Abstract
SecA is an essential multifunctional protein for the translocation of proteins across bacterial membranes. Though SecA is known to function in the membrane, the detailed mechanism for this process remains unclear. In this study we constructed a series of SecA N-terminal deletions and identified two specific domains crucial for initial SecA/membrane interactions. The first small helix, the linker and part of the second helix (Δ2–22) were found to be dispensable for SecA activity in complementing the growth of a SecA ts mutant. However, deletions of N-terminal aminoacyl residues 23–25 resulted in severe progressive retardation of growth. Moreover, a decrease of SecA activity caused by N-terminal deletions correlated to the loss of SecA membrane binding, formation of lipid-specific domains and channel activity. All together, the results indicate that the N-terminal aminoacyl residues 23–25 play a critical role for SecA binding to membranes and that the N-terminal limit of SecA for activity is at the 25th amino acid.
Keywords: SecA N-termini, Complementation, Membrane binding, Channel activity, Protein translocation
1. Introduction
SecA is a multifunctional and an essential component of Sec-dependent protein translocation across bacterial membranes. SecA exists in soluble and membrane-bound forms in the cells [1–3]. It contains two nucleotide binding domains which are required for ATPase activities in response to various ligands [4–7]. In addition, SecA contains several binding sites to facilitate its role in protein translocation in the membranes [8,9]. These include domains for interactions with anionic phospholipids [10,11], SecYEG [12,13], pre-proteins [14], and SecB [12].
Current data show that two fractions of SecA are functional in the membranes [7,15]: a fraction of SecA inserts and de-inserts from the membrane as it hydrolyzes ATP and pushes pre-proteins across cytoplasmic membranes [8,16], and the other fraction integrates and permanently embeds into the membrane [15,17]. The dynamics of how SecA inserts or embeds into the cell membrane is not well known. SecA requires the presence of anionic phospholipids for the membrane integration [11,15,18–20]. There are multiple sites of SecA interact with phospholipids [10,21–23]. There is evidence that Escherichia coli SecA membrane insertion is affected in a SecA mutant lacking N-terminal eight amino acid residues [24]. SecAN-8 also affects translocation ATPase activity and/or the topology inversion of SecG which couples with the membrane insertion-de-insertion of SecA [24]. On the other hand, there is controversy on whether SecAΔ2–10 is functional [25–28]. Moreover, although nucleotide binding domains remain intact, a SecA mutant lacking N-terminal 63 residues (C95) is inactive for ATP binding [29] and protein translocation [20].
In this study, we examined the functions of SecA N-termini derived from non-functional C95 [20,29], and determined the maximal N-terminus deletion for SecA to remain functional. We found that the first 25 amino acids appear to be essential for complementation in the cells, and for SecA stability and its initial interactions with the membrane for functions.
2. Materials and methods
2.1. Bacteria strains and media
E. coli DH5α was used for general DNA preparation, transformation, and cloning. E. coli BL21.19 (λDE3) from Don Oliver [7,8] was used as a host for secA containing plasmids. Luria–Bertani (LB) containing 0.5% glucose, 5 µM thiamine, and 100 µg/ml ampicillin in both liquid and solid forms was used.
2.2. Construction of SecA N-terminal deletion mutants
Plasmid pMAN789/secAΔ2–63 [29] containing a truncated secA lacking N-terminal 63 amino acids (from S. Mizushima) was used to construct pET5a/(secA N-terminal deletion (STable 1).
2.3. Complementation assays
Complementation assays were carried out with SecA ts-mutant E. coli BL21.19 at 42 °C. Transformants were grown in LB broth at 30 °C until OD600 = 0.5. The cells were then serially diluted and spotted (1 µl) on LB agar plates and incubated at 30 °C and 42 °C overnight for observing growth or no growth.
2.4. Membrane fractionation of SecA mutants
BL21.19 cells containing the pET5a/SecAΔ2–63 series of plasmids were grown in LB medium until OD600 = 1.0 at 30 °C. The cultures were diluted to OD600 = 0.1 in LB medium at 42 °C for 2.5 h to allow the detection of mutant SecA distribution in the membrane at 42 °C. Cells were harvested by centrifugation, resuspended in TKMD buffer (25 mM Tris-OAc, pH 7.5; 25 mM KCl; 1 mM Mg(OAc)2; 1 mM DTT), and then lysed by French Press Cell [15]. Cellular debris was removed and the supernatant was centrifuged at 86,000 rpm for 40 min. The pelleted membranes were resuspended to the original volume of the lysates. Equal volume of membrane and supernatant fractions was applied to Western blotting with SecA antibodies, followed by quantitation [15,20].
2.5. Purification of SecA and truncated SecA proteins
BL21.19 (λD3) cells containing the appropriate plasmid were grown in LB medium and induced by 0.5 mM IPTG as described [15,30]. Over-expressed wild-type SecA and SecA deletion truncates were purified as described previously [15,30].
2.6. In vitro formation of lipid-specific 48 kDa domain
E. coli total dried lipid extracts were resuspended in TKMD buffer and sonicated to prepare liposomes as described [20]. To determine the formation of lipid-specific domain, purified SecA and deletion truncated proteins were incubated with liposomes on ice for 15–20 min, and treated with 1–10 µg/ml of trypsin for 20 min on ice [20]. The lipid-specific 48-kDa domain were identified by SecA region-specific antibody [19,20,31] and quantified.
2.7. Biochemicals
All chemicals are reagent grades from commercial sources. E. coli total lipid extract was from Avanti Polar Lipids, Inc.
3. Results and discussion
3.1. In vivo complementation activity for truncated SecA: N-terminal 22 aminoacyl residues are dispensable
We constructed systematically a series of N-terminus deletion mutants (STable 1). Mutant plasmids were then introduced into a secAts amber mutant BL21.19 to test their ability for complementation of the secAts mutation at the 42 °C non-permissive temperature. The results showed that the SecA missing aminoacyl residues 2–13 (SecAΔ2–13) was able to efficiently complement just like the wild-type SecA, and even the SecAΔ2–14 to Δ2-–22 could complement albeit with slightly lower efficiency than the intact SecA (Table 1). The Δ2–25 deletion appeared to be the limit for complementation activity (Table 1). Surprisingly, SecAΔ2–15 and SecAΔ2–16 did not complement BL21.19, however, SecAΔ2–17 regained the activity (Table 1).
Table 1.
Complementation of N-deletion mutations.
|
1MLIKLLTKVF 11GSRNDRTLRR 21MRKVVNIINA |
Complementation at 42°C Dilution factor |
|||
|---|---|---|---|---|
| 10−1 | 10−2 | 10−3 | 10−4 | |
| SecA | + | + | + | + |
| SecAΔ2−9 to Δ2−13 | + | + | + | + |
| SecAΔ2−14 | + | + | + | − |
| SecAΔ2−15, Δ2−16 | − | − | − | − |
| SecAΔ2−17 to Δ2−23 | + | + | + | − |
| SecAΔ2−24 | + | + | − | − |
| SecAΔ2−25 | + | − | − | − |
| SecAΔ2−26 to Δ2−30 | − | − | − | − |
| SecAD15−16 | + | + | + | + |
| SecAΔ2−14R16X* | − | − | − | − |
| SecAΔ2−15R16Y** | − | − | − | − |
| SecAR16E | + | + | + | + |
| SecAΔ2−16T17Z*** | + | + | + | + |
| SecAΔ2−16T17A,T17S | − | − | − | − |
The upper left sequence of SecA N-terminal 30 amino acids are listed. Complementation at 42 °C was described in Section 2.3. +/− indicates growth or no growth on spot tests at serial dilutions of 10−1 to 10−4 dilution.
Experiments were repeated 2–4 times.
R16X tested were S,E,V,K.
R16Y tested were R16S,E,V.
R17Z tested were N,D,V,K,Y,C,G.
As the reported E. coli SecA (EcSecA) crystal structure [32] is missing the N-terminal 12 and 232–366 aminoacyl residues, we utilized X-ray structure of Bacillus subtilis SecA (BsSecA) [33] as the template to build a homology model of EcSecA (SFig. 1A). In the N-terminal MLIKLLTKVF10-GSRNDRTLRR20-MRKVVNIINA30 MEPEIEKLSD40, aminoacyl residues 5–11th form a helix that connects to the next helix formed by aminoacyl residues 16–37th via a linker region of aminoacyl residues 12–15th (SFig. 1B). The model reveals conformational changes of SecAΔ2–15 and SecAΔ2–16 that may be responsible for the loss of SecA activity in these mutants. Interesting, SecAΔ15–16 is fully functional in complementation as the wild-type SecA (Table 1). The modeling reveals that the deletion of both arginine–threonine at 15–16th compensates the changes to restore activity (SFig. 2B).
Helical wheel plot of SecA N-terminal 20 aa (SFig. 2) shows an amphipathic helix of SecA that lines positively charged amino acids on one side and hydrophobic amino acids on the other, suggesting that this helix interacts the negatively charged hydrophobic phospholipids in the membrane [34]. The model shows that R and K residue side chains make up one side of the helix; these residues may be required for SecA interaction with the polar lipid head groups of the lipid bi-layer or the polar environment of the cytoplasm. Similar arrangements are observed for SecA Δ15–16, and even for aminoacyl residues 17–37 (SecAΔ2–16), but not residues 24–44 (SecAΔ2–23) (SFig. 2).
3.2. Roles of AA residue 15N and 16R for SecA mutants in vivo function
To determine whether loss of SecAΔ2–15 and SecAΔ2–16 functionalities is due to the 15th or 16th AA, point mutations of the deletions were constructed at positions 15N, 16R or 17T. Substitutions at the amino acid 15N retained the in vivo activity of SecAΔ2–14 (not shown). Deletion mutations at 16R with or without 15N lost the complementation activity with various substitutions: polar hydrophilic, hydrophilic charged or hydrophobic amino acids (Table 1). The intolerance of substitution for deletion mutant at 16R suggests that the arginine residue at the beginning of the second helix is functionally as well as structurally important for the truncated SecA. However, when SecA is intact the change of this residue SecAR16E was able to complement BL21.19 to the same extent as wild-type SecA, just like SecAΔ15–16 deletion (Table 1). These SecA mutations at 16R apparently were tolerable for complementation.
Point mutations at inactive were also tested. Contrary to 16R mutations, most mutations regained activity to complement BL21.19, except for the smaller serine and alanine (Table 1). These data indicate that the size of aminoacyl residue 17T is functionally and structurally important for SecA mutant, SecAΔ2–16, as more bulky but not smaller A/S were able to complement BL21.19. SecAΔ2–16T17G was able to complement; however, glycine is flexible so perhaps be able to overcome constraints of this position (Table 1).
3.3. Complementation and structural stability
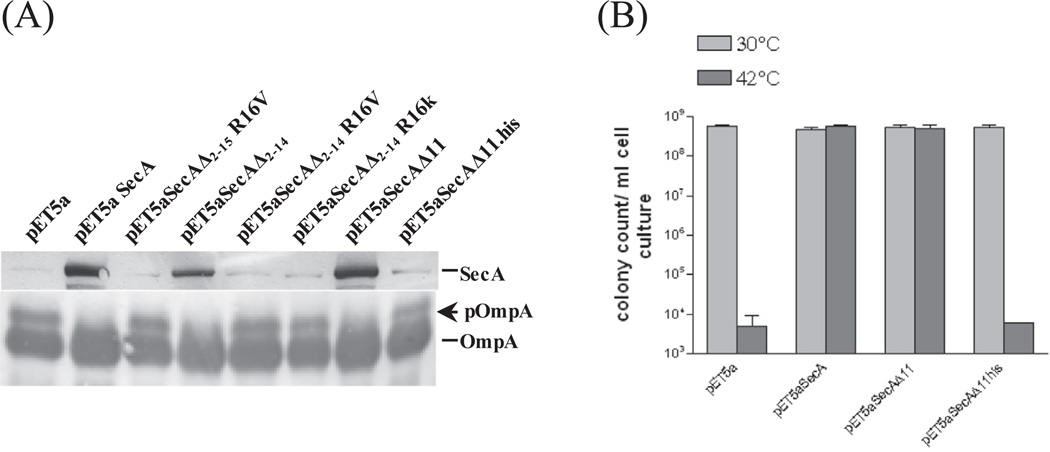
The molecular modeling suggested that some SecA constructs in this region underwent major conformational changes. We examined the steady state amount of truncated SecA in these mutants and the ability to complement. We found that the ability to complement correlated with the SecA stability and the precursor processing (Fig. 1A and B), which is consistent with the report that transmembrane α-helices strongly influence the folding or association of integral membrane proteins [35]. These results suggest that aminoacyl residues 15D and 16R play critical roles for stability and functionality of SecA, especially in the absence of Nterminal aminoacyl residues 2–14 (SecAΔ2–14).
Fig. 1.
(A) The stability of SecA in SecA deletion mutants. Wild-type SecA and SecA truncated mutants strains as indicated were grown at 30 °C and diluted to OD600 = 0.1 before shifted to 42 °C for an additional 2 h incubation. Cell pellets were resuspended in SDS sample buffer, boiled and detected by Western blot using SecA and OmpA antibodies; the latter detected both pOmpA and OmpA. (B) The plating efficiency of SecAΔ11 and SecAΔ11-his was carried out as colony forming units (CFU) at 30 °C and 42 °C with appropriate dilutions.
The SecA stability has also been attributed for the lack of complementation for SecAΔ11-his, and plating efficiency ([25] and Fig. 1B). SecAΔ11(SecAΔ2–11), but not SecAΔ11-his is stable in vivo (Fig. 1A). It has been reported that the complementation by SecAΔ11-his can be achieved with increased induction [25]. However, we have found that the over-produced purified SecAΔ2–11 and SecAΔ2–11-his have same lipid-interacting ATPase activity (SFig. 3) and for ion channel activity (data not shown; see below, Table 2). Thus stability account for the in vivo difference (Fig. 1B), though there is also difference on the monomer– dimer equilibrium [25]. It is worth noting that the introduction of his-tag may alter the stability and function of a protein in the cells.
Table 2.
SecA mutant growth and membrane interactions
| % plating efficiency | Doubling time(min) | % in vivo membrane binding | % 48-kDa formation | Ion channel activity(lA) | |
|---|---|---|---|---|---|
| SecA | 100 | 35 ± 6.1 | 50.9 ± 4.8 | 100 | 6.8 ± 0.1 |
| SecAΔ2−9 to Δ2−14 | 48.3 ± 4.9 | 88 ± 1.4 | 5.2 ± 0.3 | ||
| SecAΔ2−15, Δ2−16 | 0 | N/A | Trace | – | – |
| SecAΔ2−18 | ND | 38.3 ± 1.4 | 84 ± 0.0 | 2.2 ± 0.3 | |
| SecAΔ2−21 | 102 ± 9.5 | 55 ± 5.3 | 37.0 ± 9.8 | 42 ± 8.5 | 1.4 ± 0.4 |
| SecAΔ2−22 | 106 ± 10.1 | 45 ± 7.1 | 42.5 ± 1.0 | 33 ± 1.0 | – |
| SecAΔ2−23 | 96 ± 7.4* | 164 ± 15.6 | 33.0 ± 9.7 | 27 ± 3.5 | 0.2 ± 0.1 |
| SecAΔ2−24 | 93 ± 5.1* | 260 ± 21.2 | 18.3 ± 4.8 | Trace | – |
| SecAΔ2−25 | 101 ± 8.5* | Variable | 20.2 ± 4.2 | None | – |
| SecAΔ2−26−Δ2−62 | 0 | N/A | – | None | 0 |
Plating efficiency, measured in triplicates, represents the CFUs ratio at 42 °C/30 °C. Experiments were repeated 2–4 times.
The doubling times were from cells at steady state exponential phase in LB broth at 42 °C.
The % membrane distribution of SecA in the cells at 42 °C was determined as the fraction of membrane-bound SecA in the cells at 42 °C as described in Section 2.4.
The % 48-kDa domain of the lipid-specific interaction was determined by trypsin treatment as in Section 2.7 and quantified using intact SecA as 100%.
The ion current activities of SecAs protein-conducting channel were determined with SecA-depleted BA13 membranes in the oocytes as described[36,37].n = 45.
Cells were grown on LB plates for 48 h before countable cells were visible
3.4. Correlation of complementation and lipid-interaction: membrane binding, lipid-specific domain and ion channel activity
To substantiate that SecA with deletion of more than N-terminus 25 AA could no longer complement BL21.19 (Table 1), the plating efficiency and the growth of the SecA mutants were determined. Cells were grown and the plating efficiency was determined by the ratio of the number of colony at both 30 °C and 42 °C. All SecA mutants that exhibit various degree of complementation (Table 1), have similar plating efficiency as the wild-type SecA (Table 2). However, SecAΔ2–24 and SecAΔ2–25 with reduced complementation showed significant retardation of growth in forming colony and steady state growth doubling time as SecAΔ2–23 or wild-type SecA (Table 2). Together, the results show that while the N-terminal 25 residues are dispensable for SecA activity, the coordinate growth impairment starts from the deletion of aminoacyl residue 23R, resulting in growth retardation. We note that the deletion of up to 22R–23K minimizes the N-terminal positive charges and the 16R–30A helix (SFig. 2B).
It has been reported that SecA contains a membrane binding determinate in the first 239 amino acids and that SecA membrane interactions require anionic phospholipids interactions [1,19]. To test the hypothesis that N-terminal 25 AA are involved in SecA membrane interactions, in vivo membrane distribution and in vitro integration experiments were performed. In wild-type cells, SecA is normally distributed equally in cytosolic and membrane fraction [1–3]; Table 2). As more residues from the SecA N-terminus were deleted, significant decreases in SecA amount were observed in membrane fractions (Table 2). The loss of binding to membrane corresponded to the loss of complementation: SecAΔ2–24 and SecAΔ2–25 only partially complemented and SecAΔ2–26 did not complement (Table 1).
SecA forms a membrane/lipid specific 48-kDa domain in the presence of anionic phospholipids [19,20]. We used it as another test for examining N-terminal truncated SecA variants/membrane interactions. Purified proteins incubated with liposomes for binding, then digested with low concentrations of trypsin [20]. The induced 48-kDa domain formation of the truncated SecA was determined compared to wild-type intact SecA. The results showed that there is a significant decrease in 48-kDa domain formation between SecAΔ2–18 (84%) and SecAΔ2–21 (42%), indicating there is a stepwise loss of SecA membrane integration as the N-terminal limit of SecA is approached. SecAΔ2–23 and SecAΔ2–24, which were defective of in vivo membrane binding, showed a more than 50% decrease in 48-Da domain formation (Table 2). There was no detectable amount of 48-kDa domain formation for SecAΔ2–24-SecAΔ2–26 indicating that these proteins formed little lipid-specific conformation (Table 2; [20]).
It has been shown that SecA elicits ion channel activities with membranes that are phospholipid-specific [36,37]. We examined the ion channel activities of these SecAs in the oocytes. The complementation of SecA variants also corresponded to the ability to elicit ion channel activity: There is almost no effect for up to SecAΔ2–14; the channel activities for SecAΔ2–18 and SecAΔ2–21 were lower, for SecAΔ2–23 was almost lost; and there was no activity for SecAΔ2–26 (Table 2).
These data together suggest that the N-terminal 25 amino acids play a crucial role in initial interactions of SecA with phospholipids for SecA to function in the membrane. The complementation of the N-terminal 25 residues corresponds to the ability to interact with phospholipids. However, there are other additional domains related to SecA-lipid-binding [10,21–23,34]. It is possible that the SecA N-terminal 25 amino acids facilitate the initial binding and integration to the anionic phospholipids, which lead to further interaction of other SecA domains with membranes. This would suggest that SecA interaction with phospholipids is sequential, starting with the N-terminal binding and about half ends up with permanent integration into the membrane [15,17]. On the other hand, some SecA mutations result in the accumulation of SecA in the membrane [17].
3.5. Disproportional correlation of in vitro SecA activities and in vivo complementation
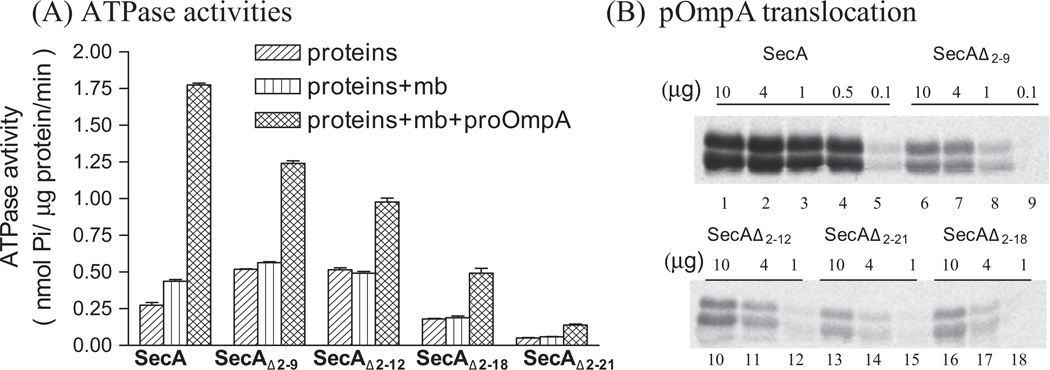
We noted that though the trend of decreasing SecA channel activities generally correspond to cell growth, the SecAΔ2–18- SecAΔ2–21 which are fully functional in complementation, have lost significant activities in forming functional channel (Table 2). To further correlate the in vitro SecA activities and in vivo complementation, we used the purified truncated SecA proteins to assess their in vitro ATPase and protein translocation activities. It has been shown [6,18] that SecA possesses intrinsic ATPase activity which is stimulated by the membrane/lipids (membrane ATPase), and the ATPase is further stimulated by the precursor with signal peptides and SecYEG (Translocation ATPase); the latter presumably reflects the SecA function on the protein translocation in the membrane. Surprisingly, the ATPase activities were reduced progressively for SecA with longer deletions (Fig. 2A), even though complementation and doubling times showed these mutant SecA variants function like intact SecA in the cells. There were several-fold reductions of translocation ATPase activities of SecAΔ2–18-SecAΔ2–21 with no corresponding decrease of in vivo complementation efficiency (Fig. 2A and Table 1).
Fig. 2.
ATPase activities and translocation of SecA variants. (A) The intrinsic ATPase, membrane ATPase (+BA13 SecA-depleted membrane) and translocation ATPase (+BA13 SecA-depleted membrane + pOmpA precursor) activities of SecAs were determined as described [20,26]. (B) The SecA-dependent protein translocation activities of pOmpA were carried out at 37 °C with BA13 SecA-depleted membranes as described [19].
The protein translocation activities into the membrane vesicles were also determined. We found that the pOmpA protein translocation activities of SecAΔ2–9-SecAΔ2–21 were significantly affected and were reduced about 10-fold for SecAΔ2–21 (Fig. 2B). These data suggested that the requirement of SecA function in the cells to complement is not proportionally related to the in vitro SecA activities. It is well known that SecA is present in excess in bacterial cells (see Refs. [2,3,37]. Moreover, the SecA-SecYEG protein-conducting channels are more efficient than SecA-alone channels [38]. Thus it is likely that the reduced activity of the SecA N-terminal deletions can be tolerated in the cells to provide full complementation activity.
Taken all data together, the N-terminal 22 residues of SecA (SecAΔ2–22) are dispensable, though with reduced activity. However, the additional deletions of 23–25 (SecAΔ2–25) results in loss of binding and interaction with membranes/liposomes, thus the activities. In this regard, the C-terminal 70 residues of the full length SecA 901 residues are also dispensable; but unlike N-terminal deletions (SecA901Δ2–22), the SecA1–831(N95) retains almost full ATPase activities and protein translocation activity [20,25,27,29,31]. Moreover, SecA Δ2–11N95831 can still complement [25,27]. It would be interesting to determine the minimal length of N- and C-terminal truncated SecA that still retains activity to complement.
Supplementary Material
Acknowledgments
This work was supported in part by a NIH Grant GM34766. J.H. and Y.H.H. were fellows of the Molecular Basis of Diseases Program at Georgia State University. The Georgia State University Biology Core Facilities were partly supported by Georgia Research Alliance.
Appendix A. Supplementary data
Supplementary data associated with this article can be found, in the online version, at http://dx.doi.org/10.1016/j.bbrc.2014.09.080.
References
- 1.Cabelli R, Dolan K, Qian L, Oliver D. Characterization of membrane-associated and soluble states of SecA protein from wild-type and SecA51(TS) mutant strains of Escherichia coli. J. Biol. Chem. 1991;266:24420–24427. [PubMed] [Google Scholar]
- 2.Oliver DB, Beckwith J. Identification of a new gene (secA) and gene product involved in the secretion of envelope proteins in Escherichia coli. J. Bacteriol. 1982;150:686–691. doi: 10.1128/jb.150.2.686-691.1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Oliver DB, Beckwith J. Regulation of a membrane component required for protein secretion in Escherichia coli. Cell. 1982;30:311–319. doi: 10.1016/0092-8674(82)90037-x. [DOI] [PubMed] [Google Scholar]
- 4.Chen L, Tai PC. ATP is essential for protein translocation into Escherichia coli membrane vesicles. PNAS. 1985;82:4384–4388. doi: 10.1073/pnas.82.13.4384. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Manting EH, Driessen AJ. Escherichia coli translocase: the unravelling of a molecular machine. Mol. Microbiol. 2000;37:226–238. doi: 10.1046/j.1365-2958.2000.01980.x. [DOI] [PubMed] [Google Scholar]
- 6.Lill R, Cunningham K, Brundage LA, Ito K, Oliver D, Wickner W. SecA protein hydrolyzes ATP and is an essential component of the protein translocation ATPase of Escherichia coli. EMBO J. 1989;8:961–966. doi: 10.1002/j.1460-2075.1989.tb03458.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Mitchell C, Oliver D. Two distinct ATP-binding domains are needed to promote protein export by Escherichia coli SecA ATPase. Mol. Microbiol. 1993;10:483–497. doi: 10.1111/j.1365-2958.1993.tb00921.x. [DOI] [PubMed] [Google Scholar]
- 8.Rajapandi T, Oliver D. Integration of SecA protein into the Escherichia coli inner membrane is regulated by its amino-terminal ATP-binding domain. Mol. Microbiol. 1996;20:43–51. doi: 10.1111/j.1365-2958.1996.tb02487.x. [DOI] [PubMed] [Google Scholar]
- 9.Driessen AJ, Nouwen N. Protein translocation across the bacterial cytoplasmic membrane. Annu. Rev. Biochem. 2008;77:643–667. doi: 10.1146/annurev.biochem.77.061606.160747. [DOI] [PubMed] [Google Scholar]
- 10.Breukink E, Keller RC, de Kruijff B. Nucleotide and negatively charged lipid-dependent vesicle aggregation caused by SecA. Evidence that SecA contains two lipid-binding sites. FEBS Lett. 1993;331:19–24. doi: 10.1016/0014-5793(93)80289-7. [DOI] [PubMed] [Google Scholar]
- 11.Hendrick JP, Wickner W. SecA protein needs both acidic phospholipids and SecY/E protein for functional high-affinity binding to the Escherichia coli plasma membrane. J. Biol. Chem. 1991;266:24596–24600. [PubMed] [Google Scholar]
- 12.Hartl FU, Lecker S, Schiebel E, Hendrick JP, Wickner W. The binding cascade of SecB to SecA to SecY/E mediates preprotein targeting to the E. coli plasma membrane. Cell. 1990;63:269–279. doi: 10.1016/0092-8674(90)90160-g. [DOI] [PubMed] [Google Scholar]
- 13.Snyders S, Ramamurthy V, Oliver D. Identification of a region of interaction between Escherichia coli SecA and SecY proteins. J. Biol. Chem. 1997;272:11302–11306. doi: 10.1074/jbc.272.17.11302. [DOI] [PubMed] [Google Scholar]
- 14.Kimura E, Akita M, Matsuyama S, Mizushima S. Determination of a region in SecA that interacts with presecretory proteins in Escherichia coli. J. Biol. Chem. 1991;266:6600–6606. [PubMed] [Google Scholar]
- 15.Chen X, Xu H, Tai PC. A significant fraction of functional SecA is permanently embedded in the membrane. SecA cycling on and off the membrane is not essential during protein translocation. J. Biol. Chem. 1996;271:29698–29706. doi: 10.1074/jbc.271.47.29698. [DOI] [PubMed] [Google Scholar]
- 16.Economou A, Wickner W. SecA promotes preprotein translocation by undergoing ATP-driven cycles of membrane insertion and deinsertion. Cell. 1994;78:835–843. doi: 10.1016/s0092-8674(94)90582-7. [DOI] [PubMed] [Google Scholar]
- 17.Schmidt MG, Rollo EE, Grodberg J, Oliver DB. Nucleotide sequence of the secA gene and secA(Ts) mutations preventing protein export in Escherichia coli. J. Bacteriol. 1988;170:3404–3414. doi: 10.1128/jb.170.8.3404-3414.1988. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lill R, Dowhan W, Wickner W. The ATPase activity of SecA is regulated by acidic phospholipid, SecY, and the leader and mature domains of precursor protein. Cell. 1990;60:271–280. doi: 10.1016/0092-8674(90)90742-w. [DOI] [PubMed] [Google Scholar]
- 19.Chen X, Brown T, Tai PC. Identification and characterization of proteaseresistant SecA fragments: secA has two membrane-integral forms. J. Bacteriol. 1998;180:527–537. doi: 10.1128/jb.180.3.527-537.1998. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.You Z, Liao M, Zhang H, Yang H, Pan X, Houghton JE, Sui SF, Tai PC. Phospholipids induce conformational changes of SecA to form membranespecific domains: AFM structures and implication on protein-conducting channels. PLoS One. 2013;8:e72560. doi: 10.1371/journal.pone.0072560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Hu HJ, Holley J, He J, Harrison RW, Yang H, Tai PC, Pan Y. To be or not to be: predicting soluble SecAs as membrane proteins. IEEE Trans. Nanobioscience. 2007;6:168–179. doi: 10.1109/tnb.2007.897486. [DOI] [PubMed] [Google Scholar]
- 22.Dapic V, Oliver D. Distinct membrane binding properties of N- and C-terminal domains of Escherichia coli SecA ATPase. J. Biol. Chem. 2000;275:25000–25007. doi: 10.1074/jbc.M001100200. [DOI] [PubMed] [Google Scholar]
- 23.Keller RC. The prediction of novel multiple lipid-binding regions in protein translocation motor proteins: a possible general feature. Cell. Mol. Biol. Lett. 2011;16:40–54. doi: 10.2478/s11658-010-0036-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Mori H, Sugiyama H, Yamanaka M, Sato K, Tagaya M, Mizushima S. Aminoterminal region of SecA is involved in the function of SecG for protein translocation into Escherichia coli membrane vesicles. J. Biochem. (Tokyo) 1998;124:122–129. doi: 10.1093/oxfordjournals.jbchem.a022070. [DOI] [PubMed] [Google Scholar]
- 25.Das S, Stivison E, Folta-Stogniew E, Oliver D. Reexamination of the role of the amino terminus of SecA in promoting its dimerization and functional state. J. Bacteriol. 2008;190:7302–7307. doi: 10.1128/JB.00593-08. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Wang H, Na B, Yang H, Tai PC. Additional in vitro and in vivo evidence for SecA functioning as dimers in the membrane: dissociation into monomers is not essential for protein translocation in Escherichia coli. J. Bacteriol. 2008;190:1413–1418. doi: 10.1128/JB.01633-07. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Or E, Boyd D, Gon S, Beckwith J, Rapoport T. The bacterial ATPase SecA functions as a monomer in protein translocation. J. Biol. Chem. 2005;280:9097–9105. doi: 10.1074/jbc.M413947200. [DOI] [PubMed] [Google Scholar]
- 28.Jilaveanu LB, Zito CR, Oliver D. Dimeric SecA is essential for protein translocation. Proc Natl. Acad. Sci. U.S.A. 2005;102:7511–7516. doi: 10.1073/pnas.0502774102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Matsuyama S, Kimura E, Mizushima S. Complementation of two overlapping fragments of SecA, a protein translocation ATPase of Escherichia coli allows ATP binding to its amino-terminal region. J. Biol. Chem. 1990;265:8760–8765. [PubMed] [Google Scholar]
- 30.Cabelli RJ, Chen L, Tai PC, Oliver DB. SecA protein is required for secretory protein translocation into E. coli membrane vesicles. Cell. 1988;55:683–692. doi: 10.1016/0092-8674(88)90227-9. [DOI] [PubMed] [Google Scholar]
- 31.Ramamurthy V, Oliver D. Topology of the integral membrane form of Escherichia coli SecA protein reveals multiple periplasmically exposed regions and modulation by ATP binding. J. Biol. Chem. 1997;272:23239–23246. doi: 10.1074/jbc.272.37.23239. [DOI] [PubMed] [Google Scholar]
- 32.Papanikolau Y, Papadovasilaki M, Ravelli RB, McCarthy AA, Cusack S, Economou A, Petratos K. Structure of dimeric SecA, the Escherichia coli preprotein translocase motor. J. Mol. Biol. 2007;366:1545–1557. doi: 10.1016/j.jmb.2006.12.049. [DOI] [PubMed] [Google Scholar]
- 33.Hunt JF, Weinkauf S, Henry L, Fak JJ, McNicholas P, Oliver DB, Deisenhofer J. Nucleotide control of interdomain interactions in the conformational reaction cycle of SecA. Science. 2002;297:2018–2026. doi: 10.1126/science.1074424. [DOI] [PubMed] [Google Scholar]
- 34.Jones MK, Anantharamaiah GM, Segrest JP. Computer programs to identify and classify amphipathic alpha helical domains. J. Lipid Res. 1992;33:287–296. [PubMed] [Google Scholar]
- 35.Zhou FX, Cocco MJ, Russ WP, Brunger AT, Engelman DM. Interhelical hydrogen bonding drives strong interactions in membrane proteins. Nat. Struct. Biol. 2000;7:154–160. doi: 10.1038/72430. [DOI] [PubMed] [Google Scholar]
- 36.Lin BR, Gierasch LM, Jiang C, Tai PC. Electrophysiological studies in Xenopus oocytes for the opening of Escherichia coli SecA-dependent protein-conducting channels. J. Membr. Biol. 2006;214:103–113. doi: 10.1007/s00232-006-0079-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Hsieh YH, Zhang H, Wang H, Yang H, Jiang C, Sui SF, Tai PC. Reconstitution of functionally efficient SecA-dependent protein-conducting channels: transformation of low-affinity SecA-liposome channels to high-affinity SecASecYEG-SecDF.YajC channels. Biochem. Biophys. Res. Commun. 2013;431:388–392. doi: 10.1016/j.bbrc.2013.01.042. [DOI] [PubMed] [Google Scholar]
- 38.Hsieh YH, Zhang H, Lin BR, Cui N, Na B, Yang H, Jiang C, Sui SF, Tai PC. SecA alone can promote protein translocation and ion channel activity: SecYEG increases efficiency and signal peptide specificity. J. Biol. Chem. 2011;286:44702–44709. doi: 10.1074/jbc.M111.300111. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.