Abstract
Chronic alcohol (ethanol) intake alters fundamental properties of the circadian clock. While previous studies have reported significant alterations in free-running circadian period during chronic ethanol access, these effects are typically subtle and appear to require high levels of intake. In the present study we examined the effects of long-term voluntary ethanol intake on ethanol consumption and free-running circadian period in male and female, selectively bred ethanol-preferring P and HAD2 rats. In light of previous reports that intermittent access can result in escalated ethanol intake, an initial 2-week water-only baseline was followed by either continuous or intermittent ethanol access (i.e., alternating 15-day epochs of ethanol access and ethanol deprivation) in separate groups of rats. Thus, animals were exposed to either 135 days of continuous ethanol access or to five 15-day access periods alternating with four 15-day periods of ethanol deprivation. Animals were maintained individually in running-wheel cages under continuous darkness throughout the experiment to allow monitoring of free-running activity and drinking rhythms, and 10% (v/v) ethanol and plain water were available continuously via separate drinking tubes during ethanol access. While there were no initial sex differences in ethanol drinking, ethanol preference increased progressively in male P and HAD2 rats under both continuous and intermittent-access conditions, and eventually exceeded that seen in females. Free-running period shortened during the initial ethanol-access epoch in all groups, but the persistence of this effect showed complex dependence on sex, breeding line, and ethanol-access schedule. Finally, while females of both breeding lines displayed higher levels of locomotor activity than males, there was little evidence for modulation of activity level by ethanol access. These results are consistent with previous findings that chronic ethanol intake alters free-running circadian period, and show further that the development of chronobiological tolerance to ethanol may vary by sex and genotype.
Keywords: alcohol, preference, circadian rhythms, wheel-running, sex differences
Introduction
Several lines of evidence have revealed reciprocal interactions between alcohol intake and the circadian timing system in both humans and experimental animals (Danel & Touitou, 2004; Hasler, Smith, Cousins, & Bootzin, 2012; Rosenwasser, 2001; Spanagel, Rosenwasser, Schumann, & Sarkar, 2005). Human alcoholics exhibit significant disturbances of sleep-wake cycles and other circadian rhythms (Conroy et al., 2012; Hasler et al., 2012; Kühlwein, Hauger, & Irwin, 2003), while in turn, chronobiological disruption promotes relapse in abstinent alcoholics and may contribute to excessive drinking in non-dependent populations (Brower, 2003; Drummond, Gillin, Smith, & DeModena, 1998; Landolt & Gillin, 2001). In animal experiments, ethanol alters fundamental aspects of circadian pacemaker function (Brager, Ruby, Prosser, & Glass, 2010; Mistlberger & Nadeau, 1992; Rosenwasser, Fecteau, & Logan, 2005; Rosenwasser, Logan, & Fectau, 2005; Seggio, Fixaris, Reed, Logan, & Rosenwasser, 2009; Seggio, Logan, & Rosenwasser, 2007). Systemically administered ethanol reaches the suprachiasmatic nucleus (SCN), site of the “master” circadian pacemaker (Brager, Ruby, Prosser, & Glass, 2011; Ruby, Brager, DePaul, Prosser, & Glass, 2009; Ruby, Prosser, DePaul, Roberts, & Glass, 2009), and alters circadian function in part via effects on SCN neural signaling (McElroy, Zakaria, Glass, & Prosser, 2009; Prosser & Glass, 2009; Prosser, Mangrum, & Glass, 2008) and gene expression (Chen, Kuhn, Advis, & Sarkar, 2004; Madeira et al., 1997). Conversely, both environmental (Clark, Fixaris, Belanger, & Rosenwasser, 2007; Gauvin et al., 1997; Rosenwasser, Clark, Fixaris, Belanger, & Foster, 2010) and genetic (Brager, Prosser, & Glass, 2011; Ozburn et al., 2013; Spanagel et al., 2005) disruption of circadian function alters voluntary ethanol intake.
While previous experiments indicate that chronic ethanol intake can alter free-running circadian period (Mistlberger & Nadeau, 1992; Rosenwasser, Fecteau, & Logan, 2005; Seggio et al., 2009), these effects have generally been subtle and apparently require very high levels of intake. In the present study, therefore, we examined the effects of long-term free-choice ethanol access on free-running circadian activity rhythms and voluntary ethanol intake in selectively bred ethanol-preferring P (Preferring) and HAD2 (High Alcohol Drinking, replicate-2) rat lines. P and HAD2 rats were bred using identical selection criteria, but were derived from genetically distinct progenitor stock (Murphy et al., 2002). Since we did not test the corresponding Non-Preferring (NP) and Low Alcohol Drinking (LAD2) lines, this study was not designed to detect phenotypic correlates of high ethanol preference, but rather to take advantage of the high ethanol intake seen reliably in P and HAD2 rats. It should be noted, however, that we previously demonstrated differences in circadian phenotype between ethanol-naïve high- and low-preferring rat lines (Rosenwasser, Fecteau, Logan, Reed, et al., 2005).
The “alcohol deprivation effect” (ADE) refers to a temporary increase in voluntary ethanol intake following a period of ethanol deprivation (Sinclair, 1972; Sinclair & Senter, 1967, 1968), an effect which may be potentiated by repeated deprivation intervals (Spanagel & Hölter, 1999). Indeed, schedules of intermittent ethanol exposure have been shown to increase ethanol intake and to exacerbate withdrawal-associated seizures and affective disturbances, relative to continuous ethanol exposure. Such effects are frequently attributed to “kindling-like” effects of repeated ethanol withdrawals and to the stressfulness of withdrawal itself (Ballenger & Post, 1978; Becker, 1998, 2012; Breese, Overstreet, & Knapp, 2005; Sanchis-Segura & Spanagel, 2006). The ADE has been studied in several lines of selectively bred high-drinking rats, with mixed results. Thus, while P rats displayed a significant ADE following a single 2-week deprivation period (Rodd-Henricks, McKinzie, Shaikh, et al., 2000; Vengeliene et al., 2006), similar effects were not seen in either HAD rats or in the high-drinking AA (Alko-Alcohol) line (McKinzie et al., 1998; Rodd-Henricks, McKinzie, Murphy, et al., 2000; Sinclair & Li, 1989; Vengeliene et al., 2006). Nevertheless, HAD rats do show an ADE following multiple deprivations, and both P and HAD2 rats exhibit a more prolonged ADE with increasing numbers of deprivations (Rodd-Henricks, McKinzie, Murphy, et al., 2000; Rodd-Henricks, McKinzie, Shaikh, et al., 2000). In the present experiment, therefore, we compared the effects of intermittent and continuous long-term ethanol access, using an ethanol access schedule closely matching that used in previous ADE studies in P and HAD rats (Rodd-Henricks, McKinzie, Murphy, et al., 2000; Rodd-Henricks, McKinzie, Shaikh, et al., 2000).
Female rodents are commonly reported to consume more ethanol and to display higher levels of ethanol preference relative to males (Adams, 1995; Juárez & Barrios de Tomasi, 1999; Lancaster & Spiegel, 1992). Nevertheless, this sex difference is not seen in all studies, and appears to depend on a number of factors including genotype, estrous phase, and ethanol access conditions. In particular, P and HAD rats show little evidence for sex differences in initial ethanol preference (Bell et al., 2003, 2004, 2006; Dhaher, McConnell, Rodd, McBride, & Bell, 2012; Li, Lumeng, & Thomasson, 1995), and in fact, males may display greater ethanol preference than females under extended ethanol access (Bell et al., 2004, 2006). Since few studies have examined sex differences in ethanol intake under long-term ethanol access, and no prior studies have investigated possible sex differences in the chronobiological effects of ethanol, we included both male and female rats in the present study.
Materials and methods
Animals and apparatus
Male and female P and HAD2 rats (n = 12 for each combination of line and sex) were obtained from the Indiana University Alcohol Research Center at about 8 weeks of age. The animals were housed individually in commercial running-wheel cages (wheel diameter, 34 cm) equipped with contact-sensing lickometer circuits (Mini-Mitter Co., Bend, OR). Animals had continuous access to 2 drinking spouts throughout the experiment, but only 1 lickometer circuit was available for each cage. Thus, under water-only conditions, animals had access to 2 waterspouts but only 1 was monitored, whereas, under free-choice ethanol access, the lickometer circuit was always used to monitor the ethanol spout. Due to the physical configuration of the apparatus, the lickometer could only monitor the drinking spout to the animal’s right; thus, it was not possible to switch the positions of the ethanol and water bottles during ethanol access periods, as is typically done in studies of preference drinking.
Cages were maintained within ventilated light- and sound-attenuating cabinets, either 6 or 12 cages per cabinet, and cabinet position was balanced across both sex and line such that each cabinet contained an equal number of males and females. Wheel turns and drinking-spout licks were monitored and stored in 1-min bins for subsequent analysis using the ClockLab interface system (Actimetrics Co., Wilmette, IL). The animals were maintained in constant darkness throughout the study to allow for assessment of free-running circadian activity and licking rhythms, and routine maintenance was performed at irregular times of day under dim red light, using the minimal amount of light necessary.
Procedures
Ethanol intake
After a 15-day water-only baseline, 10% (v/v) ethanol solution was offered in free choice with water for 15 days. Following this initial ethanol-access period, animals on the intermittent schedule were exposed to alternating 15-day periods of ethanol deprivation and ethanol access, for a total of 5 ethanol-access periods (i.e., 4 deprivation periods). In contrast, animals under continuous ethanol access were simply maintained under free-choice ethanol for an equivalent total number of days (135). This resulted in 8 separate groups (P vs. HAD, male vs. female, intermittent vs. continuous) with 6 rats per group. Since only 24 running-wheel cages were available for this experiment, we first tested male and female P and HAD rats under intermittent ethanol access, after which an additional 24 animals were obtained and tested under continuous ethanol access.
Ethanol and water intakes were recorded every 5 days and collapsed into 15-day bins for statistical analysis. Since the intermittent-access groups experienced a total of five 15-day time blocks in which ethanol was available, only the first 5 (of 9) 15-day ethanol blocks for the continuous-access groups were included in the statistical analyses; this allowed the continuous- and intermittent-access groups to be compared directly. Ethanol drinking is reported as g/kg/day and as ethanol preference, determined by dividing the volume of 10% ethanol intake by the total volume of fluid consumed (ethanol plus water). Water intake was analyzed as mL/day, since correction of water intake for body weight had no effect on the statistical outcomes.
Circadian rhythms
Circadian patterns of running-wheel activity and licking were assessed qualitatively by inspection of standard raster-style actograms. Free-running circadian period was estimated for each 15-day period using both the Lomb-Scargle periodogram routine included in ClockLab, and by computer-assisted detection of activity onset times; these 2 values were averaged to yield the period estimates reported here. Free-running period data were analyzed as change from baseline values, in order to remove small line and sex differences in baseline period that were not the focus of this investigation. ClockLab activity data were also used to determine the mean number of daily wheel turns for each group and experimental condition.
Statistical analysis
All dependent measures were initially analyzed using 4-factor repeated-measures ANOVA, with time blocks as a within-groups factor and line, sex, and access schedule as between-groups factors. This initial analysis was followed by 3-factor and 2-factor ANOVAs in order to better elucidate specific interactions, as described below. For clarity, we generally do not describe or interpret main effects when these are conditioned by significant interactions.
Ethical considerations
Experimental procedures were approved by the University of Maine Animal Care and Use Committee, and were conducted in accordance with the Guide for the Care and Use of Laboratory Animals, 8th edition (National Research Council, 2011).
Results
Activity and drinking patterns
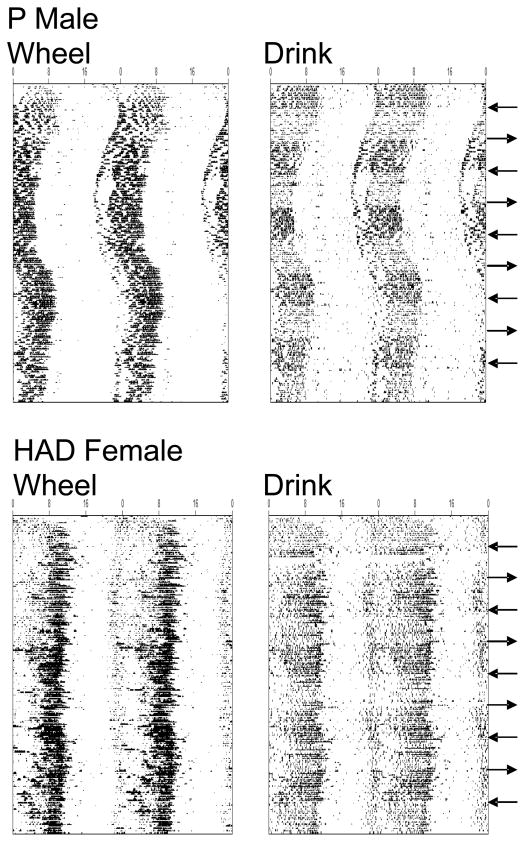
Fig. 1 shows activity (wheel-running) and drinking (licking) patterns throughout the entire 150-day experiment for 1 representative P male and 1 HAD female, both from the intermittent-access groups. As we reported previously (Rosenwasser, Fecteau, Logan, Reed, et al., 2005), there was a notable line difference in circadian patterning, in that HAD rats typically showed the most intense activity near the end of the circadian active phase. Nevertheless, both behaviors displayed robust circadian rhythmicity, with clearly defined “active” and “rest” phases throughout the experiment. Further, activity and drinking patterns were quite similar to one another and showed essentially identical long-term trends in free-running period within individual animals. Finally, the circadian patterning of water and ethanol intake was also very similar, although most animals showed higher levels of water-licking relative to ethanol-licking (note, however, that these lick records reflect drinkometer monitoring of only 1 of the 2 available bottles). Thus, as has been reported previously in high-drinking rat lines (Aalto, 1986; Agabio et al., 1996), ethanol intake appeared to follow the normal circadian pattern of water ingestion.
Figure 1.
Representative raster-style circadian actograms displaying wheel-running (“Wheel”, left) and licking (“Drink”, right) patterns for one male P rat (top) and one female HAD rat (bottom). Both animals were maintained under intermittent ethanol access, and arrows located along the right-hand y-axis indicate the beginning (leftward-pointing) or end (rightward-pointing) of 15-day ethanol access periods. Records are double-plotted on a 48-h time base (x-axis) and consecutive days are represented from top to bottom along the y-axis. A symbol is plotted for every 10-min time bin, with symbol height proportionate to the amount of running or licking occurring in that bin.
Ethanol intake
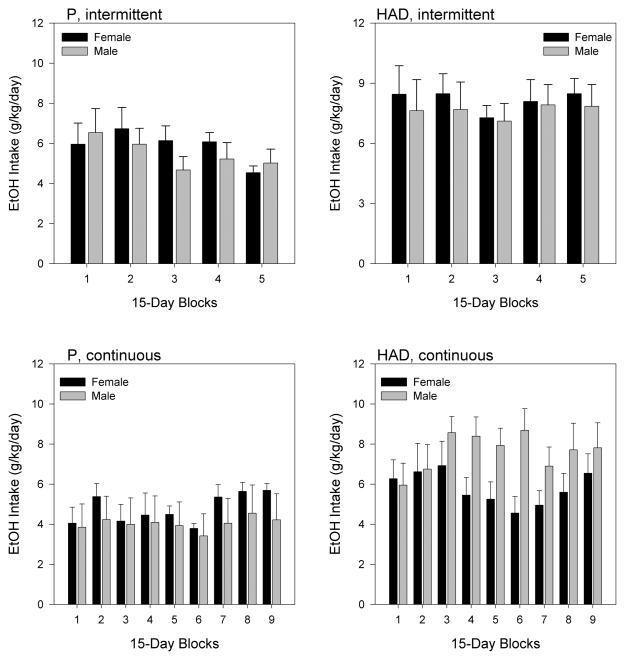
Analysis of ethanol intake data (Fig. 2) revealed a significant main effect of line [F(1,36) = 14.640, p < 0.001] (HAD rats consumed more ethanol per body weight than P rats) and a non-significant (p = 0.053) trend for higher intake under intermittent than continuous access, but no interactions among any of the factors. Despite the lack of significant interactions, we performed exploratory 2-factor time blocks × sex analyses for each of the 4 combinations of line and access conditions, which showed a significant blocks × sex interaction only in HAD rats under continuous ethanol access [F(4,40) = 3.294, p = 0.020]. Thus, male HAD rats showed an approximately 30% increase in daily ethanol intake over time, but only under continuous-access conditions.
Figure 2.
Mean (+SEM) ethanol intake (g/kg/day) in female (black bars) and male (gray bars) P (left panels) and HAD (right panels) rats, under either intermittent (top panels) or continuous (bottom panels) ethanol access.
Ethanol preference
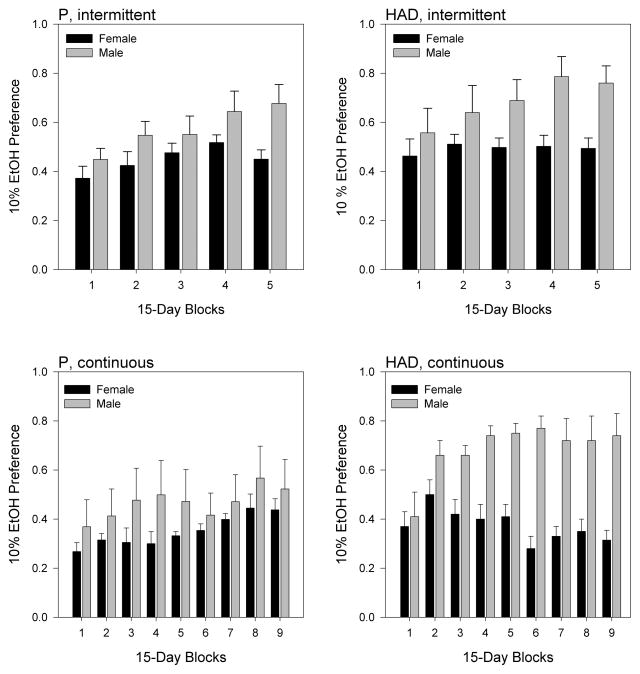
Similar to the effects seen for ethanol intake, analysis of ethanol preference (Fig. 3) showed significant main effects of line [F(1,36) = 14.640, p = 0.017] and access schedule [F(1,36) = 4.238, p = 0.047], indicating that ethanol preference was generally higher in HAD rats than in P rats and higher under intermittent relative to continuous ethanol access. In contrast to the effects for ethanol intake, however, analysis of ethanol preference data revealed a significant blocks × sex interaction [F(4,144) = 5.577, p < 0.001], indicating that male rats of both lines displayed progressively increasing levels of ethanol preference under both intermittent and continuous access conditions. We then conducted separate 2-factor blocks × sex analyses for each of the 4 combinations of line and access schedule, which revealed a significant blocks × sex interaction only for HAD rats under continuous access [F(4,40) = 4.682, p = 0.030]. Thus, while there was a general tendency for male rats of both lines to show increasing ethanol preference over time, this effect was most robust in HAD rats under continuous ethanol access, as it was for ethanol intake. Indeed, the increase in ethanol preference was clearly more robust (approximately 100%) than the corresponding increase in ethanol intake (approximately 30%).
Figure 3.
Mean (+SEM) ethanol preference (10% ethanol intake as a proportion of total fluid intake) in female (black bars) and male (gray bars) P (left panels) and HAD (right panels) rats, under either intermittent (top panels) or continuous (bottom panels) ethanol access.
Water intake
ANOVA revealed significant main effects of blocks [F(4,144) = 16.680, p < 0.001], access schedule [F(1,36) = 5.253, p = 0.028], and sex [F(1,36) = 49.306, p < 0.001] on water intake (data not shown). Water intake declined progressively over the course of the study, was higher under continuous than under intermittent ethanol access, and was higher in females than in males. In addition, we observed a significant blocks × line interaction [F(4,144) = 2.971, p = 0.022] and a 3-way blocks × line × access schedule interaction [F(4,144) = 4.359, p = 0.002]. These interactions show that water intake declined most dramatically in HAD rats under continuous ethanol access, thus accounting in part for the increased ethanol preference seen under this condition. Nevertheless, blocks × sex analyses for each of the 4 combinations of line and access schedule revealed significant effects of both block and sex for each treatment combination.
Free-running period
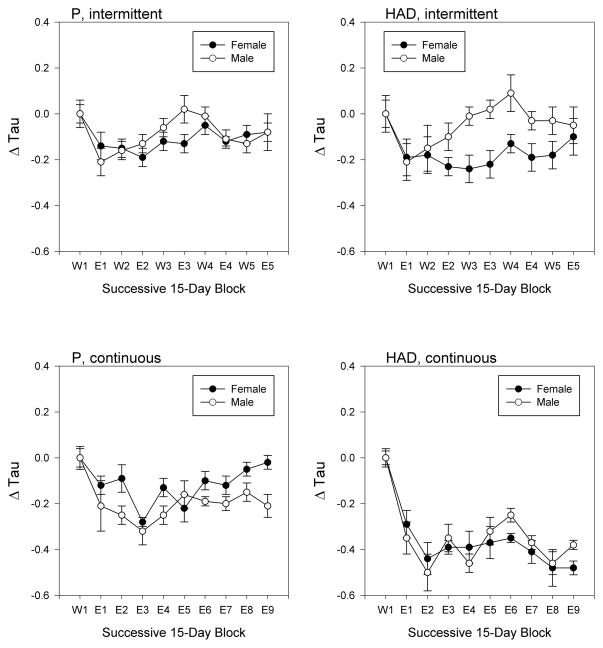
All groups showed similar shortening of free-running period during the initial 15-day ethanol exposure relative to baseline conditions (Fig. 4). Across subsequent time blocks, however, free-running period displayed complex variations as a function of line, sex, and ethanol access schedule. Thus, ANOVA detected significant time block × sex [F(9,315) = 3.411, p = 0.001], time block × line [F(9,315) = 2.592, p = 0.007], time block × access schedule [F(9,315) = 5.506, p < 0.001], and sex × access schedule interactions [F(1,35) = 4.543, p = 0.041], as well as a time block × line × access schedule interaction [F(9,315) = 4.026, p < 0.001]. We next conducted separate blocks × line × sex analyses for each access schedule, which revealed a significant blocks × sex interaction only under intermittent ethanol access [F(9,171) = 5.537, p < 0.001], indicating that males adapted to intermittent ethanol access more rapidly than females. In contrast, a significant blocks × line interaction [F(9,144) = 5.537, p < 0.001] was seen only under continuous access, indicating that P rats adapted to continuous ethanol access more rapidly than HAD rats. Finally, we conducted separate blocks × sex analyses for each of the 4 combinations of line and access schedule, which detected significant blocks × sex interactions for both HAD and P rats, but only under intermittent ethanol access [HAD rats: F(9,81) = 3.771, p = 0.018; P rats: F(9,90) = 2.188, p = 0.030].
Figure 4.
Mean (± SEM) free-running period in female (filled symbols) and male (open symbols) P (left panels) and HAD (right panels) rats, under either intermittent (top panels) or continuous (bottom panels) ethanol access. Successive 15-day epochs are labeled as either “W” (water-only; W1, W2, etc.) or as “E” (ethanol-access; E1, E2, etc.). Note that data are plotted as “delta tau” (change in period) normalized to the initial water-only epoch for all animals to remove small differences in initial period across sexes and lines. Nevertheless, statistical analyses reported in the text are based on the raw data.
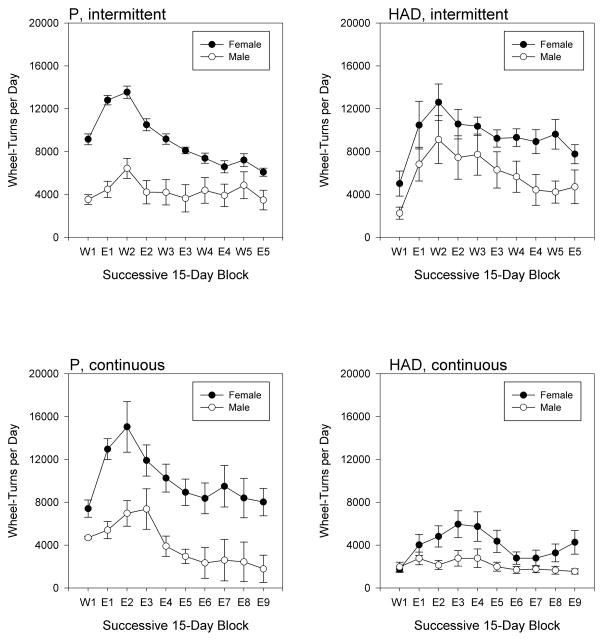
Running-wheel activity
As has been typically reported following initial exposure to running wheels, animals generally showed increasing daily wheel-turns over the first three to four 15-day time blocks, with gradual decreases thereafter (Fig. 5). Beyond that general trend, however, activity levels showed complex variations as a function of sex, line, and ethanol access schedule. Thus, ANOVA revealed 2-way interactions of time blocks and sex [F(9,306) = 2.952, p = 0.002], time blocks and line [F(9,306) = 7.189, p < 0.001] and time blocks and access schedule [F(9,306) = 2.096, p = 0.030], 3-way interactions of time blocks, sex, and access schedule [F(9,306) = 2.187, p = 0.023], and time blocks, line, and access schedule [F(9,306) = 2.952, p = 0.018], as well as a significant 4-way interaction [F(9,306) = 2.379, p =0.013]. We next performed separate 3-way analyses for each access schedule, which showed significant time block × sex [F(9,135) = 2.824, p = 0.004], time block × line [F(9,135) = 7.002, p < 0.001], and sex × line [F(1,15) = 6.523, p = 0.022] interactions under continuous ethanol access, and time block × sex [F(9,171) = 2.547, p = 0.009], time block × line [F(9,171) = 4.539, p < 0.001], and time block × sex × line [F(9,171) = 2.797, p = 0.004] interactions under intermittent access. Finally, we conducted separate blocks × sex analyses for each of the 4 combinations of line and access schedule, which detected significant blocks × sex interactions for P rats under both continuous [F(9,54) = 2.312, p = 0.028] and intermittent ethanol access [F(9,90) = 8.573, p < 0.001].
Figure 5.
Mean (± SEM) activity level (wheel-turns per day) in female (filled symbols) and male (open symbols) P (left panels) and HAD (right panels) rats, under either intermittent (top panels) or continuous (bottom panels) ethanol access. Successive 15-day epochs are labeled as either “W” (water-only; W1, W2, etc.) or as “E” (ethanol-access; E1, E2, etc.).
Discussion
Ethanol consumption, circadian period, and running-wheel activity all showed complex variations during long-term ethanol access as a function of sex, line, and ethanol-access schedule. Despite the relatively small number of animals studied, ANOVA detected several significant interactions among these variables. Nevertheless, the specific pattern of effects found differed across the dependent measures, as described below.
Initial levels of ethanol intake and ethanol preference for P and HAD2 rats seen in this study were similar to, if slightly lower than, those reported by Murphy et al. (2002). Of course, conditions in the present study – specifically the use of constant darkness and running wheels – were quite different from typical studies of preference drinking. While the present study was not designed to detect such effects, both wheel access and lighting conditions may influence voluntary ethanol intake. Running wheel access has been reported to reduce ethanol intake in hamsters (Brager & Hammer, 2012; Hammer, Ruby, Brager, Prosser, & Glass, 2010), mice (Ehringer, Hoft, & Zunhammer, 2009), and rats (McMillan, McClure, & Hardwick, 1995), although one study found that this effect was specifically associated with the initial introduction of the wheel (Ozburn, Harris, & Blednov, 2008). In addition, while a number of studies have noted increased ethanol intake under constant darkness relative to standard light-dark cycles in both rats and hamsters (Geller, 1971; Geller & Hartmann, 1977; Reiter, Blum, Wallace, & Merritt, 1974), one study using rats reported reduced ethanol intake in both constant darkness and constant light (Goodwin, Amir, & Amit, 1999). It is thus possible that overall levels of ethanol drinking in the present study may have been influenced by the use of these specific environmental conditions.
Few previous studies have examined changes in ethanol preference over the time scale employed in the present study. In P rats, both decreases (Waller, McBride, Lumeng, & Li, 1982) and increases (Kampov-Polevoy, Matthews, Gause, Morrow, & Overstreet, 2000) in ethanol intake have been reported under long-term continuous ethanol access (30 weeks and 6 weeks, respectively). Unfortunately, however, Waller et al. (1982) did not report ethanol preference data, while Kampov-Polevoy et al. (2000) failed to indicate the sex of their animals. In a series of experiments, Bell and colleagues studied male and female P and HAD rats during 4 weeks of continuous ethanol access. While Bell et al. (2003) saw no sex differences in adolescent P rats (Bell et al., 2003, 2006), adult P males and both adolescent and adult HAD males displayed progressive increases in ethanol preference that were not seen in females; indeed, within 4 weeks males displayed significantly higher ethanol preference than females (Bell et al., 2004, 2006; Dhaher et al., 2012). Similarly, P and HAD2 males in the present study showed progressive increases in ethanol preference under both continuous- and intermittent-access schedules that eventually exceeded that of females. Furthermore, both in the present study and in those of Bell et al. (2004, 2006), escalation of ethanol preference in males was far more dramatic than changes in ethanol intake per se, and was instead due mainly to progressive decreases in water intake over time. Thus, in contrast to frequent reports that female rodents show higher levels of ethanol preference than males, the present results confirm previous studies indicating that selectively bred ethanol-preferring rats show little or no initial sex difference, and that males display sex-specific escalation of ethanol preference to a level exceeding that of females within a few weeks. These results suggest an underlying interaction between genetic ethanol preference and sex that could account for the higher prevalence of alcohol abuse and dependence in human males relative to females (Hasin, Stinson, Ogburn, & Grant, 2007).
Despite previous experiments demonstrating that both P and HAD rats exhibit the ADE following multiple deprivation periods (Rodd-Henricks, McKinzie, Murphy, et al., 2000; Rodd-Henricks, McKinzie, Shaikh, et al., 2000), we found no evidence for greater escalation of intake under intermittent relative to continuous ethanol access. Indeed, while females showed no escalation under either condition, males actually showed more robust escalation under continuous than under intermittent ethanol access. It should be noted, however, that the significant ADE reported by Rodd-Henricks et al. (Rodd-Henricks, McKinzie, Murphy, et al., 2000; Rodd-Henricks, McKinzie, Shaikh, et al., 2000) persisted for only about 2–3 days even after repeated deprivations, so it is not surprising that we were unable to detect such an effect with the coarser intake measurements (i.e., every 5 days) employed in the present study. Thus, while the ADE may be a useful model of transient relapse-like drinking, it does not appear to produce sustained increases in ethanol intake.
Previous studies demonstrated alterations in circadian period during chronic ethanol drinking in hamsters (Mistlberger & Nadeau, 1992), mice (Seggio et al., 2009), and rats (Rosenwasser, Fecteau, & Logan, 2005). Nevertheless, these effects have been somewhat variable, both between and within experiments. Thus, similar to the effects seen in studies with antidepressants (Rosenwasser, 2001), both lengthening and shortening of circadian period have been reported during ethanol access (Rosenwasser, Fecteau, & Logan, 2005). Further, the most consistent effects have been seen under conditions of forced ethanol intake, presumably due to the higher levels of intake engendered under such conditions (Seggio et al., 2009). These observations could reflect the development of ethanol tolerance at the level of the suprachiasmatic circadian pacemaker (Lindsay, Glass, Amicarelli, & Prosser, 2014; Prosser & Glass, 2009). In the present study, all animals showed shortening of circadian period during the initial 15-day block of voluntary ethanol intake relative to water-only baseline conditions. Under continuous access, HAD rats of both sexes displayed sustained period shortening throughout the approximately 19 weeks of ethanol drinking, while P rats showed less robust and less consistent effects. Under intermittent access, in contrast, HAD females showed a modest but persistent effect on circadian period, while this effect appeared to have dissipated by the third ethanol-access block in HAD males. P rats showed a similar sex difference under intermittent access, but as under continuous access, appeared to be less affected than HAD rats overall. Taken together, these results confirm that ethanol intake can alter circadian period under some conditions, but these effects differ by sex and strain, and seem to require sustained high intake levels. Further, male rats may show more chronobiological tolerance to chronic ethanol intake relative to female rats.
The reported effects of ethanol intake on wheel-running activity levels are also somewhat inconsistent. While ethanol access has been reported to reduce running in hamsters (Hammer et al., 2010; Mistlberger & Nadeau, 1992) and C57BL/6 mice (Ozburn et al., 2008), other studies found no effect in C57BL/6 mice (Rosenwasser & Fixaris, 2013; Seggio et al., 2009), and one study reported increased running during ethanol access in DBA/2 mice (Rosenwasser & Fixaris, 2013). As expected from many prior reports, animals in the present study generally showed increasing activity over the first few weeks of running-wheel access followed by a more gradual decline, while females displayed generally higher levels of wheel running than males. In addition, P rats showed a more robust sex difference in activity levels than did HAD rats, while HAD rats of both sexes were generally less active under continuous than under intermittent ethanol access. On the other hand, the effect of ethanol access schedule on activity level in HAD rats was already apparent even during the first ethanol-access block, suggesting that this may have been due to sampling error rather than reflecting an effect of access schedule per se.
In conclusion, we found long-term changes in ethanol drinking, circadian period, and locomotor activity during long-term ethanol access. These effects exhibited complex interactions with genotype, sex, and ethanol access schedule that varied among the dependent measures. Consistent with previous studies in P and HAD rats – but in contrast to a number of other studies using unselected lines – males and females did not differ in their initial ethanol preference, while males showed a sex-specific escalation of ethanol preference over extended access. Also consistent with previous studies, circadian period was shortened during continuous ethanol access in both males and females. In contrast, male rats showed a sex-specific development of tolerance to this effect under intermittent access. In general, the effects on ethanol preference and circadian period were both more robust in male HAD rats, suggesting some overlap in the mechanisms regulating these 2 variables. Finally, and as expected from previous studies, females of both lines displayed higher levels of locomotor activity than males, while this sex difference was more prominent in P than in HAD rats. Variations in activity over time generally resembled those expected from previous studies of wheel-running, and there was no clear evidence that locomotor activity was affected by ethanol access schedule.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Aalto J. Circadian drinking rhythms and blood alcohol levels in two rat lines developed for their alcohol consumption. Alcohol. 1986;3:73–75. doi: 10.1016/0741-8329(86)90074-1. [DOI] [PubMed] [Google Scholar]
- Agabio R, Cortis G, Fadda F, Gessa GL, Lobina C, Reali R, et al. Circadian drinking pattern of Sardinian alcohol-preferring rats. Alcohol and Alcoholism. 1996;31:385–388. doi: 10.1093/oxfordjournals.alcalc.a008166. [DOI] [PubMed] [Google Scholar]
- Adams N. Sex differences and the effects of tail pinch on ethanol drinking in Maudsley rats. Alcohol. 1995;12:463–468. doi: 10.1016/0741-8329(95)00032-m. [DOI] [PubMed] [Google Scholar]
- Ballenger JC, Post RM. Kindling as a model for alcohol withdrawal syndromes. The British Journal of Psychiatry. 1978;133:1–14. doi: 10.1192/bjp.133.1.1. [DOI] [PubMed] [Google Scholar]
- Becker HC. Kindling in alcohol withdrawal. Alcohol Health and Research World. 1998;22:25–33. [PMC free article] [PubMed] [Google Scholar]
- Becker HC. Effects of alcohol dependence and withdrawal on stress responsiveness and alcohol consumption. Alcohol Research: Current Reviews. 2012;34:448–458. [PMC free article] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Hsu CC, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on ethanol intake by periadolescent high-alcohol-drinking rats. Alcohol. 2004;33:107–115. doi: 10.1016/j.alcohol.2004.06.001. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd ZA, Sable HJ, Schultz JA, Hsu CC, Lumeng L, et al. Daily patterns of ethanol drinking in peri-adolescent and adult alcohol-preferring (P) rats. Pharmacology, Biochemistry, and Behavior. 2006;83:35–46. doi: 10.1016/j.pbb.2005.12.004. [DOI] [PubMed] [Google Scholar]
- Bell RL, Rodd-Henricks ZA, Kuc KA, Lumeng L, Li TK, Murphy JM, et al. Effects of concurrent access to a single concentration or multiple concentrations of ethanol on the intake of ethanol by male and female periadolescent alcohol-preferring (P) rats. Alcohol. 2003;29:137–148. doi: 10.1016/s0741-8329(03)00022-3. [DOI] [PubMed] [Google Scholar]
- Brager AJ, Hammer SB. Impact of wheel running on chronic ethanol intake in aged Syrian hamsters. Physiology & Behavior. 2012;107:418–423. doi: 10.1016/j.physbeh.2012.09.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Prosser RA, Glass JD. Circadian and acamprosate modulation of elevated ethanol drinking in mPer2 clock gene mutant mice. Chronobiology International. 2011;28:664–672. doi: 10.3109/07420528.2011.601968. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Chronic ethanol disrupts circadian photic entrainment and daily locomotor activity in the mouse. Alcoholism: Clinical and Experimental Research. 2010;34:1266–1273. doi: 10.1111/j.1530-0277.2010.01204.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brager AJ, Ruby CL, Prosser RA, Glass JD. Acute ethanol disrupts photic and serotonergic circadian clock phase-resetting in the mouse. Alcoholism: Clinical and Experimental Research. 2011;35:1467–1474. doi: 10.1111/j.1530-0277.2011.01483.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breese GR, Overstreet DH, Knapp DJ. Conceptual framework for the etiology of alcoholism: a “kindling”/stress hypothesis. Psychopharmacology. 2005;178:367–380. doi: 10.1007/s00213-004-2016-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brower KJ. Insomnia, alcoholism and relapse. Sleep Medicine Reviews. 2003;7:523–539. doi: 10.1016/s1087-0792(03)90005-0. [DOI] [PubMed] [Google Scholar]
- Chen CP, Kuhn P, Advis JP, Sarkar DK. Chronic ethanol consumption impairs the circadian rhythm of pro-opiomelanocortin and period genes mRNA expression in the hypothalamus of the male rat. Journal of Neurochemistry. 2004;88:1547–1554. doi: 10.1046/j.1471-4159.2003.02300.x. [DOI] [PubMed] [Google Scholar]
- Clark JW, Fixaris MC, Belanger GV, Rosenwasser AM. Repeated light-dark phase shifts modulate voluntary ethanol intake in male and female high alcohol-drinking (HAD1) rats. Alcoholism: Clinical and Experimental Research. 2007;31:1699–1706. doi: 10.1111/j.1530-0277.2007.00476.x. [DOI] [PubMed] [Google Scholar]
- Conroy DA, Hairston IS, Arnedt JT, Hoffmann RF, Armitage R, Brower KJ. Dim light melatonin onset in alcohol-dependent men and women compared with healthy controls. Chronobiology International. 2012;29:35–42. doi: 10.3109/07420528.2011.636852. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Danel T, Touitou Y. Chronobiology of alcohol: from chronokinetics to alcohol-related alterations of the circadian system. Chronobiology International. 2004;21:923–935. doi: 10.1081/cbi-200036886. [DOI] [PubMed] [Google Scholar]
- Dhaher R, McConnell KK, Rodd ZA, McBride WJ, Bell RL. Daily patterns of ethanol drinking in adolescent and adult, male and female, high alcohol drinking (HAD) replicate lines of rats. Pharmacology, Biochemistry, and Behavior. 2012;102:540–548. doi: 10.1016/j.pbb.2012.04.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drummond SP, Gillin JC, Smith TL, DeModena A. The sleep of abstinent pure primary alcoholic patients: natural course and relationship to relapse. Alcoholism: Clinical and Experimental Research. 1998;22:1796–1802. [PubMed] [Google Scholar]
- Ehringer MA, Hoft NR, Zunhammer M. Reduced alcohol consumption in mice with access to a running wheel. Alcohol. 2009;43:443–452. doi: 10.1016/j.alcohol.2009.06.003. [DOI] [PubMed] [Google Scholar]
- Gauvin DV, Baird TJ, Vanecek SA, Briscoe RJ, Vallett M, Holloway FA. Effects of time-of-day and photoperiod phase shifts on voluntary ethanol consumption in rats. Alcoholism: Clinical and Experimental Research. 1997;21:817–825. [PubMed] [Google Scholar]
- Geller I. Ethanol preference in the rat as a function of photoperiod. Science. 1971;173:456–459. doi: 10.1126/science.173.3995.456. [DOI] [PubMed] [Google Scholar]
- Geller I, Hartmann RJ. Alteration of ethanol preference in hamsters: effects of photoperiod and 5-hydroxytryptophan. Advances in Experimental Medicine and Biology. 1977;85B:223–233. doi: 10.1007/978-1-4615-9038-5_15. [DOI] [PubMed] [Google Scholar]
- Goodwin FL, Amir S, Amit Z. Environmental lighting has a selective influence on ethanol intake in rats. Physiology & Behavior. 1999;66:323–328. doi: 10.1016/s0031-9384(98)00302-3. [DOI] [PubMed] [Google Scholar]
- Hammer SB, Ruby CL, Brager AJ, Prosser RA, Glass JD. Environmental modulation of alcohol intake in hamsters: effects of wheel running and constant light exposure. Alcoholism: Clinical and Experimental Research. 2010;34:1651–1658. doi: 10.1111/j.1530-0277.2010.01251.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hasin DS, Stinson FS, Ogburn E, Grant BF. Prevalence, correlates, disability, and comorbidity of DSM-IV alcohol abuse and dependence in the United States: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Archives of General Psychiatry. 2007;64:830–842. doi: 10.1001/archpsyc.64.7.830. [DOI] [PubMed] [Google Scholar]
- Hasler BP, Smith LJ, Cousins JC, Bootzin RR. Circadian rhythms, sleep, and substance abuse. Sleep Medicine Reviews. 2012;16:67–81. doi: 10.1016/j.smrv.2011.03.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Juárez J, Barrios de Tomasi E. Sex differences in alcohol drinking patterns during forced and voluntary consumption in rats. Alcohol. 1999;19:15–22. doi: 10.1016/s0741-8329(99)00010-5. [DOI] [PubMed] [Google Scholar]
- Kampov-Polevoy AB, Matthews DB, Gause L, Morrow AL, Overstreet DH. P rats develop physical dependence on alcohol via voluntary drinking: changes in seizure thresholds, anxiety, and patterns of alcohol drinking. Alcoholism: Clinical and Experimental Research. 2000;24:278–284. [PubMed] [Google Scholar]
- Kühlwein E, Hauger RL, Irwin MR. Abnormal nocturnal melatonin secretion and disordered sleep in abstinent alcoholics. Biological Psychiatry. 2003;54:1437–1443. doi: 10.1016/s0006-3223(03)00005-2. [DOI] [PubMed] [Google Scholar]
- Lancaster FE, Spiegel KS. Sex differences in pattern of drinking. Alcohol. 1992;9:415–420. doi: 10.1016/0741-8329(92)90041-8. [DOI] [PubMed] [Google Scholar]
- Landolt HP, Gillin JC. Sleep abnormalities during abstinence in alcohol-dependent patients. Aetiology and management. CNS Drugs. 2001;15:413–425. doi: 10.2165/00023210-200115050-00006. [DOI] [PubMed] [Google Scholar]
- Li T-K, Lumeng L, Thomasson H. Genetic and gender differences in alcohol consumption and metabolism. In: Zakhari S, Hunt WA, Hunt VA, editors. Stress, Gender, and Alcohol-Seeking Behavior. Bethesda, MD: NIH; 1995. pp. 87–99. [Google Scholar]
- Lindsay JH, Glass JD, Amicarelli M, Prosser RA. The mammalian circadian clock in the suprachiasmatic nucleus exhibits rapid tolerance to ethanol in vivo and in vitro. Alcoholism: Clinical and Experimental Research. 2014;38:760–769. doi: 10.1111/acer.12303. [DOI] [PubMed] [Google Scholar]
- Madeira MD, Andrade JP, Lieberman AR, Sousa N, Almeida OF, Paula-Barbosa MM. Chronic alcohol consumption and withdrawal do not induce cell death in the suprachiasmatic nucleus, but lead to irreversible depression of peptide immunoreactivity and mRNA levels. The Journal of Neuroscience. 1997;17:1302–1319. doi: 10.1523/JNEUROSCI.17-04-01302.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McElroy B, Zakaria A, Glass JD, Prosser RA. Ethanol modulates mammalian circadian clock phase resetting through extrasynaptic GABA receptor activation. Neuroscience. 2009;164:842–848. doi: 10.1016/j.neuroscience.2009.08.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKinzie DL, Nowak KL, Yorger L, McBride WJ, Murphy JM, Lumeng L, et al. The alcohol deprivation effect in the alcohol-preferring P rat under free-drinking and operant access conditions. Alcoholism: Clinical and Experimental Research. 1998;22:1170–1176. [PubMed] [Google Scholar]
- McMillan DE, McClure GY, Hardwick WC. Effects of access to a running wheel on food, water and ethanol intake in rats bred to accept ethanol. Drug and Alcohol Dependence. 1995;40:1–7. doi: 10.1016/0376-8716(95)01162-5. [DOI] [PubMed] [Google Scholar]
- Mistlberger RE, Nadeau J. Ethanol and circadian rhythms in the Syrian hamster: effects on entrained phase, reentrainment rate, and period. Pharmacology, Biochemistry, and Behavior. 1992;43:159–165. doi: 10.1016/0091-3057(92)90652-v. [DOI] [PubMed] [Google Scholar]
- Murphy JM, Stewart RB, Bell RL, Badia-Elder NE, Carr LG, McBride WJ, et al. Phenotypic and genotypic characterization of the Indiana University rat lines selectively bred for high and low alcohol preference. Behavior Genetics. 2002;32:363–388. doi: 10.1023/a:1020266306135. [DOI] [PubMed] [Google Scholar]
- Ozburn AR, Falcon E, Mukherjee S, Gillman A, Arey R, Spencer S, et al. The role of clock in ethanol-related behaviors. Neuropsychopharmacology. 2013;38:2393–2400. doi: 10.1038/npp.2013.138. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ozburn AR, Harris RA, Blednov YA. Wheel running, voluntary ethanol consumption, and hedonic substitution. Alcohol. 2008;42:417–424. doi: 10.1016/j.alcohol.2008.04.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Glass JD. The mammalian circadian clock exhibits acute tolerance to ethanol. Alcoholism: Clinical and Experimental Research. 2009;33:2088–2093. doi: 10.1111/j.1530-0277.2009.01048.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prosser RA, Mangrum CA, Glass JD. Acute ethanol modulates glutamatergic and serotonergic phase shifts of the mouse circadian clock in vitro. Neuroscience. 2008;152:837–848. doi: 10.1016/j.neuroscience.2007.12.049. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reiter RJ, Blum K, Wallace JE, Merritt JH. Pineal gland: evidence for an influence on ethanol preference in male Syrian hamsters. Comparative Biochemistry and Physiology A, Comparative Physiology. 1974;47:11–16. doi: 10.1016/0300-9629(74)90045-0. [DOI] [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Murphy JM, McBride WJ, Lumeng L, Li TK. The expression of an alcohol deprivation effect in the high-alcohol-drinking replicate rat lines is dependent on repeated deprivations. Alcoholism: Clinical and Experimental Research. 2000;24:747–753. [PubMed] [Google Scholar]
- Rodd-Henricks ZA, McKinzie DL, Shaikh SR, Murphy JM, McBride WJ, Lumeng L, et al. Alcohol deprivation effect is prolonged in the alcohol preferring (P) rat after repeated deprivations. Alcoholism: Clinical and Experimental Research. 2000;24:8–16. [PubMed] [Google Scholar]
- Rosenwasser AM. Alcohol, antidepressants, and circadian rhythms. Human and animal models. Alcohol Research & Health. 2001;25:126–135. [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Clark JW, Fixaris MC, Belanger GV, Foster JA. Effects of repeated light-dark phase shifts on voluntary ethanol and water intake in male and female Fischer and Lewis rats. Alcohol. 2010;44:229–237. doi: 10.1016/j.alcohol.2010.03.002. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW. Effects of ethanol intake and ethanol withdrawal on free-running circadian activity rhythms in rats. Physiology & Behavior. 2005;84:537–542. doi: 10.1016/j.physbeh.2005.01.016. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fecteau ME, Logan RW, Reed JD, Cotter SJ, Seggio JA. Circadian activity rhythms in selectively bred ethanol-preferring and nonpreferring rats. Alcohol. 2005;36:69–81. doi: 10.1016/j.alcohol.2005.07.001. [DOI] [PubMed] [Google Scholar]
- Rosenwasser AM, Fixaris MC. Chronobiology of alcohol: studies in C57BL/6J and DBA/2J inbred mice. Physiology & Behavior. 2013;110–111:140–147. doi: 10.1016/j.physbeh.2013.01.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rosenwasser AM, Logan RW, Fecteau ME. Chronic ethanol intake alters circadian period-responses to brief light pulses in rats. Chronobiology International. 2005;22:227–236. doi: 10.1081/cbi-200053496. [DOI] [PubMed] [Google Scholar]
- Ruby CL, Brager AJ, DePaul MA, Prosser RA, Glass JD. Chronic ethanol attenuates circadian photic phase resetting and alters nocturnal activity patterns in the hamster. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;297:R729–737. doi: 10.1152/ajpregu.00268.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ruby CL, Prosser RA, DePaul MA, Roberts RJ, Glass JD. Acute ethanol impairs photic and nonphotic circadian phase resetting in the Syrian hamster. American Journal of Physiology Regulatory, Integrative and Comparative Physiology. 2009;296:R411–418. doi: 10.1152/ajpregu.90782.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sanchis-Segura C, Spanagel R. Behavioural assessment of drug reinforcement and addictive features in rodents: an overview. Addiction Biology. 2006;11:2–38. doi: 10.1111/j.1369-1600.2006.00012.x. [DOI] [PubMed] [Google Scholar]
- Seggio JA, Fixaris MC, Reed JD, Logan RW, Rosenwasser AM. Chronic ethanol intake alters circadian phase shifting and free-running period in mice. Journal of Biological Rhythms. 2009;24:304–312. doi: 10.1177/0748730409338449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Seggio JA, Logan RW, Rosenwasser AM. Chronic ethanol intake modulates photic and non-photic circadian phase responses in the Syrian hamster. Pharmacology, Biochemistry, and Behavior. 2007;87:297–305. doi: 10.1016/j.pbb.2007.05.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sinclair JD. The alcohol-deprivation effect. Influence of various factors. Quarterly Journal of Studies on Alcohol. 1972;33:769–782. [PubMed] [Google Scholar]
- Sinclair JD, Li TK. Long and short alcohol deprivation: effects on AA and P alcohol-preferring rats. Alcohol. 1989;6:505–509. doi: 10.1016/0741-8329(89)90059-1. [DOI] [PubMed] [Google Scholar]
- Sinclair JD, Senter RJ. Increased preference for ethanol in rats following deprivation. Psychonomic Science. 1967;8:11–12. [Google Scholar]
- Sinclair JD, Senter RJ. Development of an alcohol-deprivation effect in rats. Quarterly Journal of Studies on Alcohol. 1968;29:863–867. [PubMed] [Google Scholar]
- Spanagel R, Hölter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol and Alcoholism. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- Spanagel R, Rosenwasser AM, Schumann G, Sarkar DK. Alcohol consumption and the body’s biological clock. Alcoholism: Clinical and Experimental Research. 2005;29:1550–1557. doi: 10.1097/01.alc.0000175074.70807.fd. [DOI] [PubMed] [Google Scholar]
- Vengeliene V, Leonardi-Essmann F, Perreau-Lenz S, Gebicke-Haerter P, Drescher K, Gross G, et al. The dopamine D3 receptor plays an essential role in alcohol-seeking and relapse. FASEB Journal. 2006;20:2223–2233. doi: 10.1096/fj.06-6110com. [DOI] [PubMed] [Google Scholar]
- Waller MB, McBride WJ, Lumeng L, Li TK. Induction of dependence on ethanol by free-choice drinking in alcohol-preferring rats. Pharmacology, Biochemistry, and Behavior. 1982;16:501–507. doi: 10.1016/0091-3057(82)90459-2. [DOI] [PubMed] [Google Scholar]