Abstract
Despite the high prevalence of prescription opioid dependence in the U.S., little is known about the course of this disorder and long-term response to treatment. We therefore examined 18-month post-randomization outcomes of participants in the Prescription Opioid Addiction Treatment Study, a multi-site, randomized controlled trial examining varying durations of buprenorphine-naloxone treatment and different intensities of counseling for prescription opioid dependence. Thus the current follow-up study provides a unique contribution to the field by reporting longer-term outcomes of a well-characterized population of treatment-seeking prescription opioid dependent patients. Participants from the treatment trial (N=252/653) completed an 18-month follow-up telephone assessment. Multivariable analyses examined associations between participant characteristics and key indicators of month-18 status: opioid abstinence, DSM-IV opioid dependence, and opioid agonist treatment. Overall, participants showed improvement from baseline to month 18: 49.6% were abstinent in the previous 30 days, with only 16.3% opioid-dependent. Some participants, however, had initiated past-year heroin use (n=9) or opioid injection (n=17). Most participants (65.9%) engaged in substance use disorder treatment during the past year, most commonly opioid agonist therapy (48.8%). Of particular interest in this population, multivariable analysis showed that greater pain severity at baseline was associated with opioid dependence at 18 months. In conclusion, although opioid use outcomes during the treatment trial were poor immediately following a buprenorphinenaloxone taper compared to those during 12 weeks of buprenorphine-naloxone stabilization, opioid use outcomes at 18-month follow-up showed substantial improvement over baseline and were comparable to the rate of successful outcomes during buprenorphine-naloxone stabilization in the treatment trial.
Keywords: buprenorphine, prescription opioids, substance use disorder, treatment, follow-up, clinical trial
1 Introduction
Abuse of prescription opioids is a well-recognized public health problem. Currently, prescription opioid use disorders are four times more common than heroin-related disorders (Substance Abuse and Mental Health Services Administration, 2012). Among treatment-seeking opioid users, the most recent treatment in the past year was 1.7 times as likely to be for prescription opioids than heroin (Substance Abuse and Mental Health Services Administration, 2012); 10% of admissions for substance use disorders (SUDs) are attributed to prescription opioid use, a fivefold increase from 2001-2011 (Substance Abuse and Mental Health Services Administration & Center for Behavioral Health Statistics and Quality, 2013).
Because the increased prevalence of prescription opioid use disorders is relatively recent, most research on opioid use disorders has focused on heroin-dependent patients. It remains unclear whether the treatment response and course of the disorder among opioid-dependent heroin users can be generalized to those using prescription opioids. For example, studies suggest a greater likelihood of successful buprenorphine treatment outcomes among those dependent on prescription opioids vs. heroin (Moore et al., 2007; Nielsen, Hillhouse, Thomas, Hasson, & Ling, 2013; Potter et al., 2013). However, the Prescription Opioid Addiction Treatment Study (POATS), conducted by the National Drug Abuse Treatment Clinical Trials Network, found rates of successful outcome for patients with DSM-IV dependence on prescription opioids to be similar to those of heroin-dependent populations in other trials (Weiss et al., 2011).
To date, POATS is the only large-scale randomized, controlled trial of treatment for prescription opioid dependence (Weiss et al., 2011). POATS examined different intensities of counseling and different durations of buprenorphine-naloxone (bup-nx) to treat patients with DSM-IV prescription opioid dependence. Several key findings emerged: 1) treatment response was similar for participants receiving individual drug counseling in addition to bup-nx and standard medical management; 2) treatment response to brief bup-nx treatment (4-week taper) was overwhelmingly poor: only 7% had successful opioid use outcomes; and 3) treatment outcomes after 12 weeks of bup-nx was considerably better: 49% of participants were successful. Pre-specified secondary analyses demonstrated that even a limited history of heroin use predicted poor treatment outcome; chronic pain was unrelated to outcome (Weiss et al., 2011).
POATS offers an important, unique opportunity to examine longer-term outcomes for prescription opioid dependence in a well-characterized cohort of treatment-seeking individuals. Participants were assessed three times, approximately 18, 30, and 42 months post-randomization. This report presents outcomes at the first of these three follow-up assessments. We examined the following questions: 1) What is the extent of substance use, particularly prescription opioids and heroin? 2) How many participants are engaged in SUD treatment? 3) Can participants’ 18-month substance use outcomes be predicted from baseline characteristics, treatment condition, or study outcomes?
2 Methods
2.1 Trial design
POATS was conducted from 2006-2009 at ten United States sites. The primary research question was whether adding individual drug counseling to bup-nx and standard medical management improved opioid use outcomes a) after a brief (4-week) bup-nx taper or b) at the end of a subsequent 12-week bup-nx stabilization regimen for those who relapsed to opioids during brief treatment (for details, see Potter et al., 2010; Weiss, Potter, Copersino, et al., 2010; Weiss et al., 2011; Weiss, Potter, Provost, et al., 2010).
The study utilized a two-phase, adaptive design: participants were randomized in each phase to receive standard medical management (Fiellin, Pantalon, Schottenfeld, Gordon, & O'Connor, 1999) alone or in addition to individual opioid drug counseling (Pantalon, Fiellin, Schottenfeld, Gordon, & O'Connor, 1999). Standard medical management consisted of a 45-minute initial visit followed by weekly 15-minute medical counseling sessions with a physician. Opioid drug counseling consisted of twice weekly visits in weeks 1-4 plus weekly visits in weeks 6 and 8 in the brief treatment phase; in the extended phase, participants assigned to counseling had two sessions a week in weeks 1-6 and weekly visits in weeks 7-12. . In the brief treatment phase, participants were inducted and stabilized on bup-nx for 2 weeks, tapered over 2 weeks, then followed for 8 weeks. Outcome for this phase was classified as the presence or absence of treatment success, i.e., finishing the 12-week study period with a) ≤4 days of urine-confirmed, self-reported opioid use in a 30-day period; b) no consecutive weeks with opioid-positive urine tests; c) no additional formal SUD treatment; and d) ≤1 missing urine sample. Only participants who were unsuccessful with brief treatment were eligible for randomization to the extended treatment: 12 weeks of bup-nx stabilization. Successful outcome for this phase was opioid abstinence in week 12 and ≥2 of the 3 previous weeks. Participants were tapered from bup-nx during weeks 13-16 and followed for another 8 weeks. Participants then had no further contact with the study until they were re-contacted by a staff member at their site in December 2008 (when the follow-up study was approved), asking them to participate in the follow-up study. For the brief treatment phase, participants were stratified according to the presence of chronic pain and a lifetime history of heroin use. For the extended treatment phase, participants were stratified by their treatment condition in the brief treatment phase.
The primary outcome measure for the trial was success or failure at the end of buprenorphine-naloxone stabilization, i.e., during weeks 9-12 of the extended treatment phase. Secondary outcomes include the likelihood of success after a brief buprenorphine-naloxone taper at the beginning of the trial and 8 weeks after a second taper following 12 weeks of buprenorphine-naloxone stabilization in the extended treatment phase.
2.2. Trial sample
Eligible participants were ≥18 years old and met DSM-IV criteria for current opioid dependence. Participants were excluded if they used heroin >4 of the past 30 days; had a lifetime DSM-IV opioid-dependence diagnosis due solely to heroin; had ever injected heroin; required continued pain management with opioids; had experienced a traumatic pain event in the previous 6 months; were psychiatrically unstable; required immediate medical attention for dependence on other substances; or had liver function tests >5 times the upper limit of normal. Participants prescribed opioids for pain could be enrolled only if their prescribing physician gave permission for them to discontinue use of these opioids and to enter the study. The study enrolled 653 outpatients, 360 of whom entered extended treatment.
2.3. Follow-up procedures
Institutional Review Board approval was obtained from each site for the follow-up study; one site did not participate. For efficiency and oversight, data were collected by the lead investigative team at McLean Hospital, via telephone by trained interviewers. Telephone interviews have been used in other SUD trials, having been shown to yield valid data similar to that of face-to-face interviews (Kramer et al., 2009; Midanik & Greenfield, 2003).
For the follow-up component, the focus of this report, the target date was 18 months after baseline, with a window from month 17 until one month before the 30-month assessment target date. Most (74%) were completed by month 24 (mean=month 21; details in Table 1). Data were entered directly into a web-based, electronic data capture system maintained by an independent data management center, in compliance with 21 Code of Federal Regulations Part 11 (National Archives and Records Administration, Revised as of April 1, 2013). To maintain fidelity, study coordinators monitored interviewers and provided feedback.
Table 1.
Baseline and treatment study characteristics by participation in the 18-month follow-up (N=653)
| Participants (252a) | Non-participants (401) | |
|---|---|---|
| Baseline sociodemographic characteristics | ||
| Female sex, % | 42.9 | 38.2 |
| Age, mean (sd) | 33.2 (9.8) | 33.2 (10.4) |
| White race, % | 88.9 | 93.0 |
| Never married, % | 51.2 | 49.1 |
| Employed full-time, % | 66.7 | 60.6 |
| Years education, mean (sd) | 12.9 (2.0) | 13.1 (2.3) |
| Baseline clinical characteristics | ||
| Substance use | ||
| Nonopioid substance dependence diagnoses, % | ||
| Alcohol | ||
| Past year | 2.4 | 4.5 |
| Lifetime | 28.6 | 25.2 |
| Cannabis | ||
| Past year | 4.8 | 5.5 |
| Lifetime | 15.1 | 15.7 |
| Cocaine | ||
| Past year | 2.0 | 4.0 |
| Lifetime | 17.5 | 18.2 |
| Other stimulants | ||
| Past year | 3.2 | 1.2 |
| Lifetime | 14.3 | 8.7* |
| Sedatives | ||
| Past year | 4.4 | 7.5 |
| Lifetime | 9.5 | 10.5 |
| None | ||
| Past year | 86.9 | 82.5 |
| Lifetime | 52.8 | 52.4 |
| Opioid use history | ||
| Ever used heroin, % | 23.8 | 20.2 |
| Years of opioid use, mean (sd) | 5.1 (4.6) | 5.1 (4.1) |
| Previous opioid use disorder treatment, % | 32.1 | 30.7 |
| First used opioids to relieve pain, % | 60.3 | 65.1 |
| Used extended-release oxycodone most, past 30 days, % | 35.7 | 34.9 |
| Route other than swallowing/sublingually, % | 81.7 | 79.3 |
| Chronic pain | ||
| Prevalence, % | 44.4 | 40.4 |
| Severity (SF-36), mean (sd) | 3.1 (1.4) | 3.1 (1.4) |
| Interference with work (SF-36), mean (sd) | 2.2 (1.1) | 2.2 (1.2) |
| Other psychiatric | ||
| Major depressive disorder, % | ||
| Past year | 22.6 | 20.9 |
| Lifetime | 35.3 | 34.2 |
| Post-traumatic stress disorder, % | ||
| Past year | 11.9 | 12.2 |
| Lifetime | 15.5 | 19.9 |
| Beck Depression Inventory, mean (sd) | 22.0 (11.9) | 22.3 (11.8) |
| Treatment study characteristics | ||
| Treatment condition SMM+ODC | ||
| Brief treatment phase, % | 49.6 | 50.9 |
| Extended treatment phase, % (N=360) | 49.4 | 50.6 |
| Successful outcome | ||
| Brief treatment phase, % | 5.6 | 7.2 |
| Extended treatment phase, % (N=360) | 46.1 | 52.2 |
p<0.05
Participant (252) follow-up times: 24.2% months 17-18; 31.4% months 19-21; 19.2% months 22-24; and 26.2% months 25-27
Participants received $75, similar to compensation rates in other SUD treatment studies (Festinger et al., 2005) and another $10 for keeping the first scheduled assessment. Participants who were in jeopardy of missing their assessment window (i.e., 6 weeks before month 29) were offered an additional $25 bonus as an incentive to complete their assessment before the deadline.
2.4. Measures
At month 18, a subset of treatment study measures was administered, focusing on substance use and treatment utilization, with slight modifications for telephone interviewing.
The Composite International Diagnostic Interview (CIDI; World Health Organization, 1997) was used in the main trial to diagnose SUDs, post-traumatic stress disorder, and major depressive disorder. At month 18, the CIDI assessed only opioid dependence, defined as meeting symptom criteria during the 30 days preceding the follow-up assessment. According to the DSM-IV definition, participants receiving agonist therapy who met no symptom criteria for current opioid dependence at their follow-up assessment were classified separately as having opioid dependence, on agonist therapy. The term “current opioid dependence” in this paper hereafter refers to those meeting current symptom criteria (i.e, receipt of agonist therapy is not sufficient for meeting criteria) unless otherwise specified.
The Addiction Severity Index was used at baseline and month 18 to assess substance use in the past 30 days (McLellan et al., 1992). Opioid use characteristics and patterns were collected at both times, using a measure developed for this study. Other than the use of buprenorphine or methadone taken as prescribed to treat opioid dependence, we did not differentiate between opioids that participants reported being prescribed for pain versus those obtained illicitly; this was consistent with our inclusion criteria (prescribing physicians had to give permission for participants to discontinue opioid use) and our procedures during the main trial; this eliminated the difficulty of trying to distinguish between “legitimate” opioid use and misuse. It is not clear how to make a valid distinction between the two, given our current understanding of pain and misuse, as well as the constraints of a clinical trial and reliance on self-report.
At baseline, the Beck Depression Inventory (BDI-II; Beck, Ward, Mendelson, Mock, & Erbaugh, 1961) assessed depressive symptoms; chronic pain was assessed with items from the Brief Pain Inventory (Cleeland & Ryan, 1994). General health and pain severity and interference were assessed at baseline and 18 months, using items from the Short Form Health Survey (SF-36 v.2; Ware & Sherbourne, 1992), developed to measure mental and physical quality of life. A treatment utilization history at month 18 assessed treatment for SUD, pain, and psychiatric reasons.
2.5. Data analysis
Bivariate analyses used χ2 tests for categorical variables and two-tailed independent t-tests for continuous variables, with McNemar tests or paired t-tests for change over time. Logistic regression models examined predictors of month 18-status, including sociodemographic, clinical, and treatment study characteristics; to account for potential clustering of data within study sites, these analyses also included study site as fixed effects (via the inclusion of an indicator (or dummy) variable for each site). All analyses used SPSS v.20 (SPSS Statistics for Windows, Version 20.0, 2011).
3 Results
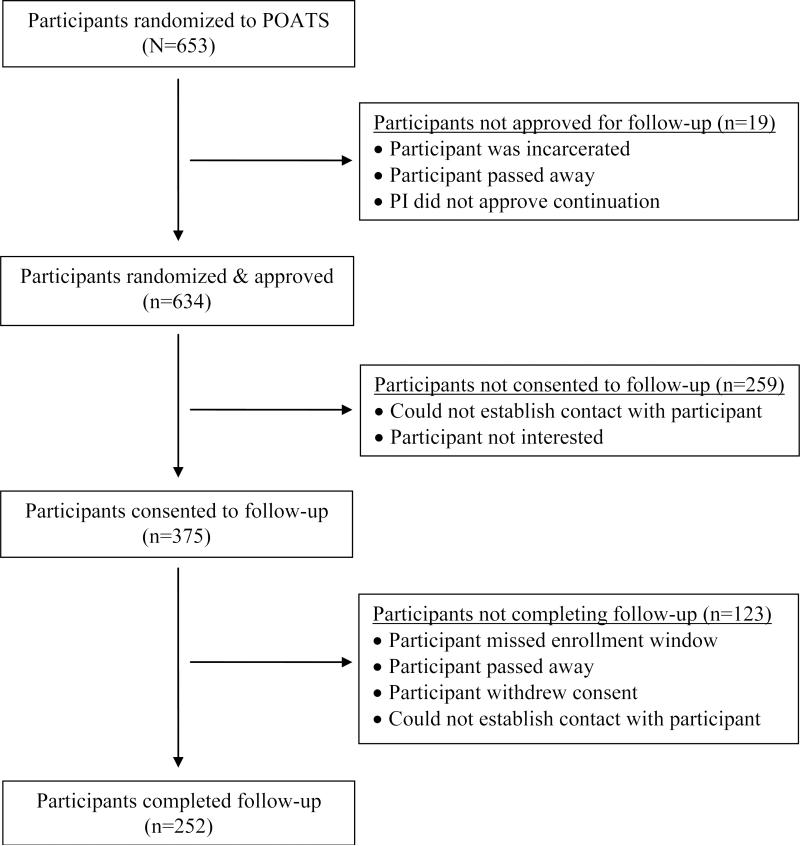
Of the 653 study participants, some were ineligible for follow-up due to site investigator judgment (n=14; for example, no longer in contact due to threatening behavior) or incarceration/death (n=5); 190 could not be contacted and 69 were no longer interested (see Figure 1). The remaining 375 participants consented. However, some could not then be located (n=70), missed the target window due to study logistics (n=50), withdrew consent (n=2), or died (n=1); 252 (38.6%) completed the 18-month follow-up assessment.
Figure 1.
Participation in follow-up 18 months post randomization
A comparison of sociodemographic and clinical characteristics by participation (Table 1) revealed a single difference: lifetime non-cocaine stimulant dependence was more common in month-18 participants than non-participants (14.3% vs. 8.7%; χ2(1)=4.93, p=0.03). Given the large number of comparisons in Table 1, this single difference could be due to chance alone and provides some reassurance that follow-up participants are representative of the study population.
Participants entering the extended treatment phase of the study were more likely to complete month-18 follow-up than those who only participated in brief treatment (50.0% vs. 24.6%; χ 2(1)=44.07, p<0.001). This was true regardless of extended phase outcomes: treatment success was not associated with month-18 participation (46.1% vs. 52.0%; χ2(1)=1.23, p=0.27). Since those who participated in the treatment trial during the first year of enrollment had been out of contact with study personnel for 1.5-2.5 years, we considered the possibility that study logistics might have contributed to non-participation. Indeed, non-participants were more likely to have been enrolled earlier in the treatment trial; i.e., the number of months from completion of participation in the treatment study until the follow-up study began was nearly twice as long for non-participants (mean=14.1 (sd=7.5) vs. 7.5 (sd=5.8), t(622.1)=12.54, p<0.001).
3.1 What is the extent of substance use at month 18
Overall opioid use
At month 18, 53.2% of participants met criteria for past-year opioid dependence, with only 16.3% meeting criteria for DSM-IV opioid dependence in the past 30 days (Table 2); 42 participants (16.7%) had opioid dependence, on agonist therapy (i.e., they met no symptom criteria for current opioid dependence). Half (48.4%) reported prescription opioid use during the past month, excluding methadone or buprenorphine used for opioid dependence treatment.
Table 2.
Change in clinical characteristics from baseline to month 18 (N=252)
| Participant characteristics | Month 0 | Month 18 |
|---|---|---|
| Substance use | ||
| Current opioid dependence, past month, % | 100 | 16.3*** |
| Days of substance use, past 30 days, mean (sd) | ||
| Prescription opioids | 28.1 (3.0) | 10.2 (12.8)*** |
| Heroin | 0.1 (0.5) | 0.5 (2.8)* |
| Cannabis | 4.9 (9.6) | 3.1 (7.5)** |
| Sedative-hypnotics, non-barbiturate | 3.1 (7.0) | 3.7 (8.3) |
| Alcohol | 2.8 (6.2) | 2.5 (5.5) |
| Amphetamines | 0.7 (3.6) | 1.0 (4.8) |
| Cocaine | 0.5 (1.9) | 0.2 (1.0)* |
| Barbiturates | 0.2 (1.8) | 0 (0.1)* |
| >1 Drug | 10.2 (11.5) | 7.2 (10.4)*** |
| Opioid use history, % | ||
| Used heroin in past 12 months ≥5 times | 10.3 | 10.3 |
| Injected in past 12 months | 4.0 | 8.7** |
| Quality of life (SF-36) itemsa, mean (sd) | ||
| General health | 2.8 (0.8) | 2.8 (1.0) |
| General health compared to 1 year ago | 3.3 (0.8) | 2.3 (1.2)*** |
| Chronic pain | ||
| Prevalence, % | 44.4 | 34.1** |
| Severity (SF-36), mean (sd)b | 4.0 (0.9) | 3.6 (1.3)** |
| Interference with normal work (SF-36), mean (sd)b | 2.9 (1.0) | 2.5 (1.3)** |
Notes: Significance from paired t-tests or McNemar tests
Higher number = worse
Based on 112 with chronic pain at baseline
p<0.05
p<0.01
p<0.001
Prescription opioid use
The average number of days of prescription opioid use in the preceding 30 days decreased from study entry to month 18 by almost two-thirds (Table 2). Most participants (76.6%) used prescription opioids less often than at baseline: 129 were abstinent during the preceding month and another 45 used <20 days (median=7); 19 used opioids on fewer days but continued to use these drugs ≥20 days. The remaining participants (23.4%) maintained baseline patterns, i.e., daily or near-daily use.
Heroin use
At month 18, past-year use of heroin ≥5 times was reported by 8.3% (n=21/252) of participants, 9 of whom reported heroin use for the first time. The average number of days using heroin in the past month increased slightly at the 18-month assessment (Table 2). While this change was significant, the number of participants using heroin (5.9%; n=15/252) and days of use (median=5) were quite low.
Injection use
Overall, the number of participants reporting past-year injection of any opioid increased from 4.0% (n=10/252) at study entry to 8.7% (n=22/252) at month 18 (McNemar χ2, p=0.02). Of these 22 participants, 6.7% (n=17/252) reported no injection of prescription opioids in the year before baseline (heroin injection was an exclusion criterion for the treatment trial, but not prescription opioid injection). Heroin use by injection ≥5 times over the past year was reported by 12 of 252 participants (4.8%) at month 18; 7 of them had not previously reported injecting.
Change in other clinical characteristics at month 18
Most clinical characteristics improved from baseline to month 18 (Table 2). Participants reported significantly fewer days of cannabis, cocaine, barbiturate, and multiple-drug use; general health and chronic pain improved, with the remaining characteristics unchanged.
3.2. How many participants were engaged in past-year SUD treatment
Opioid agonist therapy was the most commonly reported treatment during the past year (48.8%; n=123/252), primarily due to buprenorphine treatment (40.1%), rather than methadone treatment (6.0%) or both (2.8%); fewer participants received psychosocial treatment (34.1%), detoxification (11.9%), oral naltrexone (0.8%), and other SUD medications (3.2%). Self-help attendance was reported by 40.9%. The past-month rate of opioid agonist treatment was 31.7% (80/252). Approximately half (45.8%, n=38/83) of participants who were successful in extended treatment were receiving opioid agonist treatment at month 18; only 1 of 14 participants (7.1%) successful in brief treatment were receiving opioid agonist treatment. Because study results strongly supported the efficacy of agonist treatment, we examined the association at follow-up between agonist treatment and opioid use. Participants receiving agonist treatment were more likely to report opioid abstinence compared to those not in this treatment, both during the past year (62.6% vs. 38.8%, χ2(1)=14.32, p<0.001) and the past month (80.0% vs. 36.6%, χ2(1)=41.09, p<0.001).
3.3. Are baseline variables associated with substance use outcomes at month 18
Characteristics at study entry
Multivariable models examined the association between month-18 status and sociodemographic, clinical, and treatment study characteristics, also adjusted for site (Table 3). Month-18 status was assessed three ways: past-month opioid abstinence, current DSM-IV opioid-dependence diagnosis, and current agonist treatment. Past-month opioid abstinence at follow-up was more likely among participants never married and those with successful outcomes in the brief treatment phase. Those with past-month opioid dependence at month 18 reported greater pain severity at study entry. Opioid agonist treatment at month 18 was more common among those who were white, had greater baseline depressive symptoms, and participated in the extended treatment. Notably, participants who had received opioid agonist treatment prior to the main trial were no more likely to report this treatment at month 18 than participants who had received this treatment initially in the treatment study (37.8% vs. 30.4%, χ2(1)=.920, p<.34).
Table 3.
Multivariable associations of baseline and treatment study characteristics on month-18 status, past 30 days, also adjusted for site (N=252)
| Abstinence | Opioid dependence | Opioid agonist therapy | ||||
|---|---|---|---|---|---|---|
| OR | CI | OR | CI | OR | CI | |
| Sociodemographic characteristics | ||||||
| Female | 0.62 | 0.33-1.17 | 0.91 | 0.38-2.17 | 0.78 | 0.40-1.54 |
| Age | 1.03 | 0.99-1.07 | 1.01 | 0.96-1.07 | 1.01 | 0.97-1.05 |
| White race | 1.97 | 0.73-5.28 | 1.33 | 0.28-6.39 | 3.40* | 1.02-11.31 |
| Never married | 2.05* | 1.03-4.08 | 0.91 | 0.36-2.30 | 1.55 | 0.74-3.24 |
| Employed full-time | 1.08 | 0.57-2.02 | 1.05 | 0.43-2.56 | 0.60 | 0.31-1.17 |
| Years of education | 1.05 | 0.89-1.24 | 1.05 | 0.84-1.31 | 1.11 | 0.93-1.32 |
| Clinical characteristics | ||||||
| Non-opioid substance dependence diagnosis, past year | 2.02 | 0.85-4.83 | 0.26 | 0.06-1.17 | 1.68 | 0.70-4.00 |
| Opioid use history | ||||||
| Ever used heroin | 0.51 | 0.24-1.09 | 2.26 | 0.84-6.11 | 0.86 | 0.38-1.97 |
| Years of opioid use | 0.97 | 0.91-1.04 | 1.00 | 0.91-1.11 | 1.00 | 0.93-1.07 |
| Previous opioid use dependence treatment | 1.28 | 0.66-2.49 | 0.95 | 0.40-2.30 | 1.47 | 0.72-3.01 |
| First used to relieve pain | 1.45 | 0.74-2.82 | 0.55 | 0.22-1.40 | 1.96 | 0.93-4.14 |
| Used extended-release oxycodone most, past 30 days | 0.87 | 0.43-1.76 | 0.85 | 0.33-2.20 | 1.18 | 0.54-2.56 |
| Route other than swallowing/sublingually | 0.61 | 0.25-1.50 | 0.83 | 0.22-3.21 | 0.72 | 0.27-1.89 |
| Chronic pain | ||||||
| Prevalence | 1.13 | 0.54-2.39 | 0.43 | 0.16-1.17 | 0.90 | 0.41-1.98 |
| Severity (SF-36) | 0.83 | 0.63-1.10 | 1.64* | 1.09-2.47 | 1.00 | 0.74-1.36 |
| Other psychiatric | ||||||
| Major depressive disorder, past year | 1.14 | 0.52-2.53 | 0.60 | 0.20-1.77 | 0.59 | 0.25-1.38 |
| Post-traumatic stress disorder, past year | 0.80 | 0.33-1.94 | 2.44 | 0.72-8.31 | 0.85 | 0.34-2.12 |
| Beck Depression Inventorya | 1.06 | 0.91-1.22 | 1.20 | 0.98-1.46 | 1.22* | 1.04-1.43 |
| Treatment study characteristics | ||||||
| Treatment condition, Brief treatment phase | 1.04 | 0.59-1.81 | 1.23 | 0.57-2.69 | 0.98 | 0.53-1.80 |
| Success in brief treatment phase | 5.61* | 1.12-28.17 | --- | ---b | 0.20 | 0.02-1.97 |
| Participation in extended treatment phase | 1.40 | 0.70-2.81 | 0.80 | 0.30-2.09 | 2.31* | 1.07-4.96 |
| R2, % | 22.6* | 27.8* | 24.0* | |||
BDI scores are shown in units of 5, since a 1 point change in this measure is less meaningful.
Estimate of OR is on the boundary, due to n=0 successes in brief treatment phase who were opioid dependent at follow-up; likelihood-ratio test p-value>0.05 (0.0545).
OR=odds ratios; CI=95% confidence intervals
p<0.05
Treatment condition
Treatment condition, during both the brief treatment and extended treatment phases, was not associated with abstinence, opioid dependence diagnosis, or agonist treatment at follow-up, adjusted for the baseline and treatment study characteristics in Table 1.
Outcome at the end of extended treatment vs. month 18
We examined the association between outcome at the end of extended treatment and follow-up status, adjusted for the baseline and treatment study characteristics in Table 1. Results were not included in Table 3 because only a subset of the sample (n=180/252) participated in extended treatment. A successful outcome at the end of the extended treatment phase predicted abstinence (aOR=2.30, 95%CI=1.10-4.82) and not having current DSM-IV opioid dependence (aOR=4.47, 95%CI=1.26-15.87) at follow-up. In unadjusted analyses, those successful were more likely to be on opioid agonist therapy currently than those unsuccessful (45.8% vs. 27.8%, χ2(1)=6.24, p=.012); however, in adjusted analyses, end-of-treatment outcome was not related to receiving opioid agonist treatment at follow-up (aOR=1.89, 95%CI=0.87-4.13).
4 Discussion
Despite the high prevalence rate of prescription opioid dependence in the United States, little is known about the course of this disorder and long-term response to treatment. We therefore examined longer-term outcomes of participants in a multi-site, randomized controlled trial examining bup-nx and counseling for prescription opioid dependence. Since this was the first large-scale treatment outcome study for this population, the current study provides a unique contribution to the literature.
Participants’ substance use, including opioid use, DSM-IV opioid dependence diagnosis, and use of other substances, was substantially improved from study entry. For example, while trial participation required a DSM-IV opioid-dependence diagnosis, only 16.3% had current opioid dependence at follow-up. Eighteen months following treatment initiation, this represents substantial improvement. Approximately half of the participants abstained from opioids in the month prior to follow-up, and three-quarters used opioids on fewer days than at baseline; other drug use also decreased. This overall improvement in substance use was accompanied by improved perception of general health and pain.
These findings should be considered in light of engagement in opioid agonist treatment. Approximately half of the participants reported receiving agonist treatment in the year prior to the 18-month follow-up. Importantly, past-month agonist treatment at month 18 was associated with having participated in the extended study treatment phase. Thus, exposure to bup-nx maintenance for 12 weeks in the trial increased the likelihood that participants would seek this treatment again. Moreover, those engaged in agonist treatment at month 18 were over four times more likely to abstain from opioids in the past month. This positive association between current agonist treatment and opioid abstinence is consistent with strong evidence supporting the efficacy of this treatment for heroin dependence (Gowing, Ali, & White, 2009), and consistent with the short-term results of our trial.
Several sociodemographic and clinical characteristics at study entry predicted month-18 opioid use and treatment status. Notably, greater depressive symptoms at baseline were associated with current agonist treatment, whereas major depressive disorder diagnosis was not. The latter contrasts with findings our group reported in a secondary analysis of trial data, suggesting that a lifetime diagnosis of major depressive disorder was associated with treatment success in the extended phase, while depressive symptoms using the same measure were not (Dreifuss et al., 2013). Participants with greater depressive symptomatology sought bup-nx after the main study ended, consistent with reports of putative antidepressant effects of bup-nx (Bodkin, Zornberg, Lukas, & Cole, 1995; Emrich H.M., Vogt P., & Herz A., 1982). These mixed results for the relationship between mood and prescription opioid dependence will benefit from continued exploration in future research.
Not surprisingly, a successful treatment response in either phase of the trial predicted month-18 opioid abstinence. The continued overall improvement for these participants is encouraging, since longer-term substance use outcomes can differ from shorter-term reports (Blonigen, Timko, Finney, Moos, & Moos, 2011; Grella, Joshi, & Hser, 2003; Schutte, Nichols, Brennan, & Moos, 2003).
One area of particular interest is the positive month-18 opioid use outcomes for the few participants who succeeded in the brief treatment. The poor treatment outcomes typically associated with detoxification, including in POATS (Weiss et al., 2011), are well-documented (O'Connor, 2005); however, a small proportion of individuals are capable of abstaining following detoxification (Gandhi et al., 2003). The fact that those who succeeded in brief treatment were able to maintain opioid abstinence at month 18 despite the fact that only 7.1% participated in agonist treatment, suggests that their short-term ability to abstain from opioids following a taper was sustained over the long-term. Our finding of an association between being never married and successful outcomes at follow-up contributes to a mixed literature, with some research finding that becoming married is associated with better SUD outcomes (Curran, Muthen, & Harford, 1998), while a recent study found the opposite (Satre, Chi, Mertens, & Weisner, 2012). It is possible that our sample contained an overrepresentation of spouses with SUDs, which could account for our finding; unfortunately, we did not collect these data.
Participants with more severe pain at baseline were more likely to be opioid-dependent at follow-up. The presence of chronic pain was not associated with outcome during the treatment study, but study participants had, on average, only moderate pain severity. It may be that severity rather than the mere presence of chronic pain has greater prognostic significance in this population.
The racial difference in participation in agonist treatment is consistent with a previous study reporting that African-Americans were approximately half as likely as Caucasians to enter methadone maintenance treatment (Lundgren, Amodeo, Ferguson, & Davis, 2001). Understanding the reasons for this finding, which now appears to extend to buprenorphine treatment, would be helpful in attempting to address this disparity.
Unlike some reports of longer-term outcomes in behavioral trials (Carroll et al., 1994; Donovan et al., 2008), we found no evidence of delayed emergence of a benefit from additional opioid counseling, i.e., a “sleeper effect” (Carroll et al., 1994; Donovan et al., 2008). This is consistent with our previously reported (Weiss et al., 2011) finding of no benefit to opioid drug counseling in addition to bup-nx and standard medical management during the trial. Identifying the optimal intensity and content of behavioral treatment interventions to augment agonist therapy awaits additional investigation to improve treatment response.
Although our follow-up results were generally encouraging, there were some cautionary findings. There were a number of participants who did not meet current opioid dependence criteria at month 18, but were using opioids. It is possible that some of these participants would subsequently relapse to opioid dependence. Clinicians have also been concerned about the possible transition from prescription opioids to heroin (Jones, 2013), corroborated by our finding that 9 participants reported heroin use for the first time at month 18. Injection use is also of concern; 17 new injectors (6.7%) were identified, 12 of whom injected heroin ≥5 times during the past year. These results provide evidence of disease progression for some, and clinicians need to be mindful of this possibility.
Our findings demonstrate the importance of longer-term follow-up. Trial outcomes showed poor results following a bup-nx taper. Moreover, although participants had better outcomes on bup-nx, half of the study population had unsuccessful outcomes during the trial while stabilized on bup-nx. Interestingly, among participants completing the month-18 follow-up, 37.2% were abstinent from opioids without current agonist treatment. While this may in part be an artifact of the subset of participants available at month 18, the finding is nevertheless encouraging. Our findings also suggest the importance of access to pharmacotherapy in this population. Those who entered the extended treatment phase were more likely to seek bup-nx treatment in the future, and agonist treatment was associated with better outcomes at follow-up. Although drug counseling, as delivered in POATS, in addition to medical management, was not associated with either short-term or longer-term outcomes, alternate models of behavioral treatment may fare better.
4.1. Limitations
Several limitations should be considered. First, the sample represents 38.6% of treatment-study participants; hence power of analyses is reduced, and, due to potential selection bias, our results may not be generalizable to the entire study population. The follow-up sample included more participants from the extended treatment phase than from the brief phase-only. It is also possible that participants who were doing particularly well were more likely to enter the follow-up study, which could produce an overly optimistic view of outcomes. Nevertheless, the study provided a unique opportunity to follow this novel population.
Second, interviews were conducted face-to-face during the treatment trial and via telephone at follow-up, without urine drug screens to confirm self-reports. This change may compromise the validity of the self-report data, perhaps inflating the rate of successful outcomes; however, prior research demonstrates that telephone assessment is widely used and valid for assessing SUDs (Kramer et al., 2009; Midanik & Greenfield, 2003).
Third, instead of the more nuanced outcome reported in the treatment trial (≥3 of 4 abstinent weeks at the end of treatment), a dichotomous outcome of complete abstinence during the preceding month was used. This stricter definition at month 18 could decrease the rate of successful outcome.
4.2. Conclusions
No prior longitudinal studies have examined patients with prescription opioid dependence. Our results represent an important first step toward understanding the course of prescription opioid dependence and identifying factors associated with longer-term recovery following treatment. Our results are consistent with research showing that patients with prescription opioid dependence likely have better outcomes than those dependent on heroin (Moore et al., 2007; Nielsen et al., 2013; Potter et al., 2013). Like studies of heroin dependence, we found that opioid agonist treatment is effective in supporting recovery from prescription opioid dependence. However, interestingly, a considerable proportion of participants (approximately one-third) reported doing well in the absence of agonist treatment. These findings are tempered by the fact that half of the participants were still using opioids to some extent at follow-up and that a small subset of participants began using heroin and/or injecting drugs for the first time after study entry. Future follow-up with this cohort will help generate guidance for treatment and further research.
Highlights.
We conducted an 18-month follow-up in the Prescription Opioid Addiction Treatment Study
Overall, participants showed improvement from baseline to month 18.
Half of those followed were abstinent in the previous 30 days, with only 16% opioid-dependent.
Some participants, though, initiated past-year heroin use (4%) or opioid injection (7%).
Acknowledgements
Funding for this study was provided by NIDA Clinical Trials Network Grants U10 DA020024 (Trivedi), K23 DA02297 (Potter), U10 DA15831 (Weiss), and K24 DA022288 (Weiss). We gratefully acknowledge the contributions of the staff who contributed to this study, including research assistants and study coordinators from the Division of Alcohol and Drug Abuse at McLean Hospital as well as at each study site and the NIDA Clinical Trials Network (CTN) Data and Statistics Center (DSC1) at the Duke Clinical Research Institute, the support and guidance from the NIDA CTN Clinical Coordinating Center (the EMMES Corporation), and especially the generosity of the study participants.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Declaration of interest: This work was supported by NIDA Clinical Trials Network Grants U10 DA020024 (Trivedi), K23 DA02297 (Potter), U10 DA15831 (Weiss), and K24 DA022288 (Weiss). There were no contractual constraints on publishing.
Clinical Trial Registration: ClinicalTrials.gov; registration number NCT00316277; http://clinicaltrials.gov/ct2/show/NCT00316277.
References
- Beck AT, Ward CH, Mendelson M, Mock J, Erbaugh J. An inventory for measuring depression. Arch Gen Psychiatry. 1961;4:561–571. doi: 10.1001/archpsyc.1961.01710120031004. [DOI] [PubMed] [Google Scholar]
- Blonigen DM, Timko C, Finney JW, Moos BS, Moos RH. Alcoholics Anonymous attendance, decreases in impulsivity and drinking and psychosocial outcomes over 16 years: moderated-mediation from a developmental perspective. Addiction. 2011;106(12):2167–2177. doi: 10.1111/j.1360-0443.2011.03522.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bodkin JA, Zornberg GL, Lukas SE, Cole JO. Buprenorphine treatment of refractory depression. J Clin Psychopharmacol. 1995;15(1):49–57. doi: 10.1097/00004714-199502000-00008. [DOI] [PubMed] [Google Scholar]
- Carroll KM, Rounsaville BJ, Nich C, Gordon LT, Wirtz PW, Gawin F. One-year follow-up of psychotherapy and pharmacotherapy for cocaine dependence. Delayed emergence of psychotherapy effects. Arch Gen Psychiatry. 1994;51(12):989–997. doi: 10.1001/archpsyc.1994.03950120061010. [DOI] [PubMed] [Google Scholar]
- Cleeland CS, Ryan KM. Pain assessment: global use of the Brief Pain Inventory. Ann Acad Med Singapore. 1994;23(2):129–138. [PubMed] [Google Scholar]
- Curran PJ, Muthen BO, Harford TC. The influence of changes in marital status on developmental trajectories of alcohol use in young adults. J Stud Alcohol. 1998;59(6):647–658. doi: 10.15288/jsa.1998.59.647. [DOI] [PubMed] [Google Scholar]
- Donovan DM, Anton RF, Miller WR, Longabaugh R, Hosking JD, Youngblood M. Combined pharmacotherapies and behavioral interventions for alcohol dependence (The COMBINE Study): Examination of posttreatment drinking outcomes. J Stud Alcohol Drugs. 2008;69(1):5–13. doi: 10.15288/jsad.2008.69.5. [DOI] [PubMed] [Google Scholar]
- Dreifuss JA, Griffin ML, Frost K, Fitzmaurice GM, Potter JS, Fiellin DA, et al. Patient characteristics associated with buprenorphine/naloxone treatment outcome for prescription opioid dependence: Results from a multisite study. Drug Alcohol Depend. 2013;131(1-2):112–118. doi: 10.1016/j.drugalcdep.2012.12.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Emrich HM, Vogt P, Herz A. Possible antidepressive effects of opioids: action of buprenorphine. Ann N Y Acad Sci. 1982;398:108–112. doi: 10.1111/j.1749-6632.1982.tb39483.x. [DOI] [PubMed] [Google Scholar]
- Festinger DS, Marlowe DB, Croft JR, Dugosh KL, Mastro NK, Lee PA, et al. Do research payments precipitate drug use or coerce participation? Drug Alcohol Depend. 2005;78(3):275–281. doi: 10.1016/j.drugalcdep.2004.11.011. [DOI] [PubMed] [Google Scholar]
- Fiellin DA, Pantalon MV, Schottenfeld RS, Gordon LT, O'Connor PG. Manual for standard medical management of opioid dependence with buprenorphine. 1999.
- Gandhi DH, Jaffe JH, McNary S, Kavanagh GJ, Hayes M, Currens M. Short-term outcomes after brief ambulatory opioid detoxification with buprenorphine in young heroin users. Addiction. 2003;98(4):453–462. doi: 10.1046/j.1360-0443.2003.00334.x. [DOI] [PubMed] [Google Scholar]
- Gowing L, Ali R, White JM. Buprenorphine for the management of opioid withdrawal. Cochrane Database Syst Rev. 2009;(3):CD002025. doi: 10.1002/14651858.CD002025.pub4. [DOI] [PubMed] [Google Scholar]
- Grella CE, Joshi V, Hser YI. Followup of cocaine-dependent men and women with antisocial personality disorder. J Subst Abuse Treat. 2003;25(3):155–164. doi: 10.1016/s0740-5472(03)00127-2. [DOI] [PubMed] [Google Scholar]
- Jones CM. Heroin use and heroin use risk behaviors among nonmedical users of prescription opioid pain relievers - United States, 2002-2004 and 2008-2010. Drug Alcohol Depend. 2013;132(1-2):95–100. doi: 10.1016/j.drugalcdep.2013.01.007. [DOI] [PubMed] [Google Scholar]
- Kramer JR, Chan G, Kuperman S, Bucholz KK, Edenberg HJ, Schuckit MA, et al. A comparison of diagnoses obtained from in-person and telephone interviews, using the Semi-Structured Assessment for the Genetics of Alcoholism (SSAGA). J Stud Alcohol Drugs. 2009;70(4):623–627. doi: 10.15288/jsad.2009.70.623. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lundgren LM, Amodeo M, Ferguson F, Davis K. Racial and ethnic differences in drug treatment entry of injection drug users in Massachusetts. J Subst Abuse Treat. 2001;21(3):145–153. doi: 10.1016/s0740-5472(01)00197-0. [DOI] [PubMed] [Google Scholar]
- McLellan AT, Kushner H, Metzger D, Peters R, Smith I, Grissom G, et al. The Fifth Edition of the Addiction Severity Index. J Subst Abuse Treat. 1992;9(3):199–213. doi: 10.1016/0740-5472(92)90062-s. [DOI] [PubMed] [Google Scholar]
- Midanik LT, Greenfield TK. Telephone versus in-person interviews for alcohol use: results of the 2000 National Alcohol Survey. Drug Alcohol Depend. 2003;72(3):209–214. doi: 10.1016/s0376-8716(03)00204-7. [DOI] [PubMed] [Google Scholar]
- Moore BA, Fiellin DA, Barry DT, Sullivan LE, Chawarski MC, O'Connor PG, et al. Primary care office-based buprenorphine treatment: comparison of heroin and prescription opioid dependent patients. J Gen Intern Med. 2007;22(4):527–530. doi: 10.1007/s11606-007-0129-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- National Archives and Records Administration Code of Federal Regulations. 21CFR11.1, Electronic Records, Electronic Signatures (Revised as of April 1, 2013)
- Nielsen S, Hillhouse M, Thomas C, Hasson A, Ling W. A comparison of buprenorphine taper outcomes between prescription opioid and heroin users. J Addict Med. 2013;7(1):33–38. doi: 10.1097/ADM.0b013e318277e92e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor PG. Methods of detoxification and their role in treating patients with opioid dependence. JAMA. 2005;294(8):961–963. doi: 10.1001/jama.294.8.961. [DOI] [PubMed] [Google Scholar]
- Pantalon MV, Fiellin DA, Schottenfeld RS, Gordon LT, O'Connor PG. Manual for enhanced medical management of opioid dependence with buprenorphine. 1999.
- Potter JS, Prather K, Kropp F, Byrne M, Sullivan CR, Mohamedi N, et al. A method to diagnose opioid dependence resulting from heroin versus prescription opioids using the Composite International Diagnostic Interview. Contemp Clin Trials. 2010;31(2):185–188. doi: 10.1016/j.cct.2010.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potter JS, Marino EN, Hillhouse MP, Nielsen S, Wiest K, Canamar CP, Martin JA, Ang A, Baker R, Saxon AJ, Ling W. Buprenorphine/naloxone and methadone maintenance treatment outcomes for opioid analgesic, heroin, and combined users: Findings from Starting Treatment with Agonist Replacement Therapies (START). J Stud Alcohol Drugs. 2013;74:605–613. doi: 10.15288/jsad.2013.74.605. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Satre DD, Chi FW, Mertens JR, Weisner CM. Effects of age and life transitions on alcohol and drug treatment outcome over nine years. J Stud Alcohol Drugs. 2012;73(3):459–468. doi: 10.15288/jsad.2012.73.459. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schutte KK, Nichols KA, Brennan PL, Moos RH. A ten-year follow-up of older former problem drinkers: risk of relapse and implications of successfully sustained remission. J Stud Alcohol. 2003;64(3):367–374. doi: 10.15288/jsa.2003.64.367. [DOI] [PubMed] [Google Scholar]
- SPSS Statistics for Windows, Version 20.0. IBM Corp.; Armonk, NY: 2011. [Google Scholar]
- Substance Abuse and Mental Health Services Administration . Results from the 2011 National Survey on Drug Use and Health: Summary of National Findings. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2012. [Google Scholar]
- Substance Abuse and Mental Health Services Administration, & Center for Behavioral Health Statistics and Quality . Treatment Episode Data Set (TEDS): 2001-2011. National Admissions to Substance Abuse Treatment Services. Substance Abuse and Mental Health Services Administration; Rockville, MD: 2013. [Google Scholar]
- Ware JE, Jr., Sherbourne CD. The MOS 36-item short-form health survey (SF-36). I. Conceptual framework and item selection. Med Care. 1992;30(6):473–483. [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Copersino ML, Prather K, Jacobs P, Provost S, et al. Conducting clinical research with prescription opioid dependence: defining the population. Am J Addict. 2010;19(2):141–146. doi: 10.1111/j.1521-0391.2009.00017.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Fiellin DA, Byrne M, Connery HS, Dickinson W, et al. Adjunctive counseling during brief and extended buprenorphine-naloxone treatment for prescription opioid dependence: a 2-phase randomized controlled trial. Arch Gen Psychiatry. 2011;68(12):1238–1246. doi: 10.1001/archgenpsychiatry.2011.121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weiss RD, Potter JS, Provost SE, Huang Z, Jacobs P, Hasson A, et al. A multi-site, two-phase, Prescription Opioid Addiction Treatment Study (POATS): rationale, design, and methodology. Contemp Clin Trials. 2010;31(2):189–199. doi: 10.1016/j.cct.2010.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- World Health Organization . Composite International Diagnostic Interview - Version 2.1. World Health Organization; Geneva, Switzerland: 1997. [Google Scholar]