Abstract
To improve gene delivery efficiency of PEGylated poly(amidoamine) dendrimers in livers and muscles, the roles of syndecan-4 receptor and caveolin-1 protein in the endocytosis of PEGylated generation 5 (G5-PEG) or 7 (G7-PEG) dendrimers and plasmid DNA polyplexes were explored in C2C12 and HepG2 cells. Expression levels of syndecan-4 for both cell lines were downregulated by transfection of the cells with syndecan-4 specific siRNA. Caveolin-1 was upregulated by infecting the cells with adenovirus vector expressed caveolin-1 (Ad-CAV-1). The impact of syndecan-4 and caveolin-1 on endocytosis of G5-PEG/DNA or G7-PEG/DNA polyplexes was then measured by flow cytometry. Our results demonstrate that downregulation of syndecan-4 and upregulation of caveolin-1 significantly improved internalization of PEG-PAMAM dendrimer polyplexes in HepG2 cells; however, in C2C12 cells, downregulation of syndecan-4 decreased the internalization of the polyplexes while upregulation of caveolin-1 had no effect on internalization. Gene expression results for G5-PEG/pGFP on the two cell lines exhibited the same trends for syndecan-4 and caveolin-1 as was observed for endocytosis of the polyplexes. This study gives a clue how to take strategies by up- or down-regulation of the expressions of Syndecan-4 and Caveolin-1 to improve in vivo gene delivery efficiency of the PEG-PAMAM dendrimers in clinical transgenic therapy.
Keywords: PEGylated PAMAM dendrimer, polyplexes, endocytosis mechanism, syndecan-4, caveolin-1
1. Introduction
Poly(amidoamine) (PAMAM) dendrimers are synthetic nano-scale polymers with well-defined structure, high degree of intermolecular uniformity, low degree of polydispersity, and multiple terminal functional groups. The sum of these properties makes cationic PAMAM dendrimer exceptionally promising for gene transfection, and these materials have proven to be as effective as cationic liposomes or other cationic polymers [1, 2] for in vitro delivery and even more effective for in vivo DNA [3] or siRNA [4, 5] delivery. For these reasons, PAMAM dendrimer-based polyplexes also serve as a good model system for exploring binding and uptake mechanisms of polyplexes into cells [6].
Modification of cationic polymer with PEG at an appropriate ratio can give higher transfaction and expression efficiency [7], and lower immune-recognition response and cytotoxicity [8] than unmodified polymer. With 10 mole% PEG substitution, expression efficiency of PLL increased by 30 folds in HepG2 cells, although it decreased when the PEG modification ratio was increased to 25 mole% [7]. Appropriate PEG modification increases the water solubility of polymer/DNA polyplexes; however, PEG modification can serve to inhibit polyplex interaction with the cell membrane, thereby blocking the transfection process. Even most of polycations are much less effective than virus vectors in terms of in vivo gene expression, our previous studies demonstrated that G5 and G6 PAMAM dendrimers with 10 mole% PEG modification were more efficient for both in vitro and in vivo gene expression (pDNA) [3] and gene silencing (siRNA) [5] as compared to unmodified dendrimers, jet-PEI and Lipofectamine 2000.
Mechanisms of cellular internalization for cationic polyplexes have been intensely studied with clathrin-dependent, caveolae-dependent, and pinocytosis mechanisms reported [9]. Direct fusion with the cell membrane and/or fluid phase endocytosis may also contribute to the internalization process suggesting many factors are involved in the process of gene delivery into cells.
Caveolae/lipid raft mediated endocytosis (LRME) process is a type of cholesterol and dynamin dependent receptor mediated pathway [10]. Caveolae structures in cells include cholesterol, sphingolipids, and a class of membrane proteins named caveolins, especially caveolin-1 (CAV-1) [11]. Rejman, et al. demonstrated using inhibitors that PEI/DNA polyplexes were taken up partially by caveolae-mediated LRME, allowing them to escape the lysosomal compartment fate of the clathin-mediated pathway, and thus giving efficient gene expression [12]. By way of contrast, our previous studies illustrated that GM1/CAV-1 LRME mechanism was not followed for G5 and G7 PAMAM polyplexes [6]. These experiments controlled GM1 levels by choice of cell line, incubation with GM1, and inhibition with ganglioside inhibitor PPMP. Caveolin-1 levels were adjusted by viral-based upregulation of CAV-1.
Syndecans [13] are heparan sulphate proteoglycans consisting of a type I transmembrane core protein modified by heparan sulphate and sometimes chondroitin sulphate chains [14]. They have been reported to be involved in the endocytosis of PLL/DNA and PEI/DNA polyplexes [15]. In addition, studies by Behr, et al. [16] indicated that binding to adhesion receptors such as integrins and syndecans connected to actin networks appeared to be a general means for particle engulfment, including cationic PEI. Syndecan-4 (Syn-4) is expressed in virtually every cell type [17] and has become the most extensively studied member of the syndecan family.
Previous works suggest that both CAV-1 and Syn-4 could be critical in the uptake of the polyplexes. To date, no studies have tested these two factors for PEG-PAMAM dendrimer based polyplexes. Therefore, in this study, we explored the importance of CAV-1 and Syn-4 in the internalization of G5-PEG and G7-PEG polyplexes formed with plasmid DNA (pDNA).
C2C12 (mouse myoblast) and HepG2 (human hepatocellular carcinoma) cells were chosen as model cell lines because this study was designed to guide future in vivo studies of gene therapy for lipid metabolism disorders. C2C12 and HepG2 cells are related to the muscular delivery of lipoprotein lipase (LPL) gene and hepatic delivery of ApoE gene for treatment of hyperlipidemia in LPL−/− and ApoE−/− mice, respectively. LPL, a rate-limiting enzyme in hydrolysis of triglycerides in chylomicrons (CMs) and very low density lipoprotein (VLDL) in plasma, is expressed abundantly in muscle tissue. ApoE is the ligand for low density lipoprotein receptor (LDLR) as well as the ligand for receptor of CM and VLDL in hepatocytes. Both ApoE and LPL play crucial roles in atherosclerosis and lipid metabolic disorders [18, 19].
2. Materials and Methods
2.1. Materials
Generation 5 and 7 PAMAM dendrimers were purchased from Dendritech Inc. (Midland, MI, USA). Plasmid DNA encoding luciferase gene was proliferated and purified by our lab. LabelIt tracker Cy5 intracellular nucleic acid localization kit was purchased from Mirus Bio Corporation (Madison, WI, USA). Lipofectamine 2000 was purchased from Invitrogen (Carlsbad, CA, USA). Syn-4 siRNA and control siRNA were purchased from Santa Cruz (Santa Cruz, CA, USA). Ad-CAV-1 and vector Ad-Null were obtained from Vector Biolabs (Philadelphia, PA, USA). C2C12 and HepG2 cell lines were purchased from the American Type Culture Collection (ATCC, Manassas, VA, USA). Rabbit anti-Syn-4 polyclonal antibody (H-140), rabbit anti-CAV-1 polyclonal antibody (N-20), goat anti-GAPDH polyclonal antibody (A-14), mouse anti-β-actin monoclonal antibody (C4), and the relevant secondary antibodies were purchased from Santa Cruz (Santa Cruz, CA, USA). All other chemicals were purchased from Sigma-Aldrich (agent at Beijing, China).
2.2. PEGylation of Dendrimers
G5 and G7 dendrimers were purified before use according to published procedures [20]. Terminal amine groups of the two dendrimers were modified by PEG 5000 at a ratio of 8% (molar ratio of PEG to surface amine per dendrimer) according to a procedure published previously [3]. The products of the PEG-PAMAM dendrimers were purified by dialysis and obtained by lyophilization. The final products were analyzed by 1H NMR, HPLC, GPC, and potentiometric titration. (Characteristic data for PEG-PAMAM dendrimers are shown in Figure S1)
2.3. Plasmid Amplification and Cy5 Labeled Plasmid
Luciferase and GFP genes were proliferated in Escherichia coli DH5-2α and then purified using QIAGEN plasmid purification kits. The resulting plasmid was identified by restriction enzymes, and DNA concentration was determined by UV spectrophotometry. A LabelIt tracker Cy5 kit was used to label the Luciferase plasmid.
2.4. Preparation and Characterization of Polyplexes
To form polyplexes, equal volumes of plasmid solution (with 1 μg of DNA) and G5-PEG or G7-PEG solution were mixed in phosphate buffer solution (PBS) at an N/P ratio of 10. The resulting mixture was incubated for 30 min at room temperature to form polyplexes. Particle size of polyplexes was determined by dynamic light scattering. To determine condensability of pDNA after forming polyplexes with PEG-PAMAM dendrimer, agarose gel electrophoresis was performed.
2.5. Cell Culture and Cytotoxicity Assays
C2C12 and HepG2 cells were grown continuously to a monolayer in complete medium (DMEM containing 10% FBS, 100 U/mL penicillin and 100 μg/mL streptomycin) at 37°C and 5% CO2. With COS7 cells as a control cell line, cytotoxicity of PEG-PAMAM dendrimers was evaluated by MTT assay [3].
2.6. Effects of Syn-4 Knockdown on Endocytosis and Gene Expression of the Polyplexes
Cells were seeded onto 6-well plates at a density of 2×105 per well in complete medium and allowed to grow until 50–60% confluence. Then, Syn-4 specific siRNA and control siRNA were transfected by lipofectamine 2000 according to a standard protocol recommended by the manufacturer. Western blot was used to detect the protein expression of Syn-4 (rabbit-anti-syndecan-4 polyclonal antibody 1:200). After 48h knock-down of Syn-4 expression, the cells were incubated with the PEG-PAMAM/DNA-Cy5 for 6h or G5-PEG/pGFP polyplexes for 48h. Cell-associated fluorescence intensity of Cy5 from the internalized polyplexes or GFP expressed in transfected cells was analyzed by flow cytometry. Results were compared with those from control siRNA treated cells from parallel experiments. Three repeats were conducted for each experiment (n=3).
2.7. Effects of Upregulation of CAV-1 on Endocytosis and Gene Expression of Polyplexes
Cells were seeded on 6-well plates at a density of 3×105 per well, and infected by Ad-CAV-1 (titer: 1.2×1010 pfu/mL) or Ad-null (titer: 1×1010 pfu/mL) at MOI 30 (300 for C2C12 cells) in 1 mL of fresh DMEM at 37 °C for 90 min. Then, 1 mL complete medium was supplied into each well, and the cells were incubated for 48h. Western blot was used to detect the protein expressions of CAV-1 (rabbit-anti-Caveolin-1 polyclonal antibody 1:1000).
The cells were then incubated with polyplexes according to the same procedure as described in 2.6. Cell-associated fluorescence intensity of Cy5 from the internalized polyplexes or GFP expressed in transfected cells was determined by flow cytometry, and results were compared with those from Ad-null treated cells from parallel experiments. Three repeats were conducted for each experiment (n=3).
2.8. Statistical analysis
All experiments were repeated three times and data were analyzed by unpaired one-tailed t-tests. P < 0.05 was considered statistically significant.
3. Results and Discussion
3.1. Average Size, Gel Retardation Assay and Cytotoxicity of Polyplexes
Many factors are involved in the selection of polyplex uptake pathways, including polyplex content, i.e. the nature of the polymer and oligonucleotide, and the cell type. It was reported that clathrin-coated pits were involved in the internalization of particles with a diameter <200 nm and that caveolae-mediated, cholesterol dependant internalization became favored for particles with a size >200 nm [21]. Fig. 1A shows that particle size of the PEG-PAMAM dendrimer/pDNA polyplexes formed at N/P ratio of 10 is within a range of 200–250 nm and are likely to be internalized by cholesterol-dependent caveolae-mediated endocytosis.
Fig. 1.
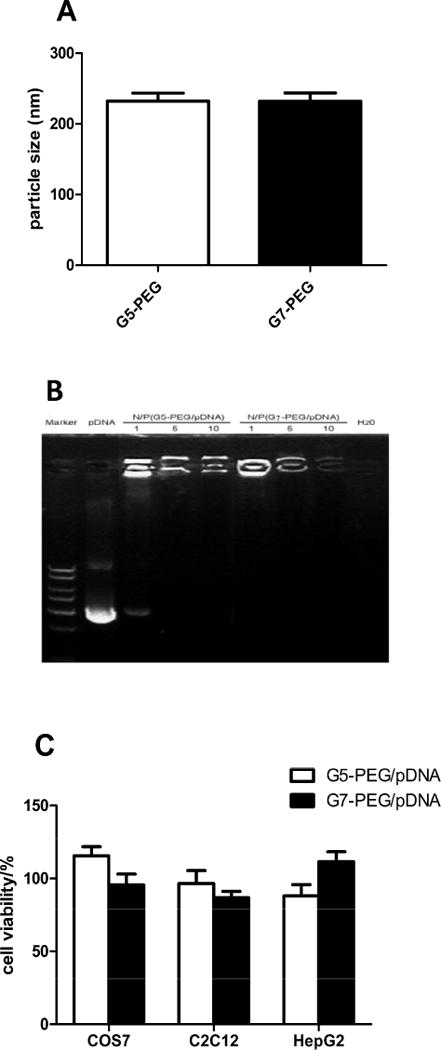
Characterization polyplexes formed by G5-PEG or G7-PEG with plasmid DNA and cytotoxicity of PEG-PAMAM dendrimers with COS7, C2C12, and HepG2 cells. (A) Average size of PEG-PAMAM dendrimer/pDNA polyplexes. (B) Retardation assay of PEG-PAMAM dendrimer to DNA mobility. From left to right: DNA ladder, Luciferase pDNA alone, G5-PEG/pDNA polyplexes, G7-PEG/pDNA polyplexes, and H2O. The polyplexes were formed at different N/P ratios of 1, 5, and 10, respectively. (C) Cell viability was determined by MTT assay after incubating the cells for 6 h with the dendrimers at the concentration used in the transfection/expression experiments (at N/P ratio of 10 with 1 μg pDNA).
When cationic dendrimers are used for gene delivery, the positively charged terminal amine groups bind to phosphate groups of DNA, and eventually condense DNA into polyplexes. In order to determine condensability of pDNA when mixed with PEG-PAMAM dendrimer, a gel retardation assay was performed. As shown in Fig. 1B, the mobility of plasmid DNA was fully retarded by both G5-PEG and G7-PEG when the N/P ratio was more than 5, indicating that the DNA was tightly combined with the dendrimer. In the following endocytosis experiments, an N/P ratio of 10 was used to form the polyplexes to ensure overall positive charge and effective interaction of the polyplexes with negatively charged cell membrane [22].
Fig. 1C shows that the viability of both C2C12 and HepG2 cells as determined by MTT assay is above 80% after incubation for 6h with the PEG-PAMAM dendrimer polyplexes (N/P = 10) at the same concentration used for the transfection and expression studies. Cytotoxicity of dendrimer mainly derives from its high net positive charge following protonation at physiological pH condition [8]; however, cytotoxicity decreases after polyplex formation because the negative charge on DNA partially neutralizes the positive charge and the particles are also screened by the PEG.
3.2. Effects of Syn-4 on Polyplex Endocytosis
Western blot results showed that Syn-4 expression was downregulated by fifty percent as compared to control after transfection with Syn-4 siRNA in C2C12 or HepG2 cells (Fig 2A and 3A). The maximum effect was observed at 48h. According to the literature, the half-life of Syn-4 mRNA is 16h [23] and changes in protein expression occurr 24–48h after RNA interference. Control siRNA had no significant effect on Syn-4 expression for the two cell lines indicating that the decrease of Syn-4 expression was caused by its specific siRNA. Upon Syn-4 downregulation, the fluorescence intensity of Cy5 from internalized polyplexes increased 1.5 folds for both G5-PEG and G7-PEG polyplexes in HepG2 cells (Fig. 3B, p<0.01 and p<0.001, respectively), while it decreased by half for G5-PEG polyplexes and by one third for G7-PEG polyplexes in C2C12 cells (Fig. 2B, p<0.001), as compared to control siRNA treated cells (n=3).
Fig. 2.
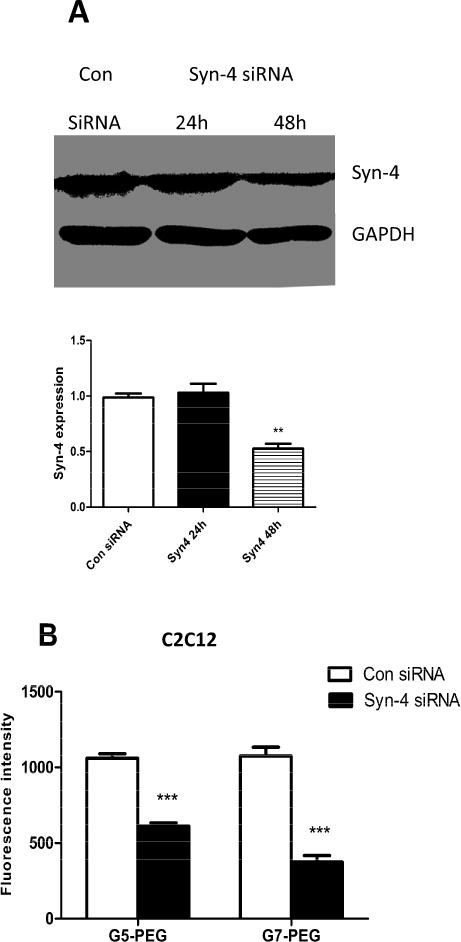
Effect of Syn-4 siRNA treatment on endocytosis of the polyplexes in C2C12 cells. (A) Western blot indicating that expression of Syn-4 was significantly knocked down after 48h. (n=3, **p<0.01, compared with the con-siRNA treated group). (B) Fluorescence intensity of the polyplexes in C2C12 cells measured by flow cytometer before and after Syn-4 siRNA treatment. (n = 3, *** p < 0.001, compared with the con-siRNA treated group).
Fig. 3.
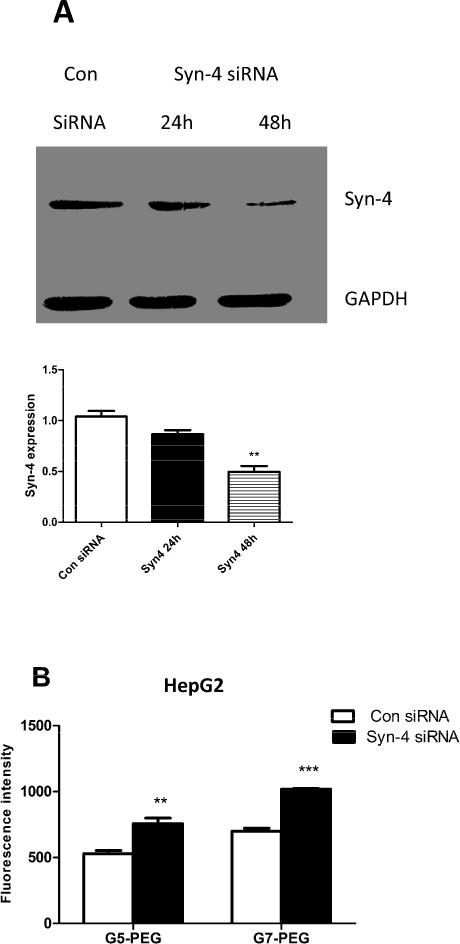
Effect of Syn-4 siRNA treatment on endocytosis of the polyplexes in HepG2 cells. (A) Western blot indicating that expression of Syn-4 was significantly knocked down after 48h (n=3, **p<0.01, compared with the con-siRNA treated group). (B) Fluorescence intensity of the polyplexes in HepG2 cells measured by flow cytometer before and after Syn-4 siRNA treatment. The polyplexes were formed by Cy5 labeled pDNA and PEG-PAMAM dendrimers. (n = 3, ** p<0.01, *** p < 0.001, compared with the con-siRNA treated group).
It is generally accepted that electrostatic interaction between cationic gene delivery carriers and negatively charged cell surfaces [24–26] drives the initial interaction and internalization of polyplexes. Negative charges on cell membrane mainly arise from proteoglycans [27, 28], particularly heparan sulfate proteoglycans (HSPGs) in which transmembrane proteins like syndecans are considered as major binding sites for cationic polymers [15, 16, 29]. Our results indicate that Syn-4, the most broadly expressed member in the syndecan family (Fig. S2), plays a quite different role in endocytosis of PEG-PAMAM dendrimer based polyplexes for HepG2 and C2C12 cells. Downregulation of Syn-4 increases uptake of PEG-PAMAM dendrimer polyplexes in HepG2 but decreases uptake in C2C12 cells. This data emphasizes that cell type influences pathway selection of polymer/DNA polyplexes and demonstrates that surface properties as well as endocytic activity of different cell types influence polyplex internalization pathways [30–34].
3.3. Effect of CAV-1 on Endocytosis of Polyplexes
Western blot results illustrate that expression of CAV-1 in both C2C12 cells (Fig. 4A) and HepG2 cells (Fig. 5A) was upregulated at 48h after infection by Ad-CAV-1. In addition, the initial expression of CAV-1 in C2C12 cells was higher than that in HepG2 cells (Fig. S3). There was almost no expression of CAV-1 in HepG2 cells and this is consistent with our previous results [6]. The C2C12 cell was not very sensitive to the adenovirus vector and a 10-fold higher dose of Ad-CAV-1 was required upregulate CAV-1 expression in C2C12 cells as compared to HepG2 cells. This results is not surprising as genetic manipulation of cultured muscle cells, especially undifferentiated muscle cell lines (myoblasts) or primary muscle stem cells (myosatellites), is notoriously difficult [35]. Fortunately, even at a high dose of Ad-CAV-1 up to MOI 400, significant cytotoxicity was not observed in C2C12 cells, which maintained normal morphology.
Fig. 4.
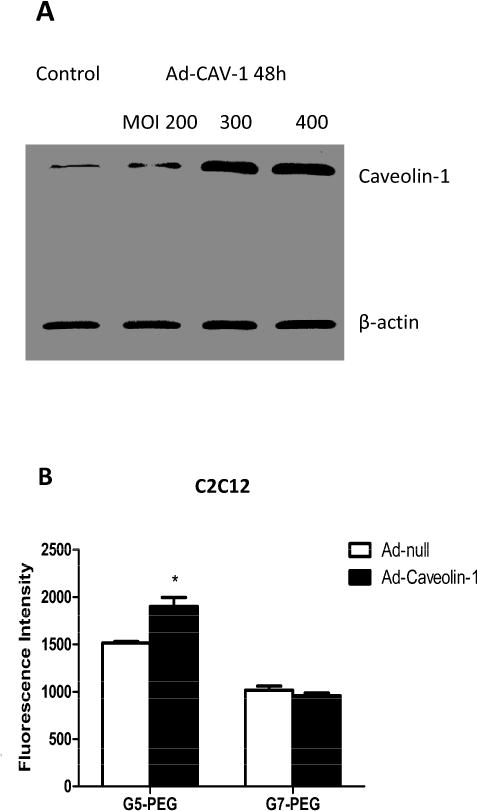
Effect of CAV-1 on endocytosis of the polyplexes in C2C12 cells. (A) Western blot indicating upregulation of CAV-1 expression 48h after infection by Ad-CAV-1 at MOI 300 and 400. MOI 300 was used as a dose of Ad-CAV-1 to infect the cells in the following endocytosis experiments. (B) Fluorescence intensity of polyplex transfected C2C12 cells measured by flow cytometer after treatment with Ad-null or Ad-CAV-1. (n = 3, * p<0.05, compared with the Ad-null treated group).
Fig. 5.
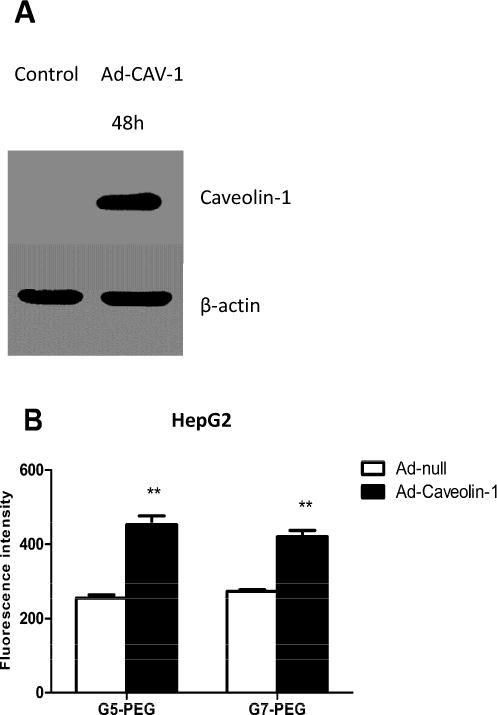
Effect of CAV-1 on endocytosis of the polyplexes in HepG2 cells. (A) Western blot blot indicating upregulation of CAV-1 expression 48h after infection by Ad-CAV-1 at MIO 30. (B) Fluorescence intensity of polyplex transfected HepG2 cells measured by flow cytometer after treatment with Ad-null or Ad-CAV-1. (n = 3, ** p<0.01, compared with the Ad-null treated group).
Compared with Ad-null treated cells, the fluorescence intensity of HepG2 cells increased 2-fold and 1.5-fold, respectively, after treatment with G5-PEG and G7-PEG (Fig. 5B, p<0.01). In previous studies, we had demonstrated that the CAV-1 pathway was not followed for G5 and G7 based polyplexes in HepG2 cells [6]. Remarkably, we observe that when employing PEGylated G5 and G7 polyplexes upregulation of CAV-1 by Ad-CAV-1 improves internalization. These results indicate that the impact of PEG goes beyond solubilization and protection of polyplexes from non-specific protein absorption and can have an important impact on the mechanism of internalization. In previous reports, it has been noted that PEGylation could alter the overall size of polyplexes, and for this reason and others, cause changes in uptake mechanism and/or gene expression efficiency [9]. For cationic lipid-based and poly-L-lysine polyplexes[36], it is also noted that the PEG changes the internal structure of the polyplex thus having potential impact on both cellular uptake and oligonucleotide release. It has also been noted that PEGylation can reduce acute inflammatory responses, which may help to minimize the impact of nucleases on gene expression efficiency [20, 37].
Fig. 4B shows that overexpression of CAV-1 in C2C12 cells results in an increase (p<0.05) of internalization for G5-PEG polyplexes; however, a similar increase is not observed for G7-PEG polyplexes. Despite the increase in internalization of G5-PEG polyplexes upon upregulation of CAV-1 expression in C2C12, the gene expression efficiency of the polyplexes did not change (Fig. 6A), suggesting that the caveolae mediated endocytosis pathway is not an important gene transfection/expression pathway in C2C12 cells. By way of contrast, both uptake and gene expression increase for G5-PEG polyplex upon upregulation of CAV-1 in HepG2 cells suggesting the pathway is important for this cell line for the PEGylated materials.
Fig. 6.
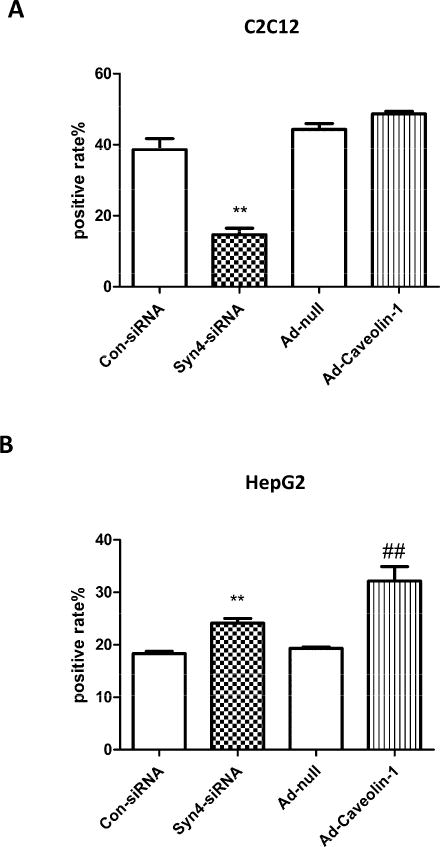
Effects of Syn-4 and CAV-1 on the gene transfection and expression efficiency of G5-PEG based polyplexes. The gene transfection and expression efficiency were measured by fluorescence of GFP protein expressed in (A) C2C12 and (B) HepG2 cells after Syn-4 downregulation or CAV-1 overexpression. (n=3, ** p<0.01, compared with the con-siRNA treated groups; ## p<0.01, compared with the Ad-null treated group).
3.4. Effects of Syn-4 and CAV-1 on Gene Expression Efficiency of G5-PEG Based Polyplexes
The gene transfection and expression process are affected by many factors. Endocytosis of polyplexes into cells is just the first step of a complicated process of that ultimately results in gene expression. To explore the influence of polyplex endocytosis mechanisms on gene expression, the impacts of Syn-4 and CAV-1 were explored using GFP as the expression gene. GFP expression efficiency of G5-PEG polyplexes decreased by half (Fig. 6A, p<0.01) upon downregulation of Syn-4; however, overexpression of CAV-1 resulted in no change for C2C12 cells. As for HepG2 cells, both downregulation of Syn-4 and overexpression of CAV-1 increased GFP expression efficiency of G5-PEG polyplexes (Fig. 6B, p<0.01). These changes in gene expression efficiency were consistent with the changes observed in G5-PEG polyplex internalization.
These studies have important implications for in vivo transgenic therapy of lipid metabolism disorders. Downregulation of Syn-4 and upregulation of CAV-1 may improve gene delivery efficacy of PEG-PAMAM dendrimers to ApoE gene in liver, and upregulation of Syn-4 will benefit gene delivery efficacy of PEG-PAMAM dendrimers to LPL gene in muscular tissues. Research on additional mechanisms involved in the endocytosis of the PEG-PAMAM based polyplexes is underway.
4. Conclusion
Syn-4 and CAV-1 play different roles in endocytosis of PEG-PAMAM/pDNA polyplexes in different cell lines. Both Syn-4 and CAV-1 were involved in the internalization process of the PEG-PAMAM polyplexes in HepG2 cells, and downregulation of Syn-4 and upregulation of CAV-1 might improve gene transfection and expression efficacy of PEG-PAMAM dendrimers. For C2C12 cells, uptake of the PEG-PAMAM polyplexes was affected by Syn-4 but not CAV-1, and upregulation of Syn-4 would contribute to gene delivery.
Supplementary Material
Fig. S1. Characteristic data for PEG-PAMAM dendrimers. (A) Characteristic data for G5-PEG-PAMAM dendrimer and G7-PEG-PAMAM dendrimer. (B) 1H NMR data for G5-PEG-PAMAM dendrimer. (C) 1H NMR data for G7-PEG-PAMAM dendrimer.
Fig. S2. Comparison of the initial expression of Syn-4 on HepG2 and C2C12 cells.
Fig. S3. Comparison of the initial expression of CAV-1 on HepG2 and C2C12 cells.
Highlights.
Downregulation of Syn-4 improves internalization of PEG-PAMAM based DNA polyplexes in HepG2 cells.
Downregulation of Syn-4 decreases internalization of PEG-PAMAM based DNA polyplexes in C2C12 cells.
Upregulation of CAV-1 improves internalization of PEG-PAMAM based DNA polyplexes in HepG2 cells.
CAV-1 does not involve in the internalization of PEG-PAMAM based DNA polyplexes in C2C12 cells.
Acknowledgments
This study was supported by National Natural Science Foundation of China (No. 30971241 and 81270368), and federal funds from the National Institute of Biomedical Imaging and Bioengineering of the U.S.A. (RO1-EB005028).
Abbreviations
- G5-PEG
PEGylated generation 5 poly(amidoamine) dendrimers
- G7-PEG
PEGylated generation 7 poly(amidoamine) dendrimers
- Syn-4 siRNA
syndecan-4 specific siRNA
- Ad-CAV-1
adenovirus vector expressed caveolin-1
- Ad-null
the empty adenovirus vector
- PLL
Poly L Lysine
- PEI
Polyethyeneimine
- LPL
lipoprotein lipase
- CM
chylomicrons
- VLDL
very low density lipoprotein
- LDLR
low density lipoprotein receptor
- ApoE
apolipoprotein E
- 1 H NMR
nuclear magnetic resonance spectroscopy
- HPLC
high-performance liquid chromatography
- GPC
gel permeation chromatography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Chemical compounds studied in this article: PAMAM dendrimer (Pubchem CID: 4140276); polyethylene glycol (Pubchem CID:174)
References
- 1.Liu H, Wang H, Yang W, Cheng Y. Disulfide cross-linked low generation dendrimers with high gene transfection efficacy, low cytotoxicity, and low cost. J Am Chem Soc. 2012;134:17680–17687. doi: 10.1021/ja307290j. [DOI] [PubMed] [Google Scholar]
- 2.Wen Y, Guo Z, Du Z, Fang R, Wu H, Zeng X, Wang C, Feng M, Pan S. Serum tolerance and endosomal escape capacity of histidine-modified pDNA-loaded complexes based on polyamidoamine dendrimer derivatives. Biomaterials. 2012;33:8111–8121. doi: 10.1016/j.biomaterials.2012.07.032. [DOI] [PubMed] [Google Scholar]
- 3.Qi R, Gao Y, Tang Y, He RR, Liu TL, He Y, Sun S, Li BY, Li YB, Liu G. PEG-conjugated PAMAM dendrimers mediate efficient intramuscular gene expression. AAPS J. 2009;11:395–405. doi: 10.1208/s12248-009-9116-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Liu X, Liu C, Laurini E, Posocco P, Pricl S, Qu F, Rocchi P, Peng L. Efficient delivery of sticky siRNA and potent gene silencing in a prostate cancer model using a generation 5 triethanolamine-core PAMAM dendrimer. Mol Pharm. 2012;9:470–481. doi: 10.1021/mp2006104. [DOI] [PubMed] [Google Scholar]
- 5.Tang Y, Li YB, Wang B, Lin RY, van Dongen M, Zurcher DM, Gu XY, Banaszak Holl MM, Liu G, Qi R. Efficient in Vitro siRNA Delivery and Intramuscular Gene Silencing Using PEG-Modified PAMAM Dendrimers. Mol Pharm. 2012;9:1812–1821. doi: 10.1021/mp3001364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Qi R, Mullen DG, Baker JR, Holl MM. The Mechanism of Polyplex Internalization into Cells:Testing the GM1/Caveolin-1 Lipid Raft Mediated Endocytosis Pathway. Mol Pharm. 2010;7:267–279. doi: 10.1021/mp900241t. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Choi YH, Liu F, Kim JS, Choi YK, Park JS, Kim SW. Polyethylene glycol-grafted poly-L-lysine as polymeric gene carrier. J Control Rel. 1998;54:39–48. doi: 10.1016/s0168-3659(97)00174-0. [DOI] [PubMed] [Google Scholar]
- 8.Jevprasesphant R, Penny J, Jalal R, Attwood D, McKeown NB, D’Emanuele A. The influence of surface modification on the cytotoxicity of PAMAM dendrimers. Int J Pharm. 2003;252:263–266. doi: 10.1016/s0378-5173(02)00623-3. [DOI] [PubMed] [Google Scholar]
- 9.Fitzsimmons RE, Uludag H. Specific effects of PEGylation on gene delivery efficacy of polyethylenimine: interplay between PEG substitution and N/P ratio. Acta Biomater. 2012;8:3941–3955. doi: 10.1016/j.actbio.2012.07.015. [DOI] [PubMed] [Google Scholar]
- 10.Nichols B. Caveosomes and endocytosis of lipid rafts. J Cell Sci. 2003;116:4707–4714. doi: 10.1242/jcs.00840. [DOI] [PubMed] [Google Scholar]
- 11.Okamoto T, Schlegel A, Scherer PE, Lisanti MP. Caveolins, a family of scaffolding proteins for organizing “preassembled signaling complexes” at the plasma membrane. J Biol Chem. 1998;273:5419–54122. doi: 10.1074/jbc.273.10.5419. [DOI] [PubMed] [Google Scholar]
- 12.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 13.Sanderson RD, Bernfield M. Molecular polymorphism of a cell surface proteoglycan: distinct structures on simple and stratified epithelia. Proc Natl Acad Sci U S A. 1988;85:9562–9566. doi: 10.1073/pnas.85.24.9562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bernfield MR, Kokenyesi MK. Biology of the syndecans: a family of transmembrane heparan sulfate proteoglycans. Annu Rev Cell Biol. 1992;8:365–393. doi: 10.1146/annurev.cb.08.110192.002053. [DOI] [PubMed] [Google Scholar]
- 15.Paris S, Burlacu A, Durocher Y. Opposing Roles of Syndecan-1 and Syndecan-2 in Polyethyleneimine-mediated Gene Delivery. J Biol Chem. 2008;283:7697–7704. doi: 10.1074/jbc.M705424200. [DOI] [PubMed] [Google Scholar]
- 16.Kopatz I, Remy JS, Behr JP. A model for non-viral gene delivery: through syndecan adhesion molecules and powered by actin. J Gene Med. 2004;6:769–776. doi: 10.1002/jgm.558. [DOI] [PubMed] [Google Scholar]
- 17.Kim CW, Goldberger OA, Gallo RL, Bernfield M. Members of the syndecan family of heparan sulfate proteoglycans are expressed in distinct cell-, tissue-, and development-specific patterns. Mol Biol Cell. 1994;5:797–805. doi: 10.1091/mbc.5.7.797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zhang X, Qi R, Xian X, Yang F, Blackstein M, Deng X, Fan J, Ross C, Karasinska J, Hayden MR, Liu G. Spontaneous atherosclerosis in aged lipoprotein lipase-deficient mice with severe hypertriglyceridemia on a normal chow diet. Circ Res. 2008;102:250–256. doi: 10.1161/CIRCRESAHA.107.156554. [DOI] [PubMed] [Google Scholar]
- 19.Jawien J, Nastalek P, Korbut R. Mouse models of experimental atherosclerosis. J Physiol Pharmacol. 2004;55:503–517. [PubMed] [Google Scholar]
- 20.Rattan R, Vaidyanathan S, Wu GS, Shakya A, Orr BG, Banaszak Holl MM. Polyplex-induced cytosolic nuclease activation leads to differential transgene expression. Mol Pharm. 2013;10:3013–3022. doi: 10.1021/mp400103f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Rejman J, Oberle V, Zuhorn IS, Hoekstra D. Size-dependent internalization of particles via the pathways of clathrin- and caveolae-mediated endocytosis. Biochem J. 2004;377:159–169. doi: 10.1042/BJ20031253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tros de Ilarduya C, Sun Y, Duzgunes N. Gene delivery by lipoplexes and polyplexes. Eur J Pharm Sci. 2010;40:159–170. doi: 10.1016/j.ejps.2010.03.019. [DOI] [PubMed] [Google Scholar]
- 23.Zhang Y, Pasparakis M, Kollias G, Simons M. Myocyte-dependent regulation of endothelial cell syndecan-4 expression. Role of TNF-alpha. J Biol Chem. 1999;274:14786–14790. doi: 10.1074/jbc.274.21.14786. [DOI] [PubMed] [Google Scholar]
- 24.Boussif O, Lezoualc’h F, Zanta MA, Mergny MD, Scherman D, Demeneix B, Behr JP. A versatile vector for gene and oligonucleotide transfer into cells in culture and in vivo: polyethylenimine. Proc Natl Acad Sci U S A. 1995;92:7297–7301. doi: 10.1073/pnas.92.16.7297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Behr JP, Demeneix B, Loeffler JP, Perez-Mutul J. Efficient gene transfer into mammalian primary endocrine cells with lipopolyamine-coated DNA. Proc Natl Acad Sci U S A. 1989;86:6982–6986. doi: 10.1073/pnas.86.18.6982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Smith KR. Gene transfer in higher animals: theoretical considerations and key concepts. J Biotechnol. 2002;99:1–22. doi: 10.1016/S0168-1656(02)00105-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Dennig J, Duncan E. Gene transfer into eukaryotic cells using activated polyamidoamine dendrimers. J Biotechnol. 2002;90:339–347. doi: 10.1016/s1389-0352(01)00066-6. [DOI] [PubMed] [Google Scholar]
- 28.Gao K, Huang L. Nonviral methods for siRNA delivery. Mol Pharm. 2009;6:651–658. doi: 10.1021/mp800134q. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Luo D, Saltzman WM. Synthetic DNA delivery systems. Nat Biotechnol. 2000;18:33–37. doi: 10.1038/71889. [DOI] [PubMed] [Google Scholar]
- 30.Simoes S, Pires P, Duzgunes N, Pedrosa de Lima MC. Cationic liposomes as gene transfer vectors: barriers to successful application in gene therapy. Curr Opin Mol Ther. 1999;1:147–157. [PubMed] [Google Scholar]
- 31.Harashima H, Shinohara Y, Kiwada H. Intracellular control of gene trafficking using liposomes as drug carriers. Eur J Pharm Sci. 2001;13:85–89. doi: 10.1016/s0928-0987(00)00211-6. [DOI] [PubMed] [Google Scholar]
- 32.Rejman J, Bragonzi A, Conese M. Role of clathrin- and caveolae-mediated endocytosis in gene transfer mediated by lipo- and polyplexes. Mol Ther. 2005;12:468–474. doi: 10.1016/j.ymthe.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 33.Chen RN, Ho HO, Yu CY, Sheu MT. Development of swelling/floating gastroretentive drug delivery system based on a combination of hydroxyethyl cellulose and sodium carboxymethyl cellulose for Losartan and its clinical relevance in healthy volunteers with CYP2C9 polymorphism. Eur J Pharm Sci. 2010;39:82–89. doi: 10.1016/j.ejps.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 34.Hillaireau H, Couvreur P. Nanocarriers’ entry into the cell: relevance to drug delivery. Cell Mol Life Sci. 2009;66:2873–2896. doi: 10.1007/s00018-009-0053-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Jackson MF, Hoversten KE, Powers JM, Trobridge GD, Rodgers BD. Genetic manipulation of myoblasts and a novel primary myosatellite cell culture system: comparing and optimizing approaches. FEBS J. 2013;280:827–839. doi: 10.1111/febs.12072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Ryuta Aono EY. Atsushi Harada, and Kenji Kono, Nanofiber Polyplex Formation. Based on the Morphology Elongation by the Intrapolyplex PEG Crowding Effect, ACS Macro Letters. 2014;3:333–336. doi: 10.1021/mz500072k. [DOI] [PubMed] [Google Scholar]
- 37.Uchida S, Itaka K, Chen Q, Osada K, Ishii T, Shibata MA, Harada-Shiba M, Kataoka K. PEGylated polyplex with optimized PEG shielding enhances gene introduction in lungs by minimizing inflammatory responses. Mol Ther. 2012;20:1196–1203. doi: 10.1038/mt.2012.20. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Fig. S1. Characteristic data for PEG-PAMAM dendrimers. (A) Characteristic data for G5-PEG-PAMAM dendrimer and G7-PEG-PAMAM dendrimer. (B) 1H NMR data for G5-PEG-PAMAM dendrimer. (C) 1H NMR data for G7-PEG-PAMAM dendrimer.
Fig. S2. Comparison of the initial expression of Syn-4 on HepG2 and C2C12 cells.
Fig. S3. Comparison of the initial expression of CAV-1 on HepG2 and C2C12 cells.


