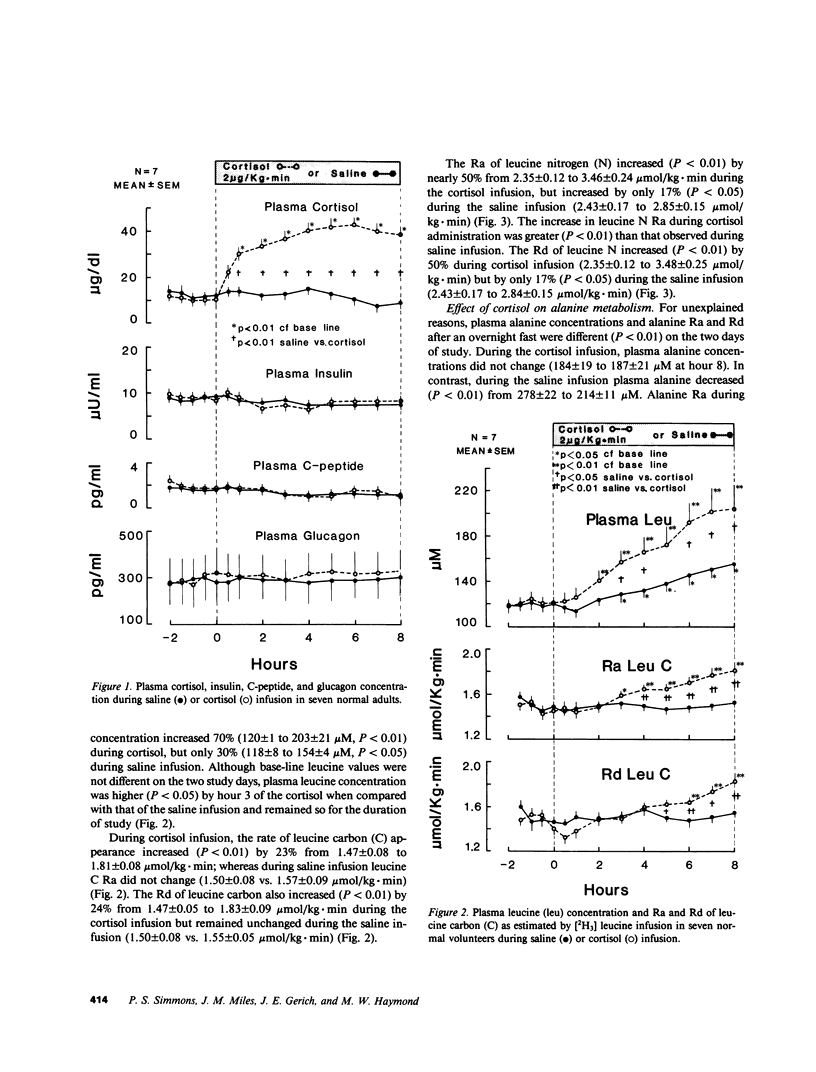
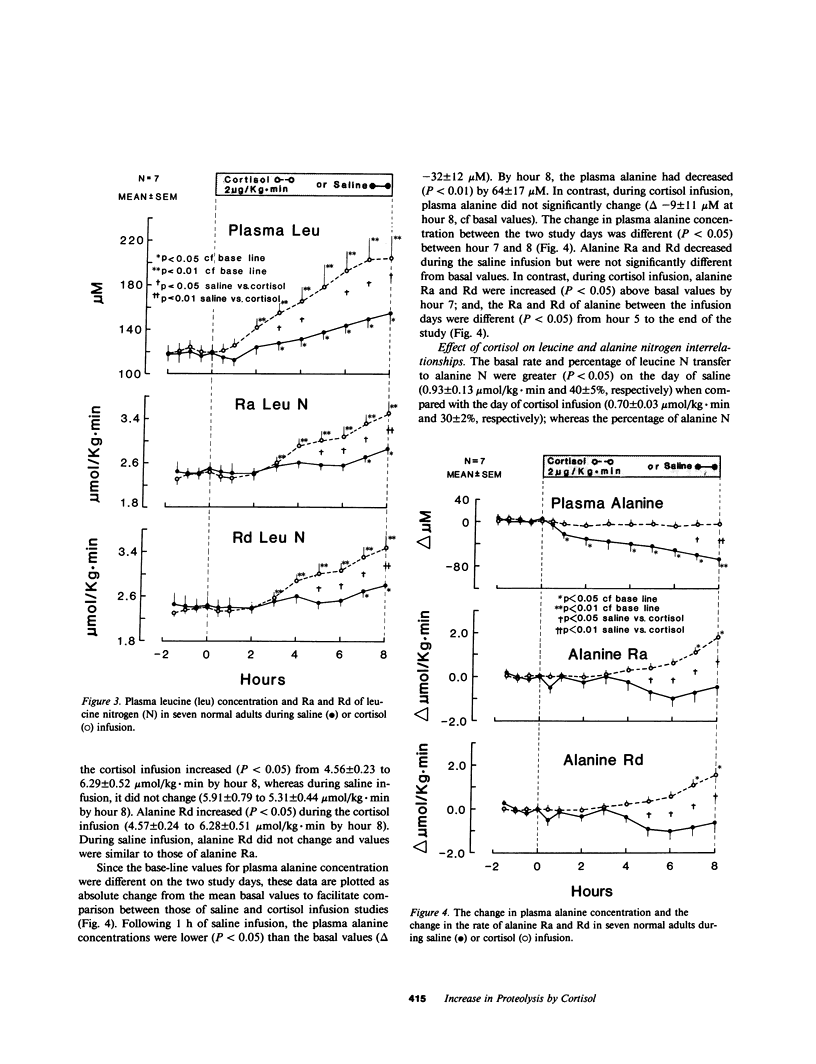
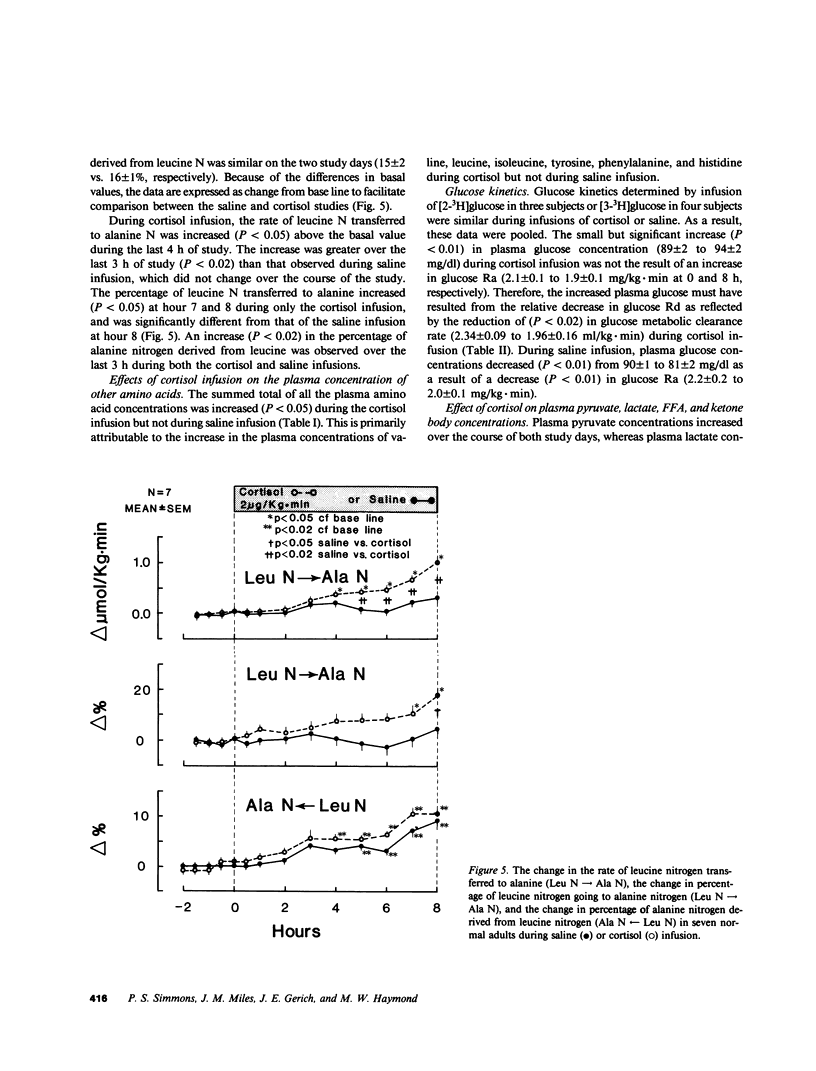
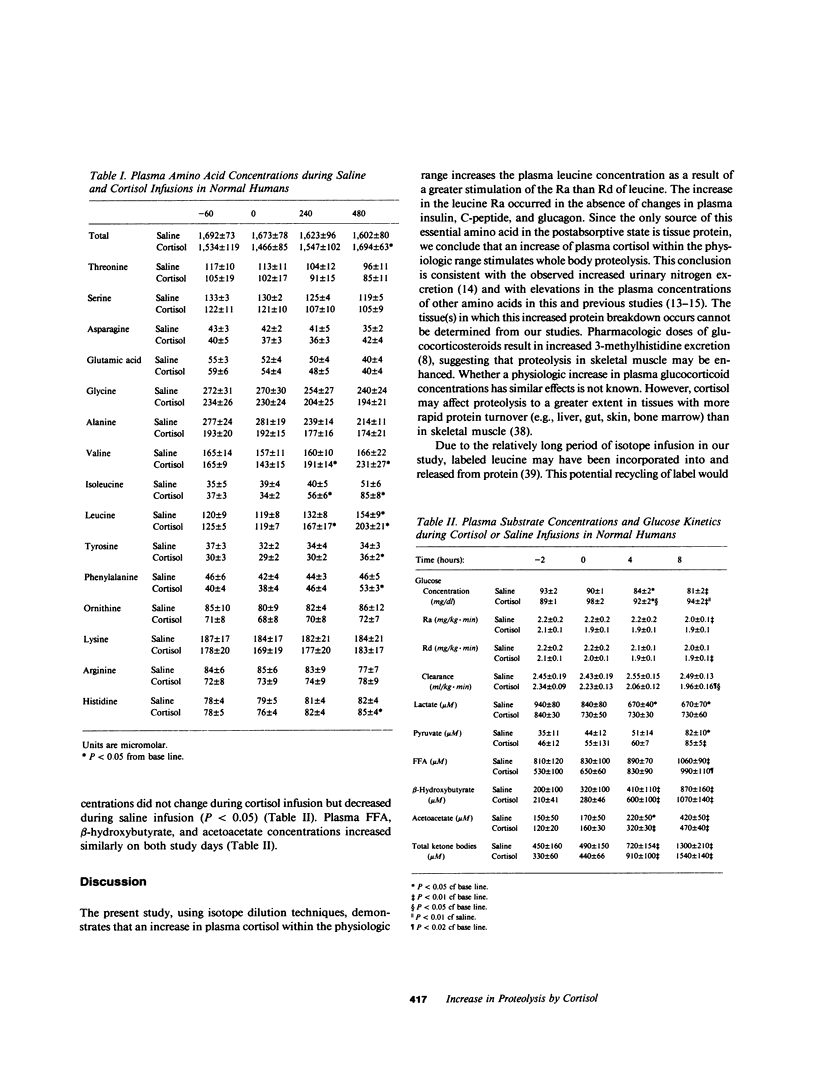
Prolonged exposure to glucocorticoids in pharmacologic amounts results in muscle wasting, but whether changes in plasma cortisol within the physiologic range affect amino acid and protein metabolism in man has not been determined. To determine whether a physiologic increase in plasma cortisol increases proteolysis and the de novo synthesis of alanine, seven normal subjects were studied on two occasions during an 8-h infusion of either hydrocortisone sodium succinate (2 micrograms/kg X min) or saline. The rate of appearance (Ra) of leucine and alanine were estimated using [2H3]leucine and [2H3]alanine. In addition, the Ra of leucine nitrogen and the rate of transfer of leucine nitrogen to alanine were estimated using [15N]leucine. Plasma cortisol increased (10 +/- 1 to 42 +/- 4 micrograms/dl) during cortisol infusion and decreased (14 +/- 2 to 10 +/- 2 micrograms/dl) during saline infusion. No change was observed in plasma insulin, C-peptide, or glucagon during either saline or cortisol infusion. Plasma leucine concentration increased more (P less than 0.05) during cortisol infusion (120 +/- 1 to 203 +/- 21 microM) than saline (118 +/- 8 to 154 +/- 4 microM) as a result of a greater (P less than 0.01) increase in its Ra during cortisol infusion (1.47 +/- 0.08 to 1.81 +/- 0.08 mumol/kg X min for cortisol vs. 1.50 +/- 0.08 to 1.57 +/- 0.09 mumol/kg X min). Leucine nitrogen Ra increased (P less than 0.01) from 2.35 +/- 0.12 to 3.46 +/- 0.24 mumol/kg X min, but less so (P less than 0.05) during saline infusion (2.43 +/- 0.17 to 2.84 +/- 0.15 mumol/kg X min, P less than 0.01). Alanine Ra increased (P less than 0.05) during cortisol infusion but remained constant during saline infusion. During cortisol, but not during saline infusion, the rate and percentage of leucine nitrogen going to alanine increased (P less than 0.05). Thus, an increase in plasma cortisol within the physiologic range increases proteolysis and the de novo synthesis of alanine, a potential gluconeogenic substrate. Therefore, physiologic changes in plasma cortisol play a role in the regulation of whole body protein and amino acid metabolism in man.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Abumrad N. N., Jefferson L. S., Rannels S. R., Williams P. E., Cherrington A. D., Lacy W. W. Role of insulin in the regulation of leucine kinetics in the conscious dog. J Clin Invest. 1982 Nov;70(5):1031–1041. doi: 10.1172/JCI110690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- BONDY P. K., INGLE D. J., MEEKS R. C. Influence of adrenal cortical hormones upon the level of plasma amino acids in eviscerate rats. Endocrinology. 1954 Sep;55(3):354–360. doi: 10.1210/endo-55-3-354. [DOI] [PubMed] [Google Scholar]
- Beitins I. Z., Shaw M. H., Kowarski A., Migeon C. J. Comparison of competitive protein binding radioassay of cortisol to double isotope dilution and Porter Silber methods. Steroids. 1970 Jun;15(6):765–776. doi: 10.1016/s0039-128x(70)80045-9. [DOI] [PubMed] [Google Scholar]
- Bier D. M., Arnold K. J., Sherman W. R., Holland W. H., Holmes W. F., Kipnis D. M. In-vivo measurement of glucose and alanine metabolism with stable isotopic tracers. Diabetes. 1977 Nov;26(11):1005–1015. doi: 10.2337/diab.26.11.1005. [DOI] [PubMed] [Google Scholar]
- Cahill G. F., Jr, Herrera M. G., Morgan A. P., Soeldner J. S., Steinke J., Levy P. L., Reichard G. A., Jr, Kipnis D. M. Hormone-fuel interrelationships during fasting. J Clin Invest. 1966 Nov;45(11):1751–1769. doi: 10.1172/JCI105481. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DEBODO R. C., STEELE R., ALTSZULER N., DUNN A., BISHOP J. S. ON THE HORMONAL REGULATION OF CARBOHYDRATE METABOLISM; STUDIES WITH C14 GLUCOSE. Recent Prog Horm Res. 1963;19:445–488. [PubMed] [Google Scholar]
- Dunn A., Katz J., Golden S., Chenoweth M. Estimation of glucose turnover and recycling in rabbits using various [3H, 14C]glucose labels. Am J Physiol. 1976 Apr;230(4):1159–1162. doi: 10.1152/ajplegacy.1976.230.4.1159. [DOI] [PubMed] [Google Scholar]
- Felig P. The glucose-alanine cycle. Metabolism. 1973 Feb;22(2):179–207. doi: 10.1016/0026-0495(73)90269-2. [DOI] [PubMed] [Google Scholar]
- Galim E. B., Hruska K., Bier D. M., Matthews D. E., Haymond M. W. Branched-chain amino acid nitrogen transfer to alamine in vivo in dogs. Direct isotopic determination with [15N]leucine. J Clin Invest. 1980 Dec;66(6):1295–1304. doi: 10.1172/JCI109981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Golden M. H., Waterlow J. C. Total protein synthesis in elderly people: a comparison of results with [15N]glycine and [14C]leucine. Clin Sci Mol Med. 1977 Sep;53(3):277–288. doi: 10.1042/cs0530277. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Howard C. P., Miles J. M., Gerich J. E. Determination of leucine flux in vivo by gas chromatography-mass spectrometry utilizing stable isotopes for trace and internal standard. J Chromatogr. 1980 Oct 10;183(4):403–409. doi: 10.1016/s0378-4347(00)81582-0. [DOI] [PubMed] [Google Scholar]
- Haymond M. W., Miles J. M. Branched chain amino acids as a major source of alanine nitrogen in man. Diabetes. 1982 Jan;31(1):86–89. doi: 10.2337/diab.31.1.86. [DOI] [PubMed] [Google Scholar]
- Herbert V., Lau K. S., Gottlieb C. W., Bleicher S. J. Coated charcoal immunoassay of insulin. J Clin Endocrinol Metab. 1965 Oct;25(10):1375–1384. doi: 10.1210/jcem-25-10-1375. [DOI] [PubMed] [Google Scholar]
- Horwitz D. L., Starr J. I., Mako M. E., Blackard W. G., Rubenstein A. H. Proinsulin, insulin, and C-peptide concentrations in human portal and peripheral blood. J Clin Invest. 1975 Jun;55(6):1278–1283. doi: 10.1172/JCI108047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson R. A., Peters N., Advani U., Perry G., Rogers J., Brough W. H., Pilkington T. R. Forearm glucose uptake during the oral glucose tolerance test in normal subjects. Diabetes. 1973 Jun;22(6):442–458. doi: 10.2337/diab.22.6.442. [DOI] [PubMed] [Google Scholar]
- KLINE D. L. A procedure for the study of factors which effect the nitrogen metabolism of isolated tissues; hormonal influences. Endocrinology. 1949 Dec;45(6):596–604. doi: 10.1210/endo-45-6-596. [DOI] [PubMed] [Google Scholar]
- Karl I. E., Garber A. J., Kipnis D. M. Alanine and glutamine synthesis and release from skeletal muscle. III. Dietary and hormonal regulation. J Biol Chem. 1976 Feb 10;251(3):844–850. [PubMed] [Google Scholar]
- Karl I. E., Pagliara A. S., Kipnis D. M. A microfluorometric enzymatic assay for the determination of alanine and pyruvate in plasma and tissues. J Lab Clin Med. 1972 Sep;80(3):434–441. [PubMed] [Google Scholar]
- Laurell S., Tibbling G. Colorimetric micro-determination of free fatty acids in plasma. Clin Chim Acta. 1967 Apr;16(1):57–62. doi: 10.1016/0009-8981(67)90269-0. [DOI] [PubMed] [Google Scholar]
- Livesey G., Lund P. Enzymic determination of branched-chain amino acids and 2-oxoacids in rat tissues. Transfer of 2-oxoacids from skeletal muscle to liver in vivo. Biochem J. 1980 Jun 15;188(3):705–713. doi: 10.1042/bj1880705. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matthews D. E., Bier D. M., Rennie M. J., Edwards R. H., Halliday D., Millward D. J., Clugston G. A. Regulation of leucine metabolism in man: a stable isotope study. Science. 1981 Dec 4;214(4525):1129–1131. doi: 10.1126/science.7302583. [DOI] [PubMed] [Google Scholar]
- Matthews D. E., Motil K. J., Rohrbaugh D. K., Burke J. F., Young V. R., Bier D. M. Measurement of leucine metabolism in man from a primed, continuous infusion of L-[1-3C]leucine. Am J Physiol. 1980 May;238(5):E473–E479. doi: 10.1152/ajpendo.1980.238.5.E473. [DOI] [PubMed] [Google Scholar]
- McGuire E. A., Helderman J. H., Tobin J. D., Andres R., Berman M. Effects of arterial versus venous sampling on analysis of glucose kinetics in man. J Appl Physiol. 1976 Oct;41(4):565–573. doi: 10.1152/jappl.1976.41.4.565. [DOI] [PubMed] [Google Scholar]
- Miles J. M., Nissen S. L., Rizza R. A., Gerich J. E., Haymond M. W. Failure of infused beta-hydroxybutyrate to decrease proteolysis in man. Diabetes. 1983 Mar;32(3):197–205. doi: 10.2337/diab.32.3.197. [DOI] [PubMed] [Google Scholar]
- Millward D. J., Garlick P. J., Nnanyelugo D. O., Waterlow J. C. The relative importance of muscle protein synthesis and breakdown in the regulation of muscle mass. Biochem J. 1976 Apr 15;156(1):185–188. doi: 10.1042/bj1560185. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Odedra B. R., Millward D. J. Effect of corticosterone treatment on muscle protein turnover in adrenalectomized rats and diabetic rats maintained on insulin. Biochem J. 1982 Jun 15;204(3):663–672. doi: 10.1042/bj2040663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- ROSE H. G., ROBERTSON M. C., SCHWARTZ T. B. Hormonal and metabolic influences on intracellular peptidase activity. Am J Physiol. 1959 Nov;197:1063–1069. doi: 10.1152/ajplegacy.1959.197.5.1063. [DOI] [PubMed] [Google Scholar]
- Robert J. J., Bier D. M., Zhao X. H., Matthews D. E., Young V. R. Glucose and insulin effects on the novo amino acid synthesis in young men: studies with stable isotope labeled alanine, glycine, leucine, and lysine. Metabolism. 1982 Dec;31(12):1210–1218. doi: 10.1016/0026-0495(82)90006-3. [DOI] [PubMed] [Google Scholar]
- STEELE R. Influences of glucose loading and of injected insulin on hepatic glucose output. Ann N Y Acad Sci. 1959 Sep 25;82:420–430. doi: 10.1111/j.1749-6632.1959.tb44923.x. [DOI] [PubMed] [Google Scholar]
- Sapir D. G., Pozefsky T., Knochel J. P., Walser M. The role of alanine and glutamine in steroid-induced nitrogen wasting in man. Clin Sci Mol Med. 1977 Sep;53(3):215–220. doi: 10.1042/cs0530215. [DOI] [PubMed] [Google Scholar]
- Shamoon H., Soman V., Sherwin R. S. The influence of acute physiological increments of cortisol on fuel metabolism and insulin binding to monocytes in normal humans. J Clin Endocrinol Metab. 1980 Mar;50(3):495–501. doi: 10.1210/jcem-50-3-495. [DOI] [PubMed] [Google Scholar]
- Shoji S., Pennington R. J. The effect of cortisone on protein breakdown and synthesis in rat skeletal muscle. Mol Cell Endocrinol. 1977 Jan;6(3):159–169. doi: 10.1016/0303-7207(77)90082-x. [DOI] [PubMed] [Google Scholar]
- Smith O. K., Long C. N. Effect of cortisol on the plasma amino nitrogen of eviscerated adrenalectomized-diabetic rats. Endocrinology. 1967 Apr;80(4):561–566. doi: 10.1210/endo-80-4-561. [DOI] [PubMed] [Google Scholar]
- Stacey-Schmidt C., Berg P., Haymond M. W. Use of D-glucosaminic acid as an internal standard in single-column accelerated amino acid analysis of physiological fluids. Anal Biochem. 1982 Jun;123(1):74–77. doi: 10.1016/0003-2697(82)90624-8. [DOI] [PubMed] [Google Scholar]
- Tomas F. M., Munro H. N., Young V. R. Effect of glucocorticoid administration on the rate of muscle protein breakdown in vivo in rats, as measured by urinary excretion of N tau-methylhistidine. Biochem J. 1979 Jan 15;178(1):139–146. doi: 10.1042/bj1780139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WOOL I. G., WEINSHELBAUM E. I. Corticosteroids and incorporation of C14-phenylalanine into protein of isolated rat diaphragm. Am J Physiol. 1960 May;198:1111–1114. doi: 10.1152/ajplegacy.1960.198.5.1111. [DOI] [PubMed] [Google Scholar]
- Wise J. K., Hendler R., Felig P. Influence of glucocorticoids on glucagon secretion and plasma amino acid concentrations in man. J Clin Invest. 1973 Nov;52(11):2774–2782. doi: 10.1172/JCI107473. [DOI] [PMC free article] [PubMed] [Google Scholar]


