Abstract
Sickle cell disease (SCD) is associated with acute vaso-occlusive crises that trigger painful episodes and frequently involves ongoing, chronic pain. Additionally, both humans and mice with SCD experience heighted cold sensitivity. However, studies have not addressed the mechanism(s) underlying the cold sensitization, nor its progression with age. Here we measured thermotaxis behavior in young and aged mice with severe SCD. Sickle mice had a marked increase in cold sensitivity measured by a cold preference test. Further, cold hypersensitivity worsened with advanced age. We assessed whether enhanced peripheral input contributes to the chronic cold pain behavior by recording from C fibers, many of which are cold-sensitive, in skin-nerve preparations. We observed that C fibers from sickle mice displayed a shift to warmer (more sensitive) cold-detection thresholds.
To address mechanisms underlying the cold sensitization in primary afferent neurons, we quantified mRNA expression levels for ion channels thought to be involved in cold detection. These included the Transient Receptor Potential Melastatin 8 (Trpm8) and TRP Ankyrin 1 (Trpa1) channels, as well as the two-pore domain potassium channels, TREK-1 (Kcnk2), TREK-2 (Kcnk4), and TRAAK (Kcnk10). Surprisingly, transcript expression levels of all of these channels were comparable between sickle and control mice. We further examined transcript expression of 83 additional pain-related genes and found increased mRNA levels for endothelin 1 and tachykinin receptor 1. These factors may contribute to hypersensitivity in sickle mice at both the afferent and behavioral levels.
Sensory neurons from sickle cell disease mice are sensitized to cold, mirroring behavioral observations, and have increased expression of endothelin 1 and tachykinin receptor 1.
Introduction
In sickle cell disease (SCD), sickle hemoglobin in erythrocytes polymerizes under low oxygen conditions, distorting the cell shape, leading to sporadic vaso-occlusive crises and tissue ischemia [21,39,54]. While the most severe pain occurs during these acute, tissue-damaging crises, many patients (50%) also develop chronic, baseline pain and suffer heightened cutaneous sensitivity during adulthood [8,54]. The lives of patients with SCD are further impacted by pain elicited by a host of innocuous, common stimuli including light wind, brushing of the skin [41], and enhanced detection of and pain sensitivity to mild cold [8,38,49]. Despite the severity and prevalence of SCD-associated pain, the underlying mechanisms remain poorly understood.
Recent evidence has shown that the Berkeley mouse model of severe sickle cell disease, in which mice express either 100% sickle or normal human hemoglobin [43], exhibits increased reflexive nocifensive behaviors in response to static cold (4°C and 20°C) [9,20]. However, the static cold temperature assay does not reflect the mouse's preferred temperature environment, nor does it offer sufficient resolution to quantify the degree of cold aversion. Therefore, there is a need to address how thermotaxis might be altered in sickle mice. Sensitization of peripheral afferent fibers may correlate to the psychophysical measurements of hyperalgesia and allodynia. Recently, we reported that primary afferent fibers in sickle mice are sensitized to mechanical stimuli, with subtypes of Aβ, Aδ and C fibers exhibiting heightened responses to both noxious mechanical and light touch stimuli [17,20]. In conjunction with altered central nervous system processing, enhanced peripheral cold detection may contribute to cold hypersensitivity in SCD.
The ability of sensory neurons to detect cold temperatures has been attributed to the cold activation properties of ion channels in peripheral nerve terminals. The Transient Receptor Potential Melastatin 8 (TRPM8) is reported to directly transduce mild cooling stimuli and is implicated in contributing to moderate and more noxious cold transduction [6,27,36]. Likewise, TRP Ankyrin 1 (TRPA1) contributes to cold sensitivity in multiple neuropathic and inflammatory states [11,12,42] and has been debated as a contributor to noxious cold transduction in naïve conditions [5,26,31,32,55]. Other channels involved in cold sensation include the two-pore domain potassium channels TREK-1 (KCNK2) [40], TREK-2 (KCNK10) [24], and TRAAK (KCNK4) [24,40]. Beyond direct ion channel gating, an overall increase in afferent excitability in SCD may also contribute to enhanced cold stimulus detection.
We first assessed cold sensitivity of sickle mice using a temperature preference assay. Since cold sensitivity relies upon the cold activation of peripheral sensory neurons, we also assessed the cold responses of C fiber nociceptors using teased fiber recordings [29,48]. Calcium imaging was used to determine if sensory neurons of sickle and control mice have altered responses to agonists of TRPM8 and TRPA1. In addition, we measured mRNA expression levels of known cold-sensitive ion channels and of 83 additional pain-related genes that may contribute to the peripheral neuron sensitization in sickle mice. To determine how the observed phenotype changes with age, we also performed behavioral analyses and mRNA expression studies using aged SCD animals.
Materials and Methods
Animals
In the Berkeley model of sickle cell disease, sickle mice (HbSS) express 100% sickle human hemoglobin in circulating erythrocytes, and this model mimics many pathological features of severe SCD in humans [43]. Control mice were either transgenic control (HbAA) mice that express 100% normal human hemoglobin on the same mixed Berkeley background or C57BL/6 wild-type mice (identified where appropriate throughout the manuscript). Cain and colleagues (2012) have previously shown that the C57BL/6 and HbAA mice have similar sensitivity to cold stimuli by assessing paw withdrawal latency and frequency [9]. Male sickle and control mice were used throughout these studies. Adult sickle mice and controls averaged 7.9 ± 0.3 months old; aged mice averaged 18.4 ± 0.4 months old, a point beyond which sickle mice show a rapid increase in mortality. Experimenters were blinded to genotype for behavioral studies, and wherever possible for teased fiber and calcium imaging studies. The care and use of animals, along with the experimental protocols used, were reviewed and approved by the Medical College of Wisconsin Institutional Animal Care and Use Committee.
Behavioral testing
For temperature preference assessment, HbSS or HbAA animals were placed in a 13″ square Plexiglas chamber in which the floor is a dual temperature-controlled metal plate allowing independent temperature control for each side (AHP1200-HCP, TECA Corp; Chicago, IL). The testing apparatus was placed in a constant location within a dedicated behavior testing room and all spatial cues remained constant throughout all experiments. All animals were habituated to the chamber (with 30°C on both plates) for 20 minutes prior to testing. For all temperature preference tests, both the time spent on each plate and the number of times the animal crossed the midline to the opposite side were recorded during a 5-minute testing period. Baseline measurements were performed on the first day of testing; animals were individually placed in the chamber with both floor plates set at 30°C (gently warm to the touch). Animals were then tested for preference between either 23°C and 30°C, or 20°C and 30°C. To control for a preferred plate side, we randomly altered both the plate where we introduced the animal (either the 20°C or 23°C and 30°C side) and the temperatures on each plate. We have found that excessive testing reduces the exploratory behavior of the animals, as determined by a decrease in the number of crosses during the later tests in preliminary studies (data not shown). Some groups of animals were also tested following experimentally-induced hypoxia (2 hours of hypoxia at 10% O2, followed by 3.5 hours at room air). Since multiple tests were performed on the same animal, including testing before and after hypoxia, the baseline preference at 30:30 was not repeated. The same animals were used before and after hypoxia to make the most direct comparison regarding the effect of hypoxia treatment. The experimenter was blinded to mouse genotype during testing.
To determine the extent of sensitivity to light mechanical touch, we used a Light Touch Behavioral Assay [16]. HbSS or HbAA mice were placed on a wire mesh platform in an enclosure, and habituated to this apparatus for an hour prior to testing. For this assay, the glabrous skin of the plantar hind paw was tested by applying a light, punctate 0.7 mN von Frey filament 10 times to each hind paw, and separately applying a dynamic light stimulus using a “puffed” cotton swab applied 5 times to each hind paw. Care was taken to avoid stimulating hair follicles during testing. For all mechanical behavior assays, we averaged the responses between both hind paws since no side difference would be expected with SCD. As before, the experimenter was blinded to mouse genotype.
Teased fiber skin-nerve recordings
The ex vivo saphenous skin-nerve preparation was utilized to determine the response properties of cutaneous primary afferent fibers in HbAA and HbSS mice following established protocols [48]. Briefly, ex vivo skin-nerve preparations were dissected from HbSS and control mice and immediately placed into a recording chamber superfused with oxygenated synthetic interstitial fluid at 32 ± 0.5°C [29]. The skin was placed in the chamber corium side up. The saphenous nerve was desheathed and teased in order to distinguish functionally discrete fibers, which were single, active fibers residing in a filament and which had a defined, isolated receptive field in the skin. A combined electrical and mechanical search of the corium side of the skin using a blunt glass rod was used to find active fibers, and an electrical stimulus was used to identify functionally single fibers.
Fibers were characterized by mechanical threshold using calibrated von Frey filaments (range 0.044-147.0 mN) and conduction velocity. Conduction velocity was measured by inserting a Teflon-coated steel stimulating electrode into the most mechanically-sensitive area of the receptive field and applying square-wave pulses (500 μs). Action potential latency and the distance between electrodes were quantified to determine conduction velocity. Functional units were classified by conduction velocity and adaptation properties, as previously described [32]. Fibers conducting ≤ 1.2 m/sec were C fibers; all recordings described herein were obtained from C fibers.
Once functionally single C fibers were isolated, a 2-minute baseline recording was taken to assess spontaneous, non stimulus-evoked activity. This activity rate was subtracted from subsequent recordings of the same fiber. A 20-second cold ramp of buffer from 32°C to 2°C was applied to the receptive field. We reasoned that such a cold ramp would be better than a static cold temperature pulse to identify a cold firing threshold and cold-induced responses to a wide range of temperatures. C fibers were considered cold sensitive if they responded to cold stimulation with at least 2 action potentials more than the average spontaneous activity rate. Action potential waveforms were visualized on an oscilloscope and audibly monitored using an audio amplifier and speaker. Extracellular recordings of action potentials were recorded and analyzed using LabChart 6 data acquisition software (ADInstruments; Colorado Springs, CO) on a PC for offline analysis.
Calcium Imaging
Lumbar 1-6 dorsal root ganglia (DRG) neurons were isolated bilaterally from HbSS and C57 mice and placed in Hank's Balanced Salt Solution (Gibco, Life Technologies; Carlsbad, CA). DRG neurons were cultured as previously described [4]. Ganglia were dissociated into single somata via enzymatic digestion and trituration through a P200 pipette tip. The neurons were plated onto laminin-coated glass coverslips and incubated overnight to allow adherence. The neurons were provided with complete cell medium consisting of DMEM/Hams-F12 medium supplemented with 10% heat-inactivated horse serum, 2 mM L-glutamine, 0.8% D-glucose, 100 units penicillin and 100 μg/ml streptomycin. No exogenous growth factors were added.
Calcium imaging was performed on small-diameter neurons (<27 μm) 18-24 hours after dissection using the dual-wavelength fluorescence indicator FURA-2 AM (Invitrogen, Life Technologies; Carlsbad, CA) as described previously [4]. Briefly, isolated DRG neurons were loaded with 2.5 μl/ml FURA-2 AM in an extracellular buffer containing 2% BSA for 45 min at room temperature, followed by a 30 min wash in extracellular buffer to ensure a more complete hydrolysis of the intracellular FURA-2-AM. Neurons were superfused with buffer at 6 ml/min using an AutoMate (Berkeley, CA) pressurized perfusion system. Recordings were conducted at room temperature (23 ± 1°C). Fluorescence images were captured with a cooled CCD camera (CoolSNAP FX, Photometrics; Tucson, AZ). MetaFluor imaging software was utilized in order to detect and analyze intracellular calcium changes throughout the experiment (Molecular Devices; Sunnyvale, CA). Baseline Fura-2 measurements were recorded during superfusion with extracellular buffer. Next, responses to a TRPM8 agonist, menthol, and separately responses to a TRPA1 agonist, cinnamaldehyde, were recorded. Previous experiments showed maximal responses to 1 mM menthol in patch clamp experiments, and observed calcium transients in response to both 10 μM and 100 μM menthol [44]. Additionally, the EC50 for cinnamaldehyde acting upon DRG neurons or transfected CHO cells is approximately 70 μM [3,4]. Thus, a submaximal concentration of either 100 μM menthol [35] or 70 μM cinnamaldehyde [3] was applied for 3 minutes. An increase in intracellular calcium of ≥20% above baseline was considered a response. At the end of each protocol, a 50 mM KCl solution was applied to depolarize neurons, thereby allowing for identification of viable small diameter sensory neurons.
Gene expression
Gene expression studies were performed on isolated dorsal root ganglia (T5-L6) removed from HbSS and HbAA mice. DRGs were dissected and stored overnight in RNAlater at -20°C. RNA was isolated using TRIzol Reagent following manufacturer's recommendations (LifeTechnologies; Carlsbad, CA). Total RNA was analyzed with a NanoDrop Lite spectrophotometer (Thermo Scientific; Wilmington, DE) and integrity assessed via visualization with ethidium bromide in a 1% agarose gel.
For assessment of individual ion channel mRNA expression levels, qPCR was performed on a Mastercycler ep Realplex2 thermal cycler (Eppendorf; Hamburg, Germany). RNA was reverse-transcribed to cDNA using SuperScript III First-Strand Synthesis System (Invitrogen; Carlsbad, CA). Real-time PCR was performed with SYBR Green using the following primers: Trpa1 (forward: GCTTTTGGCCTCAGCTTT, reverse: CTCGATAATTGATGTCTCCTAGCA), Trpm8 (forward: GTCTTCGTCCTCTTCTGTGAT, reverse: ACAATACCCGCTATGAAGTAGAAG), Trek1 (Kcnk2, IDT PrimeTime qPCR Assay Mm.PT.56a.14314703, Integrated DNA Technologies; Coralville, IA), Traak (Kcnk4, IDT assay Mm.PT.56a.7651558), and Trek2 (Kcnk10, IDT assay Mm.PT.56a.30470172). Expression of glyceraldehyde 3-phosphate dehydrogenase (Gapdh) was used as a housekeeping control gene (forward: AATGGTGAAGGTCGGTGTG, reverse: GTGGAGTCATACTGGAACATGTAG). The PCR conditions for Trpa1 and Trpm8 were 40 cycles of 15s at 95°C, 30s at 57°C, and 30s at 72°C, followed by melting curve analysis to ensure specificity of gene amplification. Conditions for Kcnk2 and Kcnk10 were 40 cycles of 15s at 95°C, 30s at 57°C, and 20s at 72°C; those for Kcnk4 were 40 cycles of 15 s at 95°C, 25 at 58°C, and 20s at 72°C. Calculations of comparative fold changes in mRNA expression levels between groups used a comparative Ct method, using ΔΔCt with normalization to Gapdh.
A PCR array of 84 genes related to neuropathic and inflammatory pain (SABiosciences; Venlo, Netherlands) was also performed on RNA extracted from DRGs (levels T5-L6) isolated from HbSS (n=6) and HbAA (n=7) mice. These PCR arrays are based on real-time PCR using optimized primer sets that have been validated by the manufacturer. For this assay, RNA was reverse transcribed using a protocol outlined by SABiosciences for use with their PCR arrays. PCR conditions were used as following the manufacturer's protocol. Gene expression was normalized to the following three housekeeping genes: Gapdh, β-actin, and heat shock protein 90α (cystosolic class B member 1) (Hsp90ab1). Also included were a mouse genomic DNA contamination control, a reverse transcription control, and a positive PCR control.
Data Analysis
Temperature preference (percent time spent on a given side) was analyzed by 2-way ANOVA, comparing young and old mice, using a Bonferroni post hoc test. An additional repeated-measures 2-way ANOVA with Bonferroni post hoc was used to compare temperature preference with or without hypoxia treatment. The number of crosses performed during thermal preference testing was analyzed in a similar manner. Baseline behavior on the thermal plate at 30°C was compared to a hypothetical value of 50% with a one way t-test for both genotypes. The percentage of cold responsive C fibers in skin-nerve recordings was analyzed with Fisher's exact tests. The percentage of neurons responding to the chemical agonists (cinnamaldehyde and menthol) in calcium imaging experiments was also analyzed with Fisher's exact tests. Expression of individual genes was compared between four groups (2 ages × 2 genotypes) using a 2-way ANOVA and a Bonferroni post hoc. Gene expression levels measured in the PCR array were analyzed with the ΔΔCt method using RT2 Profiler PCR Array Data Analysis software provided by SABiosciences (Qiagen). Data from the remaining assays were compared using two-tailed student's t-tests when two groups were considered. Data was analyzed using the Prism software (GraphPad; La Jolla, CA). Results were considered statistically significant when P < 0.05. Within the Results section, data are presented as means ± SEM.
Results
Enhanced cold avoidance in sickle mice
We have previously reported that sickle mice exhibit a heightened sensitivity to static cold temperatures (20°C) [20], and others have reported hypersensitivity to extreme cold (4°C) [9]. While this is one method used to identify cold hyperalgesia in sickle mice, it does not recapitulate the hypersensitivity that humans with SCD experience in response to smaller changes in environmental cold stimuli. Therefore, we used a temperature preference assay using a milder cold temperature with reduced deviation from skin temperature, where one plate was set to 23°C and the other to 30°C. This assay also removes possible bias from observers' interpretation of mouse responses. Our baseline measurement, with both plates set to 30°C, revealed a similar amount of time spent on each plate (Fig. 1A, left) and number of crosses between plates (Fig. 1B, left). This ensured that there was no overall baseline preference for one side of the chamber based on spatial or other cues. We then allowed HbSS or HbAA control mice to choose between the 23°C and 30°C plates. We found a marked decrease in the time that sickle mice spent on the 23°C plate (36.3 ± 4.0% spent on cold). HbAA mice exhibited no aversion to the 23°C plate, as they spent 46.1 ± 2.5% on the cold plate (Fig. 1A, left). Sickle mice and controls explored both thermal plates equally, as there was no difference in the number of crosses between genotypes (Fig. 1B, left).
Figure 1.
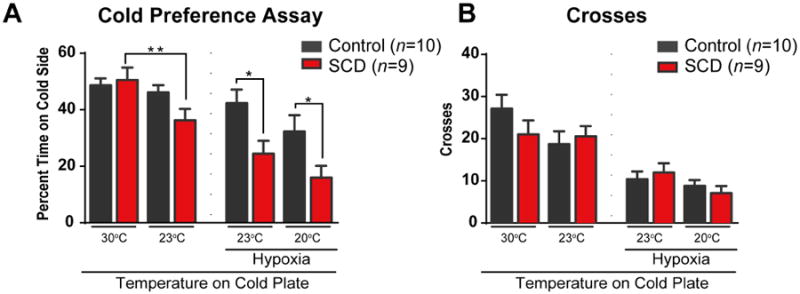
Sickle mice display cold temperature aversion, which is enhanced following induced sickling crises. (A) Both HbAA and HbSS male mice show no preference between the sides of the thermal preference plate at baseline when both sides are at 30 degrees (“baseline”), as evidenced by both groups spending time on one side at a rate not significantly different from chance during a 5 minute test (50%, P > 0.05). When the temperature on the test plate was reduced to 23°C, HbSS mice spent significantly less time on the cold plate compared to their baseline preferences (**P < 0.01). Following hypoxia and reoxygenation, sickle animals showed an enhanced avoidance of the cold plate compared to the control animals, when tested on cold plates of both 23°C (* P < 0.05) and 20°C (* P < 0.05). (B) During all behavioral thermal preference tests, there were no differences in the number of plate crosses compared between sickle and HbAA control animals (P > 0.05).
We then used 2 hours of hypoxia to further exacerbate the SCD phenotype to promote acute sickling. At 3.5 hours after returning to room air following hypoxia exposure, mouse behavior was again tested using the 23°C and 30°C plates. Sickle mice exhibited a marked aversion to the cold plate, and spent significantly less time on the cold plate than did the HbAA controls (Fig. 1A, right). Sickle mice also exhibited a trend toward a decreased time spent on the cold plate after hypoxia compared to their preference before hypoxia (p=0.08). On the other hand, control HbAA mice responded to the 23°C cold plate similarly before and after hypoxia (p=0.60). We then decreased cold plate temperature further to 20°C from 23°C to evoke a response from control mice and compare these responses with those from sickle mice. The sickle mice continued to exhibit increased cold sensitivity when compared to the HbAA controls and to their initial baseline test (Fig. 1A). There was no difference in the number of crosses between groups following hypoxia, showing that sickle mice did not have less general activity compared to control mice (Fig. 1B, right).
Sensitization of C fibers contributes to cold hypersensitivity
Chronic pain associated with disease can have both peripheral and central components. Therefore, to address one peripheral component, we asked whether peripheral afferent neurons in sickle mice were sensitized to cold. To test for increased sensitivity along the afferent axon and terminals of cutaneous C fibers, we used the ex vivo skin-saphenous nerve preparation and quantified the number of action potentials fired during a 20-second cold buffer ramp from 32°C to 2°C applied to the cutaneous receptive field. C fibers were considered cold-sensitive if they responded to the cold ramp with more than two action potentials above the fiber's average spontaneous activity rate. We found a similar percentage of cold-sensitive C fibers from sickle mice (57%) compared to HbAA controls (52%) (Fig. 2A). Of these cold-sensitive C fibers, those from sickle mice responded at higher (warmer) temperatures of the cold ramp, with a cold threshold at 19.2 ± 1.2 °C compared to 14.6 ± 1.6 °C in HbAA controls (Fig. 2B). However, the cold-sensitive C fibers from HbSS and HbAA mice fired similar numbers of cold-evoked action potentials during the cold ramp (Fig. 2C; P = 0.14). Together, these data showing warmer cold thresholds suggest that peripheral afferent terminals are sensitized to cold.
Figure 2.

C fibers from sickle mice are sensitized to cold stimuli. (A) C fibers isolated from sickle mice were activated by cold stimulation at a similar proportion compared to HbAA control C fibers. (B) Cold-activated C fibers from sickle mice had a significantly lower cold detection threshold, meaning they responded to cold stimulation at warmer temperatures than HbAA controls (P < 0.05). (C) There was no significant difference in the number of action potentials fired by cold-responsive C fibers from SCD or HbAA mice in response to cold stimulation (P = 0.1848).
Aged sickle mice exhibit increasing sensitivity to cold
In humans, sickle cell disease involves the development of chronic pain [54]. Importantly, human patients with sickle cell disease also have heightened sensitivity to cold stimulation, reporting both cold pain and cold detection at lower thresholds (warmer temperatures) [8]. In that study, older age was also associated with lowered (warmer) thresholds for both cold pain and detection (interquartile range of all study participants with SCD: 11-19 years); this was observed in both the sickle cell disease group and the healthy control group. This suggests that the cold hypersensitivity in sickle mouse populations may also increase with age. To test this, we repeated the temperature preference assay in aged sickle and control HbAA mice (average 18.4 months), at an age corresponding to a much older human population (56+ years) [14]. While we, and others, have reported increased thermal and mechanical sensitivity of younger adult sickle mice [9,17,20,28], sensitivity has not been assessed in aged sickle mice. When compared with younger adult animals (average 7.9 months; Fig. 1 and 3), we found enhanced cold aversion in old sickle mice, which spent only 15.9 ± 3.6% of the time on the 23°C cold plate, compared to 36.2 ± 4.0% in the younger HbSS adults (Fig. 3A, left). The aged HbAA mice continued to show little aversion to the 23°C cold plate (Fig. 3A). There were no differences between the number of crosses performed by HbAA and HbSS mice, showing that aged sickle mice did not have decreased general exploratory activity compared to aged control mice (Fig. 3B, right).
Figure 3.
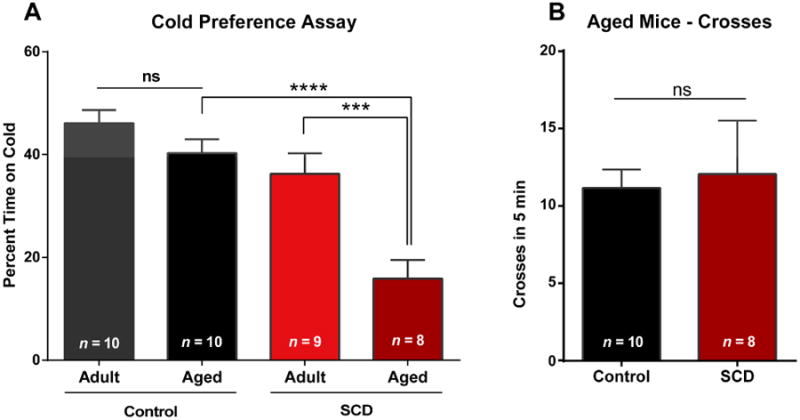
Cold aversion increases with age in sickle mice. Aged mice are on average 18.4 (± 0.4) months; younger adult mice are on average 7.9 (± 0.3) months. Mouse behavior was tested using a thermally-controlled floor with one side at 30°C and the other at 23°C, and cold aversion was measured as the percent of time spent on the colder plate during a 5 minute test. (A) Aged male HbSS mice portrayed heightened cold aversion compared with younger adult HbSS mice (*** P < 0.001). Additionally, the aged HbSS mice showed much greater cold aversion than their aged control counterparts (**** P < 0.0001). Aged male HbAA mice (controls) show no difference in cold aversion or thermal preference compared to younger adult HbAA mice (P > 0.05). (B) There were no differences in the number of crosses performed by older control or HbSS mice (P > 0.05).
The enhanced cold aversion in aged sickle mice prompted us to ask if there was a more generalized age-related behavioral change. Because human populations report increased pain in elderly sickle patient populations [54] and because we previously found hypersensitivity to light touch in sickle mice, we used a Light Touch Behavioral Assay on the aged mice. As expected, we found increased light touch sensitivity in the HbSS mice compared to age-matched HbAA mice when testing an aged population (Fig. S1A, Fig. S1B). However, unlike the enhanced age-related cold sensitivity in aged HbSS mice, there was no change in sensitivity to light mechanical touch in aged sickle mice compared to younger adult sickle mice.
Expression of known cold-sensitive channels does not change in sickle sensory neurons
The cold phenotype at both the behavioral and afferent terminal levels led us to ask what ion channels might be contributing to the increased neuronal excitability. Because transduction of the cold stimulus by the neuron may be affected, we investigated sensory neuron expression of the known cold-sensitive channel TRPM8 [36]. We also quantified expression levels of TRPA1, which has been reported to be involved in cold sensitivity [31,55], particularly in circumstances of tissue injury [11,12,42]. In addition, a change in functional expression of TRPM8 or TRPA1 could contribute to afferent activity. Therefore we used TRPM8 and TRPA1 agonists (menthol and cinnamaldehyde, respectively) to quantify both the percentage of TRPM8- and TRPA1-expressing small-diameter neurons and their response amplitude, as many of these small neurons correspond to C fibers in vivo, and they comprise a significant population of nociceptors and thermoreceptors. We expected to find an increase in responsiveness to agonists of TRPM8, TRPA1 or both in DRG neurons from sickle mice. Using calcium imaging, we found no differences in the percentage of neurons that responded to submaximal concentrations of either the TRPM8 agonist, menthol (Fig. 4A), or the TRPA1 agonist, cinnamaldehyde (Fig. 4C). This suggests that there are equal proportions of TRPM8- and TRPA1-expressing small-diameter neurons in sickle and control C57 mice. Further, we found no increase in the amplitude of response to either agonist (Fig. 4B,D). This result suggests that TRPM8- and TRPA1-independent mechanisms are likely mediating the enhanced cold response in sickle mice.
Figure 4.

Sickle and control (C57) DRGs have similar functional expression of cold-sensitive ion channels. (A) Isolated small-diameter DRG neurons from control (C57BL/6) and sickle mice were exposed to 100 μM menthol, and their responses assessed via calcium imaging. A similar percentage of small neurons from control and sickle mice responded to the chemical stimulation. (B) Control (C57) and sickle neurons responded to 100 μM menthol with a similar amplitude response of calcium influx, as measured by percent increase in Fura-2 ratio over baseline measurements. (C) Additional groups of small-diameter DRG neurons were exposed to 70 μM cinnamaldehyde. There was no significant difference in the percentage of sickle or control neurons responding to cinnamaldehyde stimulation. (D) Control and sickle neurons also responded to 70 μM cinnamaldehyde stimulation with similar amplitude increases in intracellular calcium.
We concurrently quantified expression of Trpm8 and Trpa1 mRNA expression levels in whole DRGs that contain the cell bodies of all sensory afferent subpopulations (Aβ, Aδ and C fiber neurons) by qPCR. All mRNA expression studies were performed using HbSS and HbAA mice. In agreement with our findings from the calcium imaging data with TRPM8 and TRPA1 agonists, we found no differences in mRNA expression levels between HbSS and HbAA mice (Trpa1 Fig. 5A, Trpm8 Fig. 5C). Moreover, because we found that cold hypersensitivity increased in aged sickle mice behaviorally, we quantified expression levels in aged DRG neurons. Again, we found no differences between aged sickle and control neurons (Trpa1 Fig. 5B, Trpm8 Fig. 5D). We then quantified the potassium channels Kcnk2, Kcnk4, and Kcnk10, because evidence has linked these channels to altered cold responses [24,40]. We also observed no differences in the mRNA expression levels of any of these channels (Fig. 5E).
Figure 5.
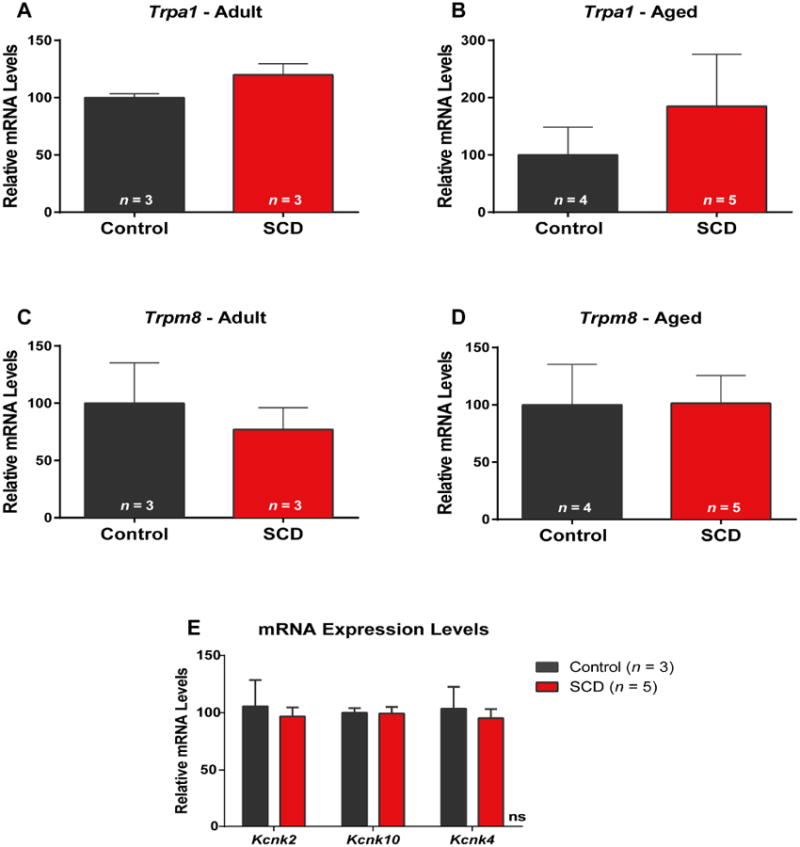
Sickle (HbSS) and HbAA control DRGs have similar mRNA expression levels of cold-sensitive ion channels. (A-D) There were no differences in the mRNA expression of either Trpa1 (A-B) or Trpm8 (C-D) between control and SCD DRGs obtained from aged or younger adult mice. (E) Similarly, there were no differences in the expression levels of Kcnk2, Kcnk10, or Kcnk4 measured from DRGs collected from younger adult SCD and HbAA mice (P > 0.05).
Sickle DRGs have increased expression of Tachkykinin 1 Receptor and Endothlelin 1
Our previous work has shown that using a TRPV1-specific antagonist results in reduced mechanical hypersensitivity in sickle mice [20]. TRPV1 is not known to be mechanically-sensitive, and therefore it is likely that there is an upregulation of a diverse set of proteins that may underlie the increased responses of primary afferents to cold and to touch by sensitizing TRPV1 and one or more cold-sensitive channels. Therefore we investigated the transcript expression levels of a broad range of genes in DRGs that have been documented to be involved in pain and inflammation and that might contribute to the SCD phenotype. Using a PCR array of 84 genes commonly implicated in pain sensation, we observed limited dysregulation of gene expression between DRGs from younger adult HbSS and HbAA control animals (Supplemental Table 1). For example, there were no changes in glial cell line derived neurotrophic factor, nerve growth factor, GTP cyclohydrolase 1, or the assessed interleukins, bradykinin receptors, purinergic receptors, cannabinoid receptors, adenosine receptors, or prostaglandin receptors. However, mRNA expression level of the tachykinin receptor 1 (neurokinin 1 receptor; substance P receptor) was increased 2.7-fold in DRGs from sickle animals compared to control animals (Supplemental Table 1). Expression of the precursor gene of the ligand of tachykinin receptor 1 (Tac1; tachykinin; preprotachykinin) was not altered between SCD and control animals.
Secondly, expression of the ligand endothelin 1 also increased 1.6-fold in sickle DRGs compared to controls (Supplemental Table 1). Conversely, we observed no differences in expression of the receptor for endothelin 1, endothelin receptor A (ETA). Also of note, we observed no differences in mRNA expression of the voltage-gated sodium channels tested (SCN3a/Nav1.3, SCN9a/Nav1.7, SCN10a/Nav1.8, and SCN11a/Nav1.9). Additionally, there was no dysregulation in expression of the remaining genes of the 84 assessed genes, including Trpa1, which we had previously investigated using traditional qPCR.
Discussion
The mechanisms contributing to cold hypersensitivity in SCD patients and sickle mice are poorly understood. Here we report increased cold aversion in sickle mice, which corresponds to previous reports of increased sensitivity to cold in both humans and mice with SCD [8,20,54]. The measurement of thermotaxis behavior via temperature preference allows higher resolution analysis of cold sensitivity and aversion than previous studies in mice using static cold plates. We report a phenotype in which younger adult sickle mice exhibited enhanced hypersensitivity to mildly cold temperatures following hypoxia/reoxygenation, which was designed to partially mimic acute sickling crises in humans. These findings were similar to our previous report showing that chronic mechanical hypersensitivity was exacerbated by oxidative stress [20]. Our findings also correlate with reports that hypoxia/reoxygenation reduces paw withdrawal latency from a static cold plate at 4°C [9]. The oxidative stress from reoxygenation may be sufficient to sensitize ion channels involved in cold sensitivity or to upregulate their expression.
Additionally, we observed that the cold-induced behavioral response increased with age selectively in sickle mice, but not in HbAA controls. These results correlate well with findings that cold sensitivity in the human SCD patient population increases with age [8]. To our knowledge, this is the only clinical study that has quantitatively addressed the age component of cold sensitivity in SCD. In general, few studies have investigated changes in somatosensation throughout normal aging [7,15], and even fewer studies have related sensation and aging in disease conditions. Thus, there is a need to better understand how chronic disease processes, like those in SCD, contribute to chronic pain and immeasurably impact the quality of life. Beyond the direct sensitivity to cold, some studies suggest that SCD patients may experience increased pain intensity and frequency in the colder months, even in the absence of sickling crisis [41,47,50,53]. This further highlights that the link between cold and pain in SCD warrants further investigation.
The behavioral cold hypersensitivity in SCD appears to be due, at least in part, to enhanced contribution of peripheral afferent fibers. Our findings demonstrate that peripheral afferent fibers from sickle mice are sensitized to cold stimuli. Further, our findings indicate that the sensitization is a stable phenotype not dependent on intact vasculature or concurrent hypoxia. This enhanced peripheral contribution in SCD may be also compounded in vivo by central mechanisms, leading to the behavioral cold hypersensitivity. Regarding the peripheral component, one possible mechanism is that the first-line, cold-sensing mechanism of the neurons is altered in either expression levels or function.
To assess whether TRPM8 and TRPA1 are sensitized or functionally upregulated in SCD, we used a functional approach (calcium imaging). Agonists for these channels linked to cold sensitivity, namely menthol for TRPM8 and cinnamaldehyde for TRPA1, were chosen because they are selective for those channels at the concentrations used. However, we found no functional evidence that either channel is sensitized or upregulated to directly contribute to the altered cold response in SCD mice.
To expand beyond these functional results, we utilized the more sensitive technique, qPCR, to evaluate expression levels of Trpa1 and Trpm8, and also of Kcnk2, Kcnk4, and Kcnk10. While we did not observe dysregulation in the mRNA expression of any of the channels tested, it is possible that these channels may be sensitized to the effects of cold through some form of posttranslational modification, covalent modification or other form of dynamic interactions. Additionally, there could be a change in localized expression, trafficking, or sensitization at the nerve terminals that is not apparent at the DRG somata.
Sickle mice appear to be sensitized to multiple modalities of somatosensory input. This study and others [9,17,20,28] demonstrate that sickle mice are hypersensitive to a range of intensities of mechanical stimuli, ranging from light to noxious, and to cold. It is possible that oxidative stress, induced by hypoxia from vaso-occlusion, may lead to dysregulation of proteins that interact with the cold receptors on sensory neurons. These changes could modulate cold sensitivity without affecting either TRPM8 or TRPA1 responses to chemical stimuli, which were used in the calcium imaging studies to assess functional expression. A similar modulatory mechanism may be occurring with other cold-sensitive channels.
We expected to find dysregulation in expression levels of genes known to be involved in neuropathic and inflammatory pain. We reasoned that although mechanical and cold sensitivity were both enhanced in afferent terminals, each modality likely relies on the involvement of different protein complexes. The skin-nerve recordings revealed differences in the threshold at which C fibers began to fire cold-evoked action potentials, but no difference in the number of cold-sensitive afferents or in action potential firing frequency between sickle and control mice. This data suggests that proteins involved in cold transduction, and not action potential propagation, likely dictate cold sensitivity in the sickle mice. However, our previous findings showed that it was the mechanically-evoked action potential firing frequency, not mechanical threshold, which increased in C fibers of SCD mice [20], suggesting a possible involvement of proteins that contribute to both mechanical amplification and action potential propagation. Therefore, we expanded our search by using the PCR array of proteins that are widely involved in pain. This search revealed that DRGs from sickle mice have increased mRNA expression of endothelin 1 and tachykinin receptor 1. Interestingly, there was also no change in expression of Nav 1.3, 1.7, 1.8, or 1.9 sodium channels known to be involved in action potential threshold or generation (Supplemental Table 1) [30].
Endothelin 1 has long been implicated in pain modulation [13,23]. Endothelin 1 may act indirectly, possibly by sensitizing vascular endothelial cells to mechanical stimulation and ATP release [22]. It may also act directly at sensory neurons by activating both endothelin receptors ETA and ETB to sensitize TRP channels; ETA and ETB are each co-expressed on ∼30% of TRPV1-immunopositive neurons [10], and endothelin 1 modulates TRPV1 through the ETA receptor [46,60]. TRPA1 may also be involved in the pain-like behavior induced by ET1 [34]. Endothelin 1 could also lead to neuronal sensitization through other mechanisms, as endothelin 1 activity can increase intracellular calcium levels, activate protein kinases, and alter activation gating of Nav1.8 and Nav1.9 [25].
Interestingly, intradermal injection of endothelin 1 rapidly induces cold hyperalgesia in humans [19]. Similarly, blockade of either ETA or ETB reduces nerve injury-induced cold allodynia in rats [59]. Not only is endothelin 1 vastly increased in plasma during a vaso-occlusive sickling event, it also remains elevated at one to three weeks following hospital discharge after a crisis [18]. Further, a study in children with SCD showed a positive correlation between plasma endothelin 1 levels and self-reported pain during acute procedural pain caused medically-required venipuncture [52]. As endothelin 1 appears to be increased in both plasma and locally at the DRGs, endothelin 1 at either location may be contributing to mechanical as well as cold hypersensitivity and pain in SCD. Further parallel studies of protein expression levels and functional assays will likely help determine the functional consequence of changes in mRNA transcript levels in vivo.
Tachykinin receptor 1 (NK1 receptor) and its primary ligand, substance P, also play a role in pain and sensation [45,56,57]. In addition to substance P release at the spinal cord during injury, substance P is also released from the sensory nerve endings into the peripheral tissues, consequently contributing to cutaneous neurogenic inflammation [51]. Cutaneous tissues, including peripheral nerves and blood vessels, of sickle mice display enhanced substance P immunoreactivity [28]. Serum levels of substance P are increased in patients with sickle cell disease, and rise further during painful crises [37]. Mirroring the findings in human patients, substance P is also increased in plasma of SCD mice [58]. The same study also showed that neurogenic inflammation occurs in mice with SCD, and that mast cell activation contributes to this inflammatory state. Interestingly, mast cell inhibition with imatinib reduces plasma substance P levels to near those found in control mice. Inhibition of mast cell activation with either cromolyn sodium or imatinib reduces both substance P and CGRP release into the skin and in DRGs. Moreover, mast cell inhibition partly ameliorates the hypoxia-induced exacerbation of hyperalgesia [58]. Thus, mast cell activation and subsequent substance P release appears to have major implications for pain in SCD.
While previous studies have focused on sensory neuron release of substance P onto NK1 receptor-expressing spinal cord neurons or into skin, [1,58] few have assessed NK1 receptor expression or function in dorsal root ganglia. The baseline expression levels of NK1 receptor may be low in DRGs [2]; however, an upregulation of expression may lead to previously-unstudied effects on sensory neuron excitability. There is evidence suggesting that NK1 receptors located in both the peripheral and central terminals of sensory neurons are involved in sensation [33]. Therefore, the upregulation of tachykinin receptor 1 expression at the DRG may also contribute to the pain and hypersensitivity in sickle mice. Consequently, examining proteins that modulate pain sensitivity in sickle populations in subsequent studies may provide insight into the mechanism of SCD-related pain.
Supplementary Material
Supplemental Figure 1. Sickle mice retain increased sensitivity to light touch with age, and remain more sensitive than HbAA controls. Mice were tested using a Dual Light Touch behavioral assay. (A) Testing with a 0.7 mN von Frey filament revealed a significant effect of genotype, as previously reported, in that adult sickle mice have hypersensitivity to punctate light touch compared to age-matched adult controls (*** P < 0.001). Aged sickle mice are also more sensitive to punctate light touch compared to aged control mice (** P < 0.01). However, the sensitivity to light touch was not different between aged and adult sickle mice or aged and adult controls (P > 0.05). (B) Testing with a cotton swab revealed that younger adult sickle mice are hypersensitive to dynamic light touch compared to young controls (* P < 0.05). Similarly, aged sickle mice remain hypersensitive to dynamic light touch compared to aged controls (**P < 0.01). Again, there were no significant differences in sensitivity to dynamic light touch between aged and younger sickle mice, or between aged and younger control mice.
Supplemental Table 1. PCR array revealed differences in gene expression in DRGs T5-L6 isolated from SCD (n=6) and HbAA (n=7) mice. Fold change in expression was determined using the ΔΔCt method. Two genes assessed in a PCR array of pain-related genes were revealed to be significantly upregulated: tachykinin receptor 1 and endothelin 1.
Acknowledgments
We thank Victoria Muller Ewald (MCW/Luther College) for assisting with the PCR experiments and Marianne Escobar-Klumph for assistance with calcium imaging experiments. We also thank Dawn Retherford for helping with hypoxia administration and Thomas Foster for assistance with generating the sickle mice. This work was completed with support from the National Institutes of Health grants NS070711 (C.L.S. and C.A.H), NS040538 (C.L.S.), HL102836 (C.A.H.) and PA-08-190 Supplemental Award to NS07011 (S.R.G.). Katherine Zappia is a member of the MCWMSTP which is partially supported by a T32 grant from NIGMS, GM080202. Funded through the Research and Education Initiative Fund, a component of the Advancing a Healthier Wisconsin endowment at the Medical College of Wisconsin and the Midwest Athletes Against Childhood Cancer Fund (CAH).
Footnotes
The authors declare no conflict of interest.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Abbadie C, Trafton J, Liu H, Mantyh PW, Basbaum AI. Inflammation increases the distribution of dorsal horn neurons that internalize the neurokinin-1 receptor in response to noxious and non-noxious stimulation. J Neurosci. 1997;17(20):8049–8060. doi: 10.1523/JNEUROSCI.17-20-08049.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Andoh T, Nagasawa T, Kuraishi Y. Expression of tachykinin NK1 receptor mRNA in dorsal root ganglia of the mouse. Brain Res Mol Brain Res. 1996;35(1-2):329–332. doi: 10.1016/0169-328x(95)00244-m. [DOI] [PubMed] [Google Scholar]
- 3.Bandell M, Story GM, Hwang SW, Viswanath V, Eid SR, Petrus MJ, Earley TJ, Patapoutian A. Noxious cold ion channel TRPA1 is activated by pungent compounds and bradykinin. Neuron. 2004;41(6):849–857. doi: 10.1016/s0896-6273(04)00150-3. [DOI] [PubMed] [Google Scholar]
- 4.Barabas ME, Kossyreva EA, Stucky CL. TRPA1 is functionally expressed primarily by IB4-binding, non-peptidergic mouse and rat sensory neurons. PLoS One. 2012;7(10):e47988. doi: 10.1371/journal.pone.0047988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Bautista DM, Jordt SE, Nikai T, Tsuruda PR, Read AJ, Poblete J, Yamoah EN, Basbaum AI, Julius D. TRPA1 mediates the inflammatory actions of environmental irritants and proalgesic agents. Cell. 2006;124(6):1269–1282. doi: 10.1016/j.cell.2006.02.023. [DOI] [PubMed] [Google Scholar]
- 6.Bautista DM, Siemens J, Glazer JM, Tsuruda PR, Basbaum AI, Stucky CL, Jordt SE, Julius D. The menthol receptor TRPM8 is the principal detector of environmental cold. Nature. 2007;448(7150):204–208. doi: 10.1038/nature05910. [DOI] [PubMed] [Google Scholar]
- 7.Bowden JL, McNulty PA. Age-related changes in cutaneous sensation in the healthy human hand. Age (Dordr) 2013;35(4):1077–1089. doi: 10.1007/s11357-012-9429-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Brandow AM, Stucky CL, Hillery CA, Hoffmann RG, Panepinto JA. Patients with sickle cell disease have increased sensitivity to cold and heat. Am J Hematol. 2013;88(1):37–43. doi: 10.1002/ajh.23341. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Cain DM, Vang D, Simone DA, Hebbel RP, Gupta K. Mouse models for studying pain in sickle disease: effects of strain, age, and acuteness. Br J Haematol. 2012;156(4):535–544. doi: 10.1111/j.1365-2141.2011.08977.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chichorro JG, Fiuza CR, Bressan E, Claudino RF, Leite DF, Rae GA. Endothelins as pronociceptive mediators of the rat trigeminal system: role of ETA and ETB receptors. Brain Res. 2010;1345:73–83. doi: 10.1016/j.brainres.2010.04.075. [DOI] [PubMed] [Google Scholar]
- 11.da Costa DS, Meotti FC, Andrade EL, Leal PC, Motta EM, Calixto JB. The involvement of the transient receptor potential A1 (TRPA1) in the maintenance of mechanical and cold hyperalgesia in persistent inflammation. Pain. 2010;148(3):431–437. doi: 10.1016/j.pain.2009.12.002. [DOI] [PubMed] [Google Scholar]
- 12.del Camino D, Murphy S, Heiry M, Barrett LB, Earley TJ, Cook CA, Petrus MJ, Zhao M, D'Amours M, Deering N, Brenner GJ, Costigan M, Hayward NJ, Chong JA, Fanger CM, Woolf CJ, Patapoutian A, Moran MM. TRPA1 contributes to cold hypersensitivity. J Neurosci. 2010;30(45):15165–15174. doi: 10.1523/JNEUROSCI.2580-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Ferreira SH, Romitelli M, de Nucci G. Endothelin-1 participation in overt and inflammatory pain. J Cardiovasc Pharmacol. 1989;13(Suppl 5):S220–222. doi: 10.1097/00005344-198900135-00065. [DOI] [PubMed] [Google Scholar]
- 14.Flurkey KCJ, C J, Harrison DE. The mouse in aging research. In: F JG, editor. Normative Biology, Husbandry, and Models. III. Burlington, MA: American College of Laboratory Animal Medicine (Elsevier); 2007. pp. 637–672. [Google Scholar]
- 15.Gagliese L. Pain and aging: the emergence of a new subfield of pain research. J Pain. 2009;10(4):343–353. doi: 10.1016/j.jpain.2008.10.013. [DOI] [PubMed] [Google Scholar]
- 16.Garrison SR, Dietrich A, Stucky CL. TRPC1 contributes to light-touch sensation and mechanical responses in low-threshold cutaneous sensory neurons. J Neurophysiol. 2012;107(3):913–922. doi: 10.1152/jn.00658.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Garrison SR, Kramer AA, Gerges NZ, Hillery CA, Stucky CL. Sickle cell mice exhibit mechanical allodynia and enhanced responsiveness in light touch cutaneous mechanoreceptors. Mol Pain. 2012;8:62. doi: 10.1186/1744-8069-8-62. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Graido-Gonzalez E, Doherty JC, Bergreen EW, Organ G, Telfer M, McMillen MA. Plasma endothelin-1, cytokine, and prostaglandin E2 levels in sickle cell disease and acute vaso-occlusive sickle crisis. Blood. 1998;92(7):2551–2555. [PubMed] [Google Scholar]
- 19.Hans G, Deseure K, Robert D, De Hert S. Neurosensory changes in a human model of endothelin-1 induced pain: a behavioral study. Neurosci Lett. 2007;418(2):117–121. doi: 10.1016/j.neulet.2007.03.008. [DOI] [PubMed] [Google Scholar]
- 20.Hillery CA, Kerstein PC, Vilceanu D, Barabas ME, Retherford D, Brandow AM, Wandersee NJ, Stucky CL. Transient receptor potential vanilloid 1 mediates pain in mice with severe sickle cell disease. Blood. 2011;118(12):3376–3383. doi: 10.1182/blood-2010-12-327429. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hillery CA, Panepinto JA. Pathophysiology of stroke in sickle cell disease. Microcirculation. 2004;11(2):195–208. doi: 10.1080/10739680490278600. [DOI] [PubMed] [Google Scholar]
- 22.Joseph EK, Green PG, Bogen O, Alvarez P, Levine JD. Vascular endothelial cells mediate mechanical stimulation-induced enhancement of endothelin hyperalgesia via activation of P2X2/3 receptors on nociceptors. J Neurosci. 2013;33(7):2849–2859. doi: 10.1523/JNEUROSCI.3229-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Joseph EK, Levine JD. Sexual dimorphism in endothelin-1 induced mechanical hyperalgesia in the rat. Exp Neurol. 2012;233(1):505–512. doi: 10.1016/j.expneurol.2011.11.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Kang D, Choe C, Kim D. Thermosensitivity of the two-pore domain K+ channels TREK-2 and TRAAK. J Physiol. 2005;564(Pt 1):103–116. doi: 10.1113/jphysiol.2004.081059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Khodorova A, Montmayeur JP, Strichartz G. Endothelin receptors and pain. J Pain. 2009;10(1):4–28. doi: 10.1016/j.jpain.2008.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Knowlton WM, Bifolck-Fisher A, Bautista DM, McKemy DD. TRPM8, but not TRPA1, is required for neural and behavioral responses to acute noxious cold temperatures and cold-mimetics in vivo. Pain. 2010;150(2):340–350. doi: 10.1016/j.pain.2010.05.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Knowlton WM, Palkar R, Lippoldt EK, McCoy DD, Baluch F, Chen J, McKemy DD. A sensory-labeled line for cold: TRPM8-expressing sensory neurons define the cellular basis for cold, cold pain, and cooling-mediated analgesia. J Neurosci. 2013;33(7):2837–2848. doi: 10.1523/JNEUROSCI.1943-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Kohli DR, Li Y, Khasabov SG, Gupta P, Kehl LJ, Ericson ME, Nguyen J, Gupta V, Hebbel RP, Simone DA, Gupta K. Pain-related behaviors and neurochemical alterations in mice expressing sickle hemoglobin: modulation by cannabinoids. Blood. 2010;116(3):456–465. doi: 10.1182/blood-2010-01-260372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Koltzenburg M, Stucky CL, Lewin GR. Receptive properties of mouse sensory neurons innervating hairy skin. J Neurophysiol. 1997;78(4):1841–1850. doi: 10.1152/jn.1997.78.4.1841. [DOI] [PubMed] [Google Scholar]
- 30.Krafte DS, Bannon AW. Sodium channels and nociception: recent concepts and therapeutic opportunities. Curr Opin Pharmacol. 2008;8(1):50–56. doi: 10.1016/j.coph.2007.09.007. [DOI] [PubMed] [Google Scholar]
- 31.Kwan KY, Allchorne AJ, Vollrath MA, Christensen AP, Zhang DS, Woolf CJ, Corey DP. TRPA1 contributes to cold, mechanical, and chemical nociception but is not essential for hair-cell transduction. Neuron. 2006;50(2):277–289. doi: 10.1016/j.neuron.2006.03.042. [DOI] [PubMed] [Google Scholar]
- 32.Kwan KY, Glazer JM, Corey DP, Rice FL, Stucky CL. TRPA1 modulates mechanotransduction in cutaneous sensory neurons. J Neurosci. 2009;29(15):4808–4819. doi: 10.1523/JNEUROSCI.5380-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Lever IJ, Grant AD, Pezet S, Gerard NP, Brain SD, Malcangio M. Basal and activity-induced release of substance P from primary afferent fibres in NK1 receptor knockout mice: evidence for negative feedback. Neuropharmacology. 2003;45(8):1101–1110. doi: 10.1016/s0028-3908(03)00298-3. [DOI] [PubMed] [Google Scholar]
- 34.Liang J, Bi H, Ji W. Involvement of TRPA1 in ET-1-induced pain-like behavior in mice. Neuroreport. 2010;21(3):201–205. doi: 10.1097/WNR.0b013e328335b3c5. [DOI] [PubMed] [Google Scholar]
- 35.Macpherson LJ, Hwang SW, Miyamoto T, Dubin AE, Patapoutian A, Story GM. More than cool: promiscuous relationships of menthol and other sensory compounds. Mol Cell Neurosci. 2006;32(4):335–343. doi: 10.1016/j.mcn.2006.05.005. [DOI] [PubMed] [Google Scholar]
- 36.McKemy DD, Neuhausser WM, Julius D. Identification of a cold receptor reveals a general role for TRP channels in thermosensation. Nature. 2002;416(6876):52–58. doi: 10.1038/nature719. [DOI] [PubMed] [Google Scholar]
- 37.Michaels LA, Ohene-Frempong K, Zhao H, Douglas SD. Serum levels of substance P are elevated in patients with sickle cell disease and increase further during vaso-occlusive crisis. Blood. 1998;92(9):3148–3151. [PubMed] [Google Scholar]
- 38.Mohan J, Marshall JM, Reid HL, Thomas PW, Hambleton I, Serjeant GR. Peripheral vascular response to mild indirect cooling in patients with homozygous sickle cell (SS) disease and the frequency of painful crisis. Clin Sci (Lond) 1998;94(2):111–120. doi: 10.1042/cs0940111. [DOI] [PubMed] [Google Scholar]
- 39.Niscola P, Sorrentino F, Scaramucci L, de Fabritiis P, Cianciulli P. Pain syndromes in sickle cell disease: an update. Pain Med. 2009;10(3):470–480. doi: 10.1111/j.1526-4637.2009.00601.x. [DOI] [PubMed] [Google Scholar]
- 40.Noel J, Zimmermann K, Busserolles J, Deval E, Alloui A, Diochot S, Guy N, Borsotto M, Reeh P, Eschalier A, Lazdunski M. The mechano-activated K+ channels TRAAK and TREK-1 control both warm and cold perception. EMBO J. 2009;28(9):1308–1318. doi: 10.1038/emboj.2009.57. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Nolan VG, Zhang Y, Lash T, Sebastiani P, Steinberg MH. Association between wind speed and the occurrence of sickle cell acute painful episodes: results of a case-crossover study. Br J Haematol. 2008;143(3):433–438. doi: 10.1111/j.1365-2141.2008.07354.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Obata K, Katsura H, Mizushima T, Yamanaka H, Kobayashi K, Dai Y, Fukuoka T, Tokunaga A, Tominaga M, Noguchi K. TRPA1 induced in sensory neurons contributes to cold hyperalgesia after inflammation and nerve injury. J Clin Invest. 2005;115(9):2393–2401. doi: 10.1172/JCI25437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Paszty C, Brion CM, Manci E, Witkowska HE, Stevens ME, Mohandas N, Rubin EM. Transgenic knockout mice with exclusively human sickle hemoglobin and sickle cell disease. Science. 1997;278(5339):876–878. doi: 10.1126/science.278.5339.876. [DOI] [PubMed] [Google Scholar]
- 44.Peier AM, Moqrich A, Hergarden AC, Reeve AJ, Andersson DA, Story GM, Earley TJ, Dragoni I, McIntyre P, Bevan S, Patapoutian A. A TRP channel that senses cold stimuli and menthol. Cell. 2002;108(5):705–715. doi: 10.1016/s0092-8674(02)00652-9. [DOI] [PubMed] [Google Scholar]
- 45.Pitcher GM, Henry JL. Nociceptive response to innocuous mechanical stimulation is mediated via myelinated afferents and NK-1 receptor activation in a rat model of neuropathic pain. Exp Neurol. 2004;186(2):173–197. doi: 10.1016/j.expneurol.2003.10.019. [DOI] [PubMed] [Google Scholar]
- 46.Plant TD, Zollner C, Mousa SA, Oksche A. Endothelin-1 potentiates capsaicin-induced TRPV1 currents via the endothelin A receptor. Exp Biol Med (Maywood) 2006;231(6):1161–1164. [PubMed] [Google Scholar]
- 47.Redwood AM, Williams EM, Desal P, Serjeant GR. Climate and painful crisis of sickle-cell disease in Jamaica. British Medical Journal. 1976;1(6001):66–68. doi: 10.1136/bmj.1.6001.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Reeh PW. Sensory receptors in a mammalian skin-nerve in vitro preparation. Prog Brain Res. 1988;74:271–276. doi: 10.1016/s0079-6123(08)63024-1. [DOI] [PubMed] [Google Scholar]
- 49.Resar LM, Oski FA. Cold water exposure and vaso-occlusive crises in sickle cell anemia. J Pediatr. 1991;118(3):407–409. doi: 10.1016/s0022-3476(05)82156-0. [DOI] [PubMed] [Google Scholar]
- 50.Rogovik AL, Persaud J, Friedman JN, Kirby MA, Goldman RD. Pediatric vasoocclusive crisis and weather conditions. J Emerg Med. 2011;41(5):559–565. doi: 10.1016/j.jemermed.2010.05.006. [DOI] [PubMed] [Google Scholar]
- 51.Roosterman D, Goerge T, Schneider SW, Bunnett NW, Steinhoff M. Neuronal control of skin function: The skin as a neuroimmunoendocrine organ. Physiol Rev. 2006;86(4):1309–1379. doi: 10.1152/physrev.00026.2005. [DOI] [PubMed] [Google Scholar]
- 52.Schlenz AM, McClellan CB, Mark TR, McKelvy AD, Puffer E, Roberts CW, Sweitzer SM, Schatz JC. Sensitization to acute procedural pain in pediatric sickle cell disease: modulation by painful vaso-occlusive episodes, age, and endothelin-1. J Pain. 2012;13(7):656–665. doi: 10.1016/j.jpain.2012.04.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Smith WR, Bauserman RL, Ballas SK, McCarthy WF, Steinberg MH, Swerdlow PS, Waclawiw MA, Barton BA Multicenter Study of Hydroxyurea in Sickle Cell A. Climatic and geographic temporal patterns of pain in the Multicenter Study of Hydroxyurea. Pain. 2009;146(1-2):91–98. doi: 10.1016/j.pain.2009.07.008. [DOI] [PubMed] [Google Scholar]
- 54.Smith WR, Scherer M. Sickle-cell pain: advances in epidemiology and etiology. Hematology Am Soc Hematol Educ Program. 2010;2010:409–415. doi: 10.1182/asheducation-2010.1.409. [DOI] [PubMed] [Google Scholar]
- 55.Story GM, Peier AM, Reeve AJ, Eid SR, Mosbacher J, Hricik TR, Earley TJ, Hergarden AC, Andersson DA, Hwang SW, McIntyre P, Jegla T, Bevan S, Patapoutian A. ANKTM1, a TRP-like channel expressed in nociceptive neurons, is activated by cold temperatures. Cell. 2003;112(6):819–829. doi: 10.1016/s0092-8674(03)00158-2. [DOI] [PubMed] [Google Scholar]
- 56.Stucky CL, Galeazza MT, Seybold VS. Time-dependent changes in Bolton-Hunter-labeled 125I-substance P binding in rat spinal cord following unilateral adjuvant-induced peripheral inflammation. Neuroscience. 1993;57(2):397–409. doi: 10.1016/0306-4522(93)90071-m. [DOI] [PubMed] [Google Scholar]
- 57.Teodoro FC, Tronco Junior MF, Zampronio AR, Martini AC, Rae GA, Chichorro JG. Peripheral substance P and neurokinin-1 receptors have a role in inflammatory and neuropathic orofacial pain models. Neuropeptides. 2013;47(3):199–206. doi: 10.1016/j.npep.2012.10.005. [DOI] [PubMed] [Google Scholar]
- 58.Vincent L, Vang D, Nguyen J, Gupta M, Luk K, Ericson ME, Simone DA, Gupta K. Mast cell activation contributes to sickle cell pathobiology and pain in mice. Blood. 2013;122(11):1853–1862. doi: 10.1182/blood-2013-04-498105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Werner MF, Trevisani M, Campi B, Andre E, Geppetti P, Rae GA. Contribution of peripheral endothelin ETA and ETB receptors in neuropathic pain induced by spinal nerve ligation in rats. Eur J Pain. 2010;14(9):911–917. doi: 10.1016/j.ejpain.2010.03.001. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto T, Ono K, Hitomi S, Harano N, Sago T, Yoshida M, Nunomaki M, Shiiba S, Watanabe S, Nakanishi O, Inenaga K. Endothelin receptor-mediated responses in trigeminal ganglion neurons. J Dent Res. 2013;92(4):335–339. doi: 10.1177/0022034513478428. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplemental Figure 1. Sickle mice retain increased sensitivity to light touch with age, and remain more sensitive than HbAA controls. Mice were tested using a Dual Light Touch behavioral assay. (A) Testing with a 0.7 mN von Frey filament revealed a significant effect of genotype, as previously reported, in that adult sickle mice have hypersensitivity to punctate light touch compared to age-matched adult controls (*** P < 0.001). Aged sickle mice are also more sensitive to punctate light touch compared to aged control mice (** P < 0.01). However, the sensitivity to light touch was not different between aged and adult sickle mice or aged and adult controls (P > 0.05). (B) Testing with a cotton swab revealed that younger adult sickle mice are hypersensitive to dynamic light touch compared to young controls (* P < 0.05). Similarly, aged sickle mice remain hypersensitive to dynamic light touch compared to aged controls (**P < 0.01). Again, there were no significant differences in sensitivity to dynamic light touch between aged and younger sickle mice, or between aged and younger control mice.
Supplemental Table 1. PCR array revealed differences in gene expression in DRGs T5-L6 isolated from SCD (n=6) and HbAA (n=7) mice. Fold change in expression was determined using the ΔΔCt method. Two genes assessed in a PCR array of pain-related genes were revealed to be significantly upregulated: tachykinin receptor 1 and endothelin 1.


