Abstract
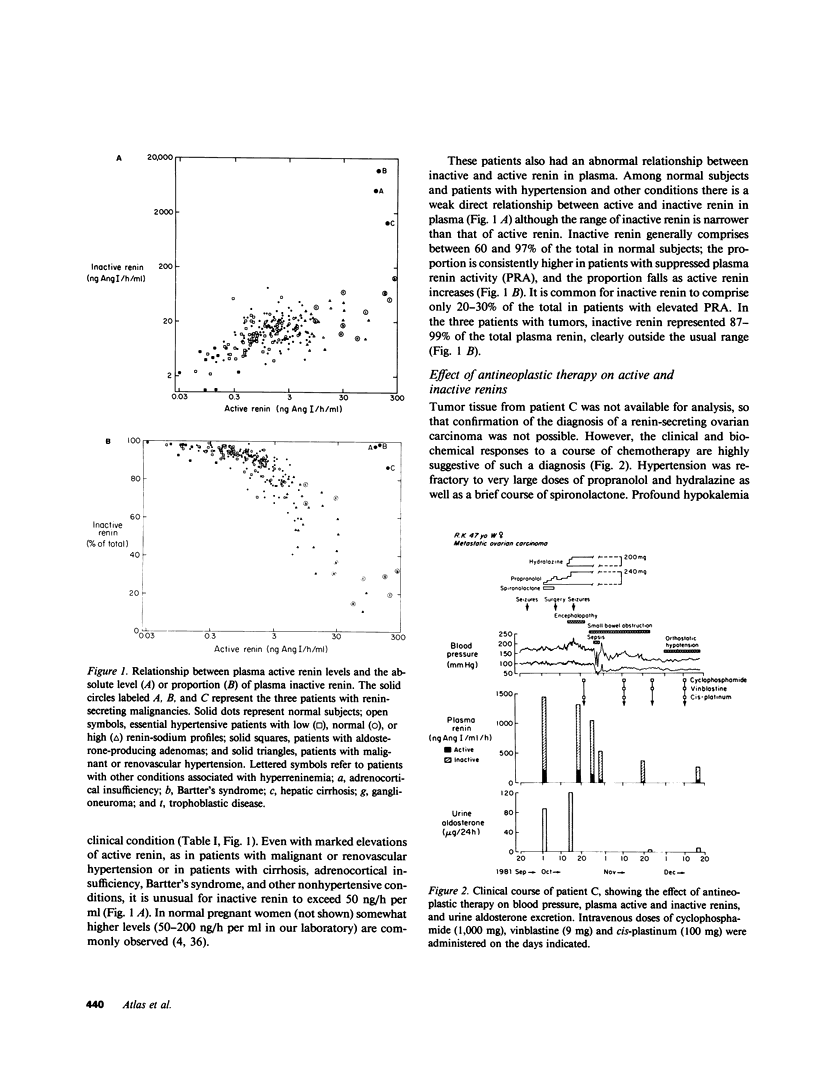
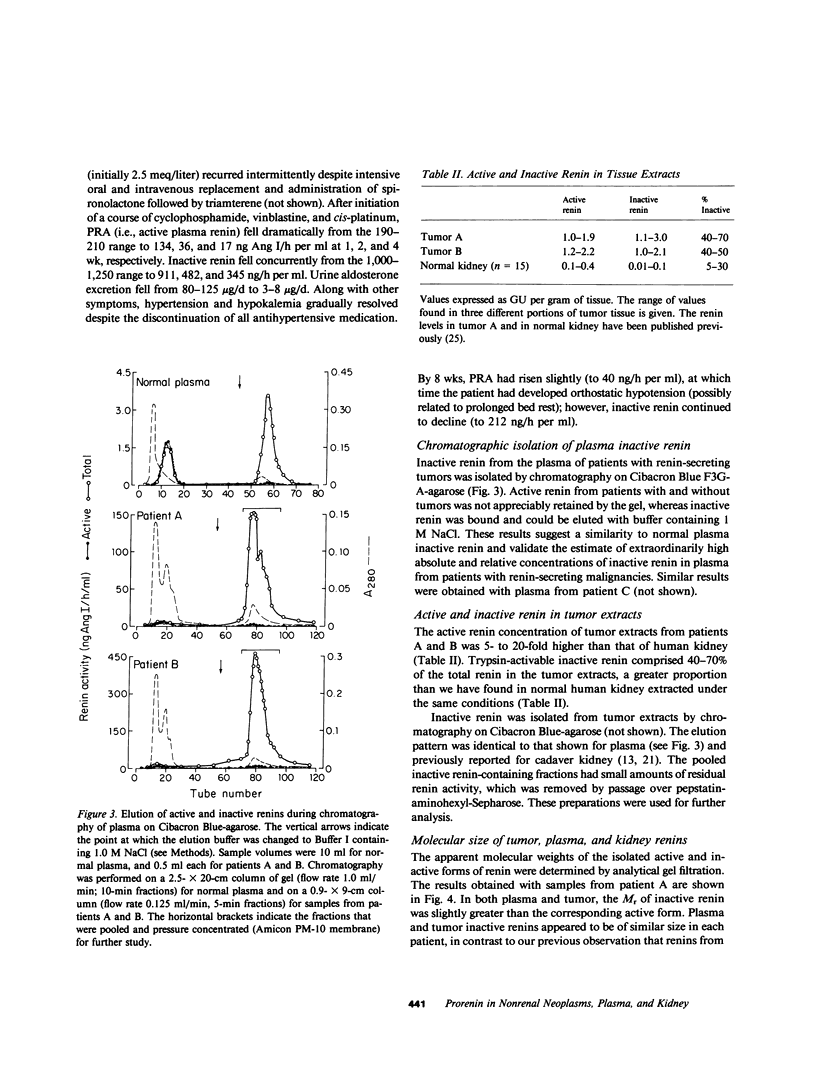
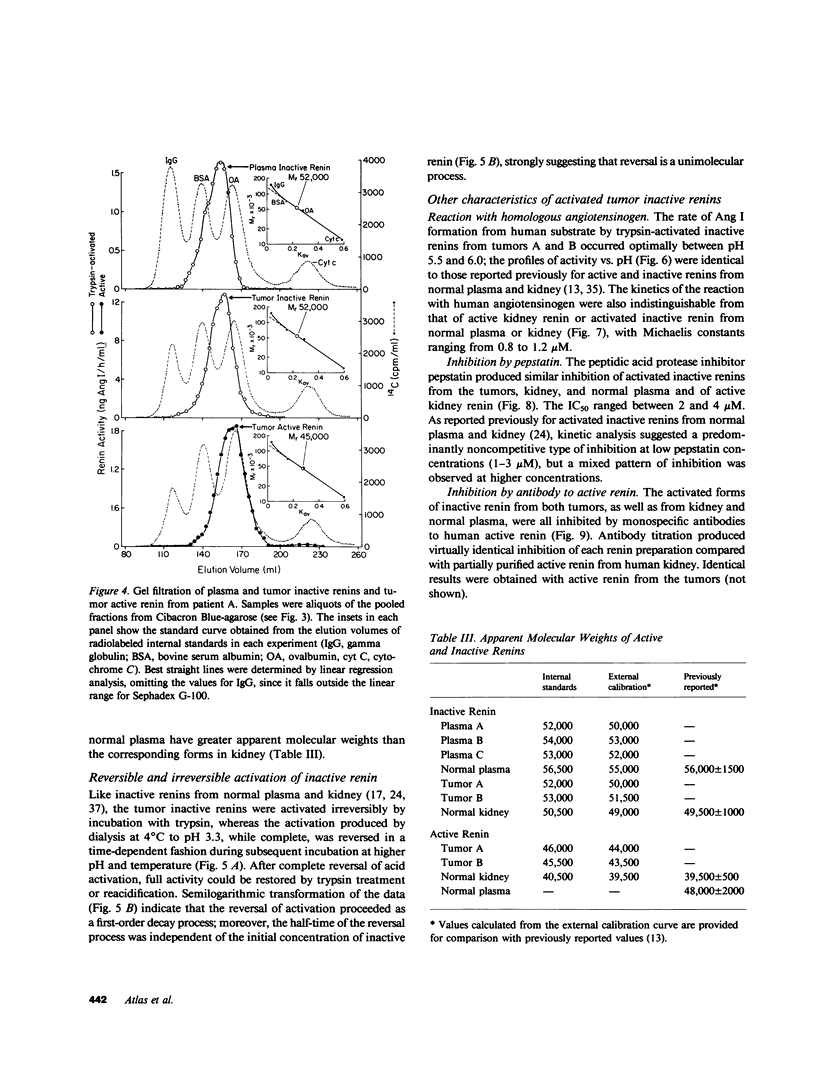
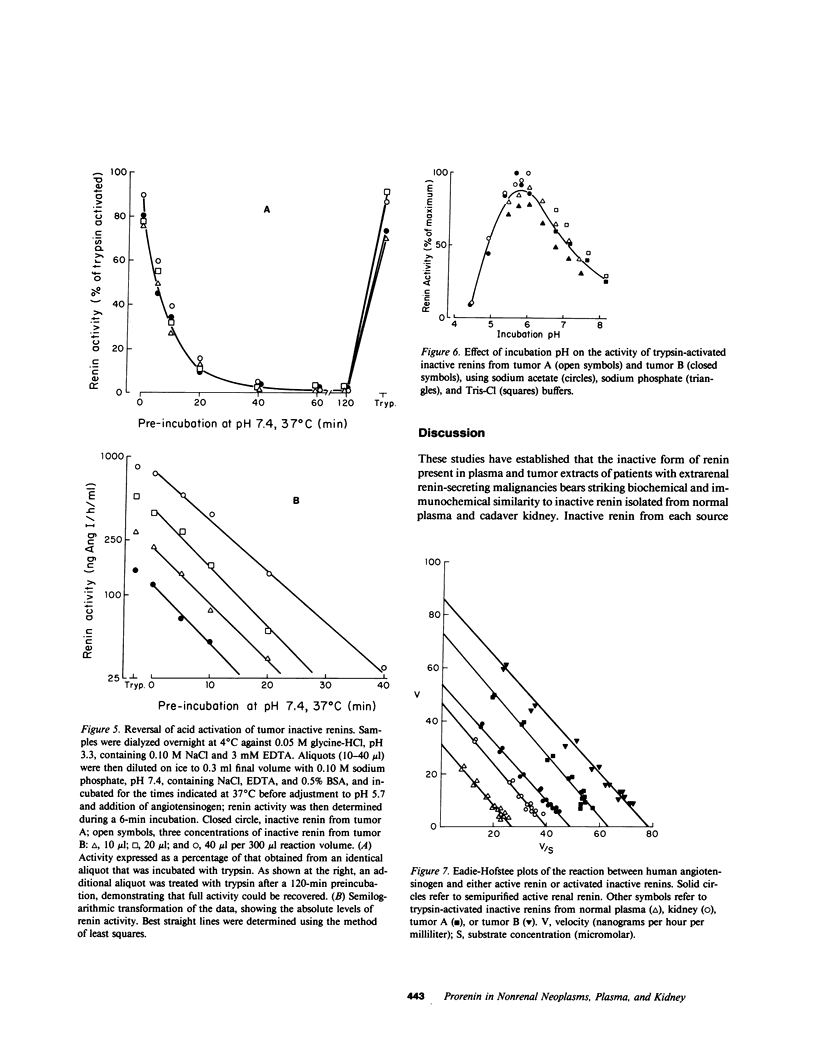
Inactive renin comprises well over half the total renin in normal human plasma. There is a direct relationship between active and inactive renin levels in normal and hypertensive populations, but the proportion of inactive renin varies inversely with the active renin level; as much as 98% of plasma renin is inactive in patients with low renin, whereas the proportion is consistently lower (usually 20-60%) in high-renin states. Two hypertensive patients with proven renin-secreting carcinomas of non-renal origin (pancreas and ovary) had high plasma active renin (119 and 138 ng/h per ml) and the highest inactive renin levels we have ever observed (5,200 and 14,300 ng/h per ml; normal range 3-50). The proportion of inactive renin (98-99%) far exceeded that found in other patients with high active renin levels. A third hypertensive patient with a probable renin-secreting ovarian carcinoma exhibited a similar pattern. Inactive renins isolated from plasma and tumors of these patients were biochemically similar to semipurified inactive renins from normal plasma or cadaver kidney. All were bound by Cibacron Blue-agarose, were not retained by pepstatin-Sepharose, and had greater apparent molecular weights (Mr) than the corresponding active forms. Plasma and tumor inactive renins from the three patients were similar in size (Mr 52,000-54,000), whereas normal plasma inactive renin had a slightly larger Mr than that from kidney (56,000 vs. 50,000). Inactive renin from each source was activated irreversibly by trypsin and reversibly by dialysis to pH 3.3 at 4 degrees C; the reversal process followed the kinetics of a first-order reaction in each instance. The trypsin-activated inactive renins were all identical to semipurified active renal renin in terms of pH optimum (pH 5.5-6.0) and kinetics with homologous angiotensinogen (Michaelis constants, 0.8-1.3 microM) and inhibition by pepstatin or by serial dilutions of renin-specific antibody. These results indicate that a markedly elevated plasma inactive renin level distinguishes patients with ectopic renin production from other high-renin hypertensive states. The co-production of inactive and active renin by extrarenal neoplasms provides strong presumptive evidence that inactive renin is a biosynthetic precursor of active renin. The unusually high proportion of inactive renin in plasma and tumor extracts from such patients is consistent with ineffective precursor processing by neoplastic tissue, suggesting that if activation of "prorenin" is involved in the normal regulation of active renin levels it more likely occurs in the tissue of origin (e.g., kidney) than in the circulation.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Atlas S. A., Laragh J. H., Sealey J. E., Hesson T. E. An inactive, prorenin-like substance in human kidney and plasma. Clin Sci (Lond) 1980 Dec;59 (Suppl 6):29s–33s. doi: 10.1042/cs059029s. [DOI] [PubMed] [Google Scholar]
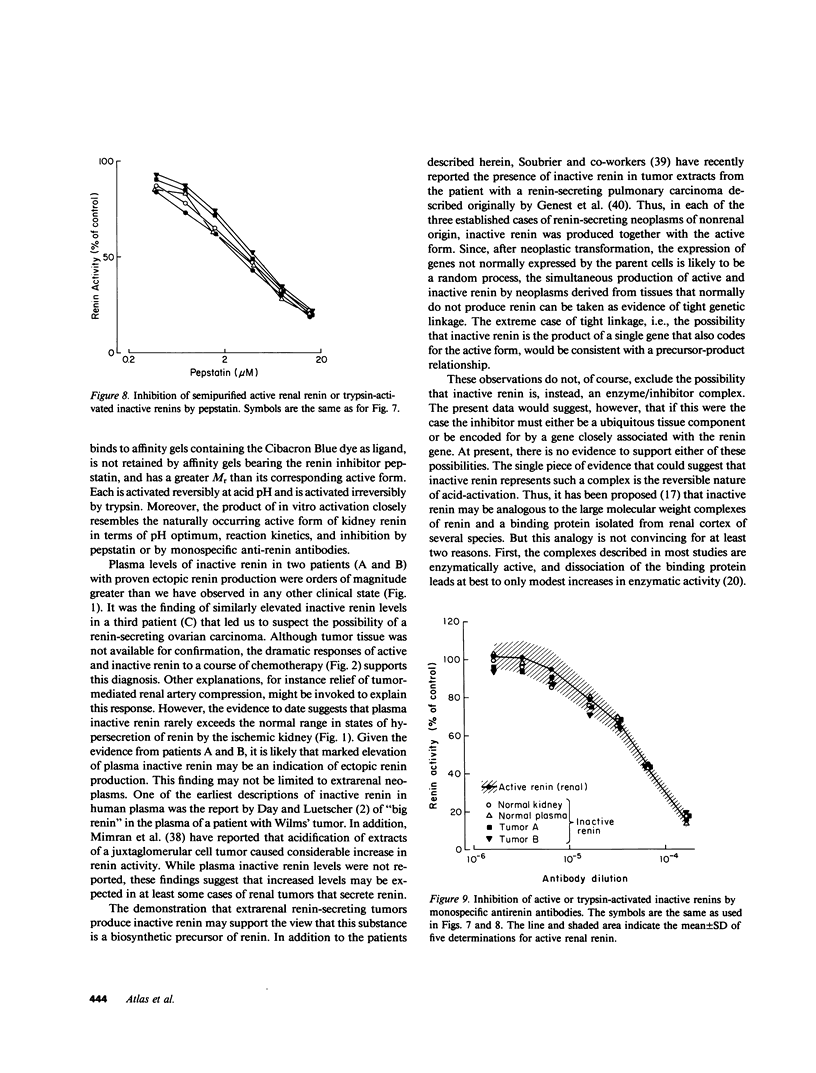
- Atlas S. A., Sealey J. E., Hesson T. E., Kaplan A. P., Ménard J., Corvol P., Laragh J. H. Biochemical similarity of partially purified inactive renins from human plasma and kidney. Hypertension. 1982 May-Jun;4(3 Pt 2):86–95. [PubMed] [Google Scholar]
- Aurell M., Rudin A., Tisell L. E., Kindblom L. G., Sandberg G. Captopril effect on hypertension in patient with renin-producing tumour. Lancet. 1979 Jul 21;2(8134):149–150. doi: 10.1016/s0140-6736(79)90031-x. [DOI] [PubMed] [Google Scholar]
- Boyd G. W. A protein-bound form of porcine renal renin. Circ Res. 1974 Sep;35(3):426–438. doi: 10.1161/01.res.35.3.426. [DOI] [PubMed] [Google Scholar]
- Chang J. J., Kisaragi M., Okamoto H., Inagami T. Isolation and activation of inactive renin from human kidney and plasma. Plasma and renal inactive renins have different molecular weights. Hypertension. 1981 Sep-Oct;3(5):509–515. doi: 10.1161/01.hyp.3.5.509. [DOI] [PubMed] [Google Scholar]
- Coulson R. L., Yazdanfar S., Rubio E., Bove A. A., Lemole G. M., Spann J. F. Recuperative potential of cardiac muscle following relief of pressure overload hypertrophy and right ventricular failure in the cat. Circ Res. 1977 Jan;40(1):41–49. doi: 10.1161/01.res.40.1.41. [DOI] [PubMed] [Google Scholar]
- Day R. P., Luetscher J. A. Big renin: a possible prohormone in kidney and plasma of a patient with Wilm's tumor. J Clin Endocrinol Metab. 1974 May;38(5):923–926. doi: 10.1210/jcem-38-5-923. [DOI] [PubMed] [Google Scholar]
- Derkx F. H., Bouma B. N., Schalekamp M. P., Schalekamp M. A. An intrinsic factor XII- prekallikrein-dependent pathway activates the human plasma renin-angiotensin system. Nature. 1979 Jul 26;280(5720):315–316. doi: 10.1038/280315a0. [DOI] [PubMed] [Google Scholar]
- Derkx F. H., Tan-Tjiong H. L., Man in't Veld A. J., Schalekamp M. P., Schalekamp M. A. Activation of inactive plasma renin by tissue kallikreins. J Clin Endocrinol Metab. 1979 Nov;49(5):765–769. doi: 10.1210/jcem-49-5-765. [DOI] [PubMed] [Google Scholar]
- Galen F. X., Devaux C., Guyenne T., Menard J., Corvol P. Multiple forms of human renin. Purification and characterization. J Biol Chem. 1979 Jun 10;254(11):4848–4855. [PubMed] [Google Scholar]
- Genest J., Rojo-Ortega J. M., Kuchel O., Boucher R., Nowaczynski W., Lefebvre R., Chrétien M., Cantin J., Granger P. Malignant hypertension with hypokalemia in a patient with renin-producing pulmonary carcinoma. Trans Assoc Am Physicians. 1975;88:192–201. [PubMed] [Google Scholar]
- Guyene T. T., Galen F. X., Devaux C., Corvol P., Menard J. Direct radioimmunoassay of human renin: comparison with renin activity in plasma and amniotic fluid. Hypertension. 1980 Jul-Aug;2(4):465–470. doi: 10.1161/01.hyp.2.4.465. [DOI] [PubMed] [Google Scholar]
- Haas E., Goldblatt H., Gipson E. C. Extraction, purification, and acetylation of human renin and the production of antirenin to human renin. Arch Biochem Biophys. 1965 Jun;110(3):534–543. doi: 10.1016/0003-9861(65)90447-9. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Carlson E. J., Luetscher J. A., Grislis G. Activation and characterization of inactive big renin in plasma of patients with diabetic nephropathy and unusual active renin. J Clin Endocrinol Metab. 1980 Sep;51(3):535–543. doi: 10.1210/jcem-51-3-535. [DOI] [PubMed] [Google Scholar]
- Hsueh W. A., Luetscher J. A., Carlson E. J., Grislis G. Inactive renin of high molecular weight (big renin) in normal human plasma. Activation by pepsin, trypsin, or dialysis to pH 3.3 and 7.5. Hypertension. 1980 Nov-Dec;2(6):750–756. doi: 10.1161/01.hyp.2.6.750. [DOI] [PubMed] [Google Scholar]
- Kawamura M., Ikemoto F., Funakawa S., Yamamoto K. Characteristics of a renin-binding substance for the conversion of renin into a higher-molecular-weight form in the dog. Clin Sci (Lond) 1979 Oct;57(4):345–350. doi: 10.1042/cs0570345. [DOI] [PubMed] [Google Scholar]
- Kotchen T. A., Welch W. J., Talwalkar R. T. Modification of the enzymatic activity of renin by acidification of plasma and by exposure of plasma to cold temperatures. Hypertension. 1979 May-Jun;1(3):190–201. doi: 10.1161/01.hyp.1.3.190. [DOI] [PubMed] [Google Scholar]
- Kourides I. A., Hoffman B. J., Landon M. B. Difference in glycosylation between secreted and pituitary free alpha-subunit of the glycoprotein hormones. J Clin Endocrinol Metab. 1980 Dec;51(6):1372–1377. doi: 10.1210/jcem-51-6-1372. [DOI] [PubMed] [Google Scholar]
- Leckie B. J., McConnell A. A renin inhibitor from rabbit kidney: conversion of a large inactive renin to a smaller active enzyme. Circ Res. 1975 Apr;36(4):513–519. doi: 10.1161/01.res.36.4.513. [DOI] [PubMed] [Google Scholar]
- Leckie B. J., McGhee N. K. Reversible activation-inactivation of renin in human plasma. Nature. 1980 Dec 25;288(5792):702–705. doi: 10.1038/288702a0. [DOI] [PubMed] [Google Scholar]
- Lumbers E. R. Activation of renin in human amniotic fluid by low pH. Enzymologia. 1971 Jun 30;40(6):329–336. [PubMed] [Google Scholar]
- Mimran A., Leckie B. J., Fourcade J. C., Baldet P., Navratil H., Barjon P. Blood pressure, renin-angiotensin system and urinary kallikrein in a case of juxtaglomerular cell tumor. Am J Med. 1978 Sep;65(3):527–536. doi: 10.1016/0002-9343(78)90780-5. [DOI] [PubMed] [Google Scholar]
- Murakami K., Inagami T., Haas E. Partial purification of human renin. Circ Res. 1977 Oct;41(4 Suppl 2):4–7. doi: 10.1161/01.res.41.4.4. [DOI] [PubMed] [Google Scholar]
- NEURATH H., DIXON G. H. Structure and activation of trypsinogen and chymotrypsinogen. Fed Proc. 1957 Sep;16(3):791–801. [PubMed] [Google Scholar]
- Poulsen K., Krøll J., Nielsen A. H., Jensenius J., Malling C. Renin binding proteins in plasma. Binding of renin to some of the plasma protease inhibitors, to lipoproteins, and to a non-trypsin-binding unidentified plasma protein. Biochim Biophys Acta. 1979 Mar 27;577(1):1–10. doi: 10.1016/0005-2795(79)90002-3. [DOI] [PubMed] [Google Scholar]
- Ruddy M. C., Atlas S. A., Salerno F. G. Hypertension associated with a renin-secreting adenocarcinoma of the pancreas. N Engl J Med. 1982 Oct 14;307(16):993–997. doi: 10.1056/NEJM198210143071606. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Oza N. B., Ryan J. W. Activation of a prorenin-like substance in human plasma by trypsin and by urinary kallikrein. Hypertension. 1979 May-Jun;1(3):179–189. doi: 10.1161/01.hyp.1.3.179. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Oza N. B., Ryan J. W. Activation of a prorenin-like substance in human plasma by trypsin and by urinary kallikrein. Hypertension. 1979 May-Jun;1(3):179–189. doi: 10.1161/01.hyp.1.3.179. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Oza N. B., Ryan J. W. Human urinary kallikrein converts inactive to active renin and is a possible physiological activator of renin. Nature. 1978 Sep 14;275(5676):144–145. doi: 10.1038/275144a0. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H. Prorenin and other large molecular weight forms of renin. Endocr Rev. 1980 Fall;1(4):365–391. doi: 10.1210/edrv-1-4-365. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Atlas S. A., Laragh J. H., Silverberg M., Kaplan A. P. Initiation of plasma prorenin activation by Hageman factor-dependent conversion of plasma prekallikrein to kallikrein. Proc Natl Acad Sci U S A. 1979 Nov;76(11):5914–5918. doi: 10.1073/pnas.76.11.5914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sealey J. E., Gerten-Banes J., Laragh J. H. The renin system: Variations in man measured by radioimmunoassay or bioassay. Kidney Int. 1972 Apr;1(4):240–253. doi: 10.1038/ki.1972.34. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Laragh J. H. "Prorenin" in human plasma? Circ Res. 1975 Jun;36(6 Suppl 1):10–16. doi: 10.1161/01.res.36.6.10. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Moon C., Laragh J. H., Alderman M. Plasma prorenin: cryoactivation and relationship to renin substrate in normal subjects. Am J Med. 1976 Nov;61(5):731–738. doi: 10.1016/0002-9343(76)90154-6. [DOI] [PubMed] [Google Scholar]
- Sealey J. E., Wilson M., Morganti A. A., Zervoudakis I., Laragh J. H. Changes in active and inactive renin throughout normal pregnancy. Clin Exp Hypertens A. 1982;4(11-12):2373–2384. doi: 10.3109/10641968209062396. [DOI] [PubMed] [Google Scholar]
- Shulkes A. A., Gibson R. R., Skinner S. L. The nature of inactive renin in human plasma and amniotic fluid. Clin Sci Mol Med. 1978 Jul;55(1):41–50. doi: 10.1042/cs0550041. [DOI] [PubMed] [Google Scholar]
- Skinner S. L., Cran E. J., Gibson R., Taylor R., Walters W. A., Catt K. J. Angiotensins I and II, active and inactive renin, renin substrate, renin activity, and angiotensinase in human liquor amnii and plasma. Am J Obstet Gynecol. 1975 Mar 1;121(5):626–630. doi: 10.1016/0002-9378(75)90463-9. [DOI] [PubMed] [Google Scholar]
- Soubrier F., Skinner S. L., Miyazaki M., Genest J., Menard J., Corvol P. Activation of renin in an anaplastic pulmonary adenocarcinoma. Clin Sci (Lond) 1981 Dec;61 (Suppl 7):299s–301s. doi: 10.1042/cs061299s. [DOI] [PubMed] [Google Scholar]
- Takii Y., Inagami T. Evidence for a completely inactive renin zymogen in the kidney by affinity chromatographic isolation. Biochem Biophys Res Commun. 1980 May 14;94(1):182–188. doi: 10.1016/s0006-291x(80)80204-x. [DOI] [PubMed] [Google Scholar]
- Yokosawa N., Takahashi N., Inagami T., Page D. L. Isolation of completely inactive plasma prorenin and its activation by kallikreins. A possible new link between renin and kallikrein. Biochim Biophys Acta. 1979 Aug 15;569(2):211–219. doi: 10.1016/0005-2744(79)90056-1. [DOI] [PubMed] [Google Scholar]



