Abstract
Agonists for neurotensin NTS1 receptor consistently exhibit antipsychotic effects in animal models without producing catalepsy, suggesting that NTS1 receptor agonists may be a novel class of drugs to treat schizophrenia. Moreover, studies utilizing NTS1 agonists have reported improvements in some aspects of cognitive functioning, including prepulse inhibition and learning procedures, that suggest an ability of NTS1 receptor agonists to diminish neurocognitive deficits. The present study sought to assess both baseline delay-induced memory performance and the effects of NTS1 receptor activation on learning and memory consolidation in male Long Evans and Brown Norway rats using a delayed non-match to position radial arm maze task. In the absence of drugs, Brown Norway rats displayed a significant increase in spatial memory errors following a 3, 7, and 24 hour delay, whereas Long Evans rats exhibited an increase in spatial memory errors following only a 7 and 24 hour delay. With Brown Norway rats, administration of PD149163 before or after an information trial significantly reduced errors during a retention trial after a 24 hour delay. Administration of the NTS1/2 receptor antagonist SR142948 prior to the information trial did not affect retention trial errors. These data are consistent with previous findings that Brown Norway rats have natural cognitive deficits and that they may be useful for assessing putative antipsychotic drugs for cognitive efficacy. Moreover, this study supports previous findings suggesting that NTS1 receptor agonists may improve some aspects of cognitive functioning.
Keywords: neurotensin, PD149163, SR142928, Brown Norway, Long Evans, delayed nonmatch to position, memory, learning, rat
Neurotensin is a neuropeptide neurotransmitter that produces effects similar to those produced by antipsychotic drugs in preclinical models (Richelson, Fredrickson, & Boules, 2005; Tanganelli et al., 2012), but unlike currently available antipsychotic drugs neurotensin does so through binding only to neurotensin receptors (Pettibone et al., 2002; Schotte et al., 1996). Brain-penetrant agonists for neurotensin NTS1 receptors, such as NT69L (Cusack et al., 2000) and PD149163 (Petrie et al., 2004), demonstrate antipsychotic-like efficacy in animal models including inhibition of conditioned avoidance responding (Hertel, Olsen, & Arnt, 2002; Holly, Ebrecht, & Prus, 2011), reversal of amphetamine-induced hyperactivity (Boules et al., 2001; Cusack et al., 2000; Feifel et al., 2008), and prevention of amphetamine-induced disruptions of prepulse inhibition of startle (PPI) (Feifel, Reza, Wustrow, & Davis, 1999; Shilling, Richelson, & Feifel, 2003). Moreover, several results from animal studies indicate that NTS1 receptor agonists produce effects similar to those produced by atypical antipsychotic drugs. Like atypical antipsychotic drugs, NTS1 receptor agonists reverse PPI disruption induced by NMDA and serotonin (5-HT)2A receptor antagonists (Feifel, Melendez, & Shilling, 2003; Feifel et al., 1999; Shilling, Melendez, Priebe, Richelson, & Feifel, 2004). Moreover, NTS1 receptor agonists do not elicit catalepsy (Cusack et al., 2000; Feifel, Melendez, & Shilling, 2004; Holly et al., 2011; Sarhan, Hitchcock, Grauffel, & Wettstein, 1997), which generally does not occur for atypical antipsychotic drugs at therapeutically-effective doses (Meltzer, 2004).
A particularly attractive feature of NTS1 receptor agonists may be an efficacy for cognitive deficits, which are a core feature of schizophrenia. Current antipsychotic drugs only modestly alleviate these deficits (Meltzer & McGurk, 1999; Woodward, Purdon, Meltzer, & Zald, 2005), which strongly contribute to poor functional outcomes, such as an inability to carry out daily living activities and gain employment, in patients with this disorder (Green, Kern, Braff, & Mintz, 2000; Harvey et al., 2012). Intracerebroventricular administration of PD149163 has been shown to reverse scopolamine-induced working memory deficits in novel object recognition (Azmi, Norman, Spicer, & Bennett, 2006), and systemic administration of PD149163 has been shown to improve social discrimination in Brattleboro rats (Feifel et al., 2009) and to selectively increase hippocampal-dependent aversive trace conditioning (Grimond-Billa, Norman, Bennett, & Cassaday, 2008), although improvements were not shown for appetitive trace conditioning (Norman, Grimond-Billa, Bennett, & Cassaday, 2010). Microinjection of neurotensin or PD149163 into the entorhinal cortex in rats was also shown to enhance firing rates and to improve spatial learning in a Barnes Maze test (Xiao et al., 2014). Further, Tirado-Santiago and colleagues (2006) demonstrated that administration of an NTS1 antagonist, SR48692, into the nucleus accumbens prior to training sessions was shown to increase working and reference memory errors in a radial arm maze. Li and colleagues (2011) identified two single nucleotide polyphormisms of the NTS1 receptor (rs4334545 and rs6090453) that were significantly correlated with working memory performance in humans, and based on these associations, the study authors concluded that the NTS1 receptor was important for cognitive functioning.
The present study sought to further evaluate the role of NTS1 receptors in memory function by assessing the effects of the NTS1 receptor agonist PD149163 on memory errors in a delayed non-match to position radial arm maze task in rats. Further, this study explored these effects using two different strains of rats -- Long Evans (LE) rats and Brown Norway (BN) rats. LE rats are commonly used in learning and memory studies, as well as for tasks used to study drugs of abuse, such as self administration. BN rats have been shown to exhibit innate deficits associated with schizophrenia including deficits in latent inhibition (Conti, Palmer, Vanella, & Printz, 2001) and prepulse inhibition (PPI) (Conti, Costill, Flynn, & Tayler, 2005; Feifel, Shilling, & Melendez, 2011; Palmer et al., 2000; Parwani et al., 2000). Moreover, antipsychotic drugs have significantly improved PPI in BN rats (Feifel et al., 2011). Due to evidence suggesting the involvement of NTS1 receptors in memory processing and research revealing innate cognitive deficits in BN rats, we hypothesized that BN rats would exhibit significant impairments compared to LE rats and that the NTS1 receptor agonist PD149163 would reduce memory errors. The study also evaluated the effects of the NTS1/2 receptor antagonist SR141948, which we hypothesized to increase the number of memory errors.
Method
Animals
Ten experimentally-naive male BN rats (obtained from a breeding facility at the University of California-San Diego, San Diego, CA USA) and 10 experimentally-naive male LE rats (Charles River Laboratories, Portage, MI USA) were used for these experiments, which were conducted at Northern Michigan University (Marquette, MI USA). All rats were housed in a vivarium maintained under constant temperature and humidity with rooms lights adjusted to a 12 hr light/dark cycle, with experiments conducted during the light cycle. Rats had free access to water in their home cages, but food was rationed to maintain 85% of free-feeding weights. LE rats were two months old and BN rats were four months old when the experimental procedures began. The procedures were approved by the Institutional Animal Care and Use Committee at Northern Michigan University, and were consistent with the Guide for the Care and Use of Laboratory Animals (National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, & National Academies Press, 2011).
Equipment
The radial arm maze apparatus consisted of eight 45 cm arms that projected from a central octagonal platform (Med-Associates, St. Albans, VT, USA). A food-pellet dispenser (45 mg grain pellets, Bio-Serve, Frenchtown, NJ, USA) was located at the end of each arm. The maze was controlled and behavior was recorded using Ethovision video monitoring software (Noldus, Leesburg, VA USA). The surrounding room walls were fixed with different patterns and shapes to aid in spatial navigation.
Drugs
The NTS1 receptor agonist PD149163 (Lys(CH2NH)-Lys-Pro-Trp-tLeu-Leu-OEt) and the NTS1/2 receptor antagonist SR142948 (2-[[5-(2,6-Dimethoxyphenyl)-1-(4-(3-N′,N′-dimethylaminopropyl)-N-methylcarbamoyl)-2-isopropylphenyl)-1H-pyrazole-3-carbonyl]amino]adamantane-2-carboxylic acid HCl) were generously provided by the National Institute of Mental Health Chemical Synthesis and Drug Supply Program (Bethesda, MD USA). Both drugs were in salt form. PD149163 was dissolved in 0.9% physiological saline and administered 30 min prior to the information trial. SR142948 was dissolved in water by sonication and was administered 1 hr prior to the information trial. Drugs were prepared in a 1 mg/ml volume and administered subcutaneously. Pretreatment times were based upon preliminary studies in this laboratory.
Delayed nonmatch to position procedure
Rats were habituated to the maze for two consecutive days. During habituation sessions, each rat was placed in the center starting platform and allowed to explore all of the arms for 10 minutes. Food pellets were scattered throughout the maze to encourage exploration. Following habituation, delayed non-match to position training began.
Each daily session of training consisted of two trials, an information and retention trial. In the first trial (the information trial), four randomly selected arms were baited with a single pellet from the dispenser and then the doors to only these four arms were raised. The information trial ended after rats retrieve food from these arms; however, the trial was terminated if the food was not retrieved within 5 min.
Following successful completion the information trial, rats were placed in a holding cage, and then following a delay (initially a 1 min delay), were returned to the maze for the retention trial. During this trial the remaining/opposite four arms were baited with food and a rat was now allowed access to all eight arms. Memory errors were recorded during the retention trial; a memory error was defined as an entry into an arm baited with food in the information trial or re-entry into a baited arm in the retention trial. No rat was given the same arm configuration on the same day and arm configurations were randomized for each rat daily. Retention trials ended after a rat obtained all four food pellets or after 5 minutes elapsed. The maze was wiped and cleaned with a 20% isopropanol alcohol solution in between trials and animals to attenuate potential olfactory cues. During training, the delay between the information and retention trial began with 1 min and was extended to 5 min and eventually 30 min as rats met the training criteria of no more than one retention trial memory error over two consecutive days.
Test sessions were conducted every 2–3 days. The first test sessions consisted of an assessment of retention trial errors occurring after a delay of either a 0, 0.5, 1, 3, 7, or 24 hours after completing the information trial. A 24 hr delay was selected following these tests to evaluate the ability of PD149163 to reduce retention trial errors given that a significant number of errors occurred during the retention trial after this delay. After the completion of delay testing, animals were tested with PD149163 (both post- and pre-information trial administration) and, after a one-week washout period with PD149163, rats were then tested with SR141948. In order to see if the neurotensin antagonist was effective in inducing memory errors, shorter delays of 1 and 3 hours, where fewer errors occurred, were used for testing SR141948.
Data analysis
The data were reported as means (+/− the standard error of the mean [SEM]). Dependent variables in this study consisted of the number of errors occurring in a retention trial and the duration time needed to complete a retention trial. Analyses were conducted to determine 1) the number of sessions required for each strain to meet the training criteria, 2) the effects of different delays between the information and retention trial on retention trial errors and trial duration, 3) effects of doses of PD149163 on retention trial errors and trial duration, and 4) effects of SR142948 on retention trial errors and trial duration. Homogeneity of variance could not be assumed for the statistical comparison of the number of sessions to meet the training criteria between the LE and BN rats, and therefore, a Mann Whitney test was performed instead of an independent samples t test. A mixed two factor analysis of variance (ANOVA) was conducted using strain as a between groups factor and drug (with dose serving as different levels for this factor) as a within subjects factor on the number of retention trial errors or duration of time to complete retention trials. Given that this study was primarily conducted to assess the effects of an NTS1 receptor agonist on memory performance, rather than comparing LE and BN rats per se, a one factor repeated ANOVA was also conducted to assess the effects of PD149163 (as the only factor) on errors and trial duration within each strain of rats. Similarly, dependent samples t-tests were also conducted to determine the effects of SR142948 (vehicle versus 1.0 mg/kg) on retention trial errors and trial duration within each strain of rats. Statistically significant effects were further assessed using a Dunnett’s post hoc test for comparisons to a control condition or with Bonferroni’s test for simple effect mean comparisons between strains. All analyses were performed using GraphPad Prism version 6 for Windows (GraphPad Software, La Jolla, CA USA).
Results
Training
All rats met the training criteria of one error or less in a session for 2 consecutive daily sessions. LE rats required significantly fewer training sessions (M = 18.10 sessions, SEM = 1.77) than BN rats (M= 35.60 sessions, SEM = 8.57) to meet the training criteria, U = 19, p < 0.05.
Delay testing
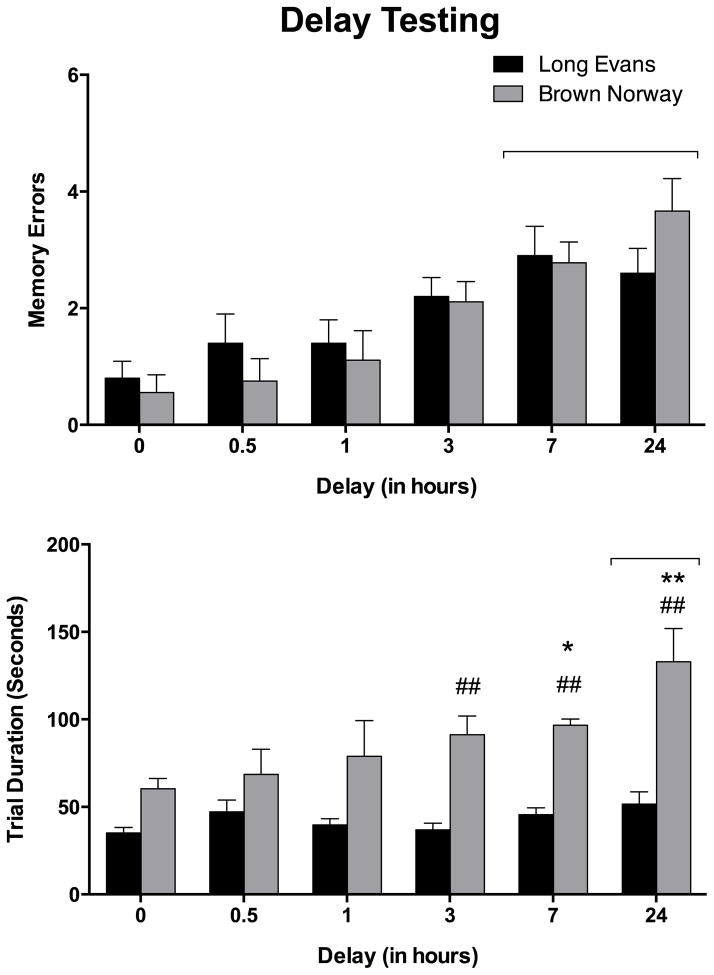
The effects of different delays (0 min, 30 min, 1 hr, 3 hr, 7 hr, and 24 hr) on retention trial errors are shown in figure 1. A mixed two-factor ANOVA on memory errors during the retention trial revealed a statistically significant effect of time, F (5, 90) = 11.72, p < 0.001, but not for strain or for an interaction between these factors. Post hoc testing indicated that significantly more errors occurred after 7 and 24 hr delays compared to a 0 hr delay. These results were the basis for selecting a 24 hr delay for drug testing with PD149163 and SR141948.
Figure 1.
The effects of inter-trial interval delays on number of memory errors (top) and trial duration (bottom) in the absence of drug during the retention trial in male Long Evans (black bars) and Brown Norway (grey bars) rats. The bars represent means (+/−SEM). * and ** indicate a significant difference (p < 0.05, and p < 0.01, respectively) compared to a 0 hr delay. ## indicates a significant difference (p < 0.01) between Brown Norway rats and Long Evans rats. The horizontal bracket indicates delays significantly different (p < 0.05) from a 0 hr delay. See text for other details.
Differences in trial duration were found for time, F = (5, 90), 4.71, p < 0.001, strain, F (1, 18) = 40.47, p < 0.0001, and an interaction between time and strain, F (5, 90) = 2.50, p < 0.05. Durations were significantly longer after a 24 hr delay compared to a 0 hr delay, and average durations were also significantly longer in the BN rats compared to the LE rats. An assessment of simple effect means indicated that trial durations increased with longer delays only in the BN rats and was significantly longer at delays of 7 and 24 hours compared to no delay. The trial duration for BN rats was significantly longer than LE rats following delays of 3, 7, and 24 hours.
PD149163
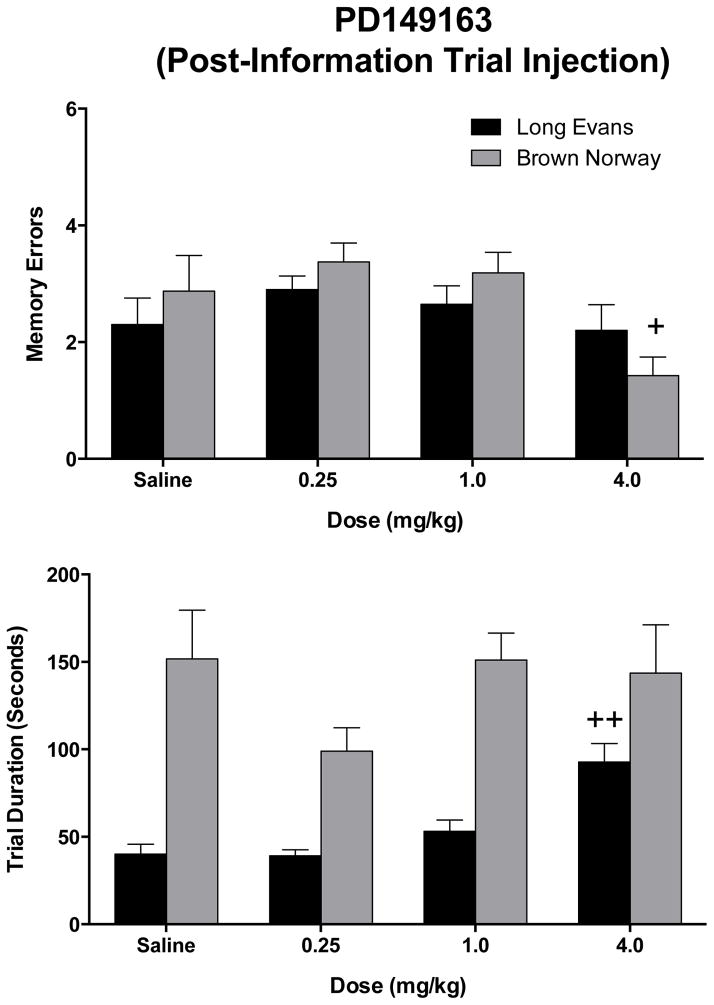
PD149163 was administered either immediately after the information trial or 30 min prior to the information trial in order to determine the effects of PD149163 on acquisition of information or consolidation of memory, respectively (figure 2, top panel). Administration of PD149163 (0.25 – 4.0 mg/kg) after the information trial led to significant main effect for PD149163, F (3, 48) = 4.92, p < 0.01, but neither for strain nor an interaction, on retention trial errors. Post hoc testing did not reveal a significant difference between doses of PD149163 versus saline, however.
Figure 2.
The effects of PD149163, injected immediately after the information trial, on retention trial memory errors (top) and retention trial duration (bottom) conducted 24 hours after the information trial in male Long Evans (black bars) and Brown Norway (grey bars) rats. The bars represent means (+/−SEM). + and ++ indicate a significant difference (p < 0.05, and p < 0.01, respectively) compared to saline within the same strain of rats. See text for other details.
One factor repeated measures ANOVAs were also conducted to assess the effects of PD149163 on retention errors and trial duration (below) within each strain of rats. While PD149163 was not shown to affect the number of memory errors occurring in the LE rats, a significant decrease in memory errors was shown in the BN rats, F (3, 21)= 4.54, p < 0.05). Post hoc testing found a significant decrease in errors for the 4.0 mg/kg dose compared to saline.
Statistically significant effects on retention trial duration were found for strain, F (1, 16) = 46.37, p < 0.0001, and dose, F (3, 48) = 4.04, p < 0.05, but not for an interaction (figure 2, bottom panel). BN rats required longer trial durations than the LE rats. While a significant effect for dose was found, neither of the doses produced a significant difference in duration compared to saline. From the one factor analysis, a significant effect on retention trial duration in the LE rats occurred, F (3, 27) = 12.51, p < 0.001, after administration of a 4.0 mg/kg dose compared to vehicle. No differences for trial duration were shown in the BN rats.
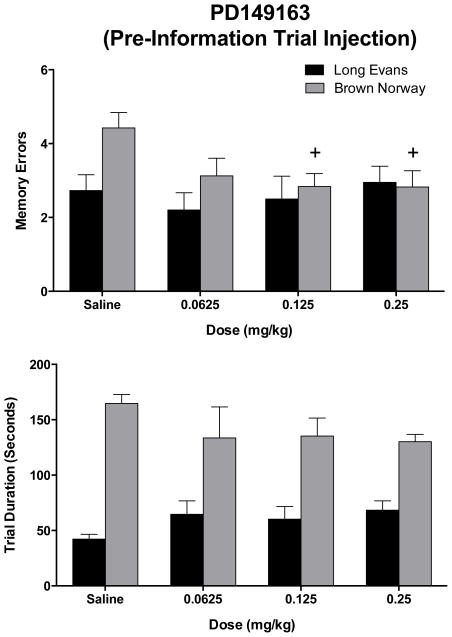
The doses selected for PD149163 were lowered to 0.0625 – 0.25 mg/kg for administration prior to the information trial, because doses above this range were found to completely suppress responding during information trial. Administration of PD149163 prior to the information trial (figure 3, top panel) did not significantly alter the number of memory errors between strains, across doses of PD149163, or for an interaction between strain and drug. An assessment of errors across doses of PD149163 within each strain only revealed that administration of PD149163 before the information trial significantly decreased memory errors in the BN rats, (F[3, 21]= 3.31, p<0.05), which occurred for a 0.125 mg/kg and 0.25 mg/kg dose compared to vehicle. BN rats required significantly more time to complete the retention trials than the LE rats, F (1, 16) = 58.06, p < 0.0001, and significant effects on duration were neither found across doses of PD149163 nor for an interaction between PD149163 and strain (figure 3, bottom panel). An analysis within each strain of rats using a repeated measures one factor ANOVA also did not reveal differences between doses of PD149163.
Figure 3.
The effects of PD149163, injected 30 min prior to the information trial, on retention trial memory errors (top) and retention trial duration (bottom) conducted 24 hours after the information trial in male Long Evans (black bars) and Brown Norway (grey bars) rats. The bars represent means (+/−SEM). + indicates a significant difference (p < 0.05) compared to saline within the same strain of rats.
SR142948
SR142948 (1.0 mg/kg) did not significantly alter memory errors or trial duration in either LE or BN rats after a 1 or 3 hour delay (table 1).
Table 1.
Effects of pre-information trial administration of SR142948 on retention trial memory errors
| Long Evans | Brown Norway | ||
|---|---|---|---|
| Drug (dose) | Intertrial interval | Mean errors (+/−SEM) | Mean errors (+/−SEM) |
| SR142948 | 1 hr | 0.67 (0.15) | 1.40 (0.39) |
| 3 hr | 1.14 (0.43) | 1.45 (0.41) | |
| Vehicle | 1 hr | 0.89 (0.41) | 1.44 (0.21) |
| 3 hr | 1.22 (0.33) | 2.00 (0.54) |
Discussion
This study provided the first known reported assessment of the BN rat in a radial arm maze delayed non-match to position task. The current findings indicate that BN rats had greater memory deficits compared to LE rats as evidenced by the fact that 1) BN rats required more trials to learn this procedure than the LE rats and 2) at the 24-hour delay BN rats exhibited a 6.6 fold increase in memory errors compared to a 3.25 fold increase in memory errors for LE rats. The BN rats in our studies were older than the LE rats (2 months vs. 4 months) but it is unlikely that this age difference contributed to poorer performance by BN rats compared to LE rats. Both 2- and 4-month old rats are considered young adults and not at a stage where significant senescent changes occur (Woldon-Hanson, 2006).
The findings from this study are consistent with previous findings that the BN strain exhibits cognitive deficits compared to other strains. Strain comparisons were carefully evaluated by van der Staay and colleagues (1990) who found that BN rats exhibited cognitive deficits between 5 weeks to 3 months of age and that these cognitive deficits persisted with limited change until after 19 months of age. Later, van der Staay and colleagues (1996) found that 3-month old BN rats exhibited slower learning in a water maze, delayed non-match to position operant task, and two-way active avoidance task compared to Wistar and Fischer-344 rats. Further, 5-week old BN rats have been shown to have reference and working memory deficits compared to albino WAG rats (van der Staay, 1999). Taken together, the current findings support previous work indicating that BN rats exhibit relatively poor cognitive functioning.
In this study the NTS1 receptor agonist PD149163 significantly improved memory in the BN, but not LE, rats. These effects occurred with the administration of PD149163 before and immediately after the information trial, suggesting an improvement in both information acquisition and memory consolidation, respectively. Lower doses of PD149163 (0.125 and 0.25 mg/kg) were effective for improving acquisition of information during the information trial, whereas a higher dose of PD149163 (4.0 mg/kg) was necessary to improve consolidation of information from the information trial. This is the first study, to our knowledge, to specifically evaluate the effects of a NTS1 receptor agonist on consolidation, and therefore, the reason for these dose differences are presently unclear. Our findings support previous studies that suggest that NTS1 receptor agonists may improve cognitive functioning in domains that are impaired in schizophrenia. The findings from the present study also complement previous findings that a NTS1 antagonist administered into the nucleus accumbens impairs the acquisition of information in spatial memory tasks (Tirado-Santiago et al., 2006; Xiao et al., 2014). A deficit in memory was not shown after systemic administration of the NTS1/2 receptor antagonist SR142948 in the present study, however, suggesting that systemic administration, rather than direct administration into a specific structure, of an NTS receptor antagonist may not impair memory. Moreover, the present study used a NTS1/2 receptor antagonist and it may be that selective antagonism of NTS1 receptors is necessary to demonstrate memory deficits.
Several lines of neuropharmacological evidence support a role for NTS1 receptor activation in cognitive functioning. Systemic administration of the NTS1 receptor agonist NT69L was shown to increase medial prefrontal cortical dopamine efflux in rats (Prus, Huang, Li, Dai, & Meltzer, 2007) possibly due to NTS1 receptor causing inhibition of D2 autoreceptors (Binder, Kinkead, Owens, & Nemeroff, 2001; Jomphe, Lemelin, Okano, Kobayashi, & Trudeau, 2006; St-Galais, Jomphe, & Trudeau, 2006). Neurotensin has also facilitated the activation of NMDA receptors when applied with sub-effective concentrations of NMDA in cortical neurons in cell culture (Antonelli et al., 2004), and in awake freely-moving animals, local application of neurotensin into the cerebral cortex led to NMDA receptor-mediated increases in cortical glutamate concentrations (Ferraro et al., 2011). Neurotensin may also affect cholinergic functioning in the prefrontal cortex, given that NTS1 receptors are also densely located on cholinergic neurons in the nucleus basalis magnocellularis (Szigethy, Wenk, & Beaudet, 1988), which project to the prefrontal cortex, and that systemic administration of NT69L increases medial prefrontal cortical acetylcholine efflux (Prus et al., 2007). Moreover, Wenk et al. (1989) suggested that NTS1 receptor loss in the nucleus basalis magnocellularis may be responsible for impaired working memory in rats performing a delayed-alternation T-maze task.
Conclusions
The present findings provide additional data for a putative pro-cognitive profile for NTS1 receptor agonism, which appears most robust in animals with naturally-occurring deficits, such as BN rats or Brattleboro rats (Feifel et al., 2009; Feifel et al., 2011). The cognitive effects appear in line with the neuropharmacologic effects of NTS1 receptor activation. Given that PD149163 exhibits putative atypical antipsychotic profile in animals models (e.g., Holly et al., 2011), PD149163 and other NTS1 receptor agonists may offer both antipsychotic and cognitive efficacy for the treatment of schizophrenia.
Acknowledgments
PD149163 was generously provided by the NIMH Drug Repository. This work was supported in part by the National Institute on Mental Health (MH-080910) to David Feifel.
Footnotes
Disclosures
All authors contributed in a significant way to the manuscript and all authors have read and approved the final manuscript.
The authors have no conflicts of interest to declare.
Contributor Information
Ashley A. Keiser, Northern Michigan University
Katelin S. Matazel, Northern Michigan University
Melissa K. Esser, Northern Michigan University
David Feifel, University of California, San Diego.
Adam J. Prus, Northern Michigan University
References
- Antonelli T, Ferraro L, Fuxe K, Finetti S, Fournier J, Tanganelli S, Tomasini MC. Neurotensin enhances endogenous extracellular glutamate levels in primary cultures of rat cortical neurons: involvement of neurotensin receptor in NMDA induced excitotoxicity. Cerebral cortex (New York, NY: 1991) 2004;14(4):466–473. doi: 10.1093/cercor/bhh008. [DOI] [PubMed] [Google Scholar]
- Azmi N, Norman C, Spicer CH, Bennett GW. Effects of a neurotensin analogue (PD149163) and antagonist (SR142948A) on the scopolamine-induced deficits in a novel object discrimination task. Behav Pharmacol. 2006;17(4):357–362. doi: 10.1097/01.fbp.0000224382.63744.20. [DOI] [PubMed] [Google Scholar]
- Binder EB, Kinkead B, Owens MJ, Nemeroff CB. Neurotensin and dopamine interactions. Pharmacol Rev. 2001;53(4):453–486. [PubMed] [Google Scholar]
- Boules M, McMahon B, Warrington L, Stewart J, Jackson J, Fauq A, Richelson E. Neurotensin analog selective for hypothermia over antinociception and exhibiting atypical neuroleptic-like properties. Brain Res. 2001;919(1):1–11. doi: 10.1016/s0006-8993(01)02981-x. [DOI] [PubMed] [Google Scholar]
- Conti LH, Costill JE, Flynn S, Tayler JE. Effects of a typical and an atypical antipsychotic on the disruption of prepulse inhibition caused by corticotropin-releasing factor and by rat strain. Behav Neurosci. 2005;119(4):1052–1060. doi: 10.1037/0735-7044.119.4.1052. [DOI] [PubMed] [Google Scholar]
- Conti LH, Palmer AA, Vanella JJ, Printz MP. Latent inhibition and conditioning in rat strains which show differential prepulse inhibition. Behav Genet. 2001;31(3):325–333. doi: 10.1023/a:1012287527438. [DOI] [PubMed] [Google Scholar]
- Cusack B, Boules M, Tyler BM, Fauq A, McCormick DJ, Richelson E. Effects of a novel neurotensin peptide analog given extracranially on CNS behaviors mediated by apomorphine and haloperidol. Brain Res. 2000;856(1–2):48–54. doi: 10.1016/s0006-8993(99)02363-x. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Murray RJ, Tina Tran DN, Rullan MA, Shilling PD. The reversal of amphetamine-induced locomotor activation by a selective neurotensin-1 receptor agonist does not exhibit tolerance. Psychopharmacology (Berl) 2008;200(2):197–203. doi: 10.1007/s00213-008-1197-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. A systemically administered neurotensin agonist blocks disruption of prepulse inhibition produced by a serotonin-2A agonist. Neuropsychopharmacology. 2003;28(4):651–653. doi: 10.1038/sj.npp.1300083. [DOI] [PubMed] [Google Scholar]
- Feifel D, Melendez G, Shilling PD. Reversal of sensorimotor gating deficits in Brattleboro rats by acute administration of clozapine and a neurotensin agonist, but not haloperidol: a potential predictive model for novel antipsychotic effects. Neuropsychopharmacology. 2004;29(4):731–738. doi: 10.1038/sj.npp.1300378. [DOI] [PubMed] [Google Scholar]
- Feifel D, Mexal S, Melendez G, Liu PY, Goldenberg JR, Shilling PD. The brattleboro rat displays a natural deficit in social discrimination that is restored by clozapine and a neurotensin analog. Neuropsychopharmacology. 2009;34(8):2011–2018. doi: 10.1038/npp.2009.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Feifel D, Reza TL, Wustrow DJ, Davis MD. Novel antipsychotic-like effects on prepulse inhibition of startle produced by a neurotensin agonist. J Pharmacol Exp Ther. 1999;288(2):710–713. [PubMed] [Google Scholar]
- Feifel D, Shilling PD, Melendez G. Clozapine and PD149163 elevate prepulse inhibition in Brown Norway rats. Behav Neurosci. 2011;125(2):268–272. doi: 10.1037/a0022691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferraro L, Beggiato S, Tomasini MC, Fuxe K, Tanganelli S, Antonelli T. Neurotensin regulates cortical glutamate transmission by modulating N-methyl-D-aspartate receptor functional activity: An in vivo microdialysis study. Journal of Neuroscience Research. 2011;89(10):1618–1626. doi: 10.1002/jnr.22686. [DOI] [PubMed] [Google Scholar]
- Green MF, Kern RS, Braff DL, Mintz J. Neurocognitive deficits and functional outcome in schizophrenia: are we measuring the “right stuff”? Schizophr Bull. 2000;26(1):119–136. doi: 10.1093/oxfordjournals.schbul.a033430. [DOI] [PubMed] [Google Scholar]
- Grimond-Billa SK, Norman C, Bennett GW, Cassaday HJ. Selectively increased trace conditioning under the neurotensin agonist PD 149163 in an aversive procedure in which SR 142948A was without intrinsic effect. J Psychopharmacol. 2008;22(3):290–299. doi: 10.1177/0269881106081528. [DOI] [PubMed] [Google Scholar]
- Harvey PD, Heaton RK, Carpenter WT, Jr, Green MF, Gold JM, Schoenbaum M. Functional impairment in people with schizophrenia: Focus on employability and eligibility for disability compensation. Schizophr Res. 2012;140(1–3):1–8. doi: 10.1016/j.schres.2012.03.025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hertel P, Olsen CK, Arnt J. Repeated administration of the neurotensin analogue NT69L induces tolerance to its suppressant effect on conditioned avoidance behaviour. Eur J Pharmacol. 2002;439(1–3):107–111. doi: 10.1016/s0014-2999(02)01414-0. [DOI] [PubMed] [Google Scholar]
- Holly EN, Ebrecht B, Prus AJ. The neurotensin-1 receptor agonist PD149163 inhibits conditioned avoidance responding without producing catalepsy in rats. Eur Neuropsychopharmacol. 2011;21(7):526–531. doi: 10.1016/j.euroneuro.2010.12.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jomphe C, Lemelin PL, Okano H, Kobayashi K, Trudeau LE. Bidirectional regulation of dopamine D2 and neurotensin NTS1 receptors in dopamine neurons. Eur J Neurosci. 2006;24(10):2789–2800. doi: 10.1111/j.1460-9568.2006.05151.x. [DOI] [PubMed] [Google Scholar]
- Li J, Chen C, Chen C, He Q, Li H, Li J, Dong Q. Neurotensin receptor 1 gene (NTSR1) polymorphism is associated with working memory. PLoS One. 2011;6(3):e17365. doi: 10.1371/journal.pone.0017365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meltzer HY. What’s atypical about atypical antipsychotic drugs? Current Opinion in Pharmacology. 2004;4(1):53–57. doi: 10.1016/j.coph.2003.09.010. [DOI] [PubMed] [Google Scholar]
- Meltzer HY, McGurk SR. The effects of clozapine, risperidone, and olanzapine on cognitive function in schizophrenia. Schizophr Bull. 1999;25(2):233–255. doi: 10.1093/oxfordjournals.schbul.a033376. [DOI] [PubMed] [Google Scholar]
- National Research Council Committee for the Update of the Guide for the Care and Use of Laboratory Animals, Institute for Laboratory Animal Research, & National Academies Press. Guide for the care and use of laboratory animals. 2011:xxv, 220. [Google Scholar]
- Norman C, Grimond-Billa SK, Bennett GW, Cassaday HJ. A neurotensin agonist and antagonist decrease and increase activity, respectively, but do not preclude discrete cue conditioning. J Psychopharmacol. 2010;24(3):373–381. doi: 10.1177/0269881108097721. [DOI] [PubMed] [Google Scholar]
- Palmer AA, Dulawa SC, Mottiwala AA, Conti LH, Geyer MA, Printz MP. Prepulse startle deficit in the Brown Norway rat: a potential genetic model. Behav Neurosci. 2000;114(2):374–388. doi: 10.1037//0735-7044.114.2.374. [DOI] [PubMed] [Google Scholar]
- Parwani A, Duncan EJ, Bartlett E, Madonick SH, Efferen TR, Rajan R, Rotrosen JP. Impaired prepulse inhibition of acoustic startle in schizophrenia. Biol Psychiatry. 2000;47(7):662–669. doi: 10.1016/s0006-3223(99)00148-1. [DOI] [PubMed] [Google Scholar]
- Petrie KA, Bubser M, Casey CD, Davis MD, Roth BL, Deutch AY. The neurotensin agonist PD149163 increases Fos expression in the prefrontal cortex of the rat. Neuropsychopharmacology. 2004;29(10):1878–1888. doi: 10.1038/sj.npp.1300494. [DOI] [PubMed] [Google Scholar]
- Pettibone DJ, Hess JF, Hey PJ, Jacobson MA, Leviten M, Lis EV, Zeng Z. The effects of deleting the mouse neurotensin receptor NTR1 on central and peripheral responses to neurotensin. J Pharmacol Exp Ther. 2002;300(1):305–313. doi: 10.1124/jpet.300.1.305. [DOI] [PubMed] [Google Scholar]
- Prus AJ, Huang M, Li Z, Dai J, Meltzer HY. The neurotensin analog NT69L enhances medial prefrontal cortical dopamine and acetylcholine efflux: Potentiation of risperidone-, but not haloperidol-, induced dopamine efflux. Brain Res. 2007;1184:354–364. doi: 10.1016/j.brainres.2007.09.092. [DOI] [PubMed] [Google Scholar]
- Richelson E, Fredrickson PA, Boules MM. Neurotensin receptor agonists and antagonists for schizophrenia. Am J Psychiatry. 2005;162(3):633–634. doi: 10.1176/appi.ajp.162.3.633-b. author reply 635. [DOI] [PubMed] [Google Scholar]
- Sarhan S, Hitchcock JM, Grauffel CA, Wettstein JG. Comparative antipsychotic profiles of neurotensin and a related systemically active peptide agonist. Peptides. 1997;18(8):1223–1227. doi: 10.1016/s0196-9781(97)00145-9. [DOI] [PubMed] [Google Scholar]
- Schotte A, Janssen PF, Gommeren W, Luyten WH, Van Gompel P, Lesage AS, Leysen JE. Risperidone compared with new and reference antipsychotic drugs: in vitro and in vivo receptor binding. Psychopharmacology (Berl) 1996;124(1–2):57–73. doi: 10.1007/BF02245606. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Melendez G, Priebe K, Richelson E, Feifel D. Neurotensin agonists block the prepulse inhibition deficits produced by a 5-HT2A and an alpha1 agonist. Psychopharmacology (Berl) 2004;175(3):353–359. doi: 10.1007/s00213-004-1835-5. [DOI] [PubMed] [Google Scholar]
- Shilling PD, Richelson E, Feifel D. The effects of systemic NT69L, a neurotensin agonist, on baseline and drug-disrupted prepulse inhibition. Behav Brain Res. 2003;143(1):7–14. doi: 10.1016/s0166-4328(03)00037-8. [DOI] [PubMed] [Google Scholar]
- St-Galais F, Jomphe C, Trudeau LE. The role of neurotensin in central nervous system pathophysiology: What is the evidence? J Psychatry Neurosci. 2006;31:229–245. [PMC free article] [PubMed] [Google Scholar]
- Szigethy E, Wenk GL, Beaudet A. Anatomical substrate for neurotensin-acetylcholine interactions in the rat basal forebrain. Peptides. 1988;9(6):1227–1234. doi: 10.1016/0196-9781(88)90186-6. [DOI] [PubMed] [Google Scholar]
- Tanganelli S, Antonelli TC, Tomasini M, Beggiato S, Fuxe K, Ferraro L. Relevance of Dopamine D2/Neurotensin NTS1 and NMDA/Neurotensin NTS1 Receptor Interaction in Psychiatric and Neurodegenerative Disorders. Current Medicinal Chemistry. 2012;19(3):304–316. doi: 10.2174/092986712803414268. [DOI] [PubMed] [Google Scholar]
- Tirado-Santiago G, Lazaro-Munoz G, Rodriguez-Gonzalez V, Maldonado-Vlaar CS. Microinfusions of neurotensin antagonist SR 48692 within the nucleus accumbens core impair spatial learning in rats. Behav Neurosci. 2006;120(5):1093–1102. doi: 10.1037/0735-7044.120.5.1093. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ. Spatial working memory and reference memory of Brown Norway and WAG rats in a holeboard discrimination task. Neurobiol Learn Mem. 1999;71(1):113–125. doi: 10.1006/nlme.1998.3860. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, Blokland A. Behavioral differences between outbred Wistar, inbred Fischer 344, brown Norway, and hybrid Fischer 344 x brown Norway rats. Physiol Behav. 1996;60(1):97–109. doi: 10.1016/0031-9384(95)02274-0. [DOI] [PubMed] [Google Scholar]
- van der Staay FJ, van Nies J, Raaijmakers W. The effects of aging in rats on working and reference memory performance in a spatial holeboard discrimination task. Behav Neural Biol. 1990;53(3):356–370. doi: 10.1016/0163-1047(90)90226-v. [DOI] [PubMed] [Google Scholar]
- Wenk GL, Markowska AL, Olton DS. Basal forebrain lesions and memory: alterations in neurotensin, not acetylcholine, may cause amnesia. Behav Neurosci. 1989;103(4):765–769. doi: 10.1037//0735-7044.103.4.765. [DOI] [PubMed] [Google Scholar]
- Woodward ND, Purdon SE, Meltzer HY, Zald DH. A meta-analysis of neuropsychological change to clozapine, olanzapine, quetiapine, and risperidone in schizophrenia. Int J Neuropsychopharmacol. 2005;8(3):457–472. doi: 10.1017/S146114570500516X. [DOI] [PubMed] [Google Scholar]
- Xiao Z, Cilz NI, Kurada L, Hu B, Yang C, Wada E, Lei S. Activation of Neurotensin Receptor 1 Facilitates Neuronal Excitability and Spatial Learning and Memory in the Entorhinal Cortex: Beneficial Actions in an Alzheimer’s Disease Model. The Journal of Neuroscience. 2014;34(20):7027–7042. doi: 10.1523/JNEUROSCI.0408-14.2014. [DOI] [PMC free article] [PubMed] [Google Scholar]